Abstract
Background
The pathophysiological process of calcific aortic valve disease (CAVD) is similar to that of atherosclerosis. Leptin accelerates the process of atherosclerosis. We sought to examine the relationship between leptin and CAVD.
Methods and Results
Serum leptin was measured in 397 consecutive patients undergoing standard transthoracic echocardiography and Doppler flow imaging. Multiple logistic regression analyses were used to assess the association between leptin and CAVD. Western blotting was performed to detect the expression of phosphorylated and total extracellular signal‐regulated kinase. Serum leptin (median) was higher in 200 patients with CAVD than that in 197 non‐CAVD controls (20.07 versus 9.03 ng/mL; P<0.01). Leptin correlated positively with age (r=0.37, P<0.01) and negatively with estimated glomerular filtration rate (r=−0.37, P<0.01). Multivariate analysis indicated that elevated leptin was an independent determinant for the presence of CAVD (P<0.01). Receiver‐operating characteristic curve analysis of leptin to detect the presence of CAVD showed that the area under the curve was 0.74 (95% CI, 0.69–0.79; P<0.01). The diagnostic value of leptin for the detection of CAVD was higher among younger patients (aged ≤65 years) or those with at least mildly reduced renal function (estimated glomerular filtration rate ≤82.06 mL/min per 1.73 m2). The activation of extracellular signal‐regulated kinase 1/2 was stronger in calcific aortic valves than in normal aortic valves.
Conclusions
Elevated leptin is associated with the presence of CAVD, especially among younger patients or those with renal dysfunction.
Keywords: age, calcific aortic valve disease, leptin, renal dysfunction
Subject Categories: Valvular Heart Disease
Clinical Perspective
What Is New?
Serum leptin level was increased in patients with calcified aortic valve disease (CAVD).
Leptin levels correlated with age and renal function; and elevated leptin was revealed as an independent risk factor for the presence of CAVD, especially in patients with younger age and lower estimated glomerular filtration rate level.
The expression of extracellular signal‐regulated kinase 1/2 was stronger in calcific than in normal aortic valves.
What Are the Clinical Implications?
Cardiovascular inflammation, mediated by leptin, might have an important role in the development of CAVD.
Increased serum leptin conferred a greater risk and independently indicated the presence of CAVD, especially for patients with younger age and reduced renal function.
These data suggest that leptin may be a novel biomarker for CAVD. Its role in CAVD prevention and caring requires further evaluation in a large prospective trial.
Introduction
Calcified aortic valve disease (CAVD) is present in ≈20% to 30% of individuals aged >65 years and 48% of individuals aged >85 years.1, 2 The late stage of CAVD, in the form of severe aortic stenosis, leads to increased mortality.3 The pathophysiological features of CAVD include the degeneration of processes related to aging4 and atherosclerosis.5 Systemic inflammation has been observed in patients with CAVD.6 The accumulation of lipids together with the infiltration of inflammatory cells have been found in early aortic valve lesions,7 and active calcification and ossification are also regarded as crucial processes in the development of CAVD.8, 9 Several randomized, placebo‐controlled trials have failed to show that the use of statins slows the progression of aortic valve stenosis,10 suggesting that despite similar risk factors and downstream mediators, there might be other pivotal pathogenic factors apart from the atherogenesis of CAVD. Thus, there is an increasing demand to further investigate the novel pathophysiological pathways and their modulation to prevent/delay the onset or progression of valve degeneration.
Leptin, a product of adipocytes regulated by obesity‐related genes, has been shown to have a proinflammatory effect, leading to cardiovascular disease, and even be associated with adverse cardiovascular events.11, 12 Interestingly, aortic valve calcification was enhanced after long‐term treatment with leptin in an animal study using an apolipoprotein E–deficient mouse model.13 In human calcified aortic valves, leptin is highly expressed and promotes the osteoblastic differentiation of vascular smooth muscle cells13, 14 and the calcification of valvular interstitial cells.15 These observations suggest that leptin may also be involved in the process of CAVD.16 In the current study, we investigated the relationship between serum leptin levels and CAVD.
Methods
The authors declare that all supporting data are available within the article.
Study Population
Of a total of 806 consecutive patients referred for coronary angiography/intervention in the Department of Cardiology, Shanghai Ruijin Hospital, from March to September 2017 because of typical anginal symptoms and/or electrocardiographic ST‐T wave changes, 754 underwent standard transthoracic echocardiography and Doppler flow imaging, according to the recommendations of the American Society of Echocardiography during hospitalization, and were entered into the screening procedure for the current study.17 Patients with acute coronary syndrome or stroke (n=208), congenital aortic valve malformation (n=5), previous aortic valve replacement (n=31), rheumatic disease (n=13), congenital heart disease (n=16), endocarditis (n=8), severe renal impairment with an estimated glomerular filtration rate (eGFR) <15 mL/min per 1.73 m2 (n=11), cancer (n=8), or autoimmune diseases (n=13) were excluded. We also excluded patients who were undergoing antiosteoporotic treatment (n=15) and those who refused to enter the study (n=29). CAVD was identified in 200 of the 397 patients who entered the study (Figure 1). All demographic and clinical information was collected prospectively, and the study flowchart is shown in Figure 1. The study protocol was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine; and written informed consent was obtained from all patients.
Figure 1.
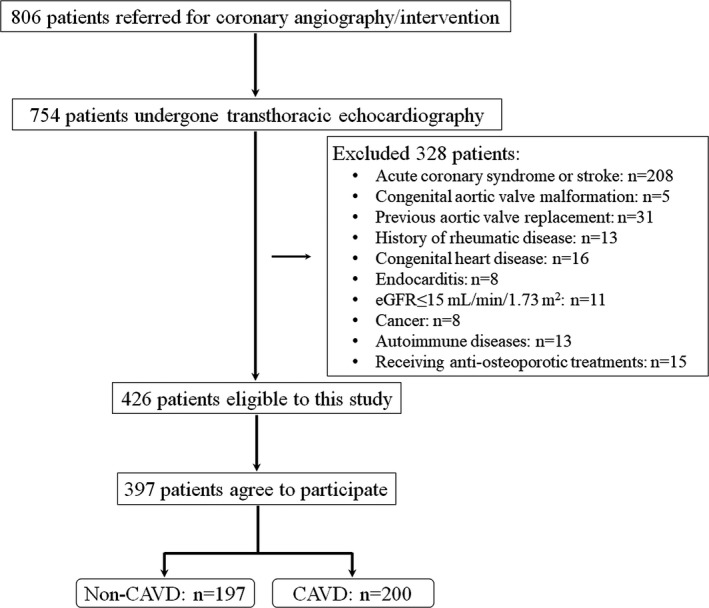
Flowchart of patient enrollment. Overall, 397 patients participated in this study (calcific aortic valve disease [CAVD]: n=200; non‐CAVD: n=197). eGFR indicates estimated glomerular filtration rate.
Definitions and Measurements
CAVD was defined as opaque leaflets with focal areas of mild thickening and increased stiffness with or without an elevated peak transaortic valve flow velocity (≥2.0 m/s)18 (Figure 2). The diagnosis of type 2 diabetes mellitus was performed according to the criteria of the American Diabetes Association,19 and hypertension was defined according to the European Society of Cardiology/European Society of Hypertension guidelines.20 Renal function was evaluated by eGFR, which was calculated using the Modification of Diet in Renal Disease equation.21 Significant coronary artery disease was diagnosed if there was ≥50% diameter stenosis in at least one major epicardial coronary artery, according to the lesion classification scheme of the American College of Cardiology/American Heart Association.22
Figure 2.
A representative echocardiography image of non–calcific aortic valve disease (CAVD) (top) and CAVD (bottom).
Fasting venous blood samples were obtained to measure the serum levels of leptin after obtaining informed consent. To avoid a diurnal variation in serum leptin concentration and dramatic fasting interval effects, all blood samples were taken at 8:00 am and were immediately transferred at a freezing temperature and centrifuged within 30 minutes at 1400g for 20 minutes to obtain platelet‐poor serum. All samples were stored at −80°C before analysis. The serum level of leptin was assessed using an ELISA kit (Human Leptin ELISA, Clinical Range; BioVendor catalog No. RD191001100), according to the manufacturer's instructions.
Experimental Study
To investigate the possible mechanism of action of leptin in the development of aortic valve calcification, we collected replaced calcific aortic valves from 3 patients after aortic valve surgery (leptin level >20 ng/mL) and normal aortic valves from 3 patients undergoing heart transplantation (leptin level <9 ng/mL). The expression of phosphorylated extracellular signal‐regulated kinase (ERK) and ERK was determined. The tissues (100 mg for each sample) were lysed with T‐PER Tissue Protein Extraction Reagent (Thermo‐Fisher, Waltham, MA), and equal concentrations of protein were electrophoretic to separate on 10% SDS/PAGE. The gels were then blotted onto a poly (vinylidene difluoride) membrane. After blotting, the membrane was labeled with antibodies with phosphorylated ERK1/2 (1:1000), tERK1/2 (1:1000), and GAPDH (1:2000). Finally, the membranes were incubated with horseradish peroxidase–conjugated secondary antibodies (1:5000) and explored with an electrochemiluminescence detection system (Millipore, MA). All antibodies were purchased from Cell Signaling (MA), and each image was captured and the intensity of each band was analyzed with Quantity One (Bio‐Rad).
Statistical Analysis
Continuous variables are expressed as the mean and SD if data were normally distributed or the median with interquartile ranges when data were not normally distributed. Categorical data are summarized as proportions and frequencies. Continuous and categorical variables were compared using independent t test or nonparametric and χ2 tests, respectively. Leptin was defined as a log‐transformed continuous variable in logistic regression model analysis and an ordinal variable divided according to its tertiles using the lowest tertile as reference to evaluate its value. Multivariable logistic regression analysis was performed to assess the independent diagnostic value of leptin for the presence of CAVD, and the Hosmer‐Lemeshow2 statistic was computed to assess model calibration (or how closely the calculated probabilities reflect actual risk). Odds ratios are presented after adjusting for age, sex, body mass index (BMI), risk factor for coronary artery disease, severity of coronary stenosis, alcohol consumption, lipid profiles, eGFR, and calcium and phosphorus levels. In addition, restricted cubic spline was used to investigate the possible association between serum leptin and CAVD.23 We selected 5%, 35%, 65%, and 95% values of leptin as knots; tested the associations between knots using a cubic function; and presented the smoothly integrated graph. A small P<0.05 of a Wald‐test type test with 2 degree of freedom indicated the nonlinear associations between leptin and CAVD. The diagnostic values of leptin and age were calculated by constructing receiver‐operating characteristic curves. Spearman correlation analysis was performed to explore the relationship between leptin and clinical data that was statistically significant at baseline. All statistical analyses were performed using SPSS software, version 19.0 (SPSS, Inc, Chicago, IL) and Stata software (Stata 13; Stata Corporation, College Station, TX). Statistical significance was set at 2 tailed, and P<0.05 was considered statistically significant. All authors had full access to the data in the study and took responsibility for the integrity of data and accuracy of data analysis.
Results
Baseline Characteristics
Patients with CAVD were older (75.00 versus 59.00 years) and had a higher proportion of hypertension (77.0% versus 64.50%) and significant coronary artery disease (88.50% versus 68.00%) (all P<0.01) than non‐CAVD patients, but the sex distribution, BMI, and proportion of smokers, individuals with diabetes mellitus, and alcohol consumers were similar in the 2 groups. Serum levels of high‐density lipoprotein cholesterol (1.03 versus 1.09 mmol/L), eGFR (72.29±18.23 versus 90.39±17.62 mL/min per 1.73 m2), calcium (2.17 versus 2.21 mg/dL), and phosphorus (1.11±0.19 versus 1.19±0.18 mg/dL) were lower in the CAVD group (all P<0.01). The 2 groups did not differ with respect to fasting glucose, triglycerides, total cholesterol, low‐density lipoprotein cholesterol, or antidiabetic and statin treatments (Table 1).
Table 1.
Baseline Characteristics of Study Participants With CAVD or Without CAVD
| Variables | CAVD Group (n=200) | Non‐CAVD Group (n=197) |
|---|---|---|
| Age, y | 75.00 (68.25–80.00) | 59.00 (53.00–64.50)* |
| Body mass index, kg/m2 | 24.82 (22.72–27.03) | 24.24 (22.49–26.50) |
| Men, n (%) | 123 (61.5) | 111 (56.3) |
| Smoking, n (%) | 48 (44.9) | 59 (55.1) |
| Alcohol drinker, n (%) | 18 (9.0) | 27 (13.7) |
| Hypertension, n (%) | 154 (77.00) | 127 (64.50)* |
| Coronary heart disease, n (%) | 177 (88.50) | 134 (68.00)* |
| Diabetes mellitus, n (%) | 61 (30.50) | 63 (32.00) |
| Fasting glucose, mg/dL | 5.02 (4.56–5.91) | 4.92 (4.51–5.66) |
| Total cholesterol, mmol/L | 3.84 (3.10–4.56) | 3.95 (3.34–4.69) |
| LDL‐C, mmol/L | 2.15 (1.66–2.85) | 2.31 (1.74–2.93) |
| HDL‐C, mmol/L | 1.03 (0.91–1.20) | 1.09 (0.94–1.29)* |
| Triglyceride, mmol/L | 1.30 (1.00–2.06) | 1.48 (1.15–2.10) |
| γ‐Glutamyl transpeptidase, mmol/L | 21.0 (15.00–29.00) | 19.0 (13.00–33.00) |
| eGFR, mL/min per 1.73 m2 | 72.29±18.23 | 90.39±17.62* |
| Serum calcium, mg/dL | 2.17 (2.10–2.26) | 2.21 (2.15–2.29)* |
| Serum phosphorus, mg/dL | 1.11±0.19 | 1.19±0.18* |
| Antidiabetic medications, n (%) | 28 (14.0) | 35 (17.8) |
| Statin, n (%) | 167 (83.5) | 163 (82.7) |
Continuous variables are expressed as the mean±SD if normally distributed; median (interquartile ranges) are provided when data were not normally distributed. CAVD indicates calcific aortic valve disease; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
P≤0.01.
Serum Leptin Levels and CAVD
The serum leptin level (median) was significantly higher in patients with CAVD than in their non‐CAVD counterparts (20.07 and 9.03 ng/mL; P<0.01) (Figure 3). Serum leptin correlated positively with age (r=0.37, P<0.01) and negatively with eGFR (r=−0.37, P<0.01) (Figure 4). However, there was no significant relationship between leptin and high‐density lipoprotein cholesterol, calcium, and phosphorus levels (all P>0.05). Because leptin has close relationships with body weight and BMI, we also explored leptin/BMI and leptin/body weight levels in the 2 groups as well as their relationships with age and eGFR. Both leptin/BMI (0.82 and 0.36; P<0.01) and leptin/body weight (0.28 and 0.13; P<0.01) were greater in patients with CAVD than in their non‐CAVD counterparts. In addition, leptin/BMI and leptin/body weight correlated positively with age (r=0.38 and r=0.38; all P<0.01) and negatively with eGFR (r=−0.36 and r=−0.37; all P<0.01).
Figure 3.
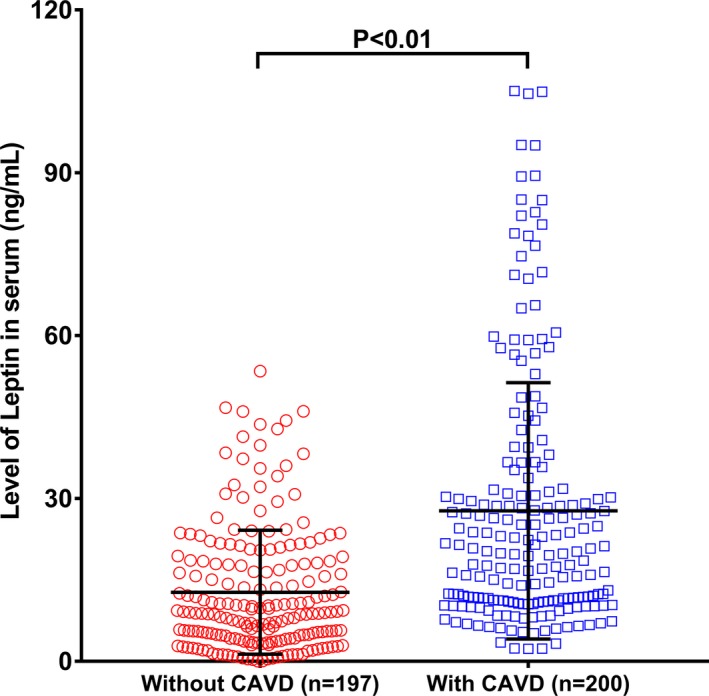
Comparison of serum leptin (ng/mL) between calcific aortic valve disease (CAVD) and non‐CAVD patients. The leptin level was higher in the CAVD group (blue circle) than in the non‐CAVD group (red circle) (P<0.01).
Figure 4.
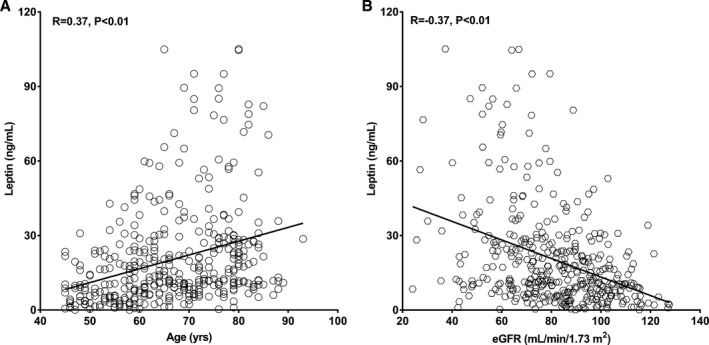
Relationship of serum leptin with age (A) and renal function (B). Spearman correlation analysis revealed a positive association trend between age and leptin (relative index=0.37, P<0.01) (A) and a negative correlation trend between estimated glomerular filtration rate (eGFR) and leptin (relative index=−0.37, P<0.01) (B).
Univariate analysis showed that CAVD was associated with age, coronary artery stenosis, lower eGFR and high‐density lipoprotein cholesterol, and elevated levels of calcium, phosphorus, and leptin. Multivariate logistic regression analyses revealed that serum levels of leptin remained an independent risk factor for the presence of CAVD (Table 2).
Table 2.
Multivariable Logistic Regression Models for Serum Leptin Level as a Predictor for CAVD
| Variable | Adjusted for Model 1 OR (95% CI) | P Value | Adjusted for Model 2 OR (95% CI) | P Value |
|---|---|---|---|---|
| Log leptin per SD | 3.20 (2.08–4.90) | <0.01 | 2.80 (1.87–4.18) | <0.01 |
| First tertile | 1 (Reference) | … | 1 (Reference) | … |
| Second tertile | 3.28 (1.60–6.70) | 0.01 | 3.83 (1.82–8.08) | <0.01 |
| Third tertile | 6.90 (3.13–15.22) | <0.01 | 5.93 (2.74–12.84) | <0.01 |
Leptin is analyzed as a log‐transformed continuous variable, an ordinal variable divided according to its tertiles, and a categorical variable using the lowest tertile as reference. Model 1, adjusted for age and sex. Model 2, adjusted for age, sex, body mass index, risk factors for coronary artery disease, level of coronary artery stenosis, alcohol drinker, lipid profiles, estimated glomerular filtration rate, levels of calcium and phosphorus, and leptin (tertile). As a continuous variable, OR is shown as per 1 SD. CAVD indicates calcific aortic valve disease; OR, odds ratio.
The logistic regression model of P/(1−P)=exp[−14.79+0.20(age)+−1.89(high‐density lipoprotein cholesterol)+1.03(lg leptin per SD)+0.01(stenosis of coronary artery)], with the Nagelkerke R2 value of 0.68, was shown to be appropriate on the basis of the Hosmer‐ Lemeshow2 statistic (calibration, 0.94). Moreover, a nonlinear association between leptin and incident CAVD was demonstrated by a restricted cubic spline curve (Figure 5). The receiver‐operating characteristic curve analysis showed that the area under the curve was 0.74 (95% CI, 0.69–0.79; P<0.01) of serum leptin and 0.90 (95% CI, 0.87–0.93; P<0.01) of age as risk factors for the presence of CAVD, with an optimal cutoff point of 9.86 ng/mL (sensitivity=84.50% and specificity=54.30%) and 63.5 years (sensitivity=90.0% and specificity=72.1%), respectively (Figure 6).
Figure 5.
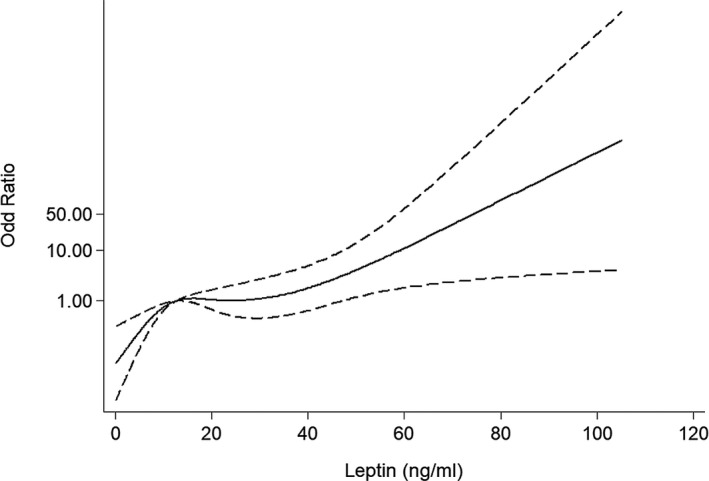
Odds ratio for the presence of calcific aortic valve disease (CAVD), according to leptin levels by restricted cubic spline. The odds ratio was adjusted for age, sex, body mass index, history of hypertension, risk factors for coronary artery disease, coronary artery stenosis, lipid profile, alcohol consumption, estimated glomerular filtration rate, and the levels of calcium and phosphorus. Knots were set at the 5%, 35%, 65%, and 95% percentiles. The median leptin level (12.53 ng/mL) was used as the reference. There was a significant nonlinear association between leptin and incident CAVD (P=0.03).
Figure 6.
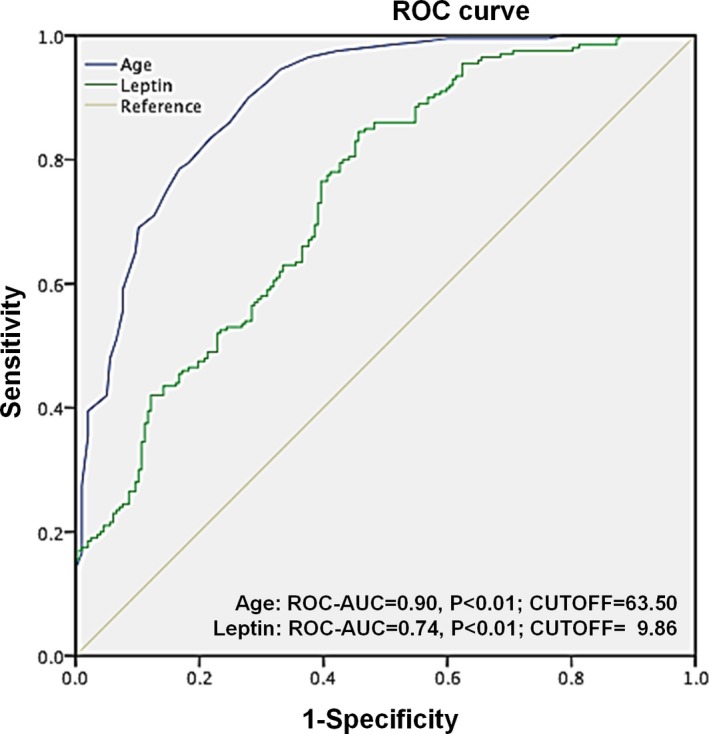
Receiver‐operating characteristic (ROC) curve of leptin and age as risk factors for calcific aortic valve disease. The blue line represents age, and the green line represents leptin. The area under curve (AUC) was 0.90 for age and 0.74 for leptin; the cutoffs with the highest sensitivity and specificity were 63.5 years (sensitivity=90.0% and specificity=72.1%) and 9.86 ng/mL (sensitivity=84.5% and specificity=54.3%), respectively.
Role of Leptin in Patients With Different Age and Renal Function
Because the correlation study revealed that leptin was correlated with age and renal function, patients were further specified on the basis of the median of age (median=65 years) and eGFR (median=82.06 mL/min per 1.73 m2). Multivariate logistic regression analysis showed that an increased serum leptin level (as a log‐transformed continuous variable) was associated with a greater risk among younger patients (odds ratio, 3.99 [95% CI, 2.12–7.50] versus 2.96 [95% CI, 1.73–5.06]; P<0.01) and patients with a lower eGFR level (odds ratio, 3.14 [95% CI, 1.84–5.34] versus 2.54 [95% CI, 1.23–5.26]; P=0.01) (Figure 7). In fact, among 187 patients whose ages were <65 years, 33 had CAVD. Serum leptin was higher in the CAVD group (20.85 [12.92–37.42] ng/mL) than in the non‐CAVD group (8.76 [3.64–19.95] ng/mL) (P<0.01). Moreover, the area under the curve values were higher among younger patients (area under the curve, 0.76 [95% CI, 0.67–0.84] versus 0.70 [95% CI, 0.61–0.79]; P<0.01) and those with at least mild renal dysfunction (area under the curve, 0.73 [95% CI, 0.65–0.81] versus 0.68 [95% CI, 0.61–0.76]; P<0.01) (Figure 8).
Figure 7.
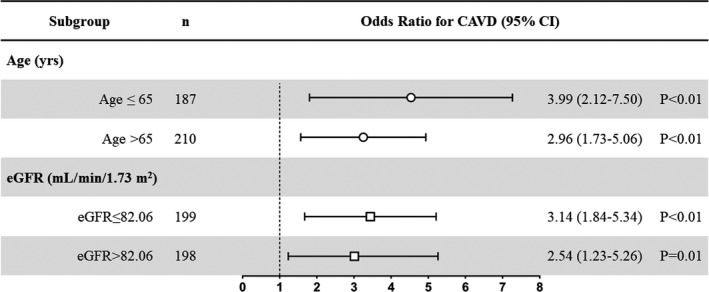
The forest plot showed the diagnostic value of serum leptin for the presence of calcific aortic valve disease (CAVD) (leptin was analyzed as a log‐transformed continuous variable). Multivariate logistic regression analysis showed that an increased serum leptin level was associated with a greater risk among younger patients and those with lower estimated glomerular filtration rate (eGFR) levels.
Figure 8.
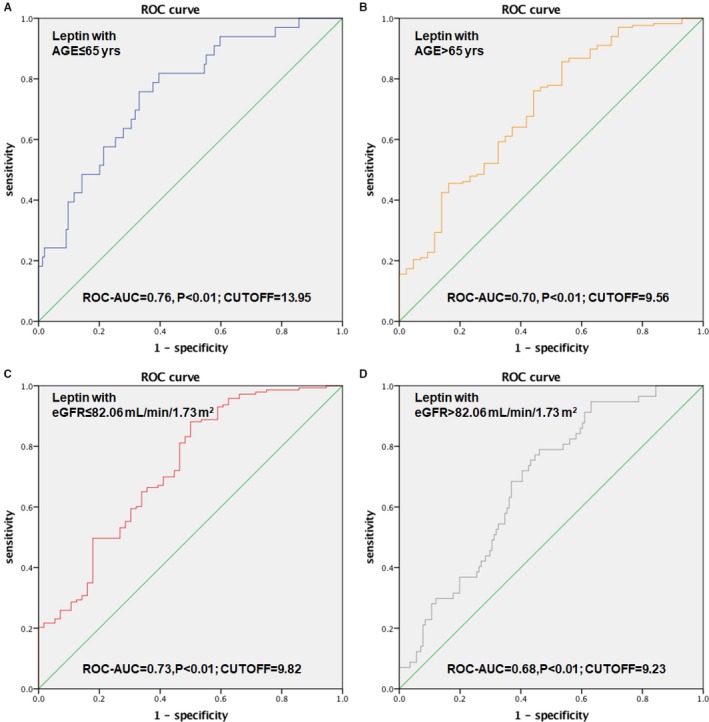
Receiver‐operating characteristic (ROC) curve of leptin as a risk factor for calcific aortic valve disease (CAVD) among patients younger (A) and older (B) than 65 years. ROC curve of leptin as a risk factor for CAVD among patients’ estimated glomerular filtration rate (eGFR) level lower (C) and higher (D) than 82.06 mL/min per 1.73 m2. AUC indicates area under the curve.
Western Blot
The activation of ERK1/2 was stronger in calcified aortic valves than in normal aortic valves (Figure 9).
Figure 9.
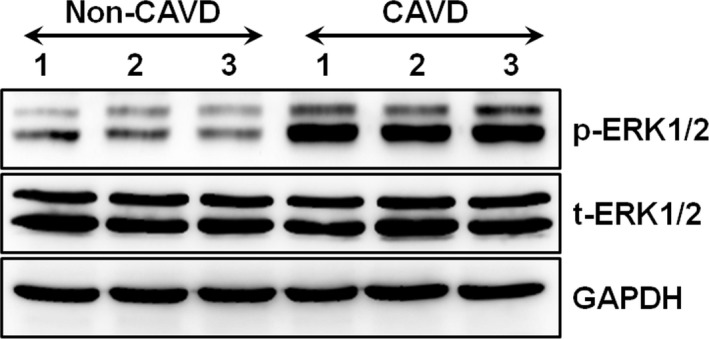
The expression of extracellular signal‐regulated kinase (ERK) 1/2 in the calcific aortic valve disease (CAVD) group with increased leptin levels and in the non‐CAVD group with normal leptin levels. The phosphorylated ERK1/2 (p‐ERK1/2) and total ERK1/2 (t‐ERK1/2) were detected by Western blot (each group included 3 samples). GAPDH was used as the internal control.
Discussion
The results of the current study showed that increased serum leptin level was associated with the presence of CAVD, particularly among patients aged <65 years and those with impaired renal function.
In general, CAVD is described as a degenerative process involving lipid deposition, inflammatory cell infiltration (predominantly macrophages), cytokine expression, and mineralization of the fibrosa layer.24 These changes are positively associated with the progression of calcification and, together with valve remodeling, the distortion of the normal structure and function of aortic valves.25 In previous basic research, leptin has been demonstrated to promote the activation and proliferation of circulating monocytes, induce the production of proinflammatory cytokines by monocytes (eg, interleukin‐1, tumor necrosis factor, or interleukin‐6), stimulate the oxidative burst, and enhance chemotactic responses by acting as a chemoattractant in monocytes and/or macrophages.26 Leptin was also reported to correlate positively with the oxidized low‐density lipoprotein level27 and play a role in CAVD by promoting the osteoblastic differentiation of human valvular interstitial cells.28 However, the clinical relationship between leptin and CAVD remains unclear. In a small cohort study, Rosa et al observed an increase in serum leptin levels in 43 patients with aortic valve calcification.28 Our study has a relatively large sample size, and all patients were selected specifically on the basis of echocardiographic determinations. The major finding of this study is that serum leptin was significantly elevated in patients with CAVD and served as an independent risk factor for the presence of CAVD. Furthermore, our findings indicated that the diagnostic value of elevated serum leptin for the risk of CAVD was greater in younger patients and those with reduced renal function. However, the clinical relevance of elevated serum leptin levels in this population still needs to be investigated in a study with a longer follow‐up.
Leptin, which protects organs against the effects of starvation,29 has been increasingly explored as a factor correlated with cardiovascular diseases,12 but its mechanism in the development of CAVD is still being investigated. In an experiment, Zeadin et al found that adipocytokine leptin promotes the calcification of both coronary atherosclerotic lesions and aortic valve leaflets.13 Rosa et al reported that leptin stimulates the osteoblast differentiation of valvular interstitial cells, the most prevalent cells found in human aortic valves composed of progenitor cells and osteoblast‐like cells, in a protein kinase B– and ERK‐dependent manner.28, 30 In our study, we found that ERK1/2 exhibited stronger activation in the context of CAVD as well. The inflammatory response is a hallmark of CAVD that has been extensively demonstrated.31, 32, 33 It has also been reported that the proinflammatory effect of leptin is associated with various cardiovascular diseases.34, 35, 36 Our study showed that the severity of coronary artery stenosis has a diagnostic value for the presence of CAVD to some extent, suggesting that the association of leptin with CAVD may be partly mediated by atherosclerosis.37 In addition, leptin correlates with C‐reactive protein.38 Therefore, cardiovascular inflammation mediated by leptin might play an important role in the development of CAVD. Recent data have highlighted a potentially important link between the toll‐like receptor–nuclear factor‐κB axis‐inflammation and CAVD pathogenesis. Accumulating evidence suggests that toll‐like receptors could be significant contributors to the pathogenesis of valvular inflammation and the ensuing inflammation‐driven calcification processes in aortic valves.39 On the other hand, previous studies have demonstrated that leptin can possibly potentiate toll‐like receptor ligand–lipopolysaccharide, inducing an increase in the expression of tumor necrosis factor and contributing to increased inflammation.40 Therefore, increased leptin may result in a more activated and proinflammatory state in individuals with CAVD.
Our results support the notion that valve calcification is a partially age‐related disease,41 as evidenced by a high prevalence of CAVD in elderly individuals.42 In fact, one third of our elderly individuals had echocardiographic evidence of aortic valve sclerosis. CAVD becomes debilitating and can progress to its most severe form of calcific aortic valve stenosis, and 2% of individuals aged >60 years experience stenosis to the extent that surgery is required to preclude death once symptoms occur.43 As CAVD predisposes patients to left ventricular diastolic and systolic dysfunction,44 its early detection and diagnosis would be expected to improve the clinical outcome of these patients. In this study, elevated serum leptin was strongly correlated with the presence of CAVD diagnosed by echocardiography, and, furthermore, the diagnostic value of serum leptin for the presence of CAVD was higher in patients aged ≤65 years, suggesting that serum leptin levels may be useful for detecting CAVD, especially in younger populations.
Numerous studies have indicated that chronic kidney disease is associated with aortic valve calcification.45, 46 Kim et al observed that the risk of CAVD was 3.01‐fold higher in predialysis patients (eGFR <60 mL/min per 1.73 m2) than in those with normal renal function.37 In a large study involving 5820 participants from the US Third National Health and Nutrition Examination Survey, elevated serum leptin concentrations were associated with chronic kidney disease after adjusting for age, sex, ethnicity, BMI, diabetes mellitus, hypertension, and serum cholesterol.47 Consistent with previous findings, our study demonstrated a lower eGFR level in patients with CAVD than in those without CAVD. In addition, we found a negative correlation between eGFR and serum leptin levels. Subgroup multiple regression analyses revealed that leptin was more correlated with CAVD in those with renal dysfunction (eGFR <82.06 mL/min per 1.73 m2). This finding implies that for patients with at least mild renal impairment, increased serum leptin probably had an improved clinical diagnostic value for the presence of CAVD.
Limitations
There are several limitations in our study. First, the study was a cross‐sectional investigation of CAVD, thereby allowing us to detect associations, not to formulate causal links. Second, all patients were Asian and were selected from a single medical center, so the generalization of the current findings may be limited. Third, CAVD was assessed by 2‐dimensional echocardiography and Doppler flow imaging in this study. Cardiac computed tomography could be useful to quantify aortic valve calcium as well. Finally, although we carefully controlled for the major known confounders, unknown factors may still have interfered in our findings.
Conclusions
The present study suggested that elevated serum leptin levels were significantly associated with the presence of CAVD, especially in younger individuals (aged ≤65 years) and in those with renal dysfunction. The potential clinical implications for the early detection of CAVD by measuring the serum leptin level and its role in CAVD prevention and treatment still need to be investigated.
Author Contributions
Study conception and design: Drs Y. Liu, Yang, Zhang, and Shen. Acquisition and analysis of data: Drs Y. Liu, Lu, Gu, Shen, Y. Li, and Lin. Drafting of a significant portion of the manuscript or figures: Drs Lin, Zhang, He, Y. Liu, Gu, and Y. Li. Revision of the manuscript for important intellectual content: Drs Y. Liu, Yang, Zhang, and Shen.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (81770384); Joint Funds for the Innovation of Science and Technology, Fujian Province (2017Y9007); the fund of Diabetic Innovation Team (2019HC002) and Clinical Endocrinology of Yunnan Province; and the Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (PWZxq2017‐05). The funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Disclosures
None.
Acknowledgments
We gratefully acknowledge all the people who participated in this study for their efforts in the conduction of the study.
(J Am Heart Assoc. 2019;8:e012495 DOI: 10.1161/JAHA.119.012495.)
Contributor Information
Qi Zhang, Email: zhangqnh@hotmail.com.
Ke Yang, Email: ykk_ykkk@126.com.
References
- 1. Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. [DOI] [PubMed] [Google Scholar]
- 2. Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. [DOI] [PubMed] [Google Scholar]
- 3. Wirrig EE, Gomez MV, Hinton RB, Yutzey KE. Cox2 inhibition reduces aortic valve calcification in vivo. Arterioscler Thromb Vasc Biol. 2015;35:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 5. Sider KL, Blaser MC, Simmons CA. Animal models of calcific aortic valve disease. Int J Inflam. 2011;2011:364310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanchez PL, Santos JL, Kaski JC, Cruz I, Arribas A, Villacorta E, Cascon M, Palacios IF, Martin‐Luengo C, Grupo A. Relation of circulating C‐reactive protein to progression of aortic valve stenosis. Am J Cardiol. 2006;97:90–93. [DOI] [PubMed] [Google Scholar]
- 7. O'Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of “degenerative” valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. [DOI] [PubMed] [Google Scholar]
- 8. Mohler ER III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. [DOI] [PubMed] [Google Scholar]
- 9. Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappab ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. [DOI] [PubMed] [Google Scholar]
- 10. Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta‐analysis of the randomized placebo‐controlled clinical trials on 2344 patients. Can J Cardiol. 2011;27:800–808. [DOI] [PubMed] [Google Scholar]
- 11. Reilly MP, Iqbal N, Schutta M, Wolfe ML, Scally M, Localio AR, Rader DJ, Kimmel SE. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3872–3878. [DOI] [PubMed] [Google Scholar]
- 12. Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation. 2001;104:3052–3056. [DOI] [PubMed] [Google Scholar]
- 13. Zeadin M, Butcher M, Werstuck G, Khan M, Yee CK, Shaughnessy SG. Effect of leptin on vascular calcification in apolipoprotein e‐deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:2069–2075. [DOI] [PubMed] [Google Scholar]
- 14. Zeadin MG, Butcher MK, Shaughnessy SG, Werstuck GH. Leptin promotes osteoblast differentiation and mineralization of primary cultures of vascular smooth muscle cells by inhibiting glycogen synthase kinase (GSK)‐3beta. Biochem Biophys Res Commun. 2012;425:924–930. [DOI] [PubMed] [Google Scholar]
- 15. Ben‐Zvi D, Savion N, Kolodgie F, Simon A, Fisch S, Schafer K, Bachner‐Hinenzon N, Cao X, Gertler A, Solomon G, Kachel E, Raanani E, Lavee J, Kotev Emeth S, Virmani R, Schoen FJ, Schneiderman J. Local application of leptin antagonist attenuates angiotensin II‐induced ascending aortic aneurysm and cardiac remodeling. J Am Heart Assoc. 2016;5:e003474 DOI: 10.1161/JAHA.116.003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glader CA, Birgander LS, Soderberg S, Ildgruben HP, Saikku P, Waldenstrom A, Dahlen GH. Lipoprotein(a), chlamydia pneumoniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur Heart J. 2003;24:198–208. [DOI] [PubMed] [Google Scholar]
- 17. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. [DOI] [PubMed] [Google Scholar]
- 18. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons , Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 20. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members . 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 22. Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease: implications for patient selection: multivessel angioplasty prognosis study group. Circulation. 1990;82:1193–1202. [DOI] [PubMed] [Google Scholar]
- 23. Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–208. [DOI] [PubMed] [Google Scholar]
- 24. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of “degenerative” valvular aortic stenosis: histological and immunohistochemical studies. Circulation. 1994;90:844–853. [DOI] [PubMed] [Google Scholar]
- 25. Charest A, Pepin A, Shetty R, Cote C, Voisine P, Dagenais F, Pibarot P, Mathieu P. Distribution of sparc during neovascularisation of degenerative aortic stenosis. Heart. 2006;92:1844–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conde J, Scotece M, Gomez R, Gomez‐Reino JJ, Lago F, Gualillo O. At the crossroad between immunity and metabolism: focus on leptin. Expert Rev Clin Immunol. 2010;6:801–808. [DOI] [PubMed] [Google Scholar]
- 27. Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. [DOI] [PubMed] [Google Scholar]
- 28. Rosa M, Paris C, Sottejeau Y, Corseaux D, Robin E, Tagzirt M, Juthier F, Jashari R, Rauch A, Vincentelli A, Staels B, Van Belle E, Susen S, Dupont A. Leptin induces osteoblast differentiation of human valvular interstitial cells via the AKT and ERK pathways. Acta Diabetol. 2017;54:551–560. [DOI] [PubMed] [Google Scholar]
- 29. Schwartz MW, Seeley RJ. Seminars in medicine of the Beth Israel Deaconess Medical Center: neuroendocrine responses to starvation and weight loss. N Engl J Med. 1997;336:1802–1811. [DOI] [PubMed] [Google Scholar]
- 30. Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more). Arterioscler Thromb Vasc Biol. 2006;26:1721–1728. [DOI] [PubMed] [Google Scholar]
- 32. Cote C, Pibarot P, Despres JP, Mohty D, Cartier A, Arsenault BJ, Couture C, Mathieu P. Association between circulating oxidised low‐density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart. 2008;94:1175–1180. [DOI] [PubMed] [Google Scholar]
- 33. Mathieu P, Bouchareb R, Boulanger MC. Innate and adaptive immunity in calcific aortic valve disease. J Immunol Res. 2015;2015:851945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Packer M. Leptin‐aldosterone‐neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation. 2018;137:1614–1631. [DOI] [PubMed] [Google Scholar]
- 35. Fukui A, Ikebe‐Ebata Y, Kondo H, Saito S, Aoki K, Fukunaga N, Shinohara T, Masaki T, Teshima Y, Takahashi N. Hyperleptinemia exacerbates high‐fat diet‐mediated atrial fibrosis and fibrillation. J Cardiovasc Electrophysiol. 2017;28:702–710. [DOI] [PubMed] [Google Scholar]
- 36. Drosos I, Chalikias G, Pavlaki M, Kareli D, Epitropou G, Bougioukas G, Mikroulis D, Konstantinou F, Giatromanolaki A, Ritis K, Munzel T, Tziakas D, Konstantinides S, Schafer K. Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery: possible role for the leptin‐inflammation‐fibrosis‐hypoxia axis. Clin Res Cardiol. 2016;105:887–900. [DOI] [PubMed] [Google Scholar]
- 37. Kim IY, Kim MJ, Lee DW, Lee SB, Shin MJ, Rhee H, Yang BY, Song SH, Seong EY, Kwak IS. Cardiac valve calcification is associated with presence and severity of coronary artery disease in patients with pre‐dialysis chronic kidney disease. Clin Exp Nephrol. 2015;19:1090–1097. [DOI] [PubMed] [Google Scholar]
- 38. Abdullah SM, Khera A, Leonard D, Das SR, Canham RM, Kamath SA, Vega GL, Grundy SM, McGuire DK, de Lemos JA. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195:404–410. [DOI] [PubMed] [Google Scholar]
- 39. Garcia‐Rodriguez C, Parra‐Izquierdo I, Castanos‐Mollor I, Lopez J, San Roman JA, Sanchez Crespo M. Toll‐like receptors, inflammation, and calcific aortic valve disease. Front Physiol. 2018;9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaedicke KM, Roythorne A, Padget K, Todryk S, Preshaw PM, Taylor JJ. Leptin up‐regulates TLR2 in human monocytes. J Leukoc Biol. 2013;93:561–571. [DOI] [PubMed] [Google Scholar]
- 41. Mohty D, Pibarot P, Despres JP, Cartier A, Arsenault B, Picard F, Mathieu P. Age‐related differences in the pathogenesis of calcific aortic stenosis: the potential role of resistin. Int J Cardiol. 2010;142:126–132. [DOI] [PubMed] [Google Scholar]
- 42. Rajamannan NM, Evans FJ, Aikawa E, Grande‐Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group: executive summary: calcific aortic valve disease—2011 update. Circulation. 2011;124:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nightingale AK, Horowitz JD. Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart. 2005;91:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pasipoularides A. Calcific aortic valve disease: part 2‐morphomechanical abnormalities, gene reexpression, and gender effects on ventricular hypertrophy and its reversibility. J Cardiovasc Transl Res. 2016;9:374–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guerraty MA, Chai B, Hsu JY, Ojo AO, Gao Y, Yang W, Keane MG, Budoff MJ, Mohler ER III; CRIC Study Investigators . Relation of aortic valve calcium to chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am J Cardiol. 2015;115:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cui L, Rashdan NA, Zhu D, Milne EM, Ajuh P, Milne G, Helfrich MH, Lim K, Prasad S, Lerman DA, Vesey AT, Dweck MR, Jenkins WS, Newby DE, Farquharson C, Macrae VE. End stage renal disease‐induced hypercalcemia may promote aortic valve calcification via annexin vi enrichment of valve interstitial cell derived‐matrix vesicles. J Cell Physiol. 2017;232:2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shankar A, Syamala S, Xiao J, Muntner P. Relationship between plasma leptin level and chronic kidney disease. Int J Nephrol. 2012;2012:269532. [DOI] [PMC free article] [PubMed] [Google Scholar]


