Abstract
Background
Circulating NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels, a well‐known indicator of atrial wall stress and remodeling, inversely correlate with body mass index. Both are strongly predictive of atrial fibrillation (AF). Their potential interaction in relation to incident AF, however, has not been explored.
Methods and Results
In total, 9556 participants of the ARIC (Atherosclerosis Risk in Communities) study who had 2 measurements of NT‐proBNP and no baseline AF or heart failure were followed from 1996 to 1998 through 2016 for the occurrence of incident AF. Participants were categorized as obese (body mass index ≥30) and nonobese (body mass index <30) and by NT‐proBNP levels (using the median of 68.2 pg/mL as the cutoff). Over a median follow‐up of 18.3 years, we identified 1806 incident cases of AF. Analysis using multivariable Cox regression models showed that obese participants with high NT‐proBNP levels at visit 4 had a higher adjusted risk of incident AF (hazard ratio: 3.64; 95% CI, 3.15–4.22) compared with nonobese individuals with low NT‐proBNP levels. The association of obesity with AF risk was not modified by NT‐proBNP levels (P=0.46 for interaction). Increasing BNP among participants from 1990–1992 to 1996–1998 was associated with increased AF risk. After further adjustment for clinical risk factors and medications, results were similar.
Conclusions
Individuals who had both elevated body mass index and NT‐proBNP and were free of clinically recognized heart failure were at higher risk of AF development. Those who experienced an increase in NT‐proBNP levels between visits 2 and 4 were at higher risk of AF.
Keywords: atrial fibrillation, brain natriuretic peptide, obesity
Subject Categories: Atrial Fibrillation, Arrhythmias
Clinical Perspective
What Is New?
In the ARIC (Atherosclerosis Risk in Communities) study, both obesity and elevated NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) were associated with future atrial fibrillation (AF) risk in individuals without heart failure, and the association of NT‐proBNP with AF risk was not modified by obesity status.
Increased NT‐proBNP over time was associated with increased risk of AF, independent of changes in body weight.
What Are the Clinical Implications?
Overweight individuals with increasing NT‐proBNP concentrations may be a group at particularly high risk of AF and might benefit from interventions targeted toward screening for and prevention of AF.
Obesity and atrial fibrillation (AF) are major public health problems in the United States, and both are associated with significant morbidity and increased risk of adverse cardiovascular events.1, 2 The prevalence of AF in the United States is expected to increase 2.5‐fold during the next 50 years.3 At the same time, an increasing trend in the prevalence of obesity has been observed in US adults.4 Obese individuals are at higher risk of developing AF.5
Natriuretic peptides (NP), such as BNP (B‐type NP) and atrial NP are produced by cardiac myocytes in response to stretching of atrial or ventricular walls,6, 7 with higher levels of BNP strongly associated with AF risk.8 BNP levels are inversely correlated with body mass index (BMI) and total body fat.9 Different hypotheses, such as the bidirectional relationship of both factors10, 11 and enhanced renal clearance of BNP in obese individuals,12, 13 have been postulated to explain the inverse relationship between obesity and circulating BNP levels. Obesity is associated with AF risk regardless of metabolic profile.14, 15 Obesity may lead to left atrial remodeling, which may provide the substrate for AF development.16 However, weight gain might not be associated with AF risk.17 These observations suggest a complex interplay among adiposity, NP physiology, and the development of AF.
NT‐proBNP (N‐terminal pro‐BNP) elevation is associated with heart failure (HF) and cardiovascular disease.18, 19, 20 Studies have found that NT‐proBNP elevation is associated with the development and progression of AF, indicating a potential underlying left atrial dysfunction.21 In this study, we investigated the independent association of obesity and elevated NT‐proBNP as a marker of atrial dysfunction and assessed their potential interaction. In addition, we took advantage of repeated measures of NT‐proBNP and BMI in the ARIC (Atherosclerosis Risk in Communities) study cohort to assess the joint effects and potential interactions of changes in these 2 variables on AF incidence. We hypothesized that elevated NT‐proBNP and obesity are independently associated with AF risk and that those with both high NT‐proBNP and obesity are at the highest risk of AF development. In addition, we hypothesized that obesity development is associated with AF risk among those who experience an increase in NT‐proBNP.
Methods
The data, analysis, and study materials are not available to other researchers for purposes of reproducing the results or replicating the analysis because of human subjects restrictions. Interested investigators may contact the ARIC Study Center at the University of North Carolina to request access to ARIC study data.22
Study Population
The ARIC study is a prospective epidemiologic cohort conducted in 4 US communities: Washington County, Maryland; Jackson, Mississippi; selected Minneapolis suburbs, Minnesota; and Forsyth County, North Carolina. A detailed description of the design and objectives of the ARIC cohort study has been published.23 Approximately 4000 individuals aged 45 to 64 years from a defined population in their community were recruited from each ARIC center. In 1987–1989, a baseline examination (visit 1) was completed for 15 792 individuals (55% women, 27% black). Participants were then followed up regularly every 3 years until 1998, with the second exam (visit 2) occurring in 1990–1992, the third (visit 3) in 1993–1995, and the fourth (visit 4) in 1996–1998. Participants who survived underwent a fifth exam (visit 5) in 2011–2013 and a sixth exam (visit 6) in 2016–2017. For this analysis, we included individuals who participated in both visits 2 and 4 and had available circulating NT‐proBNP levels and BMI recorded at both visits. Of these, we excluded participants who had developed AF or HF by visit 4 or had missing information on AF incidence (n=685; Figure 1). Study participants provided written informed consent at each visit, and the study was approved by institutional review boards at the participating institutions.
Figure 1.
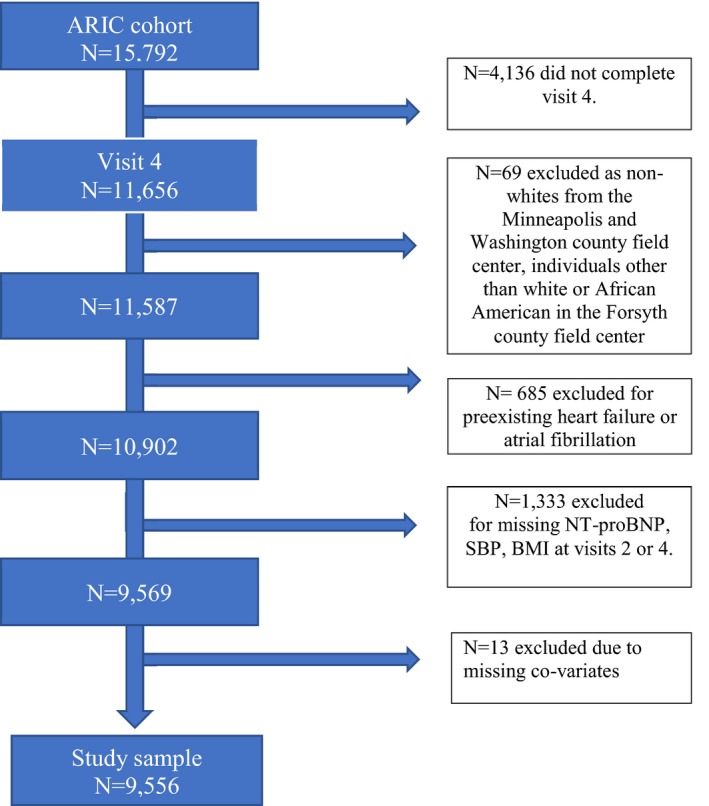
Study sample inclusion flowchart for this analysis from the ARIC (Atherosclerosis Risk in Communities) study. BMI indicates body mass index; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SBP, systolic blood pressure.
Assessment of NT‐proBNP
Blood samples were collected at each clinic visit and stored at −70°C until laboratory analysis. Assays of NT‐proBNP were conducted using stored serum from visit 2 in 2011–2013 at the University of Minnesota and using stored plasma from visit 4 in 2010 at Baylor College of Medicine. NT‐proBNP was measured in visit 2 samples with a Roche sandwich immunoassay on a Roche Elecsys 2010 analyzer; for visit 4 samples, a Roche ECLIA for proBNP II assay on a Cobas e411 analyzer was used. Based on a recalibration study in ARIC, NT‐proBNP showed excellent correlation between the visit 2 and visit 4 measurements, but serum values of NT‐proBNP at visit 4 were on average 9 pg/mL higher than visit 4 plasma values.24 Therefore, we added 9 pg/mL to the visit 4 plasma determinations to align visit 2 serum and visit 4 plasma measurements. Because we considered subtle plasma NT‐proBNP levels that might be associated with subclinical left atrial dysfunction, we dichotomized values using the median at visit 4 for the cohort as the cutoff. We also performed a sensitivity analysis considering a standard NT‐proBNP cutoff of 125 pg/mL as the upper limit of normal for NT‐proBNP in nonacute settings.25
Assessment of BMI
BMI was calculated by dividing measured weight (in kilograms) by measured height (in meters squared; kg/m2) at each visit. For the analyses of BMI, the main exposure of interest was obesity, defined as a BMI ≥30; for the analyses of BMI change, we calculated change in BMI between visits 2 and 4 and categorized it as <0 (decreased BMI) versus ≥0 (increased BMI).
Determination of Incident AF
We used 3 methods to identify AF cases in the ARIC cohort that have been described previously.26 In summary, trained abstractors collected information from participants’ hospitalizations identified by follow‐up phone calls and surveillance of local hospitals, including all discharge codes relevant for AF or atrial flutter. AF was deemed as present if International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes 427.31 (AF) or 427.32 (atrial flutter) or ICD‐10‐CM code I48.x were listed for any given hospitalization. We excluded AF cases occurring in the context of cardiac surgery. Next, 12‐lead ECGs were obtained during study exams, and data were transmitted electronically to the ARIC ECG reading center at EPICARE (Wake Forest School of Medicine, Winston‐Salem, NC) using the GE Marquette 12‐SL program for processing. A computer algorithm identified the presence of AF or atrial flutter in the ECG. Those ECGs were further reviewed by a cardiologist to confirm the computer diagnosis of AF and to overread any rhythm disorder other than AF in ECGs to reduce the possibility of missed or misreading episodes of AF. Last, AF diagnosis was identified from death certificates if ICD‐9 code 427.3 or ICD‐10 code I48 was listed as any reason of death. In a validation study, we found this approach to have a positive predictive value and sensitivity close to 90% and specificity >95%.26
Ascertainment of Other Covariates
We considered relevant covariates that were measured in both visit 2 and visit 4: sex, age, race, study center, height, systolic blood pressure (BP), diastolic BP, smoking status, alcohol drinking status, diabetes mellitus history, HF history, myocardial infarction (MI) history, use of aspirin medications, use of statin medications, ECG left ventricular hypertrophy (LVH) according to Cornell criteria and ECG P‐wave terminal force in lead V1. At visit 2, systolic and diastolic BPs were measured 3 times, and the mean of the last 2 measurements was used for our analysis; only 2 measurements were taken at visit 4, and we used the mean of these 2 for our analysis. Diabetes mellitus was defined as the use of antidiabetic medication or a self‐reported physician diagnosis of diabetes mellitus, fasting blood glucose ≥126 mg/dL, or nonfasting blood glucose >200 mg/dL. Information regarding sex, race, age, smoking status, and alcohol intake were self‐reported. Medications taken in the prior 2 weeks were brought to the clinic visit; names of the medications were recorded. HF history was derived based on the Gothenburg criteria at visit 1 and from HF‐related hospitalizations (ICD‐9 codes for HF) during follow‐up.27 MI was defined based on self‐reported physician diagnosis of MI at baseline or evidence of old MI on ECG and events adjudicated during the follow‐up.28
Statistical Analysis
The main independent variable for our analysis was the combination of BMI (<30 or ≥30) and NT‐proBNP categories (<68.2 or ≥68.2 pg/mL) at visit 4. AF risk was assessed for the 4 different categories (low NT‐proBNP/low BMI (reference), low NT‐proBNP/high BMI, high NT‐proBNP/low BMI, and high NT‐proBNP/high BMI). For our secondary analysis, we assessed AF risk among 4 different categories of participants based on the change in BMI and NT‐proBNP (decrease in BMI/increase in NT‐proBNP, increase in BMI/decrease in NT‐proBNP, and increase in BMI/increase in NT‐proBNP). We calculated cumulative incidence of AF accounting for the competing risk of death using the cumulative incidence function.29, 30 Cox proportional hazards models were used to calculate hazard ratios (HRs) for developing AF and their 95% CIs according to combined categories of BMI and NT‐proBNP and combined categories of NT‐proBNP change/BMI change among those without AF and HF at start of follow‐up (defined as the date of visit 4 for this analysis). The time of follow‐up was defined as days from visit 4 to AF incidence, death, loss to follow‐up, or December 31, 2016, whichever occurred earlier. We used the following 2 models with incremental adjustments to analyze the association of NT‐proBNP and BMI with AF risk: model 1 included adjustment for age (continuous), sex (dichotomous), education level (grade school, high school but not graduate, high school graduate, vocational school, college, graduate school), race, and ARIC study center; model 2 included further adjustment for standing height (continuous), smoking (current, former, never), drinking (current, former, never), diabetes mellitus (dichotomous), history of MI (dichotomous), aspirin use, statin use, hypnotic drugs use at visit 4, ECG LVH and P‐wave terminal force in lead V1 at visit 4 (continuous), and diastolic and systolic BP (continuous) at visit 4. We explored interaction between BMI and NT‐proBNP including interaction terms between both variables, considered as categorical and continuous measurements. In our secondary analysis, we evaluated the association of change in NT‐proBNP and change in BMI (decreases versus increases in BMI) with the incidence of AF, and we further adjusted for the following variables measured at visit 2: BMI categories, log (NT‐proBNP levels), smoking, drinking, diabetes mellitus, history of MI, aspirin use, statin use, hypnotic drugs, ECG LVH and P‐wave terminal force in lead V1, and systolic and diastolic BP. For all statistical analyses, a 2‐tailed P<0.05 was considered significant. Data analysis was conducted with SAS software v9.4 (SAS Institute ).
Results
The mean age for the study cohort of 9556 participants without HF or AF at visit 4 was 63 years, with women accounting for 57% and black participants accounting for 21% of the analyzed cohort (Table 1). Median NT‐proBNP was 68.2 pg/mL and 35% of the cohort had BMI ≥30. Mean age was relatively similar across NT‐proBNP‐BMI categories. There were more women and fewer black participants in the high NT‐proBNP groups. Similarly, systolic BP was higher in participants with high BMI or high NT‐proBNP, whereas those with high NT‐proBNP and BMI ≥30 were more likely to receive antihypertensive treatment. In addition, those with prevalent MI had higher NT‐proBNP levels, and those with diabetes mellitus were more likely to have BMI ≥30.
Table 1.
Baseline Characteristics of the ARIC Cohort Study at Visit 4 (n=9556)
| NT‐proBNP <68.2 pg/mL | NT‐proBNP ≥68.2 pg/mL | |||
|---|---|---|---|---|
| BMI <30 | BMI ≥30 | BMI <30 | BMI ≥30 | |
| Participants, % | 32.1 | 19.3 | 33.2 | 15.4 |
| Clinical variables | ||||
| Age, ya | 61.6 (5) | 61 (5.1) | 64.2 (5.7) | 63.5 (5.5) |
| Female, n (%) | 1364 (44.4) | 1018 (55.3) | 2088 (65.9) | 1022 (69.3) |
| Black, n (%) | 669 (21.8) | 627 (34.1) | 378 (11.9) | 327 (22.2) |
| BMI, kg/m2 a | 26.1 (2.6) | 34.6 (4.5) | 25.2 (2.9) | 34.8 (4.4) |
| SBP, mm Hga | 122 (17) | 127.4 (16) | 128.3 (20) | 135 (19.9) |
| ECG PTFV1, μv∙msa | 2281 (1939) | 2701 (2122) | 2440 (2277) | 2898 (2439) |
| Smoking status, n (%) | ||||
| Current smoker | 521 (17) | 182 (9.9) | 546 (17.4) | 140 (9.5) |
| Former smoker | 1320 (42.9) | 816 (44.3) | 1333 (42.1) | 647 (43.9) |
| Never smoker | 1233 (40.1) | 843 (45.8) | 1288 (40.7) | 687 (46.6) |
| Drinking status, n (%) | ||||
| Current drinker | 1689 (54.9) | 813 (44.2) | 1708 (53.9) | 614 (41.7) |
| Former drinker | 843 (27.4) | 588 (31.9) | 842 (26.6) | 481 (32.6) |
| Never drinker | 542 (17.6) | 440 (23.9) | 617 (19) | 379 (25.7) |
| Aspirin use, n (%) | 1652 (53.7) | 965 (52.4) | 1795 (56.7) | 934 (63.4) |
| Statin use, n (%) | 3090 (10.1) | 197 (10.7) | 345 (10.9) | 213 (14.5) |
| Hypnotic use, n (%) | 890 (29) | 945 (51.3) | 1251 (39.5) | 904 (61.3) |
| DM, n (%) | 370 (12) | 477 (25.9) | 287 (9.1) | 3530 (24.9) |
| MI history, n (%) | 87 (2.8) | 67 (3.6) | 225 (7.1) | 137 (9.3) |
| NT‐proBNP, pg/mLb | 43.7 (28.5–58.6) | 30.8 (17–47.2.4) | 128.4 (92.9–200.5) | 126.8 (91.2–199.6) |
Values correspond to mean (SD) unless otherwise stated. ARIC indicates Atherosclerosis Risk in Communities; BMI, body mass index; DM, diabetes mellitus; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PTFV1, P‐wave terminal force in lead V1; SBP, systolic blood pressure.
Continuous variable given as mean (SD).
Continuous variable given as median (interquartile range).
Association of NT‐proBNP and BMI Interaction at Visit 4 With Incident AF
Over a median follow up of 18.3 years, we identified 1806 incident cases of AF. Figure 2 displays the cumulative incidence of AF considering death as a competing risk by categories of NT‐proBNP and BMI. In our fully adjusted model, obese participants with high NT‐proBNP at visit 4 had the highest hazards of incident AF (HR: 3.04; 95% CI, 2.62–3.54; Table 2) compared with nonobese individuals with NT‐proBNP levels below the median. The corresponding HRs for nonobese participants with high NT‐proBNP and obese participants with low NT‐proBNP were 2.11 (95% CI, 1.84–2.42) and 1.56 (95% CI, 1.33–1.83; compared with the same reference group), respectively. In a sensitivity analysis considering the NT‐proBNP standard cutoff, the association was found to be similar. In addition, associations followed a similar pattern in analysis stratified by sex or race (Table S1). In a separate analysis using BMI and NT‐proBNP as continuous variables, the association of NT‐proBNP with AF was not modified by BMI (P=0.69 for interaction).
Figure 2.
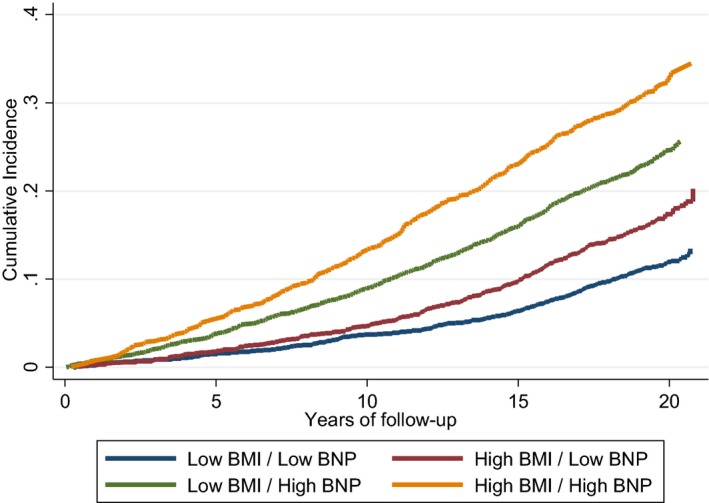
Cumulative incidence of AF, unadjusted, by categories of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide; below or above median) and body mass index (BMI; <30 or ≥30) at ARIC (Atherosclerosis Risk in Communities) visit 4, considering death as a competing risk, ARIC 1996–2016.
Table 2.
Associations of BMI and NT‐proBNP With Incident AF in the ARIC Cohort (n=9556)
| BMI<30 and Low NT‐proBNP | BMI≥30 and Low NT‐proBNP | BMI<30 and High NT‐proBNP | BMI≥30 and High NT‐proBNP | Interaction P Value | |
|---|---|---|---|---|---|
| No. of AF events | 343 | 292 | 724 | 447 | |
| Participants, n (%) | 3074 (32.2) | 1841 (19.3) | 3167 (33.1) | 1474 (15.4) | |
| Person‐years | 51 493 | 29 970 | 46 731 | 20 937 | |
| NT‐proBNP less than or greater than or equal to median <68.2 pg/mL, HR (95% CI)* | |||||
| Model 1b | Ref | 1.69 (1.45–1.98) | 2.37 (2.07–2.71) | 3.64 (3.15–4.22) | 0.34 |
| Model 2c | Ref | 1.56 (1.33–1.83) | 2.11 (1.84–2.42) | 3.04 (2.62–3.54) | 0.46 |
| NT‐proBNP <125 pg/mL or ≥125 pg/mL, HR (95% CI)a | |||||
| Model 1b | Ref | 1.63 (1.44–1.85) | 2.35 (2.07–2.66) | 3.51 (3.03–4.06) | 0.37 |
| Model 2c | Ref | 1.51 (1.33–1.71) | 2.02 (1.78–3.00) | 2.87 (2.47–3.35) | 0.21 |
AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; ref, reference.
HRs are for associations of BMI categories and pro‐BNP levels, with AF.
Model 1, adjusted for age, sex, race/center, and education.
Model 2, adjusted for age, sex, center, race, standing height, smoking, education, drinking, diabetes mellitus, myocardial infarction, aspirin, statin, hypnotic drugs, left ventricular hypertrophy by Cornell definition, P‐wave terminal force in lead V1, systolic blood pressure.
Association of NT‐proBNP and BMI Change With Incident AF
When assessing associations of AF with 6‐year joint changes in BMI and NT‐proBNP and using a Cox model adjusted for age, sex, race/center, education, height, smoking status at both visits, drinking status, diabetes mellitus, history of MI, aspirin, statin, hypnotic drugs, LVH, ECG P‐wave terminal force in lead V1, and systolic and diastolic BP at visits 2 and 4, AF risk was higher among participants who experienced both an increase in BMI and NT‐proBNP (HR: 1.67; 95% CI, 1.35–2.08) or had a decrease in BMI and an increase in NT‐proBNP (HR: 1.56; 95% CI, 1.25–1.96) compared with those who experienced decreases in both variables (Table 3 and Figure 3). However, AF risk was not increased in participants who experienced an increase in BMI with a decrease in NT‐proBNP (HR: 0.99; 95% CI, 0.78–1.25). The association of BMI change with AF risk was not modified by the change in NT‐proBNP level (P=0.56 for interaction). Associations were similar in analysis stratified by sex or race (Table S2).
Table 3.
Associations of Changes in NT‐proBNP and BMI Between Visit 2 (1990–1992) and Visit 4 (1996–1998) With Incident AF in the ARIC Cohort. (n=9556)
| BMI, HR (95%CI)b | P Interaction | |||
|---|---|---|---|---|
| Model 1a | ↓ | ↑ | ||
| NT‐proBNP | ↓ | Ref | 0.90 (0.71–1.14) | 0.32 |
| ↑ | 1.61 (1.29–2.02) | 1.66 (1.34–2.06) | ||
| Model 2c | ↓ | ↑ | ||
| NT‐proBNP | ↓ | Ref | 0.99 (0.78–1.25) | 0.56 |
| ↑ | 1.56 (1.25–1.96) | 1.67 (1.35–2.08) | ||
AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; ref, reference.
Model 1, adjusted for age, sex, BMI at visit 2, log (NT‐proBNP) at visit 2, race/center, and education.
HRs are for associations of BMI categories and pro‐BNP levels, with AF.
Model 2, adjusted for age, sex, BMI at visit 2, log (NT‐ProBNP) at visit 2, race/center, height, smoking, education, drinking, diabetes mellitus, myocardial infarction, aspirin, statin, hypnotic drugs, ECG P‐wave terminal force in lead V1, systolic blood pressure, and diastolic blood pressure.
Figure 3.
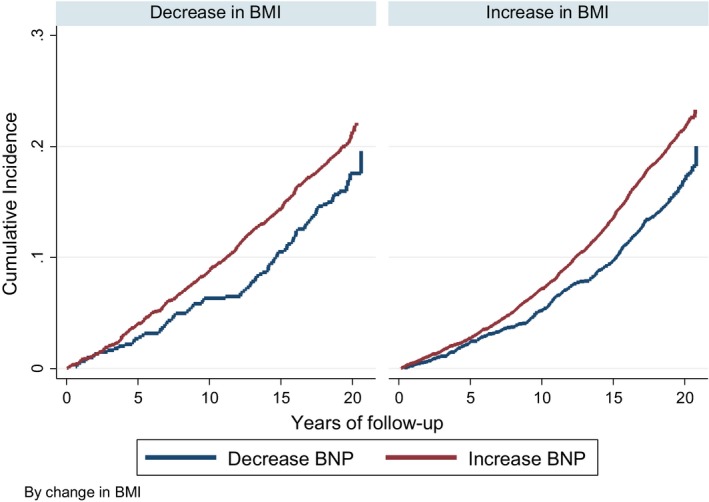
Cumulative incidence of AF by 6‐year change in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) according to 6‐year changes in body mass index (BMI), considering death as a competing risk, ARIC (Atherosclerosis Risk in Communities), 1996–2016.
Discussion
Our study demonstrated that high NT‐proBNP and obesity are associated with a higher incidence of AF among ARIC HF‐free cohort participants followed for 17.4 years. These associations were independent of other risk factors for AF and present after adjustment for hypertension and LVH. In addition, we did not find a synergistic or antagonistic interaction in the multiplicative scale between NT‐proBNP levels and BMI in relation to AF risk. However, we observed that increasing BMI was associated with AF incidence only among participants who experienced an elevation in NT‐proBNP concurrently.
To date, little is known regarding any potential interaction between circulating NT‐proBNP and BMI in relation to AF risk. Previous studies have consistently reported associations of circulating NT‐proBNP with AF incidence. In MESA (Multi‐Ethnic Study of Atherosclerosis), researchers found that a higher circulating NT‐proBNP was associated with higher incidence of AF, with differences in the predictive value of this biomarker across age and racial/ethnic groups.31 Similarly, in an analysis combining the Framingham Heart Study, the Cardiovascular Health Study, and the ARIC study, NT‐proBNP was identified as a predictor of incident AF independent of other known risk factors for AF.32, 33 At the same time, 18% of incident AF in the ARIC cohort can be attributed to elevated BMI.34 In addition, evidence shows that metabolic syndrome features, particularly visceral obesity and insulin resistance, are associated with subclinical left atrial dysfunction, providing a pathophysiological pathway to explain the association between elevated BMI and increased AF risk.35
BMI and plasma NT‐proBNP levels are known to be inversely correlated.9 NPs are thought to play an important role in cardiovascular remodeling, volume homeostasis, and response to ischemia.36 Moreover, NPs are known for their ability to enhance lipolysis and modulate energy expenditures.37, 38 The pathophysiologic mechanisms linking circulating NT‐proBNP and AF incidence are not completely elucidated. NT‐proBNP levels are usually elevated in patients with asymptomatic or symptomatic left ventricular and atrial dysfunction and in patients with overt HF.39 In addition, patients with right HF or pulmonary hypertension also have higher levels of NT‐proBNP.40, 41 Thus, NT‐proBNP can serve as a marker of adverse left atrial remodeling. Our findings suggest that this is also true in obese individuals; this result may help us identify those at the highest risk of AF among this high‐risk group.
Obesity is associated with electroanatomic remodeling of the atria and future AF risk.42 Our study found that participants with obesity at visit 4 were at higher risk of AF; however, participants who gained weight and developed obesity during follow‐up did not experience a higher risk of AF. These findings build on previous studies that long‐term obesity, particularly that acquired earlier in life, is associated with AF risk more than new weight gain and obesity development.17 These findings may help us direct AF prevention efforts toward early stages of the disease, as substantial evidence suggests that weight control may reduce the increasing incidence of AF.43
Strengths and Limitations
Our study has several strengths. First, the ARIC study includes a large and diverse population with longitudinal measures of body weight and cardiac blood biomarkers. Second, our study had extensive information on other biomarkers and clinical variables that allow adequate adjustments for potential confounders. Nevertheless, our study also has important limitations. First, we were not able to differentiate between diverse patterns of AF, such as paroxysmal AF, persistent AF, or permanent AF, and between AF and atrial flutter. Second, our approach for AF ascertainment probably led to missing asymptomatic cases and those managed exclusively in the outpatient setting. Third, our analysis was limited to participants who had a follow‐up visit 4 and repeated measures of NT‐proBNP and BMI. Consequently, our results may not be generalizable to those without repeated measures of NT‐proBNP and BMI. Finally, as an observational study, the potential for residual and unmeasured confounding remains.
Conclusions
Obesity and elevated NT‐proBNP, as a marker of left atrial dysfunction, are independently associated with AF risk. Short‐term BMI changes, however, were not associated with AF risk, highlighting the importance of weight control earlier in life to avoid long‐term atrial remodeling. Given the rising prevalence of obesity, these findings are important in AF prevention planning.
Sources of Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH; award nos. UL1TR002378, TL1TR002382). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The ARIC (Atherosclerosis Risk in Communities) study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, NIH, Department of Health and Human Services (contract nos. HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021). This work was supported by American Heart Association grant 16EIA26410001 (Dr. Alonso). Dr. Selvin was supported by NIH and National Institute of Diabetes and Digestive and Kidney Diseases grants K24DK106414 and R01DK089174.
Disclosures
None.
Supporting information
Table S1. Associations of Body Mass Index and NT‐proBNP (N‐Terminal Pro‐B‐Type Natriuretic Peptide) With Incident Atrial Fibrillation in the ARIC (Atherosclerosis Risk in Communities) Cohort by Sex and Race
Table S2. Associations of Changes in NT‐proBNP (N‐Terminal Pro‐B‐Type Natriuretic Peptide) and Body Mass Index Between Visit 2 (1990–1992) and Visit 4 (1996–1998) With Incident Atrial Fibrillation by Sex and Race in ARIC (Atherosclerosis Risk in Communities)
Acknowledgments
The authors thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study for their important contributions. Reagents for the NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) assays were donated by Roche Diagnostics Corporation.
(J Am Heart Assoc. 2019;8:e013294 DOI: 10.1161/JAHA.119.013294.)
References
- 1. Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. [DOI] [PubMed] [Google Scholar]
- 2. Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi‐Sunyer X, Hong Y, Eckel RH; American Heart Association Council on Nutrition, Physical Activity, and Metabolism . Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on nutrition, physical activity, and metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 4. STATISTICS NCFH . National health and nutrition examination survey. NCHS Fact Sheet 2017.
- 5. Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol. 2014;7:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Bold AJ, Bruneau BG, Kuroski de Bold ML. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res. 1996;31:7–18. [PubMed] [Google Scholar]
- 7. Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li L, Selvin E, Lutsey PL, Hoogeveen RC, O'Neal WT, Soliman EZ, Chen LY, Alonso A. Association of n‐terminal pro b‐type natriuretic peptide (NT‐PROBNP) change with the risk of atrial fibrillation in the ARIC cohort. Am Heart J. 2018;204:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH Jr, de Lemos JA. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas heart study. Circulation. 2005;112:2163–2168. [DOI] [PubMed] [Google Scholar]
- 10. Kalra PR, Tigas S. Regulation of lipolysis: natriuretic peptides and the development of cachexia. Int J Cardiol. 2002;85:125–132. [DOI] [PubMed] [Google Scholar]
- 11. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich‐Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation. 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 12. Tsutamoto T, Wada A, Sakai H, Ishikawa C, Tanaka T, Hayashi M, Fujii M, Yamamoto T, Dohke T, Ohnishi M, Takashima H, Kinoshita M, Horie M. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2006;47:582–586. [DOI] [PubMed] [Google Scholar]
- 13. Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294:F685–F696. [DOI] [PubMed] [Google Scholar]
- 14. Feng T, Vegard M, Strand LB, Laugsand LE, Morkedal B, Aune D, Vatten L, Ellekjaer H, Loennechen JP, Mukamal K, Janszky I. Metabolically healthy obesity and risk for atrial fibrillation: the Hunt study. Obesity (Silver Spring). 2019;27:332–338. [DOI] [PubMed] [Google Scholar]
- 15. Aune D, Sen A, Schlesinger S, Norat T, Janszky I, Romundstad P, Tonstad S, Riboli E, Vatten LJ. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose‐response meta‐analysis of prospective studies. Eur J Epidemiol. 2017;32:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 17. Feng T, Vegard M, Strand LB, Laugsand LE, Morkedal B, Aune D, Vatten L, Ellekjaer H, Loennechen JP, Mukamal K, Janszky I. Weight and weight change and risk of atrial fibrillation: the Hunt study. Eur Heart J. 2019;2589–2566. [DOI] [PubMed] [Google Scholar]
- 18. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 19. Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, Deswal A, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E. N‐terminal pro‐brain natriuretic peptide and heart failure risk among individuals with and without obesity: the atherosclerosis risk in communities (ARIC) study. Circulation. 2016;133:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anwaruddin S, Lloyd‐Jones DM, Baggish A, Chen A, Krauser D, Tung R, Chae C, Januzzi JL Jr. Renal function, congestive heart failure, and amino‐terminal pro‐brain natriuretic peptide measurement: results from the PROBNP investigation of dyspnea in the emergency department (pride) study. J Am Coll Cardiol. 2006;47:91–97. [DOI] [PubMed] [Google Scholar]
- 21. Ghazal F, Theobald H, Rosenqvist M, Al‐Khalili F. Assessment of N‐terminal pro‐B‐type natriuretic peptide level in screening for atrial fibrillation in primary health care. PLoS One. 2019;14:e0212974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collaborative studies coordinating center . Atherosclerosis risk in communities study. University of north carolina at chapel hill. Available at: https://www2.Cscc.Unc.Edu/aric/desc. Accessed February 16, 2019.
- 23. The ARIC Investigators . The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 24. Parrinello CM, Grams ME, Couper D, Ballantyne CM, Hoogeveen RC, Eckfeldt JH, Selvin E, Coresh J. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: the impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 26. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the atherosclerosis risk in communities (ARIC) study. American Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study). The American journal of cardiology. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 28. Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang C‐H, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. [DOI] [PubMed] [Google Scholar]
- 29. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 31. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N‐terminal pro‐b‐type natriuretic peptide as a predictor of incident atrial fibrillation in the multi‐ethnic study of atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 32. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation. Circulation. 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Vasan RS, Wang TJ, Agarwal SK, McManus DD, Franco OH, Yin X, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Astor BC, Ballantyne CM, Hoogeveen RC, Arai AE, Soliman EZ, Ellinor PT, Stricker BH, Gudnason V, Heckbert SR, Pencina MJ, Benjamin EJ, Alonso A. B‐type natriuretic peptide and C‐reactive protein in the prediction of atrial fibrillation risk: the charge‐AF consortium of community‐based cohort studies. Europace. 2014;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the atherosclerosis risk in communities (ARIC) study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nyman K, Granér M, Pentikäinen MO, Lundbom J, Hakkarainen A, Sirén R, Nieminen MS, Taskinen MR, Lundbom N, Lauerma K. Metabolic syndrome associates with left atrial dysfunction. Nutr Metab Cardiovasc Dis. 2018;28:727–734. [DOI] [PubMed] [Google Scholar]
- 36. Goetze JP. Biosynthesis of cardiac natriuretic peptides. Results Probl Cell Differ. 2010;50:97–120. [DOI] [PubMed] [Google Scholar]
- 37. Ricci MA, De Vuono S, Pucci G, Di Filippo F, Berisha S, Gentili A, Daviddi G, Ministrini S, Rondelli F, Boni M, Lupattelli G. Determinants of low levels of brain natriuretic peptide in morbid obesity. Clin Nutr. 2017;36:1075–1081. [DOI] [PubMed] [Google Scholar]
- 38. Khalid U, Wruck LM, Quibrera PM, Bozkurt B, Nambi V, Virani SS, Jneid H, Agarwal S, Chang PP, Loehr L, Basra SS, Rosamond W, Ballantyne CM, Deswal A. BNP and obesity in acute decompensated heart failure with preserved vs. Reduced ejection fraction: the atherosclerosis risk in communities surveillance study. Int J Cardiol. 2017;233:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muresan L, Petcu A, Muresan C, Rinzis M, Gusetu G, Pop D, Zdrenghea D, Rednic S. The role of NT‐PROBNP in the diagnosis of ventricular arrhythmias in patients with systemic sclerosis. Iranian Journal of Public Health. 2017;46:906. [PMC free article] [PubMed] [Google Scholar]
- 40. Pruszczyk P. N‐terminal pro‐brain natriuretic peptide as an indicator of right ventricular dysfunction. J Cardiac Fail. 2005;11:S65–S69. [DOI] [PubMed] [Google Scholar]
- 41. Yap LB. B‐type natriuretic peptide and the right heart. Heart Fail Rev. 2004;9:99–105. [DOI] [PubMed] [Google Scholar]
- 42. Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, Carbone A, Teo K, Agbaedeng T, Linz D, de Groot JR, Kalman JM, Lau DH, Sanders P. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol. 2018;4:1529–1540. [DOI] [PubMed] [Google Scholar]
- 43. Isakadze N, B P, B S, Patel R, Baer J, Isiadinso I, Alonso A, Lloyd M, Sperling L. Life's simple 7 approach to atrial fibrillation prevention. J Atr Fibrillation. 2018;11:2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Associations of Body Mass Index and NT‐proBNP (N‐Terminal Pro‐B‐Type Natriuretic Peptide) With Incident Atrial Fibrillation in the ARIC (Atherosclerosis Risk in Communities) Cohort by Sex and Race
Table S2. Associations of Changes in NT‐proBNP (N‐Terminal Pro‐B‐Type Natriuretic Peptide) and Body Mass Index Between Visit 2 (1990–1992) and Visit 4 (1996–1998) With Incident Atrial Fibrillation by Sex and Race in ARIC (Atherosclerosis Risk in Communities)


