Abstract
Background:
Osteochondral defects (OCDs) of the talus are found subsequent to ankle sprains and ankle fractures. With many surgical treatment strategies available, there is no clear evidence on return-to-sport (RTS) times and rates.
Purpose:
To summarize RTS times and rates for talar OCDs treated by different surgical techniques.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
The literature from January 1996 to November 2018 was screened, and identified studies were divided into 7 different surgical treatment groups. The RTS rate, with and without associated levels of activity, and the mean time to RTS were calculated per study. When methodologically possible, a simplified pooling method was used to combine studies within 1 treatment group. Study bias was assessed using the MINORS (Methodological Index for Non-Randomized Studies) scoring system.
Results:
A total of 61 studies including 2347 talar OCDs were included. The methodological quality of the studies was poor. There were 10 retrospective case series (RCSs) that investigated bone marrow stimulation in 339 patients, with a pooled mean rate of RTS at any level of 88% (95% CI, 84%-91%); 2 RCSs investigating internal fixation in 47 patients found a pooled RTS rate of 97% (95% CI, 85%-99%), 5 RCSs in which autograft transplantation was performed in 194 patients found a pooled RTS rate of 90% (95% CI, 86%-94%), and 3 prospective case series on autologous chondrocyte implantation in 39 patients found a pooled RTS rate of 87% (95% CI, 73%-94%). The rate of return to preinjury level of sports was 79% (95% CI, 70%-85%) for 120 patients after bone marrow stimulation, 72% (95% CI, 60%-83%) for 67 patients after autograft transplantation, and 69% (95% CI, 54%-81%) for 39 patients after autologous chondrocyte implantation. The mean time to RTS ranged from 13 to 26 weeks, although no pooling was possible for this outcome measure.
Conclusion:
Different surgical treatment options for talar OCDs allow for adequate RTS times and rates. RTS rates decreased when considering patients’ return to preinjury levels versus return at any level.
Keywords: ankle, OCD, return-to-sport rate, return-to-sport time, arthroscopic surgery
A talar osteochondral defect (OCD) is a combined lesion of the subchondral bone and its overlying cartilage.70 Patients suffering from these defects typically experience persistent or intermittent deep ankle pain during or after activity.20
The treatment of talar OCDs is usually initiated with a nonoperative protocol. However, surgical treatment is often required, as most talar OCDs remain symptomatic after nonoperative treatment.95 The literature reports a wide variety of surgical treatment strategies, with surgical options ranging from regenerative to replacement therapies.33,73,79,80,83 In general, all of the different surgical treatment strategies for talar OCDs have shown good results, and there is no clear evidence regarding a superior treatment strategy for either primary or secondary defects.10,45
When studying the evidence of outcomes in athletes, it is clear that even less is known in this specific patient group.81 Athletes have different needs for treatment compared with the general population. While a number of studies have focused on sports-related outcomes after the surgical treatment of talar OCDs,14,34,69,91,93 there is, to our knowledge, no available systematic review including all surgical treatment options for talar OCDs, with associated sports-specific outcomes. With the high incidence of talar OCDs seen in athletes, there is a need for an overview and comparison of sports-related outcomes of the available surgical treatment strategies.
The aim of the present review was therefore to summarize the available evidence regarding sports outcomes for different surgical treatment options by calculating the rate of return to sports (RTS), the mean RTS time, and data on other sports-related outcomes of talar OCD surgery.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement was used as a guideline for the study. The systematic review was prospectively registered at the PROSPERO database with reference number CRD42018080718.7
Search Strategy
The electronic databases of PubMed (MEDLINE), Embase, Cochrane Database of Systematic Reviews, DARE, and CENTRAL were screened for potentially suitable studies from January 1996 to November 2018. The full search strategy for all electronic databases is outlined in Appendix Table A1. Because not all titles or abstracts in these databases clearly describe whether they report any results regarding sports outcomes, narrower terms such as “sports” or “activity” were not used in our search strategy, as this would potentially exclude eligible studies.
Eligibility Criteria and Study Selection
All studies reporting sports-related outcomes after the surgical treatment of talar OCDs were included. The exclusion criteria are reported in Table 1. When necessary, authors were contacted to provide information on any patient overlap or to provide separate data for their patients when results were given for a combination of diagnoses or a combination of treatment groups. When no reply was reported, contact was sought by 2 reminder emails. If no response was recorded, the specific article was excluded. An independent evaluation of the articles and a subsequent discussion were performed by 2 reviewers (J.A.H.S. and J.D.) after title and abstract screening and full-text reading. In case of a disagreement after a discussion at any point in time in the evaluation process, the opinion from a third investigator (G.M.M.J.K.) was consulted and decisive. Studies were not blinded for author, affiliation, or source. Only studies published in English, German, French, Dutch, or Spanish were included. No limitations were put on publication status. No restrictions were set on publication date or the patient age.
Table 1.
Exclusion Criteria
| No sports outcomes reported |
| Combination of diagnoses |
| Patient overlap |
| Written in language other than English, German, French, Dutch, or Spanish |
| Fewer than 5 patients |
| Combination of treatment groups, although no separate data per group |
| No surgical treatment |
Critical Appraisal
Methodological quality was assessed for all included studies using the MINORS (Methodological Index for Non-Randomized Studies) criteria (Appendix Table A2).82 Each study was graded on methodological quality by 2 independent reviewers (J.A.H.S. and J.D.), after which any conflicting outcomes were resolved by a discussion. In case of persisting conflict, the senior author (G.M.M.J.K.) was consulted, whose opinion was decisive.
Data Extraction
To retrieve data on study characteristics, a standardized form was used. Acquired data on patient characteristics included age, sex, number of patients and ankles, stage of the defect, whether the defect was primary or secondary, preinjury activity level, mean follow-up duration, and the reported OCD classification system. Preoperative and postoperative clinical outcomes regarding sports activity were extracted and included mean scores, number of patients participating in sports activity, activity level, and RTS time. Described treatment techniques were examined per study, after which they were divided into corresponding treatment groups. In case of the presence of other sports-related clinical outcomes, these were additionally extracted from the studies and pooled as well as subsequently analyzed where possible.
Statistical and Data Analyses
To analyze the identified studies with regard to RTS and the mean time to RTS, the definition of these measures need to be clear. In this study, we distinguished 2 types of RTS: return with level specified, where the patients had to perform in the same sport at the same level as preinjury; and return without specified level, where the patients could participate in any sport at any level, regardless of their performance before their injury.2 The mean time to RTS was defined as the mean time at which patients could resume their sports activity, regardless of which sport and at what level. When studies are found to be highly different in methodological nature, a formal meta-analysis cannot be performed. Thus, a simplified pooling method was used to combine data from different studies using corresponding methodologies to provide results within 1 treatment group. Moreover, 95% confidence intervals (binomial proportion) for the RTS rates of each study and the pooled studies were calculated with the Wilson score interval. Additionally, the mean time to RTS was calculated and pooled whenever possible. A comparison of different sports outcomes by means of formal statistical tests with accompanying P values was not deemed methodologically suitable for the present review, as the specific clinical indications for specific surgical therapies were highly different from one another; that is, the prognosis of a surgical outcome for talar OCDs correlates substantially with prognostic factors such as size, primary or nonprimary nature, patient age, and location.¶
Results
Search Results
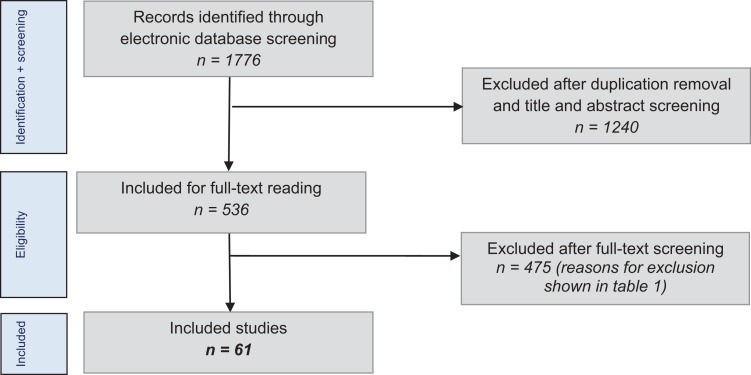
The literature search using the selected databases provided 1776 articles. After removal of duplicates and application of the eligibility criteria to the titles and abstracts, 536 articles were found to be potentially suitable and were included for a full-text review. Consequently, the full-text articles were screened, and the inclusion and exclusion criteria were applied. The authors of 15 studies were contacted through email for additional data according to our inclusion criteria. Subsequently, 2 studies were able to be included, and 13 had to be excluded attributable to this author contact process.
In total, 475 studies had to be excluded. An overview of the excluded studies by exclusion criteria is shown in Table 2. This left 61 suitable studies available for review. The literature selection algorithm according to the PRISMA guidelines is shown in Figure 1.49
Table 2.
Excluded Studies by Exclusion Criteria
| Exclusion Criteria | No. of Studies |
|---|---|
| No sports outcomes reported | 416 |
| Combination of diagnoses | 22 |
| Patient overlap | 18 |
| Written in language other than English, German, French, Dutch, or Spanish | 10 |
| Fewer than 5 patients | 5 |
| Combination of treatment groups, although no separate data per group | 3 |
| No surgical treatment | 1 |
| Total | 475 |
Figure 1.
Literature selection algorithms using PRISMA guidelines.
Characteristics of the Included Studies
A total of 2347 talar OCDs were included in the 61 studies. The mean patient age was 32 years (range, 11-72 years), and the percentage of female and male patients was 37% and 63%, respectively. The most frequently used clinical scoring system and osteochondral damage classification system were the American Orthopaedic Foot & Ankle Society (AOFAS) score and the Berndt and Harty5 classification system, respectively.40 The mean follow-up time ranged from 11 to 144 months.
Methodological Quality
A full consensus on methodological quality was reached after independent grading and a subsequent discussion by the 2 reviewers. Of the 61 studies, 52 were noncomparative prospective or retrospective studies and had an average MINORS score of 8.0 (range, 3-13) out of a possible 16 points. The remaining 9 were comparative studies and had an average MINORS score of 18.8 (range, 12-22) out of a possible 24 points. A full overview of the scores by study is shown in Appendix Table A3.
Treatment Strategies
Seven different treatment groups were formed out of the different treatment strategies (Table 3). As there were several studies that reported outcomes for multiple treatment options (ie, comparative study), the total number of treatment strategies is higher than the total number of included studies. A simplified pooling method was used for studies with the same methodology (eg, all retrospective case series together) that reported on the same outcomes after performing the same treatment technique.
Table 3.
Included Treatment Groups (61 Included Studies)a
| No. of Surgical Treatment Options | |
|---|---|
| BMS | 25 |
| BMS without additional therapies | 22 |
| BMS with platelet-rich plasma or mesenchymal stem cell injection | 2 |
| BMS with pulsed electromagnetic field therapy | 1 |
| Internal fixation | 2 |
| Drilling + fixation | 2 |
| Retrograde drilling | 1 |
| Retrograde drilling with cancellous bone grafting | 1 |
| Osteo(chondral) transplantation | 22 |
| Autograft | 13 |
| Mosaicplasty | 4 |
| Synthetic graft | 2 |
| Allograft | 1 |
| Autograft and cancellous bone allograft | 1 |
| Osteoperiosteal cylinder graft | 1 |
| Cartilage implantation | 9 |
| Autologous chondrocyte implantation | 6 |
| Matrix-induced autologous chondrocyte implantation | 2 |
| Particulated juvenile cartilage implantation | 1 |
| Chondrogenesis-inducing techniques | 10 |
| Scaffold-based therapies | 6 |
| Autologous matrix-induced chondrogenesis | 4 |
| Metal implantation | 2 |
| Metal resurfacing implant (HemiCAP) | 2 |
| Total | 71 |
aAs there were several studies that reported outcomes for multiple treatment options (ie, comparative study), the total number of treatment strategies is higher than the total number of included studies. BMS, bone marrow stimulation.
Reported Outcomes
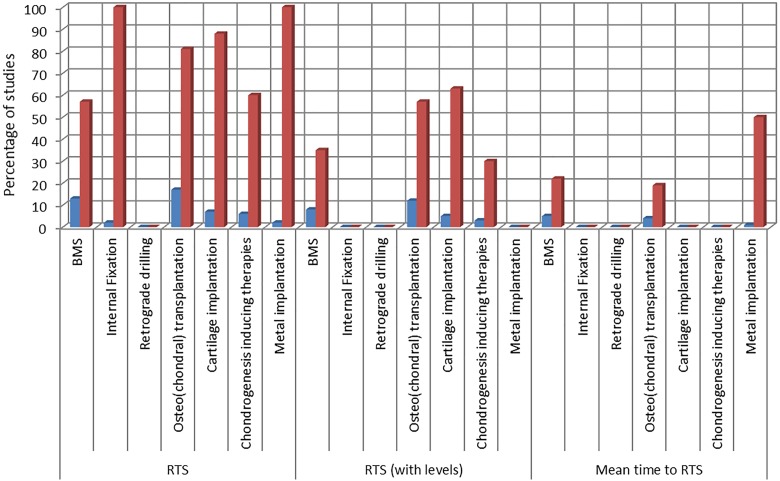
The included studies reported on sports-related outcomes by different means. Figure 2 shows an overview of the number of studies reporting on the main chosen outcome measures (RTS with and without specified level and mean time to RTS) by treatment strategy. An overview of the reported outcomes by study is shown in Appendix Table A4.
Figure 2.
Overview of reported outcomes by treatment strategy. BMS, bone marrow stimulation; RTS, return to sports.
Bone Marrow Stimulation (Debridement and/or Drilling)
Bone marrow stimulation (BMS) aims at forming local new blood vessels and stimulating fibrocartilaginous tissue.70 Additionally, platelet-rich plasma or mesenchymal stem cell injections can be used to improve cartilage regeneration.26,38 Another possibility is the use of pulsed electromagnetic field therapy.69 There were 23 studies describing the sports-related results of BMS in a total of 1243 patients.# An overview of the study characteristics, patient characteristics, and outcomes within this group is shown in Table 4.
Table 4.
Overview of Study Characteristics, Patient Characteristics, and Outcomes for Bone Marrow Stimulationa
| No. of studies | 23b |
| No. of patients | 1243 |
| Study type, n | |
| Retrospective case series | 12 |
| Randomized controlled trials | 3 |
| Retrospective comparative studies | 3 |
| Prospective comparative studies | 2 |
| Prospective case series | 2 |
| Retrospective cohort studies | 1 |
| Follow-up duration,c mo | 16-144 |
| Defects (n = 1185), n (%) | |
| Primary | 1143 (96) |
| Secondary | 42 (4) |
| Berndt and Harty classification (n = 361), n (%) | |
| Stage I | 47 (13) |
| Stage II | 155 (43) |
| Stage III | 119 (33) |
| Stage IV | 40 (11) |
| RTS rate (n = 389),c mean (95% CI), % | 76-100 (67-100) |
| RTS rate to preinjury level (n = 195),c mean (95% CI), % | 18-100 (6-100) |
| Time to RTS (n = 215),c wk | 15-26 |
A simplified pooling method was used for 10 of the 12 retrospective case series, with a total of 339 patients.** When pooled, it was calculated that 88% (95% CI, 84%-91%) of the patients were able to return to sports regardless of the level of sports activity (Table 5). There were 6 studies that specified the level of sports activity postoperatively in a total of 120 patients.18,20,43,59,60,75 Of these patients, 79% (95% CI, 70%-85%) were able to participate in sports at their preinjury level, while 18% (95% CI, 12%-26%) had some limitations and 3% (95% CI, 1%-7%) were unable to return (Table 6).
Table 5.
Mean Pooled Rates of Return to Any Level of Sportsa
| Surgical Treatment | Study Type | No. of Patients | Rate of RTS at Any Level, % |
|---|---|---|---|
| Bone marrow stimulation | 10 retrospective case series | 339 | 88 (95% CI, 84-91) |
| Internal fixation | 2 retrospective case series | 47 | 97 (95% CI, 85-99) |
| Osteochondral autograft transplant system | 5 retrospective case series | 195 | 90 (95% CI, 86-94) |
| Autologous chondrocyte implantation | 3 prospective case series | 39 | 87 (95% CI, 73-94) |
aRTS, return to sports.
Table 6.
Mean Pooled Rates of Return to Preinjury Level of Sportsa
| Surgical Treatment | Study Type | No. of Patients | Rate of RTS at Preinjury Level, % |
|---|---|---|---|
| Bone marrow stimulation | 6 retrospective case series | 120 | 79 (95% CI, 70-85) |
| Osteochondral autograft transplant system | 4 prospective case series | 67 | 72 (95% CI, 60-83) |
| Autologous chondrocyte implantation | 3 prospective case series | 39 | 69 (95% CI, 54-81) |
aRTS, return to sports.
A variety of outcome measures were reported within this group, the most commonly used being the Foot and Ankle Outcome Score (FAOS) sports score, reported by 5 studies.35,41,47,54,92 Mean follow-up times ranged from 31 to 118 months. Mean preoperative scores ranged from 35.5 to 67.6, and mean postoperative scores ranged from 48.9 to 82.2. No correlations were observed between the length of the follow-up period and the increase in the FAOS sports score. The Tegner score was also reported by 4 studies within this group.6,38,85,94 Mean follow-up times ranged from 22 to 67 months. Mean preoperative scores ranged from 1.8 to 6.2, and mean postoperative scores ranged from 3.6 to 5.9. No correlations were observed between the length of the follow-up period and the increase in the Tegner score. Other reported scores within this group were the Ankle Activity Score (AAS), reported by 2 studies,69,92 the Karlsson score, reported by 1 study,6 and the Foot and Ankle Ability Measure (FAAM) sports subscale, reported by 1 study.26 Because of low reporting, these scores were not further analyzed.
Internal Fixation
This treatment technique can be considered when large, loose osteochondral fragments are apparent in the joint, while the subchondral bone is still vital. Two studies with a total of 47 patients were identified.44,77 The mean follow-up duration ranged from 46 to 84 months. There were 13% of OCDs classified as stage I, 68% as stage II, 17% as stage III, and 2% as stage IV. Both studies were retrospective case series, reporting on RTS; therefore, the results for this treatment group were pooled. Of the 47 patients, 97% (95% CI, 85%-99%) were able to return to sports postoperatively, regardless of preinjury and postinjury sports levels, as no results regarding the specific level of sports activity or mean time to RTS were reported within this treatment group (Table 5).
Retrograde Drilling
The aim of this technique is to revascularize the subchondral bone and subsequently to achieve the formation of new bone.70 One study (a retrospective case series) was included in this treatment group,72 including 15 patients all with primary defects and a mean follow-up of 12 months.
This study solely reported results on the FAOS sports score, not on RTS rates or times. No pooling could be performed.
Osteo(chondral) Transplantation
Talar OCDs can be treated by several osteo(chondral) transplantation techniques: osteochondral autograft transplant system, mosaicplasty, autogenous bone graft transplantation, osteochondral allograft transplantation, and osteoperiosteal cylinder graft implantation.
There were 21 studies (643 patients) describing sports-related results.†† An overview of the study characteristics, patient characteristics, and outcomes within this group is shown in Table 7.
Table 7.
Overview of Study Characteristics, Patient Characteristics, and Outcomes for Osteo(chondral) Transplantationa
| No. of studies | 21b |
| No. of patients | 643 |
| Study type, n | |
| Retrospective case series | 11 |
| Prospective case series | 6 |
| Randomized controlled trials | 2 |
| Prospective comparative studies | 1 |
| Retrospective comparative studies | 1 |
| Follow-up duration,c mo | 25-151 |
| Defects (n = 589), n (%) | |
| Primary | 498 (85) |
| Secondary | 91 (15) |
| Berndt and Harty classification (n = 156), n (%) | |
| Stage I | 6 (4) |
| Stage II | 26 (17) |
| Stage III | 30 (19) |
| Stage IV | 28 (18) |
| Stage V | 66 (42) |
| RTS rate (n = 300),c mean (95% CI), % | 22-100 (6-100) |
| RTS rate to preinjury level (n = 300),c mean (95% CI), % | 21-100 (8-100) |
| Time to RTS (n = 133),c wk | 13-26 |
A simplified pooling method was used for 5 studies (all retrospective case series) reporting on RTS after treatment with autografts.21,22,46,61,63 When pooled, it was calculated that 90% (95% CI, 86%-94%) of the 194 patients could return to sports regardless of the level (Table 5). Four studies, with 67 patients, reported on the specific level of sports activity postoperatively21,22,46,61; 72% (95% CI, 60%-83%) of these patients were able to participate in sports at their preinjury level, while 13% (95% CI, 8%-27%) had some limitations and 15% (95% CI, 6%-23%) were unable to return to sports (Table 6).
The most commonly used outcome measure was the Tegner score, reported by 4 studies.39,63,65,85 Mean follow-up times ranged from 13 to 151 months. Mean preoperative scores ranged from 1.9 to 5.9, and mean postoperative scores ranged from 3.5 to 5.8. No correlations were observed between the length of the follow-up period and the increase in the Tegner score. Other reported scores within this group were the FAOS sports score, reported by 1 study,13 the FAAM sports subscale, reported by 1 study,1 and the Valderrabano activity score, reported by 1 study.86 Because of low reporting, these scores were not further analyzed.
Cartilage Implantation
The aim of cartilage implantation techniques is to regenerate damaged tissue with hyaline-like type II cartilage. There were 8 studies identified with a total of 171 patients.3,4,9,12,23,24,51,55 An overview of the study characteristics, patient characteristics, and outcomes within this group is shown in Table 8.
Table 8.
Overview of Study Characteristics, Patient Characteristics, and Outcomes for Cartilage Implantationa
| No. of studies | 8b |
| No. of patients | 171 |
| Study type, n | |
| Prospective case series | 4 |
| Retrospective case series | 4 |
| Follow-up duration,c mo | 20-67 |
| Defects (n = 172), n (%) | |
| Primary | 111 (65) |
| Secondary | 61 (35) |
| Berndt and Harty classification | N/A |
| RTS rate (n = 118),c mean (95% CI), % | 40-100 (15-100) |
| RTS rate to preinjury level (n = 88),c mean (95% CI), % | 6-100 (1-100) |
| Time to RTSc | N/A |
We were able to perform simplified pooling on 3 studies (all prospective case series) reporting on RTS after treatment with autologous chondrocyte implantation.4,23,55 It was calculated that 87% (95% CI, 73%-94%) of the patients were able to return to sports, regardless of levels, for a total of 39 patients (Table 5). All of these studies specified the level of sports activity postoperatively; of the 39 patients, 69% (95% CI, 54%-81%) were able to participate in sports activity at their preinjury levels, while 18% (95% CI, 9%-33%) had some limitations and 13% (95% CI, 6%-27%) were unable to return to sports (Table 6).
One study reported patient outcomes using the FAAM sports subscale.55 Because of low reporting, this score was not further analyzed.
Chondrogenesis-inducing Techniques
Chondrogenesis-inducing techniques can be applied for larger, cystic OCDs and aim to induce cartilage (re)generation by means of a single-step procedure. There were 9 studies with a total of 225 patients identified.‡‡ An overview of the study characteristics, patient characteristics, and outcomes within this group is shown in Table 9.
Table 9.
Overview of Study Characteristics, Patient Characteristics, and Outcomes for Chondrogenesis-Inducing Techniquesa
| No. of studies | 9b |
| No. of patients | 225 |
| Study type, n | |
| Retrospective case series | 2 |
| Prospective case series | 3 |
| Prospective cohort studies | 2 |
| Prospective comparative studies | 1 |
| Retrospective comparative studies | 1 |
| Follow-up duration,c mo | 8-60 |
| Defects (n = 146), n (%) | |
| Primary | 114 (78) |
| Secondary | 32 (22) |
| Berndt and Harty classification (n = 50), n (%) | |
| Stage III/IVd | 50 (100) |
| RTS rate (n = 79),c mean (95% CI), % | 64-92 (39-98) |
| RTS rate to preinjury level (n = 49),c mean (95% CI), % | 43-92 (16-98) |
| Time to RTSc | N/A |
A variety of outcome measures were reported within this group. The FAOS sports score was reported by 2 studies,35,54 1 study reported on the Foot and Ankle Disability Index (FADI) sports subscale,8 and 1 study reported on the German Foot Function Index (FFI-D) sports subscale.25 Because of low reporting, these scores were not further analyzed.
Metal Implantation
This technique is used for medial talar OCDs after failed primary surgical treatment. After debriding of the defect, a metal resurfacing inlay implant (HemiCAP; Arthrosurface) is introduced into the defect, thereby resurfacing the extracted subchondral bone and the cartilage layer. There were 2 studies identified with a total of 49 patients.19,96 An overview of the study characteristics, patient characteristics, and outcomes within this group is shown in Table 10.
Table 10.
Overview of Study Characteristics, Patient Characteristics, and Outcomes for Metal Implantationa
| No. of studies | 2b |
| No. of patients | 49 |
| Study type, n | |
| Prospective case series | 1 |
| Retrospective case series | 1 |
| Follow-up duration,c mo | 48-61 |
| Defects (n = 49), n (%) | |
| Secondary | 49 (100) |
| Berndt and Harty classification | N/A |
| RTS rate (n = 32),c mean (95% CI), % | 78-100 (59-100) |
| RTS rate to preinjury levelc | N/A |
| Time to RTS (n = 32),c wk | 16.5-17.5 |
One study reported outcomes using the FAOS sports score.96 Because of low reporting, this score was not further analyzed.
Discussion
To the best of our knowledge, this is the first systematic review investigating the RTS times and rates of all surgical treatment options for talar OCDs. The most important finding of the present study is that all of the surgical treatment options allowed for adequate RTS rates. However, RTS rates decreased substantially when RTS was defined as return to preinjury level. Additionally, performing simplified pooling for studies within the BMS, internal fixation, osteo(chondral) transplantation, and cartilage implantation groups yielded comparable pooled RTS rates. Furthermore, results on the mean RTS time were limited concerning quantity of reporting.
BMS was the most studied intervention; it is a commonly performed treatment worldwide, is relatively inexpensive in comparison with implantation techniques, and has low morbidity. For this treatment group, the pooled rate of RTS at any level of sport was 88%. This RTS rate is comparable to those of the pooled studies in the osteo(chondral) transplantation group describing the results of autograft (90% [95% CI, 86%-94%]) and those in the cartilage implantation group (87% [95% CI, 73%-94%]). When comparing the rates of return to preinjury level of sports between BMS (79% [95% CI, 60%-83%]), autograft (72% [95% CI, 60%-83%]), and cartilage implantation (69% [95% CI, 54%-81%]), there were no substantial differences observed either. The rate of return to preinjury level in the pooled BMS group was found to be in accordance with that in the systematic review of Ramponi et al,67 who reported an RTS rate of 76.5% after treatment with BMS. It was however not clear whether these authors reported on return to preinjury level or return to any associated level of sports. A review by Hurley et al34 showed that the rate of return to preinjury level of play for BMS was 87% for 248 patients. Although the authors provided levels of evidence for the included studies, studies with different methodological qualities were utilized to calculate this percentage, a methodological aspect that was not performed in the present study.
Return to preinjury level of sports presents the last step in the pathway of the RTS continuum, where return to participation is the first step,2 followed by RTS at any associated level.2 The rate of return to preinjury level of 79% after BMS can be considered as being relatively low when considering this continuum. However, it should be stated that is merely a short-term outcome after BMS, and the question therefore remains what the long-term sports activity level is of patients who have undergone BMS, as there are some factors that could play a vital role in the success of the long-term results. With BMS, the hyaline cartilage layer is not preserved, but the formation of a fibrin clot is promoted, after which fibrocartilaginous tissue or cartilage/collagen type I is formed. This tissue may decrease in quality over time, resulting in osteoarthritic changes.50,56,57 This type of tissue demonstrates inferior wear characteristics compared with the original hyaline cartilage (cartilage/collagen type II), which can lead to degradation of the repaired surface. Research indicates that deterioration of the natural congruency of the ankle joint can occur as a result.52,66,84 With this in mind, if possible, internal fixation could be a more appropriate treatment option for primary OCDs with a fixable radiological and intraoperative fragment.
Internal fixation aims to preserve the hyaline cartilage without causing additional clinical issues, such as donor site morbidity for the autograft group. Our pooled results for RTS at any level after internal fixation (97%) appear promising but were not substantially superior because of the lower number of patients within this treatment group. However, new data on this treatment option could be presented, as novel fixation techniques are being described, showing good short-term initial results.37
Analysis of the methodological quality of the included studies showed that most were of low methodological quality, except for 4 randomized controlled trials.1,26,69,85 This was demonstrated by the scores given for methodological quality according to the MINORS criteria (Appendix Table A3). These findings are in accordance with those of prior systematic reviews10,45,95,97 and underline the clear need for more high-quality randomized studies. Future research should be more focused on conducting randomized comparative clinical trials with extensive follow-up times and uniformity in methodology and outcome assessments. Treatment techniques from different treatment groups as well as within the same treatment group could be compared in this manner.
The present review has a number of limitations. The 2 most important limitations are the high number of methodologically low-quality studies that were included and the substantial heterogeneity in the methodology of the included studies. Heterogeneity was also observed in the used outcome measures. Some studies reported outcomes other than the RTS rate and the mean time to RTS, reporting scores from various outcome measures instead. Therefore, not all studies could be taken into account when calculating the RTS rate and the mean time to RTS, which left the results of return to preinjury level and mean time to RTS underreported and subsequently leaves a possibility for the results being subject to reporting bias.
In addition, it was not possible to perform a formal meta-analysis. Instead, a simplified pooling method was used for studies with the same methodology that reported outcomes after performing the same treatment technique. As the pooled studies were all of methodologically low quality, the evidence retrieved from this simplified pooling method was based on a lower level of evidence and may contain methodological bias. We deliberately did not choose a comparative character for the present study, as RTS rates and times were highly underreported when assessing sports outcomes, and more importantly, specific clinical indications for specific surgical therapies were highly different from one another. Therefore, it should be explicitly stated that the pooled calculated RTS rates should not be used for decision making with respect to treatment options but should merely be applied to give patients an indication on the expected RTS rates and times for the different available treatment options. The outcomes of the present study can therefore be utilized as a novel informative guideline on sports-specific prognosis for patients with primary and secondary talar OCDs aiming to return to (preinjury level of) sports.
The main strength of the present review is the extensive screening protocol, which resulted in a large number of included articles. A total of 536 articles were included for full-text reading to include all studies that reported any sports-related outcomes (Figure 1). Other strengths are the inclusion of different treatment options, the inclusion of a large number of patients, and the quality assessment of the included studies. Another strength of the present study was the extensive author contact protocol regarding additional data retrieval and further clarification on the methodology of the included studies. Last, it should be stated that the results were pooled in a methodologically correct way to retrieve relevant, unbiased findings.
The clinical relevance of the present systematic review is that the separate and pooled RTS rates and the mean times to RTS for the different surgical treatment options can be used to give patients an indication of expected outcomes when undergoing treatment for talar OCDs. The results can contribute to clinical decision-making as well as the shared decision-making process between patients and physicians, which should be an individualized approach in which close and frequent communication between surgeon, athlete, and trainer or physical therapist occurs.14
Conclusion
The different surgical treatment options for talar OCD all allowed for adequate RTS times and rates. RTS rates decreased when considering patients’ return to preinjury levels versus return at any level.
Acknowledgment
The authors thank F.S. van Etten-Jamaludin, clinical librarian, for her help with the search.
TABLE A1.
Full Electronic Search Strategy Used in This Review
| # | Search Terms | Total No. of Results |
|---|---|---|
| PubMed | ||
| 1 | “Osteochondritis Dissecans”[MeSH] | 1139 |
| 2 | osteochondritis dissecans[tiab] OR osteochondrosis dissecans[tiab] OR osteochondrolysis[tiab] OR OCD[tiab] OR OLT[tiab] | |
| 3 | (osteochondral[tiab] OR chondral[tiab] OR transchondral[tiab] OR cartilage*[tiab]) AND (defect*[tiab] OR lesion*[tiab]) | |
| 4 | #1 OR #2 OR #3 | |
| 5 | “Talus”[MeSH] | |
| 6 | talus[tiab] OR talar*[tiab] OR ankle[tiab] | |
| 7 | #5 OR #6 | |
| 8 | #4 AND #7 | |
| Embase (Ovid) | ||
| 1 | (osteochondritis dissecans/ or (osteochondritis dissecans or osteochondrosis dissecans or osteochondrolysis or OCD or OLT).ti, ab, kw. or ((osteochondral or chondral or osteochondral or transchondral or cartilage*) adj3 (defect* or lesion*)).ti, ab, kw.) and (talus/ or (talus or talar* or ankle).ti, ab, kw.) | 1580 |
| 2 | Limit 1 to yr=“1996 -Current” | 1181 |
| Cochrane Library | ||
| 1 | MeSH descriptor: [Osteochondritis Dissecans] explode all trees | 8 |
| 2 | osteochondritis dissecans or osteochondrosis dissecans or osteochondrolysis or OCD or OLT: ti, ab, kw (word variations have been searched) | 1302 |
| 3 | (osteochondral or chondral or transchondral or cartilage*) and (defect* or lesion*): ti, ab, kw (word variations have been searched) | 436 |
| 4 | #1 or #2 or #3 | 1716 |
| 5 | MeSH descriptor: [Talus] explode all trees | 36 |
| 6 | talus or talar* or ankle: ti, ab, kw (word variations have been searched) | 5877 |
| 7 | #5 or #6 | 5877 |
| 8 | #4 and #7, publication year from 1996 to 2018, in Cochrane Reviews (Reviews and Protocols), Other Reviews and Trials | 29 |
TABLE A2.
MINORS Criteriaa
| 1. A clearly stated aim: The question addressed should be precise and relevant in light of the available literature. |
| 2. Inclusion of consecutive patients: All patients potentially fit for inclusion (satisfying the criteria for inclusion) have been included in the study during the study period (no exclusion or details about the reasons for exclusion). |
| 3. Prospective collection of data: Data were collected according to a protocol established before the beginning of the study. |
| 4. Endpoints appropriate to the aim of the study: There is an unambiguous explanation of the criteria used to evaluate the main outcome, which should be in accordance with the question addressed by the study. Also, the endpoints should be assessed on an intention-to-treat basis. |
| 5. Unbiased assessment of the study endpoint: A blind evaluation of objective endpoints and a double-blind evaluation of subjective endpoints should be conducted. Otherwise, the reasons for not blinding should be stated. |
| 6. Follow-up period appropriate to the aim of the study: The follow-up should be sufficiently long to allow the assessment of the main endpoint and possible adverse events. |
| 7. Loss to follow-up less than 5%: All patients should be included in the follow-up. Otherwise, the proportion lost to follow-up should not exceed the proportion experiencing the major endpoint. |
| 8. Prospective calculation of the study size: There is information on the size of the detectable difference of interest with a calculation of the 95% CI, according to the expected incidence of the outcome event, and information about the level for statistical significance and estimates of power when comparing the outcomes. |
| Additional criteria in the case of a comparative study |
| 9. An adequate control group: There is a gold-standard diagnostic test or therapeutic intervention recognized as the optimal intervention according to the available published data. |
| 10. Contemporary groups: Control and studied groups should be managed during the same time period (no historical comparison). |
| 11. Baseline equivalence of groups: The groups should be similar regarding the criteria other than the studied endpoints. There should be an absence of confounding factors that could bias the interpretation of the results. |
| 12. Adequate statistical analyses: Statistics are in accordance with the type of study with a calculation of confidence intervals or relative risks. |
aThe items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal score is 16 for noncomparative studies and 24 for comparative studies. MINORS, Methodological Index for Non-Randomized Studies.
TABLE A3.
MINORS Scores by Studya
| First Author | Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Data Collection | Endpoints Appropriate to Aim of Study | Unbiased Assessment of Study Endpoint | Follow-up Period Appropriate to Aim of Study | <5% Loss to Follow-up | Prospective Calculation of Sample Size | Adequate Control Group | Contemporary Groups | Baseline Equivalence of Groups | Adequate Statistical Analyses | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ogut61 | 2 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 7 | ||||
| Dekker12 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Karnovsky35 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 16 |
| DeSandis13 | 1 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 6 | ||||
| Pagliazzi62 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 7 | ||||
| Sadlik74 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Vuurberg96 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 | ||||
| Ogilvie-Harris58 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Bonnin6 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 7 | ||||
| Kumai43 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 4 | ||||
| Ogilvie-Harris59 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 4 | ||||
| Ogilvie-Harris60 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| Draper17 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 14 |
| Savva75 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Ferkel20 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Ventura94 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Dunlap18 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| van Bergen88 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Kramer41 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Lee47 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Reilingh69 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 22 |
| van Eekeren92 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Kumai44 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 4 | ||||
| Schuh77 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Kim38 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Sun85 | 1 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 9 | ||||
| Guney26 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 | ||||
| Hankemeier29 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| Murphy54 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | ||||
| Saxena76 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | ||||
| Domayer15 | 2 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 7 | ||||
| Rosenberger72 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 3 | ||||
| Lee46 | 2 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 7 | ||||
| Haasper27 | 2 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 7 | ||||
| Valderrabano86 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| Hangody28 | 0 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Kennedy36 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 3 | ||||
| Paul63 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Pearce64 | 0 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Hu32 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Leumann48 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 | ||||
| Petersen65 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 9 | ||||
| Ahmad1 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Fraser21 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| Hintermann31 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 | ||||
| Zhu98 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Kim39 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Gautier22 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 4 | ||||
| Baums4 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| Giannini23 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 | ||||
| Giannini24 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| Aurich3 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | ||||
| Magnan51 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 6 | ||||
| Coetzee9 | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 5 | ||||
| Nehrer55 | 1 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 9 | ||||
| Valderrabano87 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 | ||||
| Kubosch42 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | ||||
| Gottschalk25 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 | ||||
| Clanton8 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 6 | ||||
| Richter71 | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 9 | ||||
| Ettinger19 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 8 |
aMINORS, Methodological Index for Non-Randomized Studies.
TABLE A4.
Outcome Measures Reported by Treatment Strategy and Studya
| First Author | Subcategory | Methodology | Sports Activity | Time to RTS | Tegner Score | FAOS Score | FAAM Score | FADI Score | Karlsson Score | FFI-D Score | Valderrabano Score | Martin Score | AAS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMS | |||||||||||||
| Ogilvie-Harris58 | BMS | PCS | x | ||||||||||
| Bonnin6 | BMS | RCS | x | x | |||||||||
| Kumai43 | BMS | RCS | x | ||||||||||
| Ogilvie-Harris59 | BMS | RCS | x | x | |||||||||
| Ogilvie-Harris60 | BMS | RCS | x | x | |||||||||
| Draper17 | BMS | R Comp | x | ||||||||||
| Savva75 | BMS | RCS | x | ||||||||||
| Ferkel20 | BMS | RCS | x | ||||||||||
| Ventura94 | BMS | RCS | x | x | |||||||||
| Dunlap18 | BMS | RCS | x | ||||||||||
| van Bergen88 | BMS | RCS | x | ||||||||||
| Kramer41 | BMS | RCS | x | x | x | ||||||||
| Karnovsky35 | BMS | R Comp | x | ||||||||||
| Lee47 | BMS | R Cohort | x | ||||||||||
| Reilingh69 | BMS + PEMF | RCT | x | x | x | ||||||||
| van Eekeren92 | BMS | RCS | x | x | x | ||||||||
| Kim38 | BMS + PRP/MSC | R Comp | x | x | |||||||||
| Sun85 | BMS | RCT | x | ||||||||||
| Guney26 | BMS + PRP/MSC | RCT | x | ||||||||||
| Hankemeier29 | BMS | RCS | x | ||||||||||
| Murphy54 | BMS | P Comp | x | ||||||||||
| Saxena76 | BMS | P Comp | x | x | |||||||||
| Domayer15 | BMS | PCS | x | ||||||||||
| Internal fixation | |||||||||||||
| Kumai44 | Drilling + fixation | RCS | x | ||||||||||
| Schuh77 | Drilling + fixation | RCS | x | ||||||||||
| Retrograde drilling | |||||||||||||
| Rosenberger72 | Retrograde drilling | RCS | x | ||||||||||
| Osteo(chondral) transplantation | |||||||||||||
| Saxena76 | Autograft | PCS | x | x | |||||||||
| Sun85 | Autograft | RCT | x | ||||||||||
| Draper17 | Autograft | R Comp | x | ||||||||||
| Lee46 | Autograft | RCS | x | ||||||||||
| Haasper27 | Mosaicplasty | RCS | x | ||||||||||
| Valderrabano86 | Mosaicplasty | RCS | x | x | |||||||||
| Hangody28 | Mosaicplasty | PCS | x | ||||||||||
| Kennedy36 | Autograft | PCS | x | x | |||||||||
| Paul63 | Autograft | RCS | x | x | |||||||||
| Pearce64 | Synthetic graft | PCS | x | ||||||||||
| Hu32 | Osteoperiosteal graft | RCS | x | ||||||||||
| Leumann48 | Mosaicplasty | PCS | x | ||||||||||
| Petersen65 | Autograft | PCS | x | x | |||||||||
| Ahmad1 | Autograft/allograft | RCT | x | ||||||||||
| Fraser21 | Autograft | RCS | x | x | |||||||||
| Hintermann31 | Autograft | PCS | x | x | |||||||||
| Zhu98 | Autograft + allograft | RCS | x | ||||||||||
| Kim39 | Autograft | RCS | x | ||||||||||
| Gautier22 | Autograft | RCS | x | ||||||||||
| Ogut61 | Autograft | RCS | x | ||||||||||
| DeSandis13 | Synthetic graft | RCS | x | ||||||||||
| Cartilage implantation | |||||||||||||
| Baums4 | ACI | PCS | x | ||||||||||
| Giannini23 | ACI | PCS | x | ||||||||||
| Giannini24 | ACI | RCS | x | ||||||||||
| Aurich3 | MACI | PCS | x | ||||||||||
| Magnan51 | ACI | RCS | x | ||||||||||
| Coetzee9 | ACI | RCS | x | ||||||||||
| Nehrer55 | MACI/ACI | PCS | x | ||||||||||
| Dekker12 | PJCI | RCS | x | ||||||||||
| Metal implantation | |||||||||||||
| Ettinger19 | Metal implant | RCS | x | ||||||||||
| Vuurberg96 | Metal implant | PCS | x | ||||||||||
| Chondrogenesis-inducing techniques | |||||||||||||
| Valderrabano87 | AMIC | PCS | x | ||||||||||
| Kubosch42 | AMIC | PCS | x | ||||||||||
| Gottschalk25 | AMIC | P Cohort | x | ||||||||||
| Clanton8 | Other | RCS | x | ||||||||||
| Pagliazzi62 | Other | RCS | x | ||||||||||
| Murphy54 | Other | P Cohort | x | ||||||||||
| Richter71 | Other | PCS | x | ||||||||||
| Karnovsky35 | Other | RCS | x | ||||||||||
| Sadlik74 | Other | P Comp | x | ||||||||||
aAs there were several studies that reported outcomes for multiple treatment options (ie, comparative study), the total number of treatment strategies is higher than the total number of included studies. AAS, Ankle Activity Score; ACI, autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; BMS, bone marrow stimulation; FAAM, Foot and Ankle Ability Measure; FADI, Foot and Ankle Disability Index; FAOS, Foot and Ankle Outcome Score; FFI-D, German Foot Function Index; MACI, matrix-induced autologous chondrocyte implantation; MSC, mesenchymal stem cell; P Cohort, prospective cohort study; P Comp, prospective comparative study; PCS, prospective case series; PEMF, pulsed electromagnetic field; PJCI, particulated juvenile cartilage implantation; PRP, platelet-rich plasma; R Cohort, retrospective cohort study; R Comp, retrospective comparative study; RCS, retrospective case series; RCT, randomized controlled trial; RTS, return to sports.
Footnotes
One or more of the authors declared the following potential conflict of interest or source of funding: K.T.A.L. has received research support from Marti-Keuning Eckhardt. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Ahmad J, Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent or large talar osteochondral lesions. Foot Ankle Int. 2016;37(1):40–50. [DOI] [PubMed] [Google Scholar]
- 2. Ardern CL, Glasgow P, Schneiders A, et al. 2016. consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016;50(14):853–864. [DOI] [PubMed] [Google Scholar]
- 3. Aurich M, Bedi HS, Smith PJ, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39(2):311–319. [DOI] [PubMed] [Google Scholar]
- 4. Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am. 2006;88(2):303–308. [DOI] [PubMed] [Google Scholar]
- 5. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988–1020. [PubMed] [Google Scholar]
- 6. Bonnin M, Bouysset M. Arthroscopy of the ankle: analysis of results and indications on a series of 75 cases. Foot Ankle Int. 1999;20(11):744–751. [DOI] [PubMed] [Google Scholar]
- 7. Chien PF, Khan KS, Siassakos D. Registration of systematic reviews: PROSPERO. BJOG. 2012;119(8):903–905. [DOI] [PubMed] [Google Scholar]
- 8. Clanton T, Johnson N, Matheny L. Use of cartilage extracellular matrix and bone marrow aspirate concentrate in treatment of osteochondral lesions of the talus. Tech Foot Ankle Surg. 2014;13(4):212–220. [Google Scholar]
- 9. Coetzee JC, Giza E, Schon LC, et al. Treatment of osteochondral lesions of the talus with particulated juvenile cartilage. Foot Ankle Int. 2013;34(9):1205–1211. [DOI] [PubMed] [Google Scholar]
- 10. Dahmen J, Lambers KT, Reilingh ML, van Bergen CJ, Stufkens SA, Kerkhoffs GM. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Ambrosi R, Villafane JH, Indino C, Liuni FM, Berjano P, Usuelli FG. Return to sport after arthroscopic autologous matrix-induced chondrogenesis for patients with osteochondral lesion of the talus [published online December 26, 2017]. Clin J Sport Med. doi:10.1097/JSM.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 12. Dekker TJ, Steele JR, Federer AE, Easley ME, Hamid KS, Adams SB. Efficacy of particulated juvenile cartilage allograft transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2018;39(3):278–283. [DOI] [PubMed] [Google Scholar]
- 13. DeSandis BA, Haleem AM, Sofka CM, O’Malley MJ, Drakos MC. Arthroscopic treatment of osteochondral lesions of the talus using juvenile articular cartilage allograft and autologous bone marrow aspirate concentration. J Foot Ankle Surg. 2018;57(2):273–280. [DOI] [PubMed] [Google Scholar]
- 14. D’Hooghe P, Murawski CD, Boakye LAT, et al. Rehabilitation and return to sports: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):61s–67s. [DOI] [PubMed] [Google Scholar]
- 15. Domayer SE, Welsch GH, Stelzeneder D, et al. Microfracture in the ankle: clinical results and MRI with T2-mapping at 3.0 T after 1 to 8 years. Cartilage. 2011;2(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dombrowski ME, Yasui Y, Murawski CD, et al. Conservative management and biological treatment strategies: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):9s–15s. [DOI] [PubMed] [Google Scholar]
- 17. Draper SD, Fallat LM. Autogenous bone grafting for the treatment of talar dome lesions. J Foot Ankle Surg. 2000;39(1):15–23. [DOI] [PubMed] [Google Scholar]
- 18. Dunlap BJ, Ferkel RD, Applegate GR. The “LIFT” lesion: lateral inverted osteochondral fracture of the talus. Arthroscopy. 2013;29(11):1826–1833. [DOI] [PubMed] [Google Scholar]
- 19. Ettinger S, Stukenborg-Colsman C, Waizy H, et al. Results of HemiCAP implantation as a salvage procedure for osteochondral lesions of the talus. J Foot Ankle Surg. 2017;56(4):788–792. [DOI] [PubMed] [Google Scholar]
- 20. Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750–1762. [DOI] [PubMed] [Google Scholar]
- 21. Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1272–1279. [DOI] [PubMed] [Google Scholar]
- 22. Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84(2):237–244. [DOI] [PubMed] [Google Scholar]
- 23. Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37(Suppl 1):112s–118s. [DOI] [PubMed] [Google Scholar]
- 24. Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36(5):873–880. [DOI] [PubMed] [Google Scholar]
- 25. Gottschalk O, Altenberger S, Baumbach S, et al. Functional medium-term results after autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a 5-year prospective cohort study. J Foot Ankle Surg. 2017;56(5):930–936. [DOI] [PubMed] [Google Scholar]
- 26. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384–2389. [DOI] [PubMed] [Google Scholar]
- 27. Haasper C, Zelle BA, Knobloch K, et al. No mid-term difference in mosaicplasty in previously treated versus previously untreated patients with osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2008;128(5):499–504. [DOI] [PubMed] [Google Scholar]
- 28. Hangody L, Dobos J, Balo E, Panics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38(6):1125–1133. [DOI] [PubMed] [Google Scholar]
- 29. Hankemeier S, Muller EJ, Kaminski A, Muhr G. [10-year results of bone marrow stimulating therapy in the treatment of osteochondritis dissecans of the talus]. Unfallchirurg. 2003;106(6):461–466. [DOI] [PubMed] [Google Scholar]
- 30. Hannon CP, Bayer S, Murawski CD, et al. Debridement, curettage, and bone marrow stimulation: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):16s–22s. [DOI] [PubMed] [Google Scholar]
- 31. Hintermann B, Wagener J, Knupp M, Schweizer C, Schaefer DJ. Treatment of extended osteochondral lesions of the talus with a free vascularised bone graft from the medial condyle of the femur. Bone Joint J. 2015;97(9):1242–1249. [DOI] [PubMed] [Google Scholar]
- 32. Hu Y, Guo Q, Jiao C, et al. Treatment of large cystic medial osteochondral lesions of the talus with autologous osteoperiosteal cylinder grafts. Arthroscopy. 2013;29(8):1372–1379. [DOI] [PubMed] [Google Scholar]
- 33. Hurley ET, Murawski CD, Paul J, et al. Osteochondral autograft: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):28s–34s. [DOI] [PubMed] [Google Scholar]
- 34. Hurley ET, Shimozono Y, McGoldrick NP, Myerson CL, Yasui Y, Kennedy JG. High reported rate of return to play following bone marrow stimulation for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2721–2730. [DOI] [PubMed] [Google Scholar]
- 35. Karnovsky SC, DeSandis B, Haleem AM, Sofka CM, O’Malley M, Drakos MC. Comparison of juvenile allogenous articular cartilage and bone marrow aspirate concentrate versus microfracture with and without bone marrow aspirate concentrate in arthroscopic treatment of talar osteochondral lesions. Foot Ankle Int. 2018;39(4):393–405. [DOI] [PubMed] [Google Scholar]
- 36. Kennedy JG, Murawski CD. The treatment of osteochondral lesions of the talus with autologous osteochondral transplantation and bone marrow aspirate concentrate: surgical technique. Cartilage. 2011;2(4):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kerkhoffs GM, Reilingh ML, Gerards RM, de Leeuw PA. Lift, drill, fill and fix (LDFF): a new arthroscopic treatment for talar osteochondral defects. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1265–1271. [DOI] [PubMed] [Google Scholar]
- 38. Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090–1099. [DOI] [PubMed] [Google Scholar]
- 39. Kim YS, Park EH, Kim YC, Koh YG, Lee JW. Factors associated with the clinical outcomes of the osteochondral autograft transfer system in osteochondral lesions of the talus: second-look arthroscopic evaluation. Am J Sports Med. 2012;40(12):2709–2719. [DOI] [PubMed] [Google Scholar]
- 40. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349–353. [DOI] [PubMed] [Google Scholar]
- 41. Kramer DE, Glotzbecker MP, Shore BJ, et al. Results of surgical management of osteochondritis dissecans of the ankle in the pediatric and adolescent population. J Pediatr Orthop. 2015;35(7):725–733. [DOI] [PubMed] [Google Scholar]
- 42. Kubosch EJ, Erdle B, Izadpanah K, et al. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop. 2016;40(1):65–71. [DOI] [PubMed] [Google Scholar]
- 43. Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81(9):1229–1235. [DOI] [PubMed] [Google Scholar]
- 44. Kumai T, Takakura Y, Kitada C, Tanaka Y, Hayashi K. Fixation of osteochondral lesions of the talus using cortical bone pegs. J Bone Joint Surg Br. 2002;84(3):369–374. [DOI] [PubMed] [Google Scholar]
- 45. Lambers KT, Dahmen J, Reilingh ML, van Bergen CJ, Stufkens SA, Kerkhoffs GM. No superior surgical treatment for secondary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee CH, Chao KH, Huang GS, Wu SS. Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int. 2003;24(11):815–822. [DOI] [PubMed] [Google Scholar]
- 47. Lee M, Kwon JW, Choi WJ, Lee JW. Comparison of outcomes for osteochondral lesions of the talus with and without chronic lateral ankle instability. Foot Ankle Int. 2015;36(9):1050–1057. [DOI] [PubMed] [Google Scholar]
- 48. Leumann A, Valderrabano V, Wiewiorski M, Barg A, Hintermann B, Pagenstert G. Bony periosteum-covered iliac crest plug transplantation for severe osteochondral lesions of the talus: a modified mosaicplasty procedure. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1304–1310. [DOI] [PubMed] [Google Scholar]
- 49. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lynn AK, Brooks RA, Bonfield W, Rushton N. Repair of defects in articular joints: prospects for material-based solutions in tissue engineering. J Bone Joint Surg Br. 2004;86(8):1093–1099. [DOI] [PubMed] [Google Scholar]
- 51. Magnan B, Samaila E, Bondi M, Bonetti I, Micheloni GM, Bartolozzi P. Three-dimensional matrix-induced autologous chondrocytes implantation for talus osteochondral lesions. J Orthop Traumatol. 2012;13:S76–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marsh JL, Buckwalter J, Gelberman R, et al. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002;84(7):1259–1271. [PubMed] [Google Scholar]
- 53. Mittwede PN, Murawski CD, Ackermann J, et al. Revision and salvage management: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):54s–60s. [DOI] [PubMed] [Google Scholar]
- 54. Murphy EP, McGoldrick N, Curtin M, Kearns SR. A prospective evaluation of the efficacy of bone marrow aspirate concentrate injection with microfracture in osteochondral lesions of the talus. Foot Ankle Surg. 2019;25(4):441–448. [DOI] [PubMed] [Google Scholar]
- 55. Nehrer S, Domayer SE, Hirschfeld C, Stelzeneder D, Trattnig S, Dorotka R. Matrix-associated and autologous chondrocyte transplantation in the ankle: clinical and MRI follow-up after 2 to 11 years. Cartilage. 2011;2(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505–513. [DOI] [PubMed] [Google Scholar]
- 57. O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80(12):1795–1812. [PubMed] [Google Scholar]
- 58. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564–574. [DOI] [PubMed] [Google Scholar]
- 59. Ogilvie-Harris DJ, Sarrosa EA. Arthroscopic treatment after previous failed open surgery for osteochondritis dissecans of the talus. Arthroscopy. 1999;15(8):809–812. [DOI] [PubMed] [Google Scholar]
- 60. Ogilvie-Harris DJ, Sarrosa EA. Arthroscopic treatment of osteochondritis dissecans of the talus. Arthroscopy. 1999;15(8):805–808. [DOI] [PubMed] [Google Scholar]
- 61. Ogut T, Ayhan E, Irgit K, Sarikaya AI. Endoscopic treatment of posterior ankle pain. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1355–1361. [DOI] [PubMed] [Google Scholar]
- 62. Pagliazzi G, Baldassarri M, Perazzo L, Vannini F, Castagnini F, Buda R. Tissue bioengineering in the treatment of osteochondritis dissecans of the talus in children with open physis: preliminary results. J Pediatr Orthop. 2018;38(7):375–381. [DOI] [PubMed] [Google Scholar]
- 63. Paul J, Sagstetter M, Lammle L, et al. Sports activity after osteochondral transplantation of the talus. Am J Sports Med. 2012;40(4):870–874. [DOI] [PubMed] [Google Scholar]
- 64. Pearce CJ, Gartner LE, Mitchell A, Calder JD. Synthetic osteochondral grafting of ankle osteochondral lesions. Foot Ankle Surg. 2012;18(2):114–118. [DOI] [PubMed] [Google Scholar]
- 65. Petersen W, Taheri P, Schliemann B, Achtnich A, Winter C, Forkel P. Osteochondral transplantation for the treatment of osteochondral defects at the talus with the Diamond twin system((R)) and graft harvesting from the posterior femoral condyles. Arch Orthop Trauma Surg. 2014;134(6):843–852. [DOI] [PubMed] [Google Scholar]
- 66. Qiu YS, Shahgaldi BF, Revell WJ, Heatley FW. Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthritis Cartilage. 2003;11(11):810–820. [DOI] [PubMed] [Google Scholar]
- 67. Ramponi L, Yasui Y, Murawski CD, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2016;45(7):1698–1705. [DOI] [PubMed] [Google Scholar]
- 68. Reilingh ML, Murawski CD, DiGiovanni CW, et al. Fixation techniques: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):23s–27s. [DOI] [PubMed] [Google Scholar]
- 69. Reilingh ML, van Bergen CJ, Gerards RM, et al. Effects of pulsed electromagnetic fields on return to sports after arthroscopic debridement and microfracture of osteochondral talar defects: a randomized, double-blind, placebo-controlled, multicenter trial. Am J Sports Med. 2016;44(5):1292–1300. [DOI] [PubMed] [Google Scholar]
- 70. Reilingh ML, van Bergen CJ, van Dijk CN. Diagnosis and treatment of osteochondral defects of the ankle. South Afr Orthop J. 2009;8:44–50. [Google Scholar]
- 71. Richter M, Zech S. Matrix-associated stem cell transplantation (MAST) in chondral defects of foot and ankle is effective. Foot Ankle Surg. 2013;19(2):84–90. [DOI] [PubMed] [Google Scholar]
- 72. Rosenberger RE, Bale RJ, Fink C, et al. [Computer-assisted drilling of the lower extremity: technique and indications]. Unfallchirurg. 2002;105(4):353–358. [DOI] [PubMed] [Google Scholar]
- 73. Rothrauff BB, Murawski CD, Angthong C, et al. Scaffold-based therapies: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):41s–47s. [DOI] [PubMed] [Google Scholar]
- 74. Sadlik B, Kolodziej L, Puszkarz M, Laprus H, Mojzesz M, Whyte GP. Surgical repair of osteochondral lesions of the talus using biologic inlay osteochondral reconstruction: clinical outcomes after treatment using a medial malleolar osteotomy approach compared to an arthroscopically-assisted approach. Foot Ankle Surg. 2019;25(4):449–456. [DOI] [PubMed] [Google Scholar]
- 75. Savva N, Jabur M, Davies M, Saxby T. Osteochondral lesions of the talus: results of repeat arthroscopic debridement. Foot Ankle Int. 2007;28(6):669–673. [DOI] [PubMed] [Google Scholar]
- 76. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680–1687. [DOI] [PubMed] [Google Scholar]
- 77. Schuh A, Salminen S, Zeiler G, Schraml A. [Results of fixation of osteochondral lesions of the talus using K-wires]. Zentralbl Chir. 2004;129(6):470–475. [DOI] [PubMed] [Google Scholar]
- 78. Shimozono Y, Brown AJ, Batista JP, et al. Subchondral pathology: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):48s–53s. [DOI] [PubMed] [Google Scholar]
- 79. Shimozono Y, Donders JCE, Yasui Y, et al. Effect of the containment type on clinical outcomes in osteochondral lesions of the talus treated with autologous osteochondral transplantation. Am J Sports Med. 2018;46(9):2096–2102. [DOI] [PubMed] [Google Scholar]
- 80. Shimozono Y, Hurley ET, Nguyen JT, Deyer TW, Kennedy JG. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2018;100(21):1838–1844. [DOI] [PubMed] [Google Scholar]
- 81. Shimozono Y, Yasui Y, Ross AW, Kennedy JG. Osteochondral lesions of the talus in the athlete: up to date review. Curr Rev Musculoskelet Med. 2017;10(1):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 83. Smyth NA, Murawski CD, Adams SB, Jr, et al. Osteochondral allograft: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):35s–40s. [DOI] [PubMed] [Google Scholar]
- 84. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279–286. [DOI] [PubMed] [Google Scholar]
- 85. Sun JL, Li LL, Yang JX, Chen Y, Ma WL. A comparison of surgical approaches for osteochondral lesions of the talus associated with ankle fractures. Int J Clin Exp Med. 2016;9(11):21780–21786. [Google Scholar]
- 86. Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(Suppl 1):105S–111S. [DOI] [PubMed] [Google Scholar]
- 87. Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41(3):519–527. [DOI] [PubMed] [Google Scholar]
- 88. van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GM, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95(6):519–525. [DOI] [PubMed] [Google Scholar]
- 89. van Bergen CJA, Baur OL, Murawski CD, et al. Diagnosis, history, physical examination, imaging, and arthroscopy: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):3s–8s. [DOI] [PubMed] [Google Scholar]
- 90. van Dijk PAD, Murawski CD, Hunt KJ, et al. Post-treatment follow-up, imaging, and outcome scores: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):68s–73s. [DOI] [PubMed] [Google Scholar]
- 91. van Eekeren IC, Reilingh ML, van Dijk CN. Rehabilitation and return-to-sports activity after debridement and bone marrow stimulation of osteochondral talar defects. Sports Med. 2012;42(10):857–870. [DOI] [PubMed] [Google Scholar]
- 92. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vannini F, Cavallo M, Ramponi L, et al. Return to sports after bone marrow-derived cell transplantation for osteochondral lesions of the talus. Cartilage. 2016;8(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ventura A, Terzaghi C, Legnani C, Borgo E. Treatment of post-traumatic osteochondral lesions of the talus: a four-step approach. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233–242. [DOI] [PubMed] [Google Scholar]
- 96. Vuurberg G, Reilingh ML, van Bergen CJA, van Eekeren ICM, Gerards RM, van Dijk CN. Metal resurfacing inlay implant for osteochondral talar defects after failed previous surgery: a midterm prospective follow-up study. Am J Sports Med. 2018;46(7):1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yasui Y, Ramponi L, Seow D, et al. Systematic review of bone marrow stimulation for osteochondral lesion of talus: evaluation for level and quality of clinical studies. World J Orthop. 2017;8(12):956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhu Y, Xu X. Osteochondral autograft transfer combined with cancellous allografts for large cystic osteochondral defect of the talus. Foot Ankle Int. 2016;37(10):1113–1118. [DOI] [PubMed] [Google Scholar]