Abstract
Background
Acute psychological stress and negative emotions are known risk factors for atrial fibrillation (AF). Whether exposure to chronic stress syndromes, such as posttraumatic stress disorder (PTSD), also increases susceptibility to AF is unknown.
Methods and Results
We prospectively assessed the incidence of AF over a 13‐year period among 988 090 young and middle‐aged veterans (mean age, 30.29±9.19 years; 87.8% men, 64.5% white) who first accessed care through the Veterans Health Administration from October 2001 to November 2014 and were free of AF, atrial flutter, or atrial tachycardia at baseline. Time‐varying, multivariate Cox proportional hazard models were used to examine the independent contribution of PTSD to new AF. We also tested for effect modification by sex and controlled for healthcare use. During a mean follow‐up of 4.8 years, 2491 patients were diagnosed with AF. Patients with PTSD had a higher overall incidence of AF (P<0.0001) and were more likely to develop AF at a younger age than those without PTSD (P=0.004). PTSD was significantly associated with incident AF in unadjusted models (hazard ratio, 1.31; 95% CI, 1.19–1.43) and models that adjusted for demographics, lifestyle factors, cardiovascular risk factors, and depression (hazard ratio, 1.13; 95% CI, 1.02–1.24). The interaction with sex was nonsignificant (P=0.93).
Conclusions
PTSD was associated increased risk for early incident AF after adjustment for established AF risk factors and depression in this cohort of young and middle‐aged veterans. Findings from this study require validation in more diverse populations to determine their generalizability.
Keywords: arrhythmias, atrial fibrillation, posttraumatic stress disorder, stress
Subject Categories: Atrial Fibrillation, Mental Health, Risk Factors
Clinical Perspective
What Is New?
This study is the first to examine whether exposure to extremely stressful or traumatic life events (eg, gun violence, natural disaster, sexual abuse, and military combat) and subsequent development of posttraumatic stress disorder (PTSD) is associated with risk of atrial fibrillation (AF).
In this prospective cohort of nearly 1.1 million young and middle‐aged adults, PTSD was associated with an increased risk for early incident AF after adjustment for known AF risk factors and depression.
Among those who developed AF during follow‐up, people with PTSD did so at a significantly earlier age than people without PTSD. We found no evidence of effect modification according to sex, suggesting that men and women with PTSD have a similar risk for developing AF.
What Are the Clinical Implications?
Chronic stress and more severe clinical syndromes, including PTSD, are prevalent in the general population and can significantly complicate the course of care and decrease quality of life in those with AF.
Our findings raise important questions about whether early recognition and successful treatment of PTSD can prevent or mitigate the likelihood of developing AF among those exposed to violence, severe adversity, and trauma.
Future research should evaluate whether psychological interventions that target PTSD may be an important part of a broader public health initiative to improve AF risk, management, and control.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting >33 million adults wordlwide.1, 2 This growing public health problem is costly to both patients and families, and it is associated with substantial morbidity, mortality, and healthcare expenditures.3, 4 Accordingly, efforts to identify and adequately control modifiable risk factors for AF are a top priority. Known risk factors for AF include age, hypertension, diabetes mellitus (DM), obstructive sleep apnea, and lifestyle factors.5 Emerging evidence suggests that psychological stress and negative emotions, such as acute anger and hostility, are also associated with the initiation and progression of AF.6, 7, 8 Biological data from animal studies provide further support for this potential link and show that acute social stress can induce sympathetic arousal and trigger atrial arrhythmias.9
Posttraumatic stress disorder (PTSD) is a debilitating, chronic stress syndrome that develops after direct or vicarious exposure to a life‐threatening traumatic event and is characterized by alterations in mood, intrusive memories, avoidance behavior, and hyperarousal.10 PTSD has a lifetime prevalence of 8% in the United States and is more prevalent (10%–40%) among certain populations with exposure to military deployment/combat, sexual assault, natural disaster, gun violence, and other forms of adversity.11 Like stress and negative emotions, PTSD is often accompanied by biological and behavioral factors (eg, inflammation, hypertension, smoking, alcohol consumption, physical inactivity, poor diet, and illicit drug abuse) that may adversely affect cardiovascular health.12, 13, 14 Prolonged exposure to psychological stress can also lead to alterations in autonomic tone and dysregulation of the hypothalamic‐pituitary‐adrenal axis.15, 16, 17 These autonomic changes can be arrhythmogenic and might increase risk for developing AF.
Because PTSD is a potentially treatable psychological condition, and the burden of AF is significant, assessing the relation between the 2 conditions may have important implications for treatment and prevention. The purpose of this study was to assess the prospective association of PTSD and incident AF using longitudinal electronic medical record data from a large national cohort of nearly 1.1 million young and middle‐aged US service members who deployed in support of recent conflicts in Iraq and Afghanistan (eg, Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn). This cohort provides a unique opportunity to assess the long‐term effects of PTSD on AF risk in a population that: (1) has a high rate of recent exposure to prolonged, intense stress and an elevated risk for PTSD; and (2) was young (median age, 27 years) and largely free of underlying structural heart disease at baseline enrollment. We also investigated whether the association between PTSD and AF is modified by sex, as prior work has shown sex differences in the association of PTSD with other cardiac diseases.18
Methods
Data Sources and Study Population
The data that support the findings of this study are available from the corresponding author on reasonable request. The study cohort was derived from the Veterans Health Administration (VHA) roster of Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans maintained by the Defense Manpower Data Center–Contingency Tracking System Deployment File, which contains sociodemographic and military service data for all men and women discharged from the US military since October 1, 2001 (N=1 063 973). We linked participant demographic data with VA inpatient, outpatient, pharmacy, and medical claims data from the VA Corporate Data Warehouse. Medical claims data for non‐VA care provided to veterans were also obtained from the VA Fee Basis Inpatient and Outpatient database. Clinical diagnoses were only considered present if the International Classification of Diseases, Ninth Revision (ICD‐9), code for that specific condition was recorded during hospitalization or in at least 2 outpatient encounters. This method has been used for research conducted with VHA and administrative claims data and has been shown to enhance the accuracy of patient identification in these data sources.19, 20 Study baseline was defined as the date of a patient's first VHA clinical encounter on or after October 1, 2001.
Figure 1 shows a flowchart of the sample selection for the present study. We included all patients from the roster who had at least 1 inpatient or 2 outpatient medical visits at a VHA facility between October 1, 2001, and November 1, 2014 (the most recent data available for these analyses). Patients with any history of AF, atrial flutter, or atrial tachycardia (ICD‐9 codes 427.31, 427.32, and 427.89) at their first VHA encounter were excluded (n=75 883; 7.1%), resulting in a final sample of 988 090 patients. There were no differences between patients included versus excluded on the basis of age, sex, or race/ethnicity. The project and waiver of informed consent were approved by the institutional review boards at Yale School of Medicine (New Haven, CT) and VA Connecticut Healthcare System (West Haven, CT).
Figure 1.
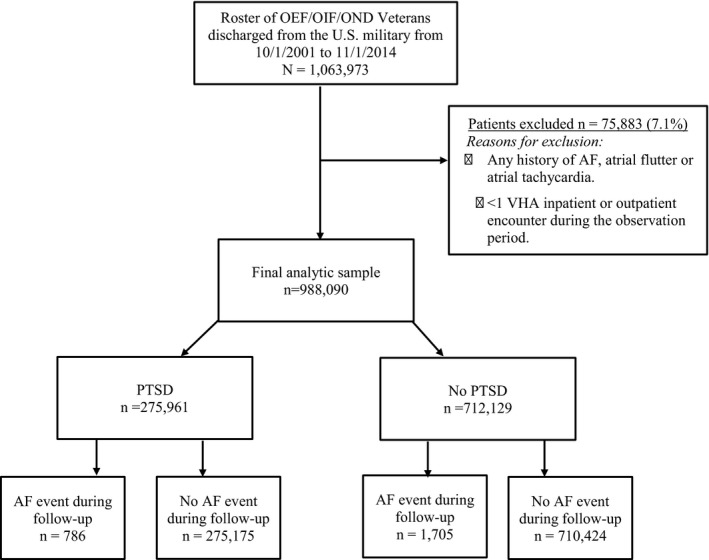
Study flowchart. AF indicates atrial fibrillation; OEF, Operation Enduring Freedom; OIF, Operation Iraqi Freedom; OND, Operation New Dawn; PTSD, posttraumatic stress disorder; VHA, Veterans Health Administration.
AF Case Ascertainment
The primary outcome was a new diagnosis of AF, defined as the presence of a new ICD‐9 code for AF (427.31) recorded during at least 1 inpatient or 2 outpatient VHA encounters during the period of observation. Patients diagnosed with atrial flutter and/or atrial tachycardia were not considered to have met the primary AF end point. Duration of AF was not assessed, and no distinction was made between paroxysmal, persistent, or permanent AF. Cases of AF and atrial flutter were not examined as a combined end point because few cases of atrial flutter were diagnosed independent of AF.
PTSD Exposure
The primary exposure was a diagnosis of PTSD (ICD‐9 code 309.81). Prior work in the VHA and elsewhere has demonstrated high agreement between the algorithm used in the current study and PTSD diagnoses obtained from a widely used screening instrument (PTSD Checklist; 82%)21 and diagnostic interview (the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; 79.4%), which are considered to be the “gold standard” in PTSD assessment.22
Assessment of AF Risk Factors
Covariates were selected a priori on the basis of their known association with AF.1, 23 Demographic variables (sex, race/ethnicity [black, Hispanic, white, or other], and marital status), smoking status (current, former, or never), and body mass index (BMI) were collected closest to the baseline date. BMI (calculated as weight in kilograms divided by height in meters squared) was dichotomized as obese (BMI ≥30.0 kg/m2) and nonobese (BMI <30 kg/m2), according to the World Health Organization/National Institutes of Health classification scheme.24 Previously validated ICD‐9 diagnostic codes were used to identify clinical diagnoses of hypertension, coronary artery disease, myocardial infarction (MI), obstructive sleep apnea, congestive heart failure, stroke (ischemic stroke/transient ischemic attacks), peripheral vascular disease, alcohol abuse, drug use, and major depressive disorder.19, 25 Prescription data for antihypertensive medications (angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β blockers, calcium channel blockers, and diuretics) and selective serotonin reuptake inhibitors (SSRIs)/serotonin and norepinephrine reuptake inhibitors were also obtained.
Statistical Analysis
With the exception of baseline age, sex, race/ethnicity, smoking status, and BMI, all covariates, including PTSD, were treated as time varying to account for the time dependence of associations. Clinical diagnoses and prescriptions occurring after the index AF event were excluded from analyses. Data analyses were performed using SAS, version 9.4 (SAS Institute, Inc, Cary, NC), and statistical significance was determined by a 2‐sided P<0.05.
Descriptive statistics were used to summarize the sample characteristics. CHA2DS2‐VASc risk scores were calculated for each patient in the overall sample (congestive heart failure, hypertension, aged ≥75 years [×2], DM, stroke/transient ischemic attack/thromboembolism [×2], vascular disease [prior MI, peripheral artery disease, or aortic plaque], aged 65–74 years, and female sex category)1 and compared among those with and without AF and PTSD, respectively. The crude incidence rate for AF in the entire sample was calculated. The cumulative incidence of AF events according to PTSD status was estimated by the Kaplan‐Meier method and compared with log‐rank test. Follow‐up was censored at the occurrence of AF or the date of the last VHA encounter, whichever occurred first.
Unadjusted and adjusted Cox proportional hazard models and 95% CIs were constructed to examine the association between PTSD and incident AF. To determine whether, and to what extent, PTSD contributed to risk for new AF over and above that of established risk factors, we constructed a series of models that serially adjusted for the following: demographics (model 1: age, sex, and race/ethnicity [white as reference group]); clinical factors (model 2: hypertension, coronary artery disease, MI, DM, and obstructive sleep apnea); and lifestyle factors (model 3: obesity, alcohol and drug abuse, and smoking status [never smoker as reference group]). The final model adjusted for all covariates and for coexisting psychiatric diagnoses (eg, major depression), as depression has been linked to both PTSD and incident cardiovascular disease in previous studies.26 A separate fully adjusted model also tested for effect modification by sex.
Although SSRIs and serotonin and norepinephrine reuptake inhibitors are frequently used in the treatment of PTSD and prescriptions for these medications were prevalent in this sample, exposure to these medications has not conferred risk for AF in prior investigations.27 As a result, these medications were not included in multivariate analyses.
Multiple imputation methods were used to account for missing covariate data (BMI=22%, and smoking status=26%), generating 5 data sets with complete covariance values. Parameter estimates for all Cox models are based on the imputed data. Analysis of Schoenfeld residuals showed no violations of the proportional hazards assumption.
Sensitivity Analyses
Several subgroup and sensitivity analyses were performed to assess the robustness of the primary findings. First, to remove any cases of prevalent AF, we applied a “washout period” that excluded AF cases diagnosed in the first 2 years of enrollment. This approach is commonly used in epidemiological research and has demonstrated reductions in incidence disease misclassification in prior studies (<10%).28 Second, because patients may be more likely to seek medical care on the basis of their PTSD symptoms, we constructed a model to control for possible surveillance bias caused by differences in healthcare use. This multivariate analysis included a term for the total number of primary care visits recorded during the first 2 years of study enrollment, as we and others have done previously.19, 29
Results
The final study sample consisted of 988 090 patients (87.8% men, 64.5% white) with a mean age of 30.29±9.19 years (median age, 27 years; range, 18–60 years); <1% were aged >55 years at baseline. Demographic information and clinical characteristics for the sample are shown in Table 1. During a mean follow‐up period of 4.8 years (SD, 2.8 years; range, 0–12 years), 2491 patients were diagnosed with AF. The crude incidence rate for AF in this sample was 0.84 events per 1000 person‐years. Of those diagnosed with AF, 50.2% had no diagnosed cardiovascular comorbidity before the index AF event (Table S1).
Table 1.
Characteristics of the Study Cohort, According to PTSD Status
| Characteristics | Overall Sample (N=988 090) | PTSD (n=275 961) | No PTSD (n=712 129) |
|---|---|---|---|
| Demographics | |||
| Age, y* | 30.29±9.19 | 29.03±8.54 | 30.63±9.39 |
| Men | 867 970 (87.84) | 247 905 (89.83) | 620 065 (87.07) |
| Race/ethnicity | |||
| White/Caucasian | 637 113 (64.48) | 179 963 (65.21) | 457 150 (64.19) |
| Black | 155 871 (15.77) | 46 218 (16.75) | 109 653 (15.40) |
| Hispanic | 109 179 (11.05) | 33 569 (12.16) | 75 610 (10.62) |
| Other | 61 793 (6.25) | 13 212 (4.79) | 48 581 (6.82) |
| Unknown | 24 134 (2.44) | 2999 (1.09) | 21 135 (2.97) |
| Married | 451 301 (45.67) | 134 902 (48.88) | 316 399 (44.43) |
| Clinical factors† | |||
| Hypertension | 125 614 (12.71) | 53 614 (19.43) | 72 000 (10.11) |
| MI | 647 (0.07) | 282 (0.10) | 365 (0.05) |
| CHF | 1072 (0.11) | 472 (0.17) | 600 (0.08) |
| CAD | 3070 (0.31) | 1379 (0.50) | 1691 (0.24) |
| DM | 27 717 (2.81) | 11 373 (4.12) | 16 344 (2.30) |
| OSA | 89 945 (9.10) | 42 922 (15.55) | 47 023 (6.60) |
| Stroke | 2397 (0.24) | 1117 (0.40) | 1280 (0.18) |
| PVD | 1638 (0.17) | 702 (0.25) | 936 (0.13) |
| CHA2DS2‐VASc score | |||
| 0 | 741 682 (75.06) | 193 954 (70.28) | 547 728 (76.91) |
| 1 | 215 608 (21.02) | 69 333 (25.12) | 146 275 (20.54) |
| ≥2 | 30 800 (3.12) | 12 674 (4.59) | 18 126 (2.55) |
| Medications† | |||
| Antihypertensive therapy | 122 029 (12.35) | 58 910 (21.35) | 63 119 (8.86) |
| SSRI/SNRI | 358 999 (36.33) | 224 539 (81.37) | 134 460 (18.88) |
| Lifestyle factors | |||
| Obesity‡ | 256 630 (33.09) | 91 103 (34.76) | 165 527 (32.24) |
| Substance use† | |||
| Alcohol abuse | 103 463 (10.47) | 71 212 (25.81) | 32 251 (4.53) |
| Drug abuse | 50 010 (5.06) | 35 867 (13.00) | 14 143 (1.99) |
| Smoking status‡ § | |||
| Current | 309 703 (42.31) | 126 312 (50.95) | 183 391 (37.89) |
| Former | 104 705 (14.31) | 33 469 (13.50) | 71 236 (14.72) |
| Never | 317 493 (43.38) | 88 139 (35.55) | 229 354 (47.39) |
| Psychiatric comorbidities | |||
| Major depression† | 89 345 (9.04) | 63 910 (23.16) | 25 435 (3.57) |
Data are presented as number (percentage), unless otherwise indicated. CAD indicates coronary artery disease; CHF, congestive heart failure; DM, diabetes mellitus; MI, myocardial infarction; OSA, obstructive sleep apnea; PTSD, posttraumatic stress disorder; PVD, peripheral vascular disease; SSRI/SNRI, selective serotonin reuptake inhibitor/serotonin and norepinephrine reuptake inhibitor.
Data are presented as mean±SD.
Data refer to diagnoses and prescriptions that occurred during the follow‐up period before the index atrial fibrillation event.
Data missing: body mass index=22%, and smoking status=26%.
Defined as positive for smoking tobacco within 180 days of baseline.
PTSD was diagnosed in 27.9% of the overall sample. The cumulative risk for AF was greater among patients with versus without PTSD (P<0.0001; Figure 2). Individuals with versus without PTSD were also more likely to develop AF at a younger age (39.79 years [SD, 11.61 years] versus 41.30 years [SD, 12.17 years]; P=0.004) and to be diagnosed with concomitant MI, coronary artery disease, congestive heart failure, DM, chronic obstructive pulmonary disease, obstructive sleep apnea, and depression during the follow‐up period. Prescriptions for antihypertensive medications and SSRIs/serotonin and norepinephrine reuptake inhibitors were also more prevalent in patients with versus without a diagnosis of PTSD.
Figure 2.
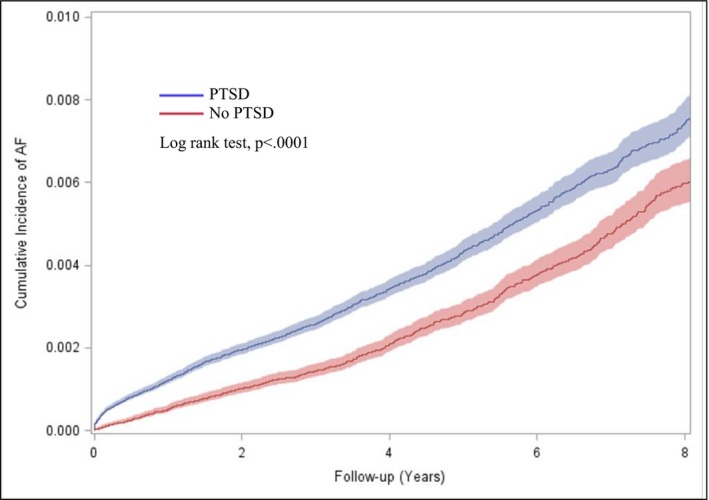
Cumulative incidence of atrial fibrillation (AF) according to posttraumatic stress disorder (PTSD) status.
PTSD and AF
PTSD was significantly associated with incident AF in the unadjusted Cox model (hazard ratio, 1.31; 95% CI, 1.19–1.43). As shown in Table 2, a significant association between PTSD and new AF persisted in models that serially adjusted for demographics (model 1), comorbid clinical factors (model 2), and lifestyle factors (model 3); however, there was a moderate reduction in the magnitude of the PTSD effect. In the final model that adjusted for all covariates and for depression (model 4), the contribution of PTSD to incident AF risk remained significant (hazard ratio, 1.13; 95% CI, 1.02–1.24).
Table 2.
Multivariate Models for the Effect of PTSD on Risk for Incident AF
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| PTSD* | 1.48 (1.36–1.62) | <0.0001 | 1.26 (1.15–1.38) | <0.0001 | 1.14 (1.04–1.26) | 0.006 | 1.13 (1.02–1.24) | 0.02 |
| Demographics | ||||||||
| Age | 1.07 (1.06–1.07) | <0.0001 | 1.05 (1.04–1.05) | <0.0001 | 1.05 (1.04–1.06) | <0.0001 | 1.05 (1.05–1.06) | <0.0001 |
| Women | 0.32 (0.26–0.39) | <0.0001 | 0.38 (0.31–0.46) | <0.0001 | 0.39 (0.32–0.48) | <0.0001 | 0.39 (0.30–0.49) | <0.0001 |
| Black | 0.99 (0.89–1.09) | 0.79 | 0.86 (0.78–0.96) | 0.005 | 0.84 (0.76–0.94) | 0.002 | 0.84 (0.76–0.93) | 0.001 |
| Hispanic | 0.71 (0.62–0.82) | <0.0001 | 0.69 (0.59–0.79) | <0.0001 | 0.68 (0.59–0.78) | <0.0001 | 0.67 (0.58–0.77) | <0.0001 |
| Other | 0.85 (0.72–1.01) | 0.06 | 0.81 (0.68–0.96) | 0.01 | 0.81 (0.69–0.96) | 0.02 | 0.81 (0.68–0.96) | 0.02 |
| Unknown | 0.66 (0.47–0.94) | 0.02 | 0.73 (0.52–1.04) | 0.08 | 0.72 (0.51–1.02) | 0.07 | 0.74 (0.52–1.04) | 0.08 |
| Clinical factors* | ||||||||
| Hypertension | … | … | 2.54 (2.30–2.81) | <0.0001 | 2.42 (2.19–2.68) | <0.0001 | 2.41 (2.18–2.66) | <0.0001 |
| CAD | … | … | 4.51 (3.67–5.55) | <0.0001 | 4.62 (3.75–5.69) | <0.0001 | 4.57 (3.71–5.63) | <0.0001 |
| MI | … | … | 4.11 (2.77–6.11) | <0.0001 | 4.13 (2.78–6.15) | <0.0001 | 4.14 (2.79–6.16) | <0.0001 |
| DM | … | … | 1.12 (0.96–1.31) | 0.15 | 1.11 (0.95–1.29) | 0.20 | 1.10 (0.94–1.29) | 0.23 |
| OSA | … | … | 1.75 (1.58–1.94) | <0.0001 | 1.70 (1.53–1.89) | <0.0001 | 1.69 (1.52–1.87) | <0.0001 |
| Lifestyle factors | ||||||||
| Obesity | … | … | … | … | 1.20 (1.11–1.31) | <0.0001 | 1.23 (1.13–1.33) | <0.0001 |
| Alcohol abuse | … | … | … | … | 1.81 (1.58–2.07) | <0.0001 | 1.81 (1.58–2.07) | <0.0001 |
| Drug abuse | … | … | … | … | 1.23 (0.99–1.53) | 0.07 | 1.20 (0.96–1.49) | 0.11 |
| Current smoker | … | … | … | … | 0.98 (0.87–1.10) | 0.71 | 0.87 (0.80–0.96) | 0.003 |
| Former smoker | … | … | … | … | 0.89 (0.81–0.97) | 0.01 | 0.99 (0.88–1.11) | 0.85 |
| Psychiatric comorbidities | ||||||||
| Major depression* | … | … | … | … | … | … | 1.11 (0.97–1.28) | 0.15 |
Data are given as hazard ratio (95% CI), per 1 year. AF indicates atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus; MI, myocardial infarction; OSA, obstructive sleep apnea; PTSD, posttraumatic stress disorder.
Diagnoses occurred before the index ischemic stroke event and are time dependent.
We also examined sex as a moderator of the association between PTSD and AF. Fewer cases of incident AF were diagnosed in women (4.0%) than in men (96%). In a separate multivariate Cox model, the interaction between sex and PTSD in predicting new‐onset AF was nonsignificant (P=0.93). Among this subgroup of patients with AF, however, a prior diagnosis of PTSD was more prevalent among women compared with men (37% versus 31%).
Subgroup and Sensitivity Analyses
In post hoc analyses, exclusion of AF cases diagnosed in the first 2 years of follow‐up (n=781) did not alter the findings (data not shown). In additional sensitivity analyses that adjusted for factors previously shown to increase AF risk, for depression, and for the effect of healthcare use (Table S2), PTSD remained significantly associated with incident AF (hazard ratio, 1.11; 95% CI, 1.00–1.23). This suggests that increased medical attention attributable to PTSD had only a modest effect on the association between PTSD and a new diagnosis of AF.
Discussion
In this nationwide study of nearly 1.1 million young and middle‐aged veterans, we found that a diagnosis of PTSD was associated with a 13% greater risk for early incident AF. This excess risk persisted after adjustment for established AF risk factors, including demographics, lifestyle factors, cardiovascular risk factors, and depression, suggesting that the contribution of PTSD to incident AF is largely independent of overlapping comorbidity. Furthermore, among individuals who developed AF during the period of observation, those with PTSD did so at a significantly earlier age than patients without PTSD.
Previous studies have demonstrated an association between PTSD and incident cardiovascular disease (eg, coronary heart disease, MI, and stroke), recurrent cardiovascular events, and all‐cause and cardiovascular mortality.14, 19, 30 Results from the current investigation extend this work by showing, for the first time, that a trauma‐induced chronic stress syndrome is significantly associated with the onset of new AF. This effect was maintained after controlling for a wide range of clinical comorbidities, coexisting psychiatric disorders, and healthcare use, supporting the validity of our findings. Furthermore, although the absolute increase in AF risk among individuals with PTSD was modest in this low‐risk cohort of initially healthy young and middle‐aged adults, it is comparable in magnitude with other traditional risk factors (eg, DM and obesity), underscoring its clinical relevance. Additional studies are needed to confirm our findings and to determine whether variation in PTSD symptom onset (eg, early versus delayed onset), expression (eg, fear, avoidance, or arousal), and trajectory (eg, active versus partial or full remission) has distinct effects on arrhythmia risk.
Another feature of this investigation was the examination of sex as a moderator of the association between PTSD and AF. Recent data have suggested that PTSD acts differentially to promote coronary artery disease in men versus women18; however, we found no evidence of effect modification according to sex, suggesting that men and women with PTSD have a similar risk for developing AF. This finding may reflect the overall lower incidence of AF among women in this study (n=100) compared with men (n=2391), which reduced statistical power to detect sex differences. Because men and women differ with respect to the causes and outcomes associated with AF, and PTSD is twice as prevalent in women,31, 32 appropriately powered studies should investigate this question further.
Our findings also provide new insight into the risk factors for AF in patients who are relatively young and largely free of underlying structural heart disease. Although AF is rare in patients aged <55 years (0.1%–0.2%),33 national data show an apparent increase in AF‐associated comorbidities (eg, cerebrovascular events) and hospitalizations in young patients over the past decade.34, 35 Despite this alarming trend, data on AF‐related risk factors and outcomes in young and early‐middle‐aged people are sparse because of the low prevalence of AF in this age group. Findings from the current study, along with those in the literature, suggest that the development of AF in young patients may be more strongly influenced by nontraditional risk factors, such as psychological characteristics (eg, PTSD, anger, and hostility) and behavioral/lifestyle factors (eg, binge drinking, smoking, and vigorous exercise/participation in sports),36, 37 than traditional cardiovascular risk factors, which may be less prevalent in this population. Indeed, in our sample of young patients (median age, 27 years), 50.2% of those who developed AF did so in the absence of any diagnosed cardiovascular comorbidity. Thus, early‐onset AF may represent a distinct phenotype that develops from a confluence of genetic, traditional, and emerging risk factors, including PTSD. Given the increasing burden, longer life expectancy, and potential socioeconomic consequences of early‐onset AF, further research is needed to improve risk stratification, prevention, and treatment in this age group.
Potential Mechanisms Linking PTSD to AF
The mechanisms by which PTSD increases vulnerability for AF have not been evaluated but may involve a combination of behavioral/lifestyle and other pathophysiologic factors or pathways yet to be fully delineated. PTSD may indirectly predispose individuals to develop AF through the onset or progression of hypertension, DM, inflammation, and/or metabolic syndrome.14, 38 Lifestyle factors and unhealthy behaviors, such as smoking, alcohol consumption, physical inactivity, poor diet, and drug abuse, are also well documented in patients with PTSD12, 13 and may contribute to AF. Yet, as in other studies,30 adjustment for these variables did not fully attenuate PTSD‐associated risk for AF, suggesting that PTSD has a more direct, independent effect. Furthermore, although long‐term endurance exercise can trigger AF in the young,37 prospective data from the Millennium Cohort indicate that most post‐9/11 veterans do not regularly engage in high‐intensity exercise and that physical activity is significantly decreased in those with PTSD.39 It is also possible that PTSD may trigger the occurrence of AF directly through chronic changes in autonomic tone (eg, increasing sympathetic activation and decreasing vagal stimulation), which can alter atrial electrophysiological characteristics (shortening the effective refractory period) and thereby facilitate AF.40 Findings from previous studies have shown that acute negative emotions can precipitate AF.6, 7, 8 PTSD tends to have a more long‐term course and is associated with greater autonomic reactivity than momentary negative affect.41 Thus, it is possible that PTSD represents a continuum of risk attributable to emotion‐related factors. More research is needed to understand the mechanisms underlying the increased AF risk in patients with PTSD and identify potential targets for therapeutic intervention.
Implications of Findings
PTSD is prevalent in the general population and can significantly complicate the course of care and decrease quality of life in those with AF.42 Although there is an ongoing debate as to whether PTSD is an independent risk factor for cardiovascular disease and not just a risk marker,14 there are plenty of data showing that therapeutic interventions (eg, cognitive‐processing therapy and prolonged exposure therapy) can be effective in treating this condition and reducing the associated physiological hyperarousal.43 Thus, findings from this investigation raise important questions about whether early detection and successful treatment of PTSD can prevent or mitigate the likelihood of developing AF among those exposed to violence, severe adversity, and trauma. If prospective trials demonstrate that therapeutic treatments for PTSD can decrease AF incidence, psychological interventions that target PTSD may be an important part of a broader public health initiative to improve AF risk factor management and control.
In addition, AF is a complex condition that occurs in a heterogeneous patient population. The causes, characteristics, and consequences of AF for young patients will likely differ from those for the “typical” patient with AF who is older and more likely to have concomitant disease. This suggests the need for a more personalized approach to risk prediction, prevention, and patient management. Programs aimed at reducing cardiovascular risk in the young may need to aggressively target traditional and emerging risk factors. Complementary medicine interventions (eg, yoga and acupuncture) have shown great promise as a tool to reduce arrhythmia burden, improve quality of life, and reduce anxiety and depression in patients with AF.44 The benefits and risks of these approaches in young at‐risk populations are not known, but developing effective management strategies for vulnerable patient groups is an important area for further study.
Strengths and Limitations
A major strength of this investigation is the large, nationally representative sample of young and middle‐aged adults and the long duration of follow‐up, which provided a biologically plausible time frame for the relationship between PTSD and AF to develop. Furthermore, because exposures and clinical covariates were prospectively recorded in the electronic medical record before development of AF, the possibility of recall bias that is often present in case‐control and cross‐sectional studies was eliminated.
Several limitations should be considered when interpreting these data. First, although clinical diagnoses and inclusion/exclusion procedures were based on previously validated ICD‐9 diagnostic codes obtained from electronic medical records and included medical claims data for non‐VA care, we cannot discount the possibility that some diagnoses were misclassified or unrecognized. This concern is lessened by the fact that prior work has demonstrated 95% sensitivity and 99% specificity for the AF ICD‐9 code (427.31) in administrative data.45 Furthermore, VHA guidelines require annual screening for PTSD in VA primary and specialty care settings, suggesting that underrecognition of PTSD may be less likely. The prevalence of PTSD in this study was also comparable to prior studies of veterans that used both diagnostic interview and checklist assessment methods,21 suggesting that our assessment of the exposures is equally valid. Whether cases were diagnosed inpatient versus outpatient cannot be determined from our data. Second, the number of atrial flutter cases in this study was insufficient to examine them as an independent end point. Similarly, we were unable to examine interactions between PTSD and AF by age (using typical age‐related cutoffs for AF of >65 years) or SSRI treatment status, as most AF cases in this study occurred before the age of 50 years and 81% of those diagnosed with PTSD also had a prescription for an SSRI. In addition, data about the effect that PTSD treatment (eg, psychotherapy) has on the relationship between PTSD and AF were not available for this analysis. Third, despite adjustment for multiple time‐varying covariates, residual confounding cannot be ruled out. Finally, as the cohort is composed of young and middle‐aged veteran patients, most of whom were men, generalizability to other populations (eg, nonveteran populations or veterans who do not use VHA services) may be limited. Whether these findings would be seen in older patients with AF is unknown, although it is possible that the effects of PTSD on AF risk might be more pronounced in a population who has a longer duration of exposure to PTSD symptoms and a higher risk factor burden. Prospective studies in more diverse populations are needed to enhance the generalizability of these findings.
Conclusions
PTSD is associated with an increased risk for early incident AF after adjustment for known AF risk factors and depression in this cohort of young and middle‐aged veterans. These findings warrant further investigation and replication in more diverse populations to determine their generalizability. Future studies are needed to investigate the mechanisms underlying this association and to identify possible interventions that target and treat vulnerable segments of the population.
Sources of Funding
This study was supported by a grant from the Department of Veterans Affairs (IIR 12‐118) to Drs Haskell and Brandt and by a grant from the National Heart, Lung, and Blood Institute (R01 HL125587) to Dr Burg.
Disclosures
None.
Supporting information
Table S1. Characteristics of the Study Cohort According to AF Status
Table S2. Results of Sensitivity Analysis Showing Hazard Ratios and 95% CIs for Full Model With Adjustment for Healthcare Utilization
(J Am Heart Assoc. 2019;8:e013741 DOI: 10.1161/JAHA.119.013741.)
References
- 1. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 4. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 5. Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PHM, Polak JF, Sutton‐Tyrrell K, Herrington DM, Price TR, Cushman M. C‐reactive protein, carotid intima‐media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. [DOI] [PubMed] [Google Scholar]
- 6. Eaker ED, Sullivan LM, Kelly‐Hayes M, D'Agostino RB Sr, Benjamin EJ. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation. 2004;109:1267–1271. [DOI] [PubMed] [Google Scholar]
- 7. Eaker ED, Sullivan LM, Kelly‐Hayes M, D'Agostino RB Sr, Benjamin EJ. Tension and anxiety and the prediction of the 10‐year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosom Med. 2005;67:692–696. [DOI] [PubMed] [Google Scholar]
- 8. Lampert R, Jamner L, Burg M, Dziura J, Brandt C, Liu H, Li F, Donovan T, Soufer R. Triggering of symptomatic atrial fibrillation by negative emotion. J Am Coll Cardiol. 2014;64:1533–1534. [DOI] [PubMed] [Google Scholar]
- 9. Sgoifo A, de Boer SF, Westenbroek C, Maes FW, Beldhuis H, Suzuki T, Koolhaas JM. Incidence of arrhythmias and heart rate variability in wild‐type rats exposed to social stress. Am J Physiol. 1997;273:H1754–H1760. [DOI] [PubMed] [Google Scholar]
- 10. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 11. Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartoli F, Crocamo C, Alamia A, Amidani F, Paggi E, Pini E, Clerici M, Carra G. Posttraumatic stress disorder and risk of obesity: systematic review and meta‐analysis. J Clin Psychiatry. 2015;76:e1253–e1261. [DOI] [PubMed] [Google Scholar]
- 13. Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, Joseph AM. Post‐traumatic stress disorder and smoking: a systematic review. Nicotine Tob Res. 2007;9:1071–1084. [DOI] [PubMed] [Google Scholar]
- 14. Edmondson D, von Känel R. Post‐traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taggart P, Boyett MR, Logantha SJRJ, Lambiase PD. Anger, emotion, and arrhythmias: from brain to heart. Front Physiol. 2011;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69:935–943. [DOI] [PubMed] [Google Scholar]
- 17. Yehuda R. Sensitization of the hypothalamic‐pituitary‐adrenal axis in posttraumatic stress disorder. Ann N Y Acad Sci. 1997;821:57–75. [DOI] [PubMed] [Google Scholar]
- 18. Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta‐analytic review. Am Heart J. 2013;166:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burg MM, Brandt C, Buta E, Schwartz J, Bathulapalli H, Dziura J, Edmondson DE, Haskell S. Risk for incident hypertension associated with posttraumatic stress disorder in military veterans and the effect of posttraumatic stress disorder treatment. Psychosom Med. 2017;79:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walkup JT, Wei W, Sambamoorthi U, Crystal S. Sensitivity of an AIDS case‐finding algorithm: who are we missing? Med Care. 2004;42:756–763. [DOI] [PubMed] [Google Scholar]
- 21. Gravely AA, Cutting A, Nugent S, Grill J, Carlson K, Spoont M. Validity of PTSD diagnoses in VA administrative data: comparison of VA administrative PTSD diagnoses to self‐reported PTSD checklist scores. J Rehabil Res Dev. 2011;48:21–30. [DOI] [PubMed] [Google Scholar]
- 22. Holowka DW, Marx BP, Gates MA, Litman HJ, Ranganathan G, Rosen RC, Keane TM. PTSD diagnostic validity in Veterans Affairs electronic records of Iraq and Afghanistan veterans. J Consult Clin Psychol. 2014;82:569–579. [DOI] [PubMed] [Google Scholar]
- 23. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey J‐Y, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2006;48:854–906. [DOI] [PubMed] [Google Scholar]
- 24. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US) . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda (MD): National Heart, Lung, and Blood Institute; 1998. Sep. Available at: https://www.ncbi.nlm.nih.gov/books/NBK2003/.
- 25. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 26. O'Donnell ML, Creamer M, Pattison P. Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am J Psychiatry. 2004;161:1390–1396. [DOI] [PubMed] [Google Scholar]
- 27. Lapi F, Azoulay L, Kezouh A, Benisty J, Matok I, Mugelli A, Suissa S. The use of antidepressants and the risk of chronic atrial fibrillation. J Clin Pharmacol. 2015;55:423–430. [DOI] [PubMed] [Google Scholar]
- 28. Griffiths RI, O'Malley CD, Herbert RJ, Danese MD. Misclassification of incident conditions using claims data: impact of varying the period used to exclude pre‐existing disease. BMC Med Res Methodol. 2013;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scherrer JF, Salas J, Cohen BE, Schnurr PP, Schneider FD, Chard KM, Tuerk P, Friedman MJ, Norman SB, van den Berk‐Clark C, Lustman PJ. Comorbid conditions explain the association between posttraumatic stress disorder and incident cardiovascular disease. J Am Heart Assoc. 2019;8:e011133 DOI: 10.1161/JAHA.118.011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew‐Blais J, Chen Q, Cerda M, Rexrode KM, Rich‐Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. [DOI] [PubMed] [Google Scholar]
- 32. Piccini JP, Simon DN, Steinberg BA, Thomas L, Allen LA, Fonarow GC, Gersh B, Hylek E, Kowey PR, Reiffel JA, Naccarelli GV, Chan PS, Spertus JA, Peterson ED. Differences in clinical and functional outcomes of atrial fibrillation in women and men: two‐year results from the ORBIT‐AF registry. JAMA Cardiol. 2016;1:282–291. [DOI] [PubMed] [Google Scholar]
- 33. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 34. Bejot Y, Daubail B, Jacquin A, Durier J, Osseby GV, Rouaud O, Giroud M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. J Neurol Neurosurg Psychiatry. 2014;85:509–513. [DOI] [PubMed] [Google Scholar]
- 35. Deshmukh A, Pothineni NV, Patel N, Badheka A, Mulpuru SK, Paydak H, Noseworthy PA. Trends in hospitalizations of young patients with atrial fibrillation: a cause for concern? Int J Cardiol. 2016;203:164–165. [DOI] [PubMed] [Google Scholar]
- 36. Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol consumption and risk of atrial fibrillation: a meta‐analysis. J Am Coll Cardiol. 2011;57:427–436. [DOI] [PubMed] [Google Scholar]
- 37. Turagam MK, Velagapudi P, Kocheril AG. Atrial fibrillation in athletes. Am J Cardiol. 2012;109:296–302. [DOI] [PubMed] [Google Scholar]
- 38. Vancampfort D, Rosenbaum S, Ward PB, Steel Z, Lederman O, Lamwaka AV, Richards JW, Stubbs B. Type 2 diabetes among people with posttraumatic stress disorder: systematic review and meta‐analysis. Psychosom Med. 2016;78:465–473. [DOI] [PubMed] [Google Scholar]
- 39. LeardMann CA, Kelton ML, Smith B, Littman AJ, Boyko EJ, Wells TS, Smith TC. Prospectively assessed posttraumatic stress disorder and associated physical activity. Public Health Rep. 2011;126:371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lampert R. Behavioral influences on cardiac arrhythmias. Trends Cardiovasc Med. 2016;26:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dennis PA, Kimbrel NA, Sherwood A, Calhoun PS, Watkins LL, Dennis MF, Beckham JC. Trauma and autonomic dysregulation: episodic—versus systemic—negative affect underlying cardiovascular risk in posttraumatic stress disorder. Psychosom Med. 2017;79:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Westcott SK, Beach LY, Matsushita F, Albert CM, Chatterjee N, Wong J, Williams DR, Vinayagamoorthy M, Buring JE, Albert MA. Relationship between psychosocial stressors and atrial fibrillation in women >45 years of age. Am J Cardiol. 2018;122:1684–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watts BV, Schnurr PP, Mayo L, Young‐Xu Y, Weeks WB, Friedman MJ. Meta‐analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74:e541–e550. [DOI] [PubMed] [Google Scholar]
- 44. Lakkireddy D, Atkins D, Pillarisetti J, Ryschon K, Bommana S, Drisko J, Vanga S, Dawn B. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. J Am Coll Cardiol. 2013;61:1177–1182. [DOI] [PubMed] [Google Scholar]
- 45. Glazer NL, Dublin S, Smith NL, French B, Jackson LA, Hrachovec JB, Siscovick DS, Psaty BM, Heckbert SR. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167:246–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the Study Cohort According to AF Status
Table S2. Results of Sensitivity Analysis Showing Hazard Ratios and 95% CIs for Full Model With Adjustment for Healthcare Utilization


