Short abstract
We previously reported that ganglioside GD3 is the predominant species in neural stem cells (NSCs) and reduced postnatal NSC pools are observed in both the subventricular zone and dentate gyrus (DG) of GD3-synthase knockout (GD3S-KO) mouse brains. Specifically, deficiency of GD3 in GD3S-KO animals revealed a dramatic reduction in cellularity in the DG of the hippocampus of the developing mouse brain, resulting in severe behavioral deficits in these animals. To further evaluate the functional role of GD3 in postnatal brain, we performed rescue experiments by intracerebroventricular infusion of ganglioside GD3 in adult GD3S-KO animals and found that it could restore the NSC pools and enhance the NSCs for self-renewal. Furthermore, 5xFAD mouse model was utilized, and GD3 restored NSC numbers and GM1 promoted neuronal differentiation. Our results thus demonstrate that exogenously administered gangliosides are capable to restore the function of postnatal NSCs. Since ganglioside expression profiles are associated not only with normal brain development but also with pathogenic mechanisms of diseases, such as Alzheimer’s disease, we anticipate that the administration of exogenous gangliosides, such as GD3 and GM1, may represent a novel and effective strategy for promoting adult neurogenesis in damaged brain for disease treatment.
Keywords: Alzheimer’s disease, cell differentiation, ganglioside, neural stem cell, self-renewal
Introduction
Adult neural stem cells (NSCs) are located primarily in the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus and the subventricular zone (SVZ) of the lateral ventricles of the brain. NSCs are fundamental cells that can differentiate into various types of cells in the developing nervous system, and, in mature brain, for adult neurogenesis. It has been suggested that NSCs contribute to repairing damaged or degenerated central nervous system (CNS). Throughout neural development, dynamic changes are observed in the composition of carbohydrate-rich molecules, including gangliosides.
Gangliosides are sialic acid-containing glycosphingolipids expressed primarily, but not exclusively, in the outer leaflet of the plasma membrane of cells of all vertebrates. Those gangliosides are particularly abundant in the nervous system. Ganglioside metabolism is closely associated with brain development. We have reported that GD3 is the predominant ganglioside species in NSCs (Nakatani et al., 2010) and modulates NSC proliferation by interacting with epidermal growth factor receptor (EGFR) signaling (Wang and Yu, 2013). GD3-synthase (sialyltransferase II, ST-II; GD3S) is responsible for catalyzing the biosynthesis of GD3, a key intermediate for the synthesis of other b- and c-series gangliosides. In GD3S gene knockout (GD3S-KO) mice, all b-series gangliosides, including GD3, GD2, GD1b, GT1b, and GQ1b, are deleted, and a-series gangliosides such as GM1, GD1a, and GM2 show accretion (Okada et al., 2002). In postnatal brain, GD3 is required for the long-term maintenance of NSCs (Wang et al., 2014). Deficiency in GD3 leads to developmental and behavior deficits, such as depression. The synthesis of GD3 is switched to the synthesis of complex, brain-type gangliosides, notably GM1, GD1a, GD1b, and GT1b, resulting in terminal differentiation and loss of “stemness” of NSCs. We have reported that efficient histone acetylation of glycosyltransferase (GT) genes contributes to the developmental alteration of ganglioside expression in mouse brain (Suzuki et al., 2011). Furthermore, we have demonstrated that acetylation of histones H3 and H4 on the GM2/GD2S gene promoter leads to recruitment of trans-activation factors Sp1 and AP-2 during neuronal differentiation (Tsai and Yu, 2014). Recently, we found that nuclear GM1 binds with acetylated histones on the promoters of the GM2/GD2S gene as well as on the neurogenic transcription factor, NeuroD1 gene, in differentiated neurons (Tsai et al., 2016). In this process, GM1 is augmented by a novel GM1-modulated epigenetic gene regulation mechanism of GTs at a later differentiation stage. Consequently, these studies suggest that stage-specific gangliosides play specific roles in maintaining NSC activities and in cell fate determination.
Neurons and glia are generated in the CNS by a defined temporal sequence in early developmental stages. Postnatally, most of the SVZ disappears except along the lateral wall of the lateral ventricles, where it is considered as a NSC niche in the adult state. Although neurogenesis mostly arises at the time of the development in most adult mammals, it continues to occur at a much slower pace and in a limited manner throughout the entire adult life. In the adult brain of mammals, neurogenesis persists primarily in two germinal zones: the SVZ of the lateral ventricles (Doetsch et al., 1997, 1999) and the SGZ in the DG of the hippocampus (Suhonen et al., 1996; Seri et al., 2001). It is hypothesized that an accelerated loss of the NSC pool is one of the mechanisms for transition from normal aging to Alzheimer’s disease (AD) (Moreno-Jimenez et al., 2019; Tobin et al., 2019). In AD brains, neurogenic impairments may underlie, at least in part, the progressive loss of memory and compromised ability to learn and process new information are characteristic of the disease. Both olfactory and hippocampal dysfunction might be enhanced by compromised neurogenesis in the SVZ and in the DG, respectively.
In this study, we provide evidence that treatment of damaged brain by gangliosides could bring about enhancement of the self-renewal capability of endogenous NSCs and to promote adult neurogenesis in vivo. Understanding the roles of gangliosides on NSC functions in living animals should pave the way for developing novel strategies for promoting neural repair in pathologically damaged brains, such as in AD, Parkinson’s disease, and other neurodegenerative diseases.
Materials and Methods
Materials
GD3 and GM1 used in this study were isolated from either bovine buttermilk or brains in our laboratory by established procedures (Ledeen and Yu, 1982; Ren et al., 1992; Ariga et al., 1994).
Animals
GD3S-knockout (KO) mice: The original GD3S-KO mice and their wild-type (WT) mates were kindly provided by the courtesy of Dr. Richard Proia (NIDDK, NIH, Bethesda, MD) and crossed to generate the heterozygous mice. Then, the heterozygous male and female mice were mated, and PCR screening was performed for genotyping. Littermates were used as controls. Six-month-old male GD3S-KO mice and their WT litter mates were used in this study. Animals were divided into three groups: (a) WT with saline infusion group; (b) GD3S-KO with saline infusion group; and (c) GD3S-KO with GD3 infusion group. AD model mice: Mice expressing five mutations in human amyloid precursor protein and presenilin1 (5xFAD) (B6SJL-Tg[APP*K670N*M671L*I716V*V717I, PSEN1*M146*L286V]6799Vas/J) under the Thy1 promoter were purchased from The Jackson Laboratory. Ten-week-old male 5xFAD mice and their WT liter mates were used in this study. Animals were divided into five groups: (a) WT with saline infusion group; (b) 5xFAD with saline infusion group; (c) 5xFAD with GD3 infusion group; (d) 5xFAD GM1 infusion group; and (e) GD3 infusion plus GM1 infusion group. Each group consisted of three to four animals (n = 3–4). Mice were anesthetized by intramuscular injection of ketamine hydrochloride (40 mg/kg body weight) and xylazine hydrochloride (4 mg/kg body weight) and then fixed on a stereotaxic apparatus. Mini-osmotic pumps (MOPs) were employed (ALZET, model #1007D, Cupertino, CA) for lateral ventricular infusion with a 7-day duration at a flow rate of 0.5 µL/hr. Mice in the GD3 or GM1 infusion group were given 5 mg/kg bodyweight/day GD3 or GM1, respectively. Mice in the saline infused group were given the same volume of sterilized saline. Bromodeoxyuridine (BrdU) was administrated intraperitoneally immediately after the MOP was installed, at 50 mg/kg body weight, 3 times a day at 4-hr intervals for 7 days.
Immunohistochemical Staining
Mice were perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). After perfusion, brain blocks with SVZ were removed and postfixed in 4% paraformaldehyde at 4°C for 24 hr. The blocks were then equilibrated in sucrose (30% in PBS) and embedded in optimal cutting temperature compound and kept frozen at −80°C. Cryosections were cut at a thickness of 30 μm. Sections were incubated in 1N HCl at 0°C for 5 min and 2N HCl at 37°C for 15 min and washed with PBS before immunohistochemical staining. Sections were then blocked in 3% normal donkey serum in PBS with 1% Triton X-100 for 30 min and further incubated in the following primary antibodies 4°C overnight: BrdU (mouse, 1:200, Developmental Studies Hybridoma Bank, G3G4), SOX2 (rabbit, 1:200, Cell Signaling Technology, #2748), doublecortin (DCX; goat,1:200, Santa Cruz Biotechnology, C-18). After three washes with PBS, the samples were incubated in an appropriate secondary antibodies coupled to Alexa488 or Alexa 568 (Invitrogen) at a dilution of 1:1,000 at room temperature for 2 hr. After washing with PBS for 3 times, the sections were mounted with Vectashield mountain medium with DAPI (Vector Laboratory).
Confocal Microscopy and Cell Counting
Confocal images were obtained using a Zeiss LSM 510 META confocal microscope. Pictures were taken under 20× and 63× Plan-Apochromat objective. Eight sections with DAPI, BrdU, and SOX2 or DCX triple staining between Bregma 0.6 mm to Bregma 0.1 mm which contained the dorsal lateral part of SVZ, or between Bregma −1.22 mm and Bregma −4.52 mm which contained the DG were used for cell counting. The number of DAPI+, BrdU+, SOX2+, and DCX+ cells in the noninfusion side SVZ was counted with Image J software. The percentage of BrdU+, SOX2+, and DCX+ cells versus DAPI+ cells, as well as BrdU, SOX2 double-labeled cells versus DAPI+ cells and BrdU DCX double-labeled cells versus DAPI+ cells were calculated.
Statistical Analysis
Data were presented as mean ± standard error of the mean. Analyses of significant difference were performed using one-way analysis of variance. A p value of .05 was considered to be statistically significant.
Results and Discussion
GD3 Restores BrdU+/SOX2+ Cells in SVZ and Hippocampus
Mammalian neurogenesis commences during early embryonic stages and is almost complete shortly after birth. Neurogenesis continues to occur at a much slower pace and in a limited manner throughout the entire adult life. Neurogenesis persists primarily in two germinal zones: the SVZ of the lateral ventricles (Doetsch et al., 1997, 1999) and the SGZ in the DG of the hippocampus (Suhonen et al., 1996; Seri et al., 2001), in the adult brain of mammals. With regard to gangliosides, we have shown that GD3 is the predominant ganglioside in NSCs, and it can serve as a convenient cell surface marker of these cells (Nakatani et al., 2010). The interaction of GD3 with EGFR plays a crucial role in maintaining the self-renewal capacity of NSCs by directing the EGFR through the recycling pathway rather than through the degradative pathway after endocytosis (Wang and Yu, 2013). The cellularity at the SVZ and DG of GD3S-KO mice were significantly reduced compared with that of their WT littermates (Wang et al., 2014). Previous findings indicated that there are progressive reductions of the NSC pool in the SVZ and DG in adult GD3S-KO mice.
GD3 is the predominant ganglioside species in NSCs and in early development of brains. The synthesis of GD3 is switched to the synthesis of complex, brain-type gangliosides, notably GM1, GD1a, GD1b, and GT1b, resulting in terminal differentiation and loss of “stemness” of NSCs (Figure 1). Compare to WT mice, GD3S-KO mice showed thinner SVZ with reduced cellularity (DAPI+ cells), consistent with our previous results (Wang et al., 2014). Although the absolute numbers of BrdU+ and SOX2+ cells were reduced in GD3S-KO mice compared with age-matched WT animals, there was no significant difference of the percentage of BrdU+ versus DAPI+ cells and SOX2+ cells versus DAPI+ cells among different groups. This observation suggested that the reduction of the number of BrdU+ and SOX2+ cells was due to the reduction of the whole cellular pool. To investigate the functional roles of GD3 in postnatal brains, GD3S-KO mice were utilized. GD3 was intracerebroventricularly (icv) infused into adult (6-month-old) GD3S-KO mice employing a mini-pump for 7 days. In the SVZ of GD3S-KO mice, BrdU+ newly generated and SOX2+ self-renewal or multipotent cells were less than that in the WT control (Figure 2). On the other hand, GD3 treatment increased SOX2+ self-renewal or multipotent cells in the SVZ of GD3S-KO mice (Figure 2). In both neurogenic regions, SVZ and DG of GD3S-KO mice, the number of BrdU+/SOX2+ newly generated multipotent cells was significantly increased following GD3 infusion (Figure 3). These data indicate that infusion of GD3 could restore NSCs in SVZ and DG to maintain their properties at early NSC stages.
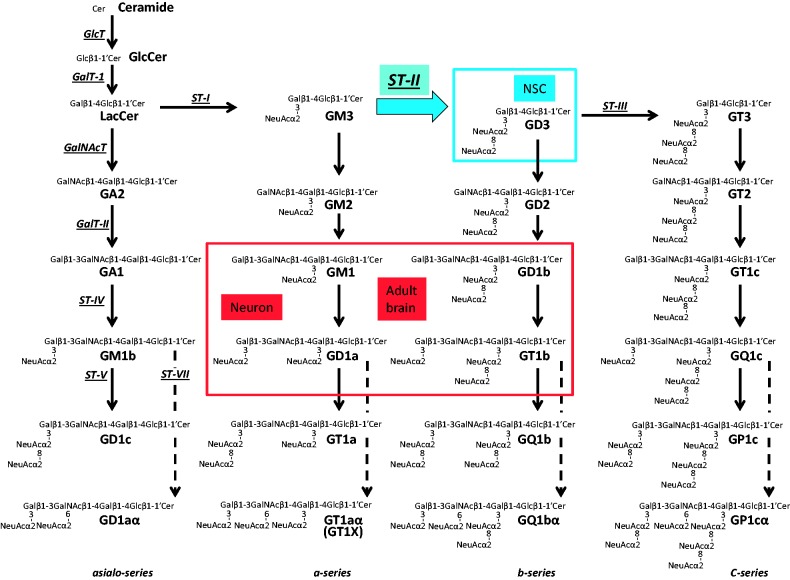
Figure 1.
Metabolic pathways and key glycosyltransferases for glycosphingolipids, including gangliosides. The nomenclature for gangliosides and their components are based on those of Svennerholm (1963) and IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (1977). GalNAc-T = N-acetylgalactosaminyltransferase I (GA2/GM2/GD2/GT2-synthase); GalT-I = galactosyltransferase I (lactosylceramide synthase); GalT-II = galactosyltransferase II (GA1/GM1/GD1b/GT1c-synthase); GlcT = glucosyl transferase (glucosylceramide synthase); ST-I = sialyltransferase I (GM3-synthase); ST-II = sialyltransferase II (GD3-synthase); ST-III = sialyltransferase III (GT3-synthase); ST-IV = sialyltransferase IV (GM1b/GD1a/GT1b/GQ1c-synthase); ST-V = sialyltransferase V (GD1c/GT1a/GQ1b/GP1c-synthase); ST-VII = sialyltransferase VII (GD1aα/GT1aα/GQ1bα/GP1cα-synthase); NSC = neural stem cell.
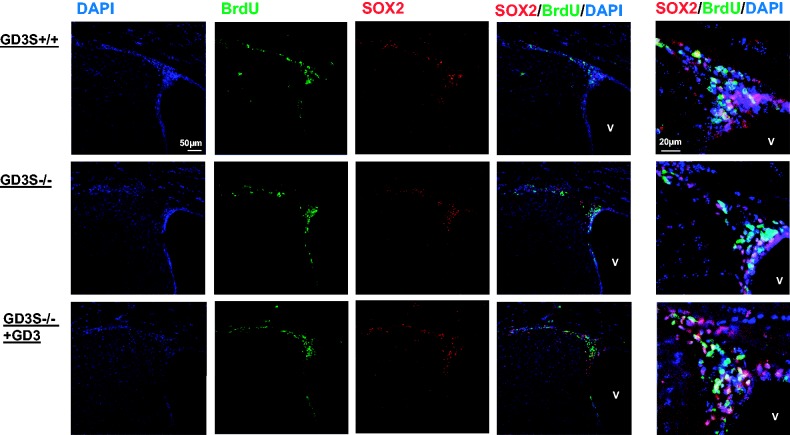
Figure 2.
GD3 infusion restores the number of BrdU+/SOX2+ cells in the SVZ of GD3S-KO mouse brain. GD3 was introduced into the brains of adult (6-month-old) GD3S-KO mice for 7 days and then injected with BrdU to assess its neurogenic potential with costaining of SOX2. Green, BrdU; blue, nuclear DAPI; and red, SOX2.
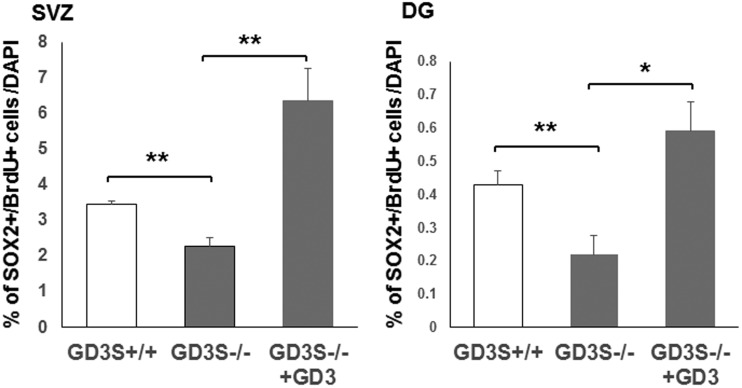
Figure 3.
Percentage of BrdU+/SOX2+ cells in the SVZ and in the DG. BrdU and SOX2 double positive cells were quantified. GD3 treatment is associated with a significant increase on BrdU+/SOX2+ cells in the SVZ and DG. V = ventricle; (n = 4), *p < .05. **p < .01. SVZ = subventricular zone; DG = dentate gyrus.
GD3 Restores BrdU+/SOX2+ Cells in AD Model Mice
The loss of NSCs is known to occur during normal aging, and it has been hypothesized that an accelerated loss of the NSC pool is one of the mechanisms for transition from normal aging to AD (Moreno-Jimenez et al., 2019; Tobin et al., 2019). Therefore, promotion of endogenous neurogenesis has been suggested as an important target for treatment and prevention of AD (Mu and Gage, 2011; Choi et al., 2018). The 5XFAD transgenic mice is an AD model with two point mutations in presenilin1 (M146L & L286V) and the Florida (I716V), London (V717I), and Swedish (KM670/671NL) mutations in the amyloid precursor protein. The 5XFAD mice are utilized as a preclinical AD mouse model extensively. 5xFAD mice showed significantly decreased percentage of BrdU and SOX2 double labeled cells versus DAPI+ cells compared to WT mice (Figure 4). To examine the physiological roles of GD3 on postnatal neurogenesis in the DG of AD model mouse, GD3 was administered into the 5XFAD mouse brain. GD3 infusion augments self-renewal and the multipotent marker, SOX2-expressing newly generated cells in the DG (Figure 4). These data suggest that icv infusion of GD3 can be an effective means to promote neurogenesis in AD model brain.
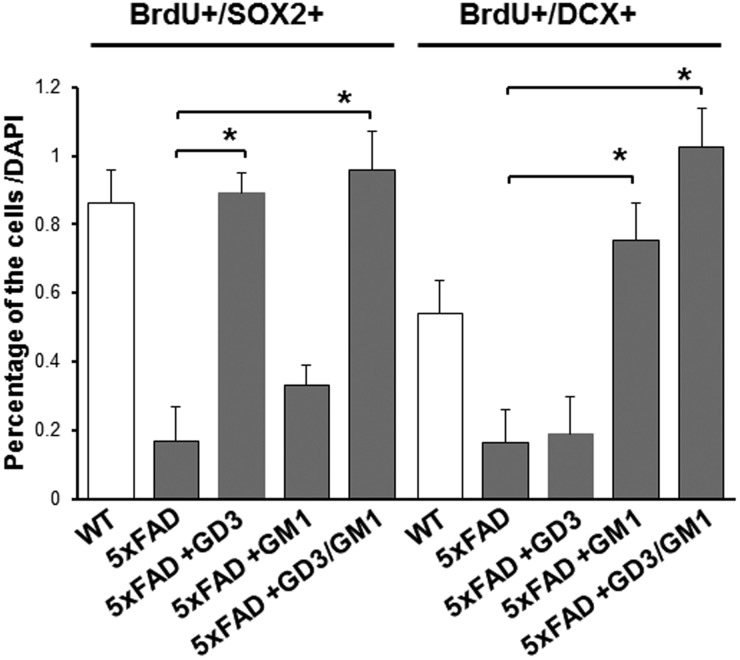
Figure 4.
GD3 restores the number of SOX2+ cells and GM1 restores DCX+ neuronal cells in the DG of 5xFAD. GD3 and GM1 were introduced into the brains of (10-week-old) 5XFAD mice (via icv for 7 days by micro-osmotic pump) and then injected with BrdU to assess its neurogenic potential with co-staining of lineage-associated markers. For the combinational experiment, GD3 was first infused for 7 days and then GM1 was infused for 7 days. (n = 3), *p < .05. WT = wild type.
GM1 Promotes Neuronal Differentiation in AD Model Mice
During neuronal differentiation, the concentration of GD3, which is the predominant ganglioside in NSCs, is rapidly decreased. Concomitantly, the levels of “brain-type” gangliosides such as GM1, GD1a, GD1b, and GT1b continuously increase in young animals, reaching a plateau during adulthood (Hirschberg et al., 1996; Ngamukote et al., 2007; Suzuki et al., 2011). These pattern changes follow closely with the upregulation of GM2/GD2S expression (Ngamukote et al., 2007). The dramatic changes in the expression profile of gangliosides clearly reflect the biological needs of GalNAc-containing ganglio-series gangliosides at particular stages of brain development. Throughout neuronal development, GM1-expressing cells are considered as neuronal progenitor cells and neurons (Maric et al., 2003; Liour et al., 2005; Androutsellis-Theotokis et al., 2010). To investigate the functional roles of GM1 on postnatal neurogenesis in the DG of AD model mouse, GM1 was administered into the 5XFAD mouse brain. GM1 increases BrdU+/doublecortin+ (DCX+) newly generated immature neuronal cells in 5XFAD mouse brains (Figure 4). As expected, the combinatorial infusion (GD3 and GM1) had a synergistic effect.
Subcutaneous or intramuscular injection of gangliosides to treat CNS disorders has been tried and failed (Svennerholm et al., 1990; Flicker et al., 1994). It is well known that gangliosides form micelles and are normally not permeable through the blood–brain barrier. Svennerholm and his collaborators described that icv administration of GM1 to AD patients could stop the continuous deterioration of nerve processes and increased the turnover of transmitter substances (Svennerholm et al., 2002). So far, icv administration is the most reliable method to deliver gangliosides into the brain of AD and other disease models (Dodge et al., 2015; Alpaugh et al., 2017). Our ganglioside infusion experiments clearly indicated that exogenous gangliosides are acting functionally on NSCs in vivo. We chose this route (icv) to ensure the successful delivery of gangliosides into the brain to modulate the cell fate of endogenous NSCs in vivo. Studies are in progress to determine whether gangliosides can also be introduced by less invasive ways, such as intranasal (Fine et al., 2015) or pulsed ultrasound (Carpentier et al., 2016).
In conclusion, we showed that icv infusion of gangliosides GD3 and GM1 simultaneously could enhance neurogenesis in adult mouse brain. For promoting adult hippocampal neurogenesis, GD3 restored NSC pools and that GM1 enhanced neuronal differentiation at DG of AD model mouse brain. We have reported that the GD3 containing microdomains initiate and facilitate the EGF signaling in cultured NSCs (Wang and Yu, 2013). We have also reported that GM1 promotes neuronal differentiation by an epigenetic regulatory mechanism (Tsai and Yu, 2014; Tsai et al., 2016). Further studies are in progress to determine whether injection of specific gangliosides, such as GD3, GM1, and their analogs, could represent a novel and effective adjuvant strategy for the treatment of AD, Parkinson’s disease, and related neurodegenerative diseases by promoting adult neurogenesis.
Acknowledgment
The authors thank Dr. Toshio Ariga for excellent technical supports.
Author Contributions
Y. I., D. L., and R. Y. K. provided conception and design of research and interpretation of the results; Y. I. and D. L. performed experiments and analyzed data; Y. I. and D. L. prepared figures; Y. I., D. L., and R. Y. K. approved the final version of manuscript; and Y. I. drafted the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a USPHS NIH grant (R01 NS100839 to R. K. Y.) and a Sheffield Memorial Grant of the CSRA Parkinson’s Disease Support Group (to R. K. Y.).
References
- Alpaugh M., Galleguillos D., Forero J., Morales L. C., Lackey S. W., Kar P., Di Pardo A., Holt A., Kerr B. J., Todd K. G., Baker G. B., Fouad K., Sipione S. (2017). Disease-modifying effects of ganglioside GM1 in Huntington’s disease models. EMBO Mol Med, 9(11), 1537–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A., Walbridge S., Park D. M., Lonser R. R., McKay R. D. (2010). Cholera toxin regulates a signaling pathway critical for the expansion of neural stem cell cultures from the fetal and adult rodent brains. PLoS One, 5(5), e10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga T., Tao R. V., Lee B. C., Yamawaki M., Yoshino H., Scarsdale N. J., Kasama T., Kushi Y., Yu R. K. (1994). Glycolipid composition of human cataractous lenses. Characterization of Lewisx glycolipids. J Biol Chem, 269(4), 2667–2675. [PubMed] [Google Scholar]
- Carpentier A., Canney M., Vignot A., Reina V., Beccaria K., Horodyckid C., Karachi C., Leclercq D., Lafon C., Chapelon J. Y., Capelle L., Cornu P., Sanson M., Hoang-Xuan K., Delattre J. Y., Idbaih A. (2016). Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med, 8(343), 343re342. [DOI] [PubMed] [Google Scholar]
- Choi S. H., Bylykbashi E., Chatila Z. K., Lee S. W., Pulli B., Clemenson G. D., Kim E., Rompala A., Oram M. K., Asselin C., Aronson J., Zhang C., Miller S. J., Lesinski A., Chen J. W., Kim D. Y., van Praag H., Spiegelman B. M., Gage F. H., Tanzi R. E. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science, 361(6406), eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J. C., Treleaven C. M., Pacheco J., Cooper S., Bao C., Abraham M., Cromwell M., Sardi S. P., Chuang W. L., Sidman R. L., Cheng S. H., Shihabuddin L. S. (2015). Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A, 112(26), 8100–8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Caille I., Lim D. A., Garcia-Verdugo J. M., Alvarez-Buylla A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell, 97(6), 703–716. [DOI] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M., Alvarez-Buylla A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci, 17(13), 5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J. M., Renner D. B., Forsberg A. C., Cameron R. A., Galick B. T., Le C., Conway P. M., Stroebel B. M., Frey W. H., II, Hanson L. R. (2015). Intranasal deferoxamine engages multiple pathways to decrease memory loss in the APP/PS1 model of amyloid accumulation. Neurosci Lett, 584, 362–367. [DOI] [PubMed] [Google Scholar]
- Flicker C., Ferris S. H., Kalkstein D., Serby M. (1994). A double-blind, placebo-controlled crossover study of ganglioside GM1 treatment for Alzheimer’s disease. Am J Psychiatry, 151(1), 126–129. [DOI] [PubMed] [Google Scholar]
- Hirschberg K., Zisling R., van Echten-Deckert G., Futerman A. H. (1996). Ganglioside synthesis during the development of neuronal polarity. Major changes occur during axonogenesis and axon elongation, but not during dendrite growth or synaptogenesis. J Biol Chem, 271(25), 14876–14882. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. (1982). Gangliosides: Structure, isolation, and analysis. Methods Enzymol, 83, 139–191. [DOI] [PubMed] [Google Scholar]
- Liour S. S., Dinkins M. B., Su C. Y., Yu R. K. (2005). Spatiotemporal expression of GM1 in murine medial pallial neural progenitor cells. J Comp Neurol, 491(4), 330–338. [DOI] [PubMed] [Google Scholar]
- Maric D., Maric I., Chang Y. H., Barker J. L. (2003). Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci, 23(1), 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jimenez E. P., Flor-Garcia M., Terreros-Roncal J., Rabano A., Cafini F., Pallas-Bazarra N., Avila J., Llorens-Martin M. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med, 25(4), 554–560. [DOI] [PubMed] [Google Scholar]
- Mu Y., Gage F. H. (2011). Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener, 6, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y., Yanagisawa M., Suzuki Y., Yu R. K. (2010). Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology, 20(1), 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamukote S., Yanagisawa M., Ariga T., Ando S., Yu R. K. (2007). Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem, 103(6), 2327–2341. [DOI] [PubMed] [Google Scholar]
- The nomenclature of lipids. Recommendations. (1976). IUPAC-IUB Commission on Biochemical Nomenclature (1977). Lipids, 12(6), 455–468. [PubMed] [Google Scholar]
- Okada M., Itoh M.i, M., Haraguchi M., Okajima T., Inoue M., Oishi H., Matsuda Y., Iwamoto T., Kawano T., Fukumoto S., Miyazaki H., Furukawa K., Aizawa S. (2002). b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J Biol Chem, 277(3), 1633–1636. [DOI] [PubMed] [Google Scholar]
- Ren S., Scarsdale J. N., Ariga T., Zhang Y., Klein R. A., Hartmann R., Kushi Y., Egge H., Yu R. K. (1992). O-acetylated gangliosides in bovine buttermilk. Characterization of 7-O-acetyl, 9-O-acetyl, and 7,9-di-O-acetyl GD3. J Biol Chem, 267(18), 12632–12638. [PubMed] [Google Scholar]
- Seri B., Garcia-Verdugo J. M., McEwen B. S., Alvarez-Buylla A. (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci, 21(18), 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen J. O., Peterson D. A., Ray J., Gage F. H. (1996). Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature, 383(6601), 624–627. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yanagisawa M., Ariga T., Yu R. K. (2011). Histone acetylation-mediated glycosyltransferase gene regulation in mouse brain during development. J Neurochem, 116(5), 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L. (1963). Chromatographic separation of human brain gangliosides. J Neurochem, 10, 613–623. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Brane G., Karlsson I., Lekman A., Ramstrom I., Wikkelso C. (2002). Alzheimer disease—Effect of continuous intracerebroventricular treatment with GM1 ganglioside and a systematic activation programme. Dement Geriatr Cogn Disord, 14(3), 128–136. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Gottfries C. G., Blennow K., Fredman P., Karlsson I., Mansson J. E., Toffano G., Wallin A. (1990). Parenteral administration of GM1 ganglioside to presenile Alzheimer patients. Acta Neurol Scand, 81(1), 48–53. [DOI] [PubMed] [Google Scholar]
- Tobin M. K., Musaraca K., Disouky A., Shetti A., Bheri A., Honer W. G., Kim N., Dawe R. J., Bennett D. A., Arfanakis K., Lazarov O. (2019). Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell, 24(6), 974–982.e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. T., Itokazu Y., Yu R. K. (2016). GM1 ganglioside is involved in epigenetic activation loci of neuronal cells. Neurochem Res, 41(1–2), 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. T., Yu R. K. (2014). Epigenetic activation of mouse ganglioside synthase genes: implications for neurogenesis. J Neurochem, 128(1), 101–110. [DOI] [PubMed] [Google Scholar]
- Wang J., Cheng A., Wakade C., Yu R. K. (2014). Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. J Neurosci, 34(41), 13790–13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yu R. K. (2013). Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc Natl Acad Sci U S A, 110(47), 19137–19142. [DOI] [PMC free article] [PubMed] [Google Scholar]