Abstract
Background
Inherited thrombophilias are well‐established predisposing factors for venous thromboembolism, but their role in arterial thrombosis, such as arterial ischemic stroke, remains uncertain. We aimed to evaluate the association between inherited thrombophilia (factor V Leiden, prothrombin G20210A mutation, protein C deficiency, protein S deficiency, and antithrombin deficiency) and risk of arterial ischemic stroke in adults.
Methods and Results
We searched PubMed, EMBASE, and Cochrane Library Databases from inception to December 31, 2018. We included case‐control or cohort studies of adults reporting the prevalence of inherited thrombophilias in those with arterial ischemic stroke and subjects without arterial ischemic stroke. Two reviewers (T.C., E.D.) independently searched the literature and extracted data. Pooled odds ratios (ORs) and 95% CIs were calculated using random‐effects model. We identified 68 eligible studies, which collectively enrolled 11 916 stroke patients and 96 057 controls. The number of studies reporting factor V Leiden, prothrombin G20210A mutation, protein C deficiency, protein S deficiency, and antithrombin deficiency were 56, 45, 15, 17, and 12, respectively. Compared with controls, patients with arterial ischemic stroke were significantly more likely to have the following inherited thrombophilias: factor V Leiden (OR, 1.25; 95% CI, 1.08–1.44; I2=0%), prothrombin G20210A mutation (OR, 1.48; 95% CI, 1.22–1.80; I2=0%), protein C deficiency (OR, 2.13; 95% CI, 1.16–3.90; I2=0%), and protein S deficiency (OR, 2.26; 95% CI, 1.34–3.80; I2=8.8%). Statistical significance was not reached for antithrombin deficiency (OR, 1.25; 95% CI, 0.58–2.67; I2=8.8%).
Conclusions
Inherited thrombophilias (factor V Leiden, prothrombin G20210A mutation, protein C deficiency, and protein S deficiency) are associated with an increased risk of arterial ischemic stroke in adults. The implications of these findings with respect to clinical management of patients with ischemic stroke require further investigation.
Keywords: hypercoagulopathy; stroke; stroke, ischemic; thrombosis
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke
Clinical Perspective
What Is New?
Inherited thrombophilias (factor V Leiden, prothrombin G20210A mutation, protein C deficiency, and protein S deficiency) are associated with an increased risk of arterial ischemic stroke in adults, particularly in younger adults.
What Are the Clinical Implications?
The role of inherited thrombophilia testing in patients with ischemic stroke as well as its influence on clinical management warrant further study.
Introduction
The inherited thrombophilias, factor V Leiden (FVL), the prothrombin G20210A mutation (PTM), protein C deficiency (PCD), protein S deficiency (PSD), and antithrombin deficiency (ATD), are well‐established predisposing factors for venous thromboembolism,1, 2 but their role in arterial thrombosis, such as arterial ischemic stroke, remains uncertain.
In patients with arterial ischemic stroke, inherited thrombophilia testing is often ordered to identify the cause of stroke. However, the benefit of screening for inherited thrombophilia is unknown and such practice is controversial.3, 4 Indeed, the 2018 American Heart Association/American Stroke Association clinical practice guideline recommends against thrombophilia testing in patients with ischemic stroke,5 although such testing remains common in clinical practice.6
Current evidence about the association of inherited thrombophilia and the risk of ischemic stroke is conflicting.7 Individual studies carry the limitations of small sample size and reduced statistical power. Therefore, we conducted a systematic review and meta‐analysis to evaluate the association of inherited thrombophilia (FVL, PTM, PCD, PSD, and ATD) and the risk of arterial ischemic stroke in adults.
Methods
The study protocol is registered on PROSPERO (CRD42018090020). We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses8 and the Meta‐Analysis of Observational Studies in Epidemiology guidelines.9 The data that support the findings of this study are available from the corresponding author on request.
Data Sources and Search Strategies
We searched PubMed, EMBASE, and the Cochrane Library Databases from inception to December 31, 2018. The following search terms were used: “stroke” OR “cerebrovascular accident” AND “factor V” OR “prothrombin” OR “antithrombin” OR “protein C” OR “protein S” OR “thrombophilia.” No language restriction was applied. The detailed search queries are presented in Data S1. Additional searches were performed by manual review of abstracts from the American Society of Hematology annual meeting, the Congress of the International Society on Thrombosis and Hemostasis, the American Academy of Neurology annual meeting, and the International Stroke Conference (from 2015 to 2018). Reference lists of relevant studies and review articles were screened for potentially eligible studies.
Study Selection
Two authors (T.C., E.D.) independently searched the literature, screened titles and abstracts, and reviewed full texts to identify potentially eligible studies. Disagreements were resolved by consensus or a third reviewer (A.C.) when necessary.
The primary outcome of interest was arterial ischemic stroke. Eligible studies included case‐control or cohort studies of adults, aged ≥15 years, that reported the prevalence of at least one of the inherited thrombophilias of interest (FVL, PTM, PCD, PSD, or ATD) in both subjects with a history of arterial ischemic stroke and subjects without arterial ischemic stroke. Both prospective and retrospective studies were included. Studies were required to have ≥10 subjects in each group. Studies that enrolled patients with transient ischemic attack, hemorrhagic stroke, cerebral venous sinus thrombosis, and other arterial thromboses were excluded unless data for arterial ischemic stroke could be disaggregated. Studies that included neonates or children were also excluded.
If multiple studies used the same or overlapping samples, we included only the one with the largest sample size in the quantitative analysis. Cohen's κ coefficient was calculated to evaluate interobserver agreement for study selection.
We did not attempt to control for method used to diagnose thrombophilia, nor did we limit how the control population was constituted, assuming it appeared to be a valid comparator group.
Data Extraction
Two authors (T.C., E.D.) independently extracted data from included studies in duplicate using a standardized evidence table. Discrepancies were resolved by consensus or a third reviewer (A.C.) when necessary. The following data were collected: study period, country of study, number of cases and controls, case and control identification method, method of stroke diagnosis, matched variables for cases and controls, baseline characteristics of cases and controls (eg, age, sex, ethnicity, and cardiovascular risk factors), type(s) of thrombophilia reported, methods and timing of thrombophilia testing, and number of cases and controls testing positive and negative for each type of thrombophilia.
Quality Assessment
Methodological quality assessment was performed independently by 2 authors (T.C., E.D.) using either the National Institutes of Health–National Heart, Lung, and Blood Institute Quality Assessment of Case‐Control Studies assessment tool10 or the National Institutes of Health–National Heart, Lung, and Blood Institute Quality Assessment for Observational Cohort and Cross‐Sectional Studies assessment tool,11 as appropriate. Studies were categorized by their risk of bias as good, fair, or poor quality. Any differences in quality rating were resolved by consensus or adjudication by a third reviewer (A.C.).
Statistical Analysis
Data analysis was performed using R, Version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria). Pooled odds ratios (ORs) and 95% CIs were calculated using the bayesian method with random‐effects model. Interstudy heterogeneity was evaluated using the Cochran Q test and I2 statistic. A Cochran Q test P<0.05 is considered significant for heterogeneity. An I2 value of 0% to 25% represents insignificant heterogeneity, 26% to 50% represents low heterogeneity, 51% to 75% represents moderate heterogeneity, and >75% represents high heterogeneity. For FVL and PTM, separate analyses for homozygosity and heterozygosity were performed if studies provided stratified data by zygosity status. Prespecified subgroup analyses were performed in young patients (aged <65 years), patients with a patent foramen ovale (PFO), and patients with cryptogenic stroke, where reported. Sensitivity analyses were performed between age‐matched versus non–age‐matched studies and studies among different continents. Funnel plots of OR versus SE and Egger's test for asymmetry were used to assess for the presence of publication bias. P<0.05 was considered statistically significant. When publication bias was detected, Copas selection model was used and adjusted pooled ORs were reported to estimate the effect of publication bias on the results.12
Results
Study Identification
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram is shown in Figure 1. A total of 1875 records were retrieved from the literature search. After screening by title and abstract, 1661 records were excluded. The remaining 214 references underwent full‐text review, 68 of which met eligibility criteria and were included in the analysis. These 68 studies collectively enrolled 11 916 stroke patients and 96 057 controls. All 68 studies were case‐control studies. We did not identify any cohort studies that met eligibility criteria. The complete list of included studies is provided in Supplemental References. The number of studies that reported on FVL, PTM, PCD, PSD, and ATD were 56, 45, 15, 17, and 12, respectively. There was excellent agreement between the 2 independent reviewers with respect to study selection (κ=0.96).
Figure 1.
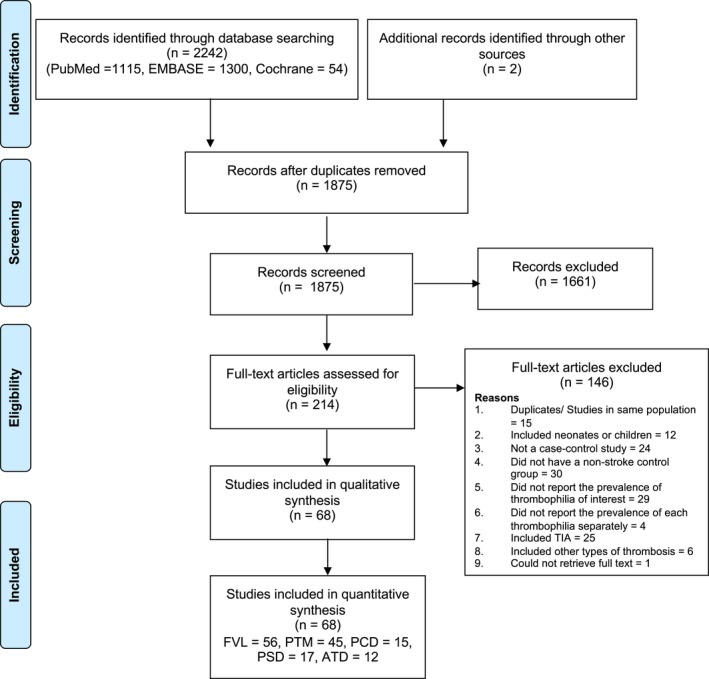
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram. ATD indicates antithrombin deficiency; FVL, factor V Leiden; PCD, protein C deficiency; PSD, protein S deficiency; PTM, prothrombin G20210A mutation; TIA, transient ischemic attack.
Study Characteristics
Characteristics of included studies are listed in Tables S1 and S2. The results from individual studies are listed in Tables S3 through S7. We included 64 case‐control and 4 nested case‐control studies. One study was published as a conference abstract.
The publication year of included studies ranged from 1993 to 2017. Twenty‐eight studies enrolled only young and middle‐aged adults, with an upper age limit ranging from 40 to 65 years. All included studies enrolled ≥20 cases and controls, with most (87% of studies) enrolling >40 subjects in each group. A few studies focused on specific subgroups with certain comorbidities, such as atrial fibrillation,13 HIV infection,14, 15 and systemic lupus erythematosus.16 Most studies recruited healthy subjects in the same geographic area as controls. In 4 studies, historical controls were used, whereas the remaining 64 studies recruited contemporaneous controls. Although most studies matched cases and controls by age and sex, only 4 studies matched by ethnicity and only 1 study matched controls for the presence of cardiovascular risk factors.17 A comparison of demographic data and clinical risk factors between cases and controls in each study is listed in Table S2. Most studies did not provide detailed information about clinical risk factors in the control group. When reported, clinical stroke risk factors, such as hypertension, diabetes mellitus, and smoking, were more frequent in cases than controls in most studies.
Ischemic stroke was diagnosed by neuroimaging in most studies. In 9 studies, the method of diagnosis was not described. One epidemiologic study used self‐reported history of stroke to define cases.18 Studies varied in terms of stroke subtypes included. Some exclusively enrolled cases with cryptogenic stroke,19, 20, 21, 22, 23, 24, 25 whereas in other studies, the proportion of cryptogenic stroke among cases ranged from 6% to 55% when reported. In 24 studies, only cases with first‐ever ischemic stroke were included. Forty‐one studies did not specify whether recurrent stroke was included, whereas 3 studies included cases of both first‐ever and recurrent stroke (16%–42% of cases), but did not provide disaggregated data for the recurrent stroke group.26, 27, 28 Almost all of the included studies reported use of standard and widely accepted test methods for the diagnosis of thrombophilia (Table S3 through S7).
Quality Appraisal
Using the National Institutes of Health–National Heart, Lung, and Blood Institute Quality Assessment of Case‐Control Studies tool, the included studies were rated as good (N=22), fair (N=43), and poor (N=3) quality. The studies with good, fair, and poor rating contributed 31%, 56%, and 13% of cases and 9%, 90%, and 1% of controls, respectively. Details of study quality assessment items for each study are reported in Table S8.
Studies with a good quality rating carry the least risk of bias. Studies were rated as fair quality when they were susceptible to some degree of bias. These included studies that did not recruit cases and controls from the same population, studies that did not match controls or did not adjust for confounders, and studies that did not specify valid and reliable methods of stroke diagnosis or thrombophilia testing. Studies were rated as poor quality when the definition of cases and controls was not explicitly described.
Genetic testing was used to identify FVL and PTM, whereas functional tests were used in most studies to identify PCD, PSD, and ATD. Protein C, protein S, and antithrombin levels may be reduced in the setting of anticoagulant therapy and acute thromboembolism. Eight of the studies excluded patients receiving anticoagulants, whereas 9 studies did not specifically mention anticoagulant use. All but 2 studies required testing at a distant time from the stroke event (with time frames ranging from 2 days to 6 months) or a second confirmatory test if the first one was abnormal. Although several studies reported blinding of exposure assessor to case/control status,17, 20, 25, 27, 29, 30, 31, 32, 33, 34, 35, 36 most did not specify whether the assessor was blinded.
Thrombophilia and Arterial Ischemic Stroke
The pooled ORs of arterial ischemic stroke for each thrombophilia are summarized in Figure 2.
Figure 2.
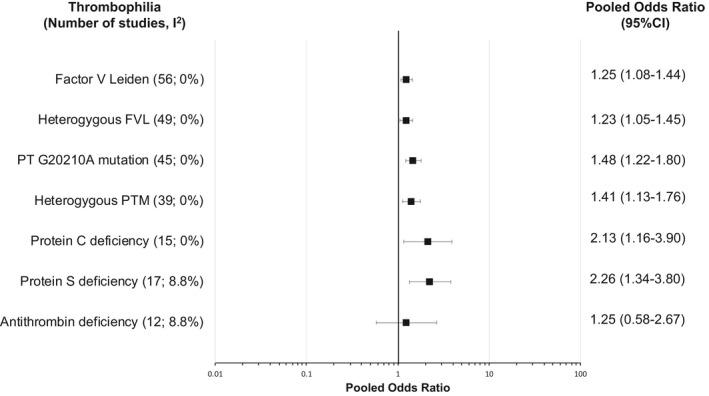
Forest plot showing association between inherited thrombophilia and risk of arterial ischemic stroke. The forest plot shows the results from the meta‐analysis for each type of thrombophilia and its association with arterial ischemic stroke. The pooled odds ratio (OR) is represented by the square box. The whiskers represent 95% CIs. The I2 statistic was used to evaluate study heterogeneity. FVL indicates factor V Leiden; PTM, prothrombin G20210A mutation.
Factor V Leiden
FVL was assessed in 56 studies (10 229 cases and 31 816 controls), 49 of which reported homozygosity and heterozygosity status. FVL, irrespective of zygosity status, was found in significantly more arterial ischemic stroke cases than controls, with a pooled OR of 1.25 (95% CI, 1.08–1.44). Heterogeneity among studies was insignificant (P=0.93; I2=0%). The forest plot is shown in Figure S1.
For homozygous FVL, the pooled OR was 0.72 (95% CI, 0.39–1.34; I2=0%) (Figure S2). Of 49 studies that tested for FVL, 33 (67%) did not identify homozygous FVL in any of the cases or controls. When such studies with zero events were excluded from the analysis, the pooled OR for homozygous FVL was 2.24 (95% CI, 1.26–4.71). For heterozygous FVL, the pooled OR was 1.23 (95% CI, 1.05–1.45; I2=0%) (Figure S3).
A funnel plot was symmetrical (Figure S4A) and Egger's test was nonsignificant (P=0.46), suggesting absence of publication bias.
Prothrombin G20210A mutation
PTM was assessed in 45 studies (7921 cases and 83 574 controls), 39 of which reported homozygosity and heterozygosity status. PTM, irrespective of zygosity status, was found in significantly more arterial ischemic stroke cases than controls, with a pooled OR of 1.48 (95% CI, 1.22–1.80). Heterogeneity among studies was insignificant (P=0.93; I2=0%). The forest plot is shown in Figure S5.
For homozygous PTM, the pooled OR was 0.31 (95% CI, 0.11–0.83; I2=35%) (Figure S6). Of 39 studies that tested for PTM, 31 (79%) did not identify homozygous PTM in any of the cases or controls. When such studies with zero events were excluded from the analysis, the pooled OR for homozygous PTM was 7.19 (95% CI, 2.47–20.94). For heterozygous PTM, the pooled OR was 1.41 (95% CI, 1.13–1.76; I2=0%) (Figure S7).
A funnel plot was symmetrical (Figure S4B) and Egger's test was nonsignificant (P=0.05), suggesting absence of publication bias.
Protein C deficiency
Protein C was measured in 15 studies (1676 cases and 11 895 controls). Of these studies, 7 excluded patients receiving anticoagulants, whereas 8 did not specifically mention anticoagulant use. PCD was found in significantly more arterial ischemic stroke cases than controls, with a pooled OR of 2.13 (95% CI, 1.16–3.90) (Figure S8). Heterogeneity among studies was insignificant (P=0.52; I2=0%). A funnel plot was symmetrical (Figure S4C) and Egger's test was nonsignificant (P=0.05), suggesting absence of publication bias.
Protein S deficiency
Protein S was measured in 16 studies (1803 cases and 6133 controls). Of these studies, 8 excluded patients receiving anticoagulants, whereas 8 did not specifically mention anticoagulant use. PSD was found in significantly more arterial ischemic stroke cases than controls, with a pooled OR of 2.26 (95% CI, 1.34–3.80) (Figure S9). Heterogeneity among studies was insignificant (P=0.31; I2=8.8%). A funnel plot was symmetrical (Figure S4D) and Egger's test was nonsignificant (P=0.45), suggesting absence of publication bias.
Antithrombin deficiency
Antithrombin was measured in 12 studies (1407 cases and 11 796 controls). Of these studies, 5 excluded patients receiving anticoagulants, whereas 7 did not specifically mention anticoagulant use. ATD was numerically more common in arterial ischemic stroke cases than controls, but statistical significance was not reached (pooled OR, 1.25; 95% CI, 0.58–2.67) (Figure S10). Heterogeneity among studies was insignificant (P=0.22; I2=8.8%). A funnel plot was asymmetrical (Figure S4E) and Egger's test was significant (P=0.01), suggesting possible publication bias. The pooled OR adjusted for publication bias using the Copas selection model was 1.39 (95% CI, 0.34–5.73).
Subgroup Analyses
We conducted prespecified subgroup analyses in young patients (aged ≤65 years), patients with a PFO, and patients with cryptogenic stroke. Results of these subgroup analyses for each thrombophilia are summarized in Figure 3.
Figure 3.
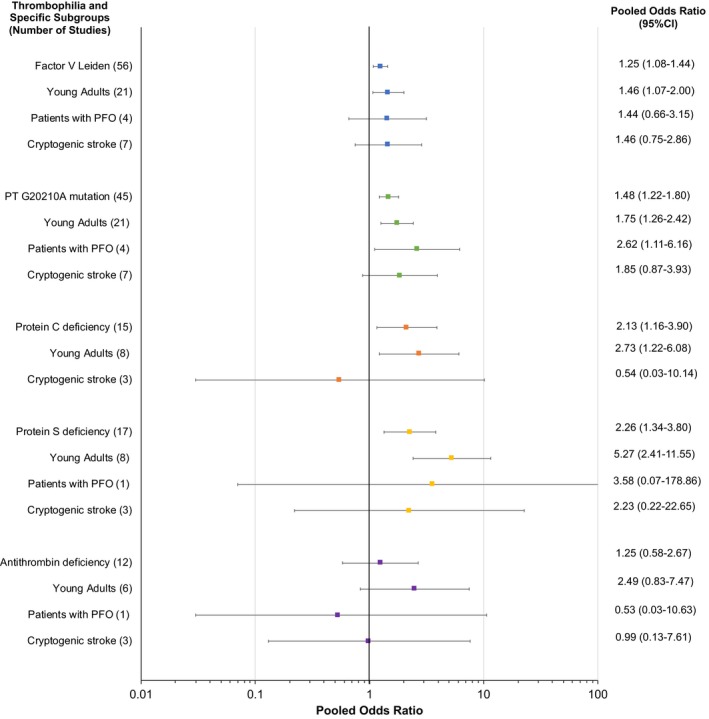
Forest plot showing pooled odds ratio (OR) for each thrombophilia in specific subgroups of patients. The forest plot shows the results from the prespecified subgroup analyses for each type of thrombophilia. The pooled ORs are represented by the square boxes. The horizontal lines represent the 95% CIs. PFO indicates patent foramen ovale.
Young patients
Twenty‐eight studies exclusively enrolled young patients (aged ≤65 years). In the subgroup of young patients, the association of FVL, PTM, PCD, and PSD and arterial ischemic stroke remained significant. In general, the pooled ORs for young patients were greater than the overall pooled ORs across all thrombophilias (Figure 3 and Figures S11 through S15).
Patients with PFO
Two studies25, 37 exclusively enrolled patients with PFO, whereas two21, 38 reported disaggregated data for cases with and without PFO. A significant association between thrombophilia and arterial ischemic stroke was not detected in the subgroups of patients with PFO, except for PTM (OR, 2.62; 95% CI, 1.11–6.16) (Figure 3 and Figures S11 through S15).
Patients with cryptogenic stroke
Seven studies19, 20, 21, 22, 23, 24, 25 exclusively enrolled patients with cryptogenic stroke. A significant association between thrombophilia and arterial ischemic stroke was not detected in the subgroups of patients with cryptogenic stroke (Figure 3 and Figures S11 through S15).
Sensitivity Analyses
We prespecified sensitivity analyses according to geographic region and whether studies used age‐matched versus non–age‐matched controls.
Age‐matched versus unmatched controls
The number of studies with and without age‐matched controls and their corresponding pooled ORs for each thrombophilia are shown in Table 1. In general, pooled ORs were similar irrespective of whether studies used age‐matched or non–age‐matched controls. However, significant associations were found in studies with age‐matched controls only.
Table 1.
Sensitivity Analysis of Studies That Used Age‐Matched versus Non–Age‐Matched Controls
| Thrombophilia | Age‐Matched Studies | Non–Age‐Matched Studies | ||
|---|---|---|---|---|
| No. of Studies | Pooled OR (95% CI) | No. of Studies | Pooled OR (95% CI) | |
| FVL | 24 | 1.58 (1.16–2.15)* | 32 | 1.09 (0.92–1.28) |
| Homozygous FVL | 19 | 1.18 (0.51–2.71) | 30 | 0.42 (0.17–1.08) |
| Heterozygous FVL | 19 | 1.69 (1.19–2.40)* | 30 | 1.07 (0.89–1.28) |
| PT G20210A mutation | 22 | 1.86 (1.38–2.49)* | 23 | 1.21 (0.90–1.61) |
| Homozygous PTM | 18 | 0.27 (0.06–1.11) | 21 | 0.33 (0.08–1.42) |
| Heterozygous PTM | 18 | 1.91 (1.35–2.70)* | 21 | 1.10 (0.79–1.53) |
| Protein C deficiency | 9 | 2.54 (1.21–5.37)* | 6 | 1.39 (0.44–4.33) |
| Protein S deficiency | 11 | 2.28 (1.21–4.33)* | 6 | 2.30 (0.95–5.59) |
| Antithrombin deficiency | 7 | 1.73 (0.70–4.28) | 5 | 0.47 (0.09–2.38) |
FVL indicates factor V Leiden; OR, odds ratio; PTM, prothrombin G20210A mutation.
Significant association.
Geographic region
Most studies were conducted in Europe (50%), Asia (19%), and North America (17%), with a smaller number from Africa (6%), Australia (3%), and South America (3%). Results were fairly consistent across geographic regions, except for the notably higher ORs for PCD and PSD in studies conducted in Asia (Table 2).
Table 2.
Sensitivity Analysis by Study Region
| Thrombophilia | Africa | Asia | Australia | Europe | North America | South America | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Pooled OR (95% CI) | No. of Studies | Pooled OR (95% CI) | No. of Studies | Pooled OR (95% CI) | No. of Studies | Pooled OR (95% CI) | No. of Studies | Pooled OR (95% CI) | No. of Studies | Pooled OR (95% CI) | |
| FVL | 1 | 0.00 (0.00–259.67) | 11 | 1.68 (1.08–2.61)* | 2 | 1.45 (0.37–5.70) | 33 | 1.31 (1.08–1.58)* | 7 | 0.78 (0.52–1.18) | 2 | 1.31 (0.39–4.33) |
| Homozygous FVL | 1 | 0.00 (0.00–197.19) | 11 | 1.15 (0.37–3.61) | 1 | 0.00 (0.00–201.44) | 29 | 0.72 (0.35–1.50) | 5 | 0.00 (0.00–23.31) | 2 | 0.00 (0.00–116.06) |
| Heterozygous FVL | 1 | 0.00 (0.00–678.42) | 11 | 1.51 (0.99–2.29) | 1 | 0.30 (0.01–6.66) | 29 | 1.32 (1.05–1.67)* | 5 | 0.68 (0.37–1.27) | 2 | 1.32 (0.40–4.36) |
| PTM | 1 | 2.32 (0.54–9.68) | 7 | 0.90 (0.45–1.78) | 2 | 2.33 (0.54–10.07) | 28 | 1.57 (1.21–2.05)* | 6 | 1.35 (0.77–2.37) | 1 | 2.00 (0.39–10.23) |
| Homozygous PTM | 1 | 0.00 (0.00–243.44) | 7 | 0.18 (0.01–2.59) | 1 | 0.00 (0.00–160.03) | 24 | 0.34 (0.10–1.13) | 5 | 0.36 (0.02–6.16) | 1 | 0.00 (0.00–160.03) |
| Heterozygous PTM | 1 | 2.29 (0.54–9.76) | 7 | 0.81 (0.39–1.67) | 1 | 8.37 (0.23–308.69) | 24 | 1.52 (1.12–2.08)* | 5 | 1.23 (0.64–2.37) | 1 | 2.04 (0.40–10.36) |
| Protein C deficiency | 2 | 3.91 (0.75–20.44) | 4 | 4.94 (1.52–16.06)* | 1 | 0.61 (0.09–4.19) | 7 | 1.28 (0.46–3.55) | ··· | ··· | ··· | ··· |
| Protein S deficiency | 3 | 1.83 (0.69–4.83) | 4 | 7.46 (2.43–22.93)* | 1 | 0.73 (0.07–7.59) | 7 | 1.96 (0.86–4.43) | 1 | 1.05 (0.28–4.02) | ··· | ··· |
| Antithrombin deficiency | 1 | 5.22 (0.91–30.00) | 3 | 0.60 (0.06–6.21) | 1 | 1.26 (0.29–5.44) | 7 | 0.75 (0.22–2.51) | ··· | ··· | ··· | ··· |
FVL indicates factor V Leiden; OR, odds ratio; PTM, prothrombin G20210A mutation.
Significant association.
First‐ever ischemic stroke
After the analysis was restricted to the 24 studies that exclusively enrolled cases with first‐ever ischemic stroke, the association with arterial ischemic stroke remained significant for PTM (OR, 1.46; 95% CI, 1.10–2.00) and PSD (OR, 3.58; 95% CI 1.12–11.42), but not for FVL (OR, 1.16; 95% CI, 0.92–1.47) or PCD (OR, 1.62; 95% CI, 0.51–5.40).
Additional sensitivity analyses
Sensitivity analyses were performed by excluding each of the following: studies with enriched case population (those who were referred for thrombophilia testing because of a clinical indication or recruited from a thrombophilia center),19, 31, 39 studies that used self‐reported history of stroke rather than imaging to define cases,18 studies that were rated as poor quality,40, 41, 42 and studies that reported inclusion of cases of recurrent ischemic stroke (but including studies that failed to report whether recurrent ischemic stroke was included or not).26, 27, 28 After each of these exclusions, the association of FVL, PTM, PCD, and PSD with arterial ischemic stroke remained significant, with similar pooled OR to the original analysis (Table S9).
Discussion
The results from our systematic review and meta‐analysis suggest that inherited thrombophilias, including FVL, PTM, PCD, and PSD, are associated with a significant but small increase in the risk of arterial ischemic stroke in adults (Figure 2), particularly in young patients (Figure 3). When studies with zero events in both groups were excluded from analysis, the association of FVL and PTM was stronger in the homozygous than in the heterozygous state, suggesting a potential dose‐response relationship and a causal role for inherited thrombophilia in arterial ischemic stroke.
Arterial ischemic stroke is a multicausal disease that involves complex interactions of genetic and environmental risk factors. Several lines of evidence implicate the coagulation pathway in the pathophysiological characteristics of arterial ischemic stroke. Increased levels of clotting proteins, such as factor VIII and factor XI, have been posited as independent risk factors for ischemic stroke.43, 44 Conversely, congenital deficiency of factors VIII, IX, and XI is protective against stroke and cardiovascular disease.45, 46 Anticoagulants reduce the risk of ischemic stroke. Compared with aspirin (the “standard of practice” in many studies for prevention of first or recurrent stroke), warfarin is noninferior for the secondary prevention of noncardioembolic ischemic stroke.47 Although rivaroxaban was not superior to aspirin in preventing recurrence after embolic stroke of undetermined source,48 the addition of rivaroxaban to aspirin reduced cardiovascular events, including stroke, in patients with stable atherosclerosis.49 Extended‐duration treatment with betrixaban for prevention of venous thrombosis among hospitalized medically ill patients reduced the risk of subsequent stroke.50
Although inherited thrombophilias have not been traditionally recognized as risk factors for arterial thrombosis,7 there are several potential mechanisms by which they could contribute to arterial ischemic stroke. First, ischemic stroke may arise in the setting of deep vein thrombosis and subsequent paradoxical embolism via a PFO. In a prespecified subgroup analysis of subjects with PFO in our study, ischemic stroke was significantly associated with PTM, but not with other thrombophilias (Figure 3), possibly because of the limited number of studies in which PFO status was assessed. A previous meta‐analysis focusing on patients with PFO yielded similar results.51 Second, the unbalanced thrombin activation in individuals with inherited thrombophilia may contribute to formation and progression of atherosclerotic lesions through various mechanisms, including platelet activation, endothelial and vascular smooth muscle cell dysregulation, and recruitment of monocytes and macrophages.52, 53
For FVL and PTM, our results are consistent with previous meta‐analyses. One report included 15 studies in FVL (pooled OR, 1.27; 95% CI, 0.86–1.87) and 10 studies in PTM (pooled OR, 1.30; 95% CI, 0.91–1.87).54 The association was more robust in young patients (aged <55 years). In another meta‐analysis,55 only studies that enrolled young adults (aged <50 years) were included. Among the 18 eligible studies, FVL was significantly associated with ischemic stroke (pooled OR, 1.89; 95% CI, 1.31–2.72). Although a meta‐analysis of PCD, PSD, and ATD has not previously been performed in adults, a meta‐analysis in children with arterial ischemic stroke identified a significant association with PCD (OR, 11.0; 95% CI, 5.13–23.59), but not with PSD (OR, 1.49; 95% CI, 0.32–6.92) or ATD (OR, 3.29; 95% CI, 0.70–15.48).56
Genome‐wide association studies have identified genetic loci associated with stroke,57, 58 many of which share associations with other cardiovascular diseases, such as hypertension, atrial fibrillation, coronary artery disease, and venous thromboembolism. In the MEGASTROKE study, the weighted genetic risk score for venous thromboembolism was significantly associated with large‐artery atherosclerotic stroke and cardioembolic stroke, but not small‐vessel stroke.57 However, none of the inherited thrombophilias we investigated in the present study was significantly associated with stroke in genome‐wide association studies. This could be, in part, because of the inadequate statistical power to detect an association with rare variants in genome‐wide association studies, allelic heterogeneity inherent in certain thrombophilias (PCD, PSD, and ATD), and/or heterogeneity in stroke subtypes and ethnicity of the study populations. Interestingly, data extracted from multiple genome‐wide association studies have shown that genetic variants indicative of high protein C level were associated with lower risk of coronary artery disease/myocardial infarction,59 suggesting a potential role for natural anticoagulants in the pathogenesis of arterial thrombosis. A similar analysis for arterial ischemic stroke would be an insightful topic for future studies.
Among the studies included in our analysis, interstudy heterogeneity was low, with I2 values ranging from 0% to 35%, suggesting that the results could appropriately be combined. Sources of heterogeneity among studies included the following: study population (number of participants, age groups, geographic region and ethnicity, baseline clinical risk of stroke, and presence of comorbidities); outcome measurement (methods of stroke diagnosis and types of stroke included); and exposure measurement (thrombophilia test methods, timing of testing after stroke in cases, and exclusion of patients taking anticoagulants).
From our sensitivity analysis by study region, the ORs for PCD and PSD were notably higher in studies conducted in Asia than other regions (Table 2). These disparities could be, in part, because of differences in the prevalence of inherited thrombophilias in different regions. For example, PCD, PSD, and ATD have been reported to be more common in the Asian population than in whites.60, 61, 62 In one included study from Taiwan,63 the prevalence of these natural anticoagulant deficiencies was distinctly high, affecting 27% of the cases.
Our study has several limitations. First, because this is a meta‐analysis of case‐control studies, the results may be affected by biases inherent to case‐control studies, including selection bias and misclassification bias. In a small number of studies, controls were not drawn from the same population as cases. For instance, cases were recruited from patients referred for clinical thrombophilia testing, whereas controls were recruited from a population without a history of thrombosis in 3 studies.19, 31, 39 In such studies, the presence of inherited thrombophilia in the cases may be overrepresented because of selection bias. In most studies in which clinical stroke risk factors were reported in both cases and controls, the risk factors were more prevalent in cases than controls. These imbalances could have confounded the results of these studies. Moreover, cases with recurrent stroke were included in a few studies,26, 27, 28 possibly resulting in overrepresentation of thrombophilia in the cases for such studies. However, a sensitivity analysis excluding these 3 studies reassuringly yielded similar results to the original analysis. Misclassification of exposure status could have arisen if the exposure assessors were not blinded or if there were confounders that influenced the results of thrombophilia testing. This is especially true in the case of natural anticoagulant deficiencies (PCD, PSD, and ATD), where thrombophilia status was defined by phenotypic assays as opposed to genetic testing. Acute thrombosis, including stroke, may cause acquired natural anticoagulant deficiencies and lead to the appearance of higher frequencies of such conditions in stroke cases. However, most studies avoided this issue by requiring repeated testing after the short‐term phase to define deficiencies. The use of anticoagulants and the presence of certain medical conditions (eg, liver disease) can also cause acquired deficiencies of natural anticoagulants. Attempts to account for these factors varied between studies. Second, we were not able to perform subgroup analyses by ethnicity or stroke subtype because of a lack of disaggregated data for these variables. Finally, although we found a significant association between inherited thrombophilia and ischemic stroke, this cannot be taken as evidence of a causal relationship nor can it be considered supportive of thrombophilia testing in clinical practice. Further studies are needed to determine whether thrombophilia testing in patients with otherwise unexplained arterial ischemic stroke is beneficial and whether and how the results should influence management.
Despite its limitations, our study has several strengths. First, our meta‐analysis included the largest number of studies and participants to date. Second, to minimize publication bias, our literature search included “gray literature,” such as conference abstracts and letters to editors. Third, the included studies originated from a wide range of geographic regions and the results may, therefore, be applicable to clinicians and patients around the world.
Conclusions
Our systematic review and meta‐analysis demonstrates an association between multiple inherited thrombophilias and the risk of arterial ischemic stroke in adults. Further studies are needed to determine whether inherited thrombophilias have an impact on clinical outcomes, such as recurrent stroke, and whether the finding of inherited thrombophilia should influence clinical management of patients with arterial ischemic stroke.
Disclosures
None.
Supporting information
Data S1. Supplemental methods: search strategies.
Table S1. Characteristics of Included Studies: Types of Thrombophilias, Numbers of Participants, and Study Population
Table S2. Characteristics of Included Studies: Demographic Data of Cases and Controls
Table S3. Results of Included Studies: Factor V Leiden
Table S4. Results of Included Studies: Prothrombin G20210A Mutation
Table S5. Results of Included Studies: Protein C Deficiency
Table S6. Results of Included Studies: Protein S Deficiency
Table S7. Results of Included Studies: Antithrombin Deficiency
Table S8. Components of Quality Assessment
Table S9. Additional Sensitivity Analyses
Figure S1. Forest plot showing pooled odds ratio for Factor V Leiden.
Figure S2. Forest plot showing pooled odds ratio for Factor V Leiden (Homozygous).
Figure S3. Forest plot showing pooled odds ratio for Factor V Leiden (Heterozygous).
Figure S4. Funnel plot of included studies.
Figure S5. Forest plot showing pooled odds ratio for prothrombin G20210A mutation.
Figure S6. Forest plot showing pooled odds ratio for prothrombin G20210A mutation (homozygous).
Figure S7. Forest plot showing pooled odds ratio for Factor V Leiden (heterozygous).
Figure S8. Forest plot showing pooled odds ratio for protein C deficiency.
Figure S9. Forest plot showing pooled odds ratio for protein S deficiency.
Figure S10. Forest plot showing pooled odds ratio for antithrombin deficiency.
Figure S11. Subgroup analyses: Factor V Leiden.
Figure S12. Subgroup analyses: prothrombin G20210A mutation.
Figure S13. Subgroup analyses: protein C deficiency.
Figure S14. Subgroup analyses: protein S deficiency.
Figure S15. Subgroup analyses: antithrombin deficiency.
(J Am Heart Assoc. 2019;8:e012877 DOI: 10.1161/JAHA.119.012877.)
References
- 1. Middeldorp S, Meinardi JR, Koopman MM, van Pampus EC, Hamulyak K, van Der Meer J, Prins MH, Buller HR. A prospective study of asymptomatic carriers of the factor V Leiden mutation to determine the incidence of venous thromboembolism. Ann Intern Med. 2001;135:322–327. [DOI] [PubMed] [Google Scholar]
- 2. Coppens M, van de Poel MH, Bank I, Hamulyak K, van der Meer J, Veeger NJ, Prins MH, Buller HR, Middeldorp S. A prospective cohort study on the absolute incidence of venous thromboembolism and arterial cardiovascular disease in asymptomatic carriers of the prothrombin 20210A mutation. Blood. 2006;108:2604–2607. [DOI] [PubMed] [Google Scholar]
- 3. Omran SS, Lerario MP, Gialdini G, Merkler AE, Moya A, Chen ML, Kamel H, DeSancho M, Navi BB. Clinical impact of thrombophilia screening in young adults with ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim K, Cox N, Witt DM. Stroke diagnosis associated with thrombophilia testing overutilization. Thromb Res. 2017;157:139–141. [DOI] [PubMed] [Google Scholar]
- 5. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie‐Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 6. Cox N, Johnson SA, Vazquez S, Fleming RP, Rondina MT, Kaplan D, Chauv S, Fontaine GV, Stevens SM, Woller S, Witt DM. Patterns and appropriateness of thrombophilia testing in an academic medical center. J Hosp Med. 2017;12:705–709. [DOI] [PubMed] [Google Scholar]
- 7. Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke: can it cause more harm than good? Stroke. 2010;41:2985–2990. [DOI] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting: meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 10. NHLBI and Research Triangle Institute International . Study quality assessment tools: quality assessment of case‐control studies. 2018. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed August 14, 2018.
- 11. NHLBI and Research Triangle Institute International . Study quality assessment tools: quality assessment tool for observational cohort and cross‐sectional studies. 2018. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed August 14, 2018.
- 12. Copas JB, Shi JQ. A sensitivity analysis for publication bias in systematic reviews. Stat Methods Med Res. 2001;10:251–265. [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Reed GL, Hylek EM, Phillips KA, Liu L, Henault LE, Selby JV, Singer DE. Factor V Leiden and risk of ischemic stroke in nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. J Thromb Thrombolysis. 2003;15:41–46. [DOI] [PubMed] [Google Scholar]
- 14. Mochan AM, Modi M, Modi G. Protein S deficiency in HIV associated ischaemic stroke: an epiphenomenon of HIV infection. J Neurol Neurosurg Psychiatry. 2005;76:1455–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zimba S, Ntanda PM, Lakhi S, Atadzhanov M. HIV infection, hypercoagulability and ischaemic stroke in adults at the University Teaching Hospital in Zambia: a case control study. BMC Infect Dis. 2017;17:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pullmann R Jr, Skerenova M, Lukac J, Hybenova J, Melus V, Kubisz P, Rovensky J, Pullmann R. Factor V Leiden and prothrombin G20210A mutations and the risk of atherothrombotic events in systemic lupus erythematosus. Clin Appl Thromb Hemost. 2004;10:233–238. [DOI] [PubMed] [Google Scholar]
- 17. Eterovic D, Titlic M, Culic V, Zadro R, Primorac D. Lower contribution of factor V Leiden or G202104 mutations to ischemic stroke in patients with clinical risk factors: pair‐matched case‐control study. Clin Appl Thromb Hemost. 2007;13:188–193. [DOI] [PubMed] [Google Scholar]
- 18. Fan AZ, Fang J, Yesupriya A, Chang M, Kilmer G, House M, Hayes D, Ned RM, Dowling NF, Mokdad AH. Gene polymorphisms in association with self‐reported stroke in US adults. Appl Clin Genet. 2010;3:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aznar J, Mira Y, Vaya A, Corella D, Ferrando F, Villa P, Estelles A. Factor V Leiden and prothrombin G20210A mutations in young adults with cryptogenic ischemic stroke. Thromb Haemost. 2004;91:1031–1034. [DOI] [PubMed] [Google Scholar]
- 20. Belvis R, Santamaria A, Marti‐Fabregas J, Cocho D, Borrell M, Fontcuberta J, Marti‐Vilalta JL. Diagnostic yield of prothrombotic state studies in cryptogenic stroke. Acta Neurol Scand. 2006;114:250–253. [DOI] [PubMed] [Google Scholar]
- 21. Favaretto E, Sartori M, Conti E, Legnani C, Palareti G. G1691A factor V and G20210A FII mutations, acute ischemic stroke of unknown cause, and patent foramen ovale. Thromb Res. 2012;130:720–724. [DOI] [PubMed] [Google Scholar]
- 22. Haeusler KG, Herm J, Hoppe B, Kasabov R, Malzahn U, Endres M, Koscielny J, Jungehulsing GJ. Thrombophilia screening in young patients with cryptogenic stroke: prevalence of gene polymorphisms compared to healthy blood donors and impact on secondary stroke prevention. Hamostaseologie. 2012;32:147–152. [DOI] [PubMed] [Google Scholar]
- 23. Halbmayer WM, Haushofer A, Schon R, Fischer M. The prevalence of poor anticoagulant response to activated protein C (APC resistance) among patients suffering from stroke or venous thrombosis and among healthy subjects. Blood Coagul Fibrinolysis. 1994;5:51–57. [DOI] [PubMed] [Google Scholar]
- 24. Karakuş Z, Gürkan E, Başlamişli F, Tanriverdi K. Prothrombotic heritable risk factors for cerebral ischemic infarction, acute myocardial infarction and venous thrombosis in young adult Turkish patients. Ann Med Sci. 2005;14:31–36. [Google Scholar]
- 25. Karttunen V, Hiltunen L, Rasi V, Vahtera E, Hillbom M. Factor V Leiden and prothrombin gene mutation may predispose to paradoxical embolism in subjects with patent foramen ovale. Blood Coagul Fibrinolysis. 2003;14:261–268. [DOI] [PubMed] [Google Scholar]
- 26. Chatterjee T, Gupta N, Choudhry VP, Behari M, Saxena R, Ashraf MZ. Prediction of ischemic stroke in young Indians: is thrombophilia profiling a way out? Blood Coagul Fibrinolysis. 2013;24:449–453. [DOI] [PubMed] [Google Scholar]
- 27. Kumar A, Misra S, Sagar R, Kumar P, Yadav A, Talwar P, Raj R, Prasad K. Relationship between factor V Leiden gene variant and risk of ischemic stroke: a case‐control study. Ann Indian Acad Neurol. 2017;20:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. They‐They TP, Battas O, Slassi I, Rafai MA, Katumbay DT, Nadifi S. Prothrombin G20210A and factor V Leiden polymorphisms in stroke. J Mol Neurosci. 2012;46:210–216. [DOI] [PubMed] [Google Scholar]
- 29. De Lucia D, d'Alessio D, Pezzella S, Maisto G, Di Mauro C, Marotta R, Del Giudice V, Iacoviello L. A hypercoagulable state in activated protein C resistant patients with ischemic stroke. Int J Clin Lab Res. 1998;28:74–75. [DOI] [PubMed] [Google Scholar]
- 30. Longstreth WT Jr, Rosendaal FR, Siscovick DS, Vos HL, Schwartz SM, Psaty BM, Raghunathan TE, Koepsell TD, Reitsma PH. Risk of stroke in young women and two prothrombotic mutations: factor V Leiden and prothrombin gene variant (G20210A). Stroke. 1998;29:577–580. [DOI] [PubMed] [Google Scholar]
- 31. Pahus SH, Hansen AT, Hvas AM. Thrombophilia testing in young patients with ischemic stroke. Thromb Res. 2016;137:108–112. [DOI] [PubMed] [Google Scholar]
- 32. Petrovic D, Milanez T, Kobal J, Bregar D, Potisk KP, Peterlin B. Prothrombotic gene polymorphisms and atherothrombotic cerebral infarction. Acta Neurol Scand. 2003;108:109–113. [DOI] [PubMed] [Google Scholar]
- 33. Rubattu S, Di Angelantonio E, Nitsch D, Gigante B, Zanda B, Stanzione R, Evangelista A, Pirisi A, Rosati G, Volpe M. Polymorphisms in prothrombotic genes and their impact on ischemic stroke in a Sardinian population. Thromb Haemost. 2005;93:1095–1100. [DOI] [PubMed] [Google Scholar]
- 34. Slooter AJC, Rosendaal FR, Tanis BC, Kemmeren JM, Van Der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005;3:1213–1217. [DOI] [PubMed] [Google Scholar]
- 35. Smiles AM, Jenny NS, Tang Z, Arnold A, Cushman M, Tracy RP. No association of plasma prothrombin concentration or the G20210A mutation with incident cardiovascular disease: results from the Cardiovascular Health Study. Thromb Haemost. 2002;87:614–621. [PubMed] [Google Scholar]
- 36. Szolnoki Z, Somogyvari F, Kondacs A, Szabo M, Fodor L, Bene J, Melegh B. Evaluation of the modifying effects of unfavourable genotypes on classical clinical risk factors for ischaemic stroke. J Neurol Neurosurg Psychiatry. 2003;74:1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wypasek E, Stepien E, Pieculewicz M, Podolec P, Undas A. Factor XIII Val34Leu polymorphism and ischaemic stroke in patients with patent foramen ovale. Thromb Haemost. 2009;102:1280–1282. [DOI] [PubMed] [Google Scholar]
- 38. Pezzini A, Grassi M, Del Zotto E, Archetti S, Spezi R, Vergani V, Assanelli D, Caimi L, Padovani A. Cumulative effect of predisposing genotypes and their interaction with modifiable factors on the risk of ischemic stroke in young adults. Stroke. 2005;36:533–539. [DOI] [PubMed] [Google Scholar]
- 39. Martinelli I, Battaglioli T, Burgo I, Di Domenico S, Mannucci PM. Oral contraceptive use, thrombophilia and their interaction in young women with ischemic stroke. Haematologica. 2006;91:844–847. [PubMed] [Google Scholar]
- 40. Halbmayer WM, Haushofer A, Hermann KM, Fischer M. The 20210A allele of the prothrombin gene: a risk factor for juvenile stroke? Result of a pilot study. Blood Coagul Fibrinolysis. 1998;9:209–210. [PubMed] [Google Scholar]
- 41. Tupitsyna TV, Bondarenko EA, Kravchenko SA, Tatarskyy PF, Shetova IM, Shamalov NA, Kuznetsova SM, Shul'Zhenko DV, Skvortsova VI, Slominsk PA, Livshits LA, Limborska SA. Comparative analysis of associations between polymorphic variants of the F2, F5, GP1BA, and ACE genes and the risk of developing stroke in Russian and Ukrainian populations. Mol Gen Microbiol Virol. 2013;28:8–14. [PubMed] [Google Scholar]
- 42. Romdhane NB, Baccouche H, Lahmar M, Mahjoub S, Manai Z. Deficiency of coagulation inhibitors in young adults with ischemic stroke. J Thromb Haemost. 2011;9:899.21342431 [Google Scholar]
- 43. Suri MF, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Cerebrovasc Dis. 2010;29:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zakai NA, Judd SE, Kissela B, Howard G, Safford MM, Cushman M. Factor VIII, protein C and cardiovascular disease risk: the reasons for geographic and racial differences in stroke study (REGARDS). Thromb Haemost. 2018;118:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salomon O, Steinberg DM, Koren‐Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. [DOI] [PubMed] [Google Scholar]
- 46. Sood SL, Cheng D, Ragni M, Kessler CM, Quon D, Shapiro AD, Key NS, Manco‐Johnson MJ, Cuker A, Kempton C, Wang TF, Eyster ME, Kuriakose P, von Drygalski A, Gill JC, Wheeler A, Kouides P, Escobar MA, Leissinger C, Galdzicka S, Corson M, Watson C, Konkle BA. A cross‐sectional analysis of cardiovascular disease in the hemophilia population. Blood Adv. 2018;2:1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP Jr, Jackson CM, Pullicino P; Warfarin‐Aspirin Recurrent Stroke Study Group . A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. [DOI] [PubMed] [Google Scholar]
- 48. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, Swaminathan B, Lavados P, Wang Y, Wang Y, Davalos A, Shamalov N, Mikulik R, Cunha L, Lindgren A, Arauz A, Lang W, Czlonkowska A, Eckstein J, Gagliardi RJ, Amarenco P, Ameriso SF, Tatlisumak T, Veltkamp R, Hankey GJ, Toni D, Bereczki D, Uchiyama S, Ntaios G, Yoon BW, Brouns R, Endres M, Muir KW, Bornstein N, Ozturk S, O'Donnell MJ, De Vries Basson MM, Pare G, Pater C, Kirsch B, Sheridan P, Peters G, Weitz JI, Peacock WF, Shoamanesh A, Benavente OR, Joyner C, Themeles E, Connolly SJ; NAVIGATE ESUS Investigators . Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378:2191–2201. [DOI] [PubMed] [Google Scholar]
- 49. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez‐Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Stork S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp‐Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 50. Gibson CM, Chi G, Halaby R, Korjian S, Daaboul Y, Jain P, Arbetter D, Goldhaber SZ, Hull R, Hernandez AF, Gold A, Bandman O, Harrington RA, Cohen AT; APEX Investigators . Extended‐duration betrixaban reduces the risk of stroke versus standard‐dose enoxaparin among hospitalized medically ill patients: an APEX trial substudy (acute medically ill venous thromboembolism prevention with extended duration betrixaban). Circulation. 2017;135:648–655. [DOI] [PubMed] [Google Scholar]
- 51. Lichy C, Padovani A, Magoni M, Grau A, Costa P, Volonghi I, Giossi A, Zotto ED, Grassi M, Pezzini A. Do common prothrombotic mutations influence the risk of cerebral ischaemia in patients with patent foramen ovale? Thromb Haemost. 2017;101:813–817. [PubMed] [Google Scholar]
- 52. Borissoff JI, Spronk HM, Heeneman S, ten Cate H. Is thrombin a key player in the “coagulation‐atherogenesis” maze? Cardiovasc Res. 2009;82:392–403. [DOI] [PubMed] [Google Scholar]
- 53. Martorell L, Martinez‐Gonzalez J, Rodriguez C, Gentile M, Calvayrac O, Badimon L. Thrombin and protease‐activated receptors (PARs) in atherothrombosis. Thromb Haemost. 2008;99:305–315. [DOI] [PubMed] [Google Scholar]
- 54. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta‐analysis of published studies. Am Heart J. 2003;146:948–957. [DOI] [PubMed] [Google Scholar]
- 55. Hamedani AG, Cole JW, Mitchell BD, Kittner SJ. Meta‐analysis of factor V Leiden and ischemic stroke in young adults: the importance of case ascertainment (provisional abstract). Stroke. 2010;41:1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kenet G, Lutkhoff LK, Albisetti M, Bernard T, Bonduel M, Brandao L, Chabrier S, Chan A, deVeber G, Fiedler B, Fullerton HJ, Goldenberg NA, Grabowski E, Gunther G, Heller C, Holzhauer S, Iorio A, Journeycake J, Junker R, Kirkham FJ, Kurnik K, Lynch JK, Male C, Manco‐Johnson M, Mesters R, Monagle P, van Ommen CH, Raffini L, Rostasy K, Simioni P, Strater RD, Young G, Nowak‐Gottl U. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta‐analysis of observational studies. Circulation. 2010;121:1838–1847. [DOI] [PubMed] [Google Scholar]
- 57. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten‐Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, et al. Multiancestry genome‐wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Network (SiGN); International Stroke Genetics Consortium (ISGC) . Loci associated with ischaemic stroke and its subtypes (SiGN): a genome‐wide association study. Lancet Neurol. 2016;15:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schooling CM, Zhong Y. Plasma levels of the anti‐coagulation protein C and the risk of ischaemic heart disease. Thromb Haemost. 2017;117:262–268. [DOI] [PubMed] [Google Scholar]
- 60. Miyata T, Maruyama K, Banno F, Neki R. Thrombophilia in East Asian countries: are there any genetic differences in these countries? Thromb J. 2016;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Angchaisuksiri P. Venous thromboembolism in Asia—an unrecognised and under‐treated problem? Thromb Haemost. 2011;106:585–590. [DOI] [PubMed] [Google Scholar]
- 62. Rojnuckarin P, Settapiboon R, Akkawat B, Teocharoen S, Suksusut A, Uaprasert N. Natural anticoagulant deficiencies in Thais: a population‐based study. Thromb Res. 2019;178:7–11. [DOI] [PubMed] [Google Scholar]
- 63. Chen WH, Lan MY, Chang YY, Chen SS, Liu JS. The prevalence of protein C, protein S, and antithrombin III deficiency in non‐APS/SLE Chinese adults with noncardiac cerebral ischemia. Clin Appl Thromb Hemost. 2003;9:155–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods: search strategies.
Table S1. Characteristics of Included Studies: Types of Thrombophilias, Numbers of Participants, and Study Population
Table S2. Characteristics of Included Studies: Demographic Data of Cases and Controls
Table S3. Results of Included Studies: Factor V Leiden
Table S4. Results of Included Studies: Prothrombin G20210A Mutation
Table S5. Results of Included Studies: Protein C Deficiency
Table S6. Results of Included Studies: Protein S Deficiency
Table S7. Results of Included Studies: Antithrombin Deficiency
Table S8. Components of Quality Assessment
Table S9. Additional Sensitivity Analyses
Figure S1. Forest plot showing pooled odds ratio for Factor V Leiden.
Figure S2. Forest plot showing pooled odds ratio for Factor V Leiden (Homozygous).
Figure S3. Forest plot showing pooled odds ratio for Factor V Leiden (Heterozygous).
Figure S4. Funnel plot of included studies.
Figure S5. Forest plot showing pooled odds ratio for prothrombin G20210A mutation.
Figure S6. Forest plot showing pooled odds ratio for prothrombin G20210A mutation (homozygous).
Figure S7. Forest plot showing pooled odds ratio for Factor V Leiden (heterozygous).
Figure S8. Forest plot showing pooled odds ratio for protein C deficiency.
Figure S9. Forest plot showing pooled odds ratio for protein S deficiency.
Figure S10. Forest plot showing pooled odds ratio for antithrombin deficiency.
Figure S11. Subgroup analyses: Factor V Leiden.
Figure S12. Subgroup analyses: prothrombin G20210A mutation.
Figure S13. Subgroup analyses: protein C deficiency.
Figure S14. Subgroup analyses: protein S deficiency.
Figure S15. Subgroup analyses: antithrombin deficiency.


