Abstract
Technological advancements have revolutionized our understanding of the complexity and importance of the human microbiome. This progress has also emphasized the need for precision therapeutics, as it has underscored the dilemmas, such as dysbiosis and increasing antibiotic resistance, associated with current, broad-spectrum treatment modalities. Dental caries remains the most common chronic disease worldwide, accompanied by a tremendous financial and social burden, despite widespread and efficacious fluoride and hygienic regimens. Over the past several decades, various precision approaches to combat dental caries, including vaccines, probiotics, and antimicrobial compounds, have been pursued. Despite the distinct overall conceptual strengths of each approach, for various reasons, there are currently no approved precision antibiotic therapeutics to prevent dental caries. Specifically targeted antimicrobial peptides (STAMPs) are synthetic molecules that combine the antibiotic moiety of a traditional antimicrobial peptide with a targeting domain to provide specificity against a particular organism. Conjoining the killing domain from the antimicrobial, novispirin G10, and a targeting domain derived from the Streptococcus mutans pheromone, CSP, the STAMP C16G2 was designed to provide targeted killing of S. mutans, widely considered the keystone species in dental caries pathogenesis. C16G2 was able to selectively eliminate S. mutans from complex ecosystems while leaving closely related, yet health-associated, oral species unharmed. This remodeling of the dental plaque community is expected to have significant advantages compared to conventional broad-spectrum mouthwashes, as the intact, surviving community is apt to prevent reinfection by pathogens. Following successful phase I clinical trials that evaluated the safety and basic microbiology of C16G2 treatments, the phase II trials of several C16G2 formulations are currently in progress. C16G2 represents an exciting advance in precision therapeutics, and the STAMP platform provides vast opportunities for both the development of additional therapeutics and the overall study of microbial ecology.
Keywords: precision antibiotics, Streptococcus mutans, human microbiome, oral health, dysbiosis, clinical trial
During the very first observation of the human microbiome, Van Leeuwenhoek noted the diversity of microbial life in his dental plaque (Willey et al. 2011). Throughout the next 3 centuries, however, it was mainly studies using reductionist approaches that accomplished scientific advancements in the field of microbiology. Recently, the development of second- and now third-generation sequencing technologies has allowed investigators to examine the complexity of the microbiome without the limitations of cultivation. The microbiome of a healthy human is now understood to be highly diverse and of prime importance, contributing significantly to immune modulation, digestion, colonization resistance against would-be pathogens, and other functions (Human Microbiome Project 2012). This enlightenment has caused a major paradigm shift from the concept of “one-germ, one-disease” to that of dysbiosis and polymicrobial diseases. Despite the historical study of a single pathogen per disease, most therapeutics currently in use to treat maladies of microbial origin have a broad spectrum of activity. The adverse effects of broad-spectrum antimicrobial therapies against many diseases are now well established (Keeney et al. 2014). Typically, administration of broad-spectrum antibiotics eliminates not only the pathogen of interest but also wide swaths of the overall microbiota. This leaves the treated body site prone to blooms of antibiotic-resistant pathogens or recolonization with a less-than-optimal, potentially even harmful, microbial community. More precise targeting of pathogenic species by treatment modalities that leave the remainder of the microbiome unharmed is an objective that has inspired a significant amount of research in recent years.
Moreover, the utility of precision antibiotics is not limited to the treatment of disease. Lack of robust model systems and, importantly, the inability to selectively examine the role of specific taxa within a clinically relevant microbial community have hampered the study of microbial ecology and its relationship to pathogenesis and disease. The complexity of the communities of the human microbiome makes it very difficult to determine the functions of individual species. In addition to use as therapeutic agents themselves, precision antibiotics would serve to accelerate the study of microbial ecology by enabling the knockout of a single taxa from a given community. Research using this strategy could be employed in a similar manner to that of single-gene deletion mutants, whose role in past and present scientific discovery and progress cannot be understated.
Two hundred years after Van Leeuwenhoek’s observation of dental plaque microbes, W. D. Miller first conceptualized that tooth decay is mediated by the production of acid by oral bacteria, as a result of their metabolism of dietary carbohydrates (Miller 1890). Although the acid produced by a dysbiotic dental plaque is indeed the etiologic agent of dental caries, the disease is now understood to be multifactorial, with diet, oral hygiene practices, and immunology also contributing to pathogenesis, or lack thereof (Pitts et al. 2017; Bowen et al. 2018). Critically, dental caries is the most common chronic disease worldwide and will afflict well over half the human population at some point in their lives. Dental caries is particularly problematic in vulnerable groups such as children and the economically deprived, while the extraordinary prevalence of caries results in a tremendous economic burden (Pitts et al. 2017).
The oral cavity contains a number of microenvironments and as such harbors one of the most diverse populations of the human microbiome (Human Microbiome Project 2012). The taxa that are thought to be the chief contributors to caries pathogenesis generally have 3 traits: prodigious production of acids as a by-product of carbohydrate metabolism, high acid tolerance, and the ability to form a biofilm on the tooth surface. Streptococcus mutans, the archetype species of the wider mutans group of streptococci, has served as the paradigm cariogenic organism for many decades due to historical isolation of S. mutans from caries lesions, its robust capacity to generate biofilms, and the ability of S. mutans to cause disease in animal models (Loesche 1986; Bowen 2016; Pitts et al. 2017; Banas and Drake 2018). This focus on S. mutans has not been without controversy, particularly in recent years (Simon-Soro and Mira 2015; Philip et al. 2018a). This is mainly due to the discordant results of various studies examining the association between S. mutans and dental caries, coupled with the fact that numerous taxa in the dental plaque milieu can produce and tolerate acid. Furthermore, a number of taxa have now been associated with dental caries based on culture-independent detection methods (reviewed in Hajishengallis et al. 2017). Nonetheless, crucially, S. mutans stands virtually alone in its ability to produce insoluble glucans from sucrose and is correlated with caries in the majority of cases, strongly supporting its role as a pivotal species in the pathogenesis of dental caries (Guo, McLean, Lux, et al. 2015; Bowen 2016; Banas and Drake 2018) and therefore a logical target for therapeutic development.
For over 50 y, fluoride treatments, including fluoridated toothpaste and drinking water, have been used to combat the disease. Mechanistically, fluoride prevents and treats dental caries by both promoting favorable remineralization of the tooth enamel and impairing bacterial metabolism (Pitts et al. 2017). In addition, incorporation of fluoride into the hydroxyapatite mineral reduces its solubility, therefore helping to prevent demineralization (ten Cate and Featherstone 1991). Although the efficacy of fluoride treatments is well documented, the extreme prevalence of dental caries, as specified above, is in the face of current fluoride-using modalities. Clearly, fluoride alone is insufficient to prevent dental caries in many situations. In addition, as with many broad-spectrum antibiotic therapies, reports of fluoride resistance in S. mutans are emerging (Liao et al. 2017). Other antimicrobial agents are available for dental use (e.g., chlorhexidine and triclosan), but all are similarly broad spectrum. Since the oral cavity serves as the portal to the external environment, the colonization resistance exhibited by the oral microbiome is crucial and has been well documented (He et al. 2014). As such, reengineering of a dysbiotic oral microbiome is likely to generate a more positive outcome than its total destruction. Precision approaches to specifically target biofilm formation and/or cariogenic species such as S. mutans remain highly attractive goals.
Historically and contemporarily, there have been a number of approaches that endeavored to combat dental caries by precisely targeting S. mutans, although none have maintained substantial traction in the long term. Research investigating the feasibility of active or passive immunization against dental caries has been sporadic. Levels of salivary IgA against immunogenic S. mutans epitopes, such as glucosyltransferases (GTFs)and glucan-binding proteins (GBPs), inversely correlate with colonization of S. mutans and caries prevalence (Taubman and Smith 1993; Nogueira et al. 2005). Readers interested in a detailed description of various vaccine approaches, the scientific challenges facing development of an anticaries vaccine, and a summary of the results of early investigations on the topic are directed to an excellent review (Taubman and Nash 2006). More recent studies have explored vaccination using a recombinant P1 adhesin antigen (Batista et al. 2017), a DNA-based vaccine against glucosyltransferases and surface proteins (Jiang et al. 2017), and a glycoconjugate vaccine based on rhamnan surface polysaccharides (St Michael et al. 2018). Unfortunately, past, present, and likely future translational efforts to move anticaries vaccine research into clinical trials face significant headwinds due to the fact that it is a non-life-threatening disease. There are currently no licensed vaccines to prevent dental caries, and to our knowledge, only one candidate vaccine has proceeded to phase II clinical trials, in 2005 (Weintraub et al. 2005). Conceptually, bacteriophage is a very attractive approach to combat S. mutans and dental caries that has received relatively little attention. Although the few phages known to infect S. mutans were lytic, and completely eliminated viable counts from single-species biofilms, the phage demonstrated a highly stringent host specificity, which was considered a significant disadvantage, particularly considering the high intraspecies diversity exhibited by S. mutans (reviewed in Szafranski et al. 2017). No testing in multispecies communities or further studies have been reported. Probiotics approaches have sought to displace S. mutans with engineered strains of S. mutans modified for reduced pathogenesis or with species that either compete with or directly antagonize S. mutans (reviewed in Hoare et al. 2017; Philip et al. 2018b). Many approaches have been unsuccessful largely due to the use of probiotic taxa ill-equipped to persist in dental plaque and compete directly with S. mutans in situ. Furthermore, many probiotic species tested were acid producers and acid tolerant (e.g., Lactobacillus spp.) and thus may not have been particularly well suited to this intended role. Although more recently discovered taxa, such as Streptococcus oralis subsp. dentisani (López-López et al. 2017) and Streptococcus A12 (Huang et al. 2016), hold promise compared to older candidates, to date, no probiotic formulations have successfully demonstrated safety and efficacy in adequately rigorous clinical trials (Gruner et al. 2016; Burne 2018). Two small molecules were recently reported to exhibit the ability to specifically disperse or inhibit S. mutans biofilms (Ren et al. 2016; Garcia et al. 2017). However, biofilm disruption alone, with minimal effect on the overall dental plaque ecology, is likely to allow rapid reestablishment of the problematic community and require constant application of the therapeutic. Recent work has identified 2 antimicrobial peptides, ZXR-2 (Chen et al. 2017) and CLP-4 (Ding et al. 2014), and a vitamin D derivative, doxercalciferol (Saputo et al. 2018), with antimicrobial activity against S. mutans, although particular specificity for S. mutans was not reported in these studies. While the various approaches discussed above have yet to bear fruit in the form of an approved therapeutic to prevent dental caries, the studies have contributed substantially to our understanding of the disease and S. mutans and are foundational in guiding current and future research.
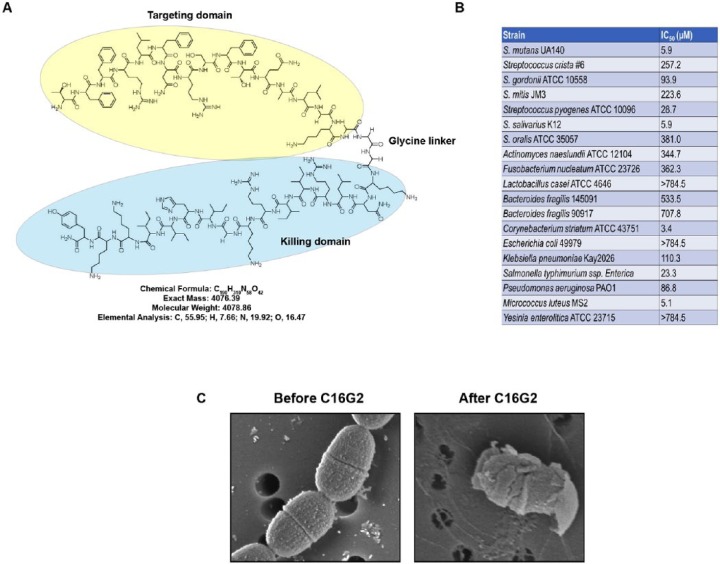
Specifically targeted antimicrobial peptides (STAMPs) were developed to address the need for a precision antibiotic therapy. STAMPs are synthetic peptides consisting of a targeting domain to invoke specificity and a killing domain to exert antimicrobial action against the targeted species (Eckert, Qi, et al. 2006). To design a STAMP targeting S. mutans, a “G2” killing domain, consisting of a 16-residue segment of the well-characterized antimicrobial peptide novispirin G10, was chosen (Eckert, He, et al. 2006). Novispirin G10 is well known to have low toxicity to mammalian cells while possessing a potent broad-spectrum antimicrobial activity (Eckert, Qi, et al. 2006). The targeting domain selected was “C16,” which consisted of the 16 C-terminal residues of the S. mutans pheromone, CSP. An earlier attempt at generating a successful S. mutans–targeting STAMP used G2 and the full-length CSP, but this version of the STAMP did not display any antimicrobial activity, likely due to steric hindrance (Eckert, He, et al. 2006). The targeting and killing moieties of C16G2 were joined by a flexible triglycine linker (Fig. 1A).
Figure 1.
Design and activity of C16G2. (A) Skeletal formula of C16G2. Targeting domain, linker region, and killing domain are denoted. (B) Table illustrating IC50 of C16G2 against the indicated bacteria following a 5-min treatment period. (C) Electron microscopy images of Streptococcus mutans before (left panel) and after (right panel) treatment with C16G2. Panels A and C are courtesy of C3J Therapeutics, and panel B is adapted from Guo et al. (2015).
Preliminary analyses of C16G2 showed that it was capable of targeted killing of S. mutans in either planktonic or biofilm settings and that it could selectively kill S. mutans in a 3-species biofilm while leaving the closely related, yet health-associated, organisms Streptococcus gordonii and Streptococcus sanguinis unharmed (Eckert, He, et al. 2006; Sullivan et al. 2011) (Fig. 1B). Further work determined that C16G2 exerted its killing effect through membrane disruption, as it induced membrane permeability and depolarization, as well as intracellular metabolite leakage (Kaplan et al. 2011) (Fig. 1C). The cytotoxic effect of C16G2 was rapid, killing S. mutans in less than 1 min of exposure, acceptably swift for application as an oral care product (Kaplan et al. 2011). The STAMP was also soluble in aqueous solutions for delivery into the oral cavity in a rinse formulation. In addition, C16G2 had negligible adverse effects on human cells in vitro and was stable in both phosphate-buffered saline (PBS) and human saliva (Sullivan et al. 2011). The same study also included a pilot clinical trial using bovine enamel chips in an intraoral retainer, which were frequently exposed to sucrose, with minimal oral hygiene, to mimic an in vivo environment very conducive to demineralization and caries development (Sullivan et al. 2011). Encouragingly, compared to placebo, C16G2 significantly reduced the number of viable S. mutans in both plaque and saliva samples, decreased lactic acid production, increased the resting pH of dental plaque, and prevented demineralization of the bovine enamel (Sullivan et al. 2011). A time course experiment illustrated that the probability of S. mutans and other species developing resistance to C16G2 was very low, as no stable change in C16G2 MIC was detected (C3J Therapeutics, unpublished data).
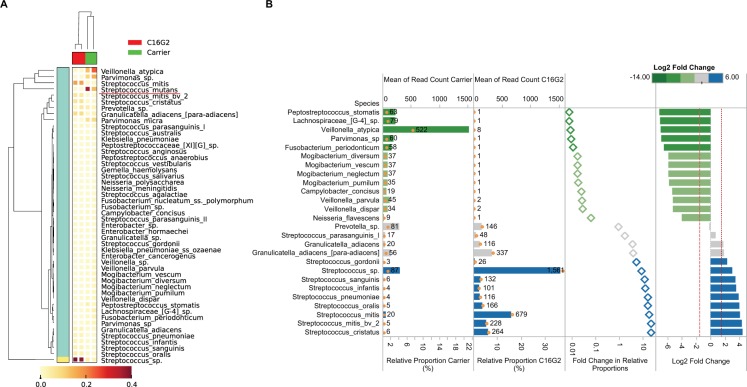
More recently, the antimicrobial capacity of C16G2 was determined against an expanded panel of both Gram-positive and Gram-negative bacteria (Guo, McLean, Yang, et al. 2015) (Fig. 1B). Only Streptococcus salivarius K12, Corynebacterium striatum ATCC 43751, and Mycobacterium luteus M52 had an IC50 that was comparable to S. mutans. The effect of C16G2 treatment was also tested on an in vitro, saliva-derived oral community, which consisted of over 100 species, to which S. mutans had been added. Compared to control, C16G2 significantly reduced the number of S. mutans in the community (Guo, McLean, Yang, et al. 2015). This was accompanied by a significant increase in the relative abundance of Streptococcus mitis and other streptococcal species associated with good dental health (Fig. 2A). In addition to the depletion of S. mutans from the community, several other species decreased in abundance as well. These included Fusobacterium periodonticum, Campylobacter concisus, and Neisseria spp. (Fig. 2B). Interestingly, F. periodonticum did not appear to be susceptible to C16G2 in IC50 testing of the monoculture. Furthermore, all 3 of these taxa appeared to form physical associations with S. mutans based upon a pull-down assay (Guo, McLean, Yang, et al. 2015). This suggests that these species may be reliant on physical and/or metabolic associations with S. mutans and likely decreased in abundance due to the reduction in S. mutans abundance rather than due to nonspecific killing by the STAMP. In support of this hypothesis, Veillonella atypica also decreased in relative abundance concurrent with S. mutans (Fig. 2B). Metabolic dependence of Veillonella species on lactic acid, which is produced by S. mutans, has been well documented (Chalmers et al. 2008). Taken together, these results suggest that C16G2 is effective at selectively eliminating S. mutans from the dental plaque milieu and remodeling the community to one that is dominated by species that are associated with good dental health.
Figure 2.
Remodeling of the dental plaque microbiome by C16G2. Adapted from Guo et al. (2015). (A) The effect of C16G2 treatment on a complex (>100 species) saliva-derived microbial community. Cluster analyses of oral taxa-weighted abundance profiles obtained from regrowth following treatment with either carrier (negative control) or C16G2. Relative proportions of the total taxa abundance are indicated in the heat map, which displays how the dominant taxa varied. (B) Oral species other than Streptococcus mutans that displayed a significant change in relative abundance following treatment with C16G2, compared to control. Relative proportions are corrected for read depth changes given that S. mutans decreased to below 0.1% following C16G2 treatment. Therefore, proportions are calculated as a relative percentage of the total population excluding S. mutans counts. Library size and normalized read counts are displayed as text and open orange circles. Differences in the relative proportions are expressed as fold changes and log2 fold changes to display the difference between carrier and C16G2 treatment. Plots are colored according to the log2 fold changes between carrier and C16G2 treatment.
Following extensive preclinical examination, C16G2 proceeded to phase I clinical trials. The randomized, double-blind, placebo-controlled studies included a dose escalation period and focused on evaluating safety and pharmacokinetics. A total of 127 subjects were enrolled, and no C16G2-related events or severe adverse events were reported in the study (C3J Therapeutics, unpublished data [NTCs: NCT02509845, NCT03004365, and NCT03052842]). Several formulations were evaluated, including a mouth rinse, an oral gel, a varnish, and a tooth strip. A single varnish application outperformed multiple tooth gel applications delivered by dental tray or brushing (C3J Therapeutics, unpublished data). The varnish formulation is a product candidate for in-office treatment, similar to fluoride varnishes commonly applied by dentists or hygienists, and is therefore particularly well suited for the treatment of high-risk, caries-prone populations. In phase II trials completed in 2016, the C16G2 varnish achieved significant reductions in S. mutans, and no adverse effects were reported (C3J Therapeutics, unpublished data [NCT02594254]). The tooth strip application was designed to become a convenient, at-home application for multiple treatments. Additional phase II clinical trials of C16G2 are ongoing.
C16G2 represents an exciting advance in precision therapeutics, with the ability to selectively eliminate a pathogen, causing remodeling of a dysbiotic microbial community to one rich in health-associated species. Dental caries remains a serious public health concern and one that could strongly benefit from a precision therapeutic, such as C16G2, to supplement current recommended fluoride and hygienic regimens. Beyond dental caries, the opportunities to use STAMP technology to treat other diseases and advance research of complex bacterial communities, such as that of the human microbiome, are vast and await discovery.
Author Contributions
J.L. Baker, contributed to conception and design, drafted and critically revised the manuscript; X. He, W. Shi, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
Research in the laboratories of the authors is supported by National Institutes of Health/National Institute of Dental and Craniofacial Research F32-DE026947 (J.L.B.) and by R01-DE020102, R01-DE023810, and R01-DE026186 (X.H. and W.S.). J.L.B. is a part-time consultant for uBiome, Inc. W.S. is a part-time chief scientific officer of C3J Therapeutics, Inc., which has licensed technologies from the University of California Regents that may be indirectly related to this research project.
The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: J.L. Baker  https://orcid.org/0000-0001-5378-322X
https://orcid.org/0000-0001-5378-322X
References
- Banas JA, Drake DR. 2018. Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health. 18(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista MT, Ferreira EL, Pereira GS, Stafford P, Maeda D, Rodrigues JF, Brady LJ, Johnston SA, Ferreira LCS, Ferreira RCC. 2017. Lt adjuvant modulates epitope specificity and improves the efficacy of murine antibodies elicited by sublingual vaccination with the N-terminal domain of Streptococcus mutans P1. Vaccine. 35(52):7273–7282. [DOI] [PubMed] [Google Scholar]
- Bowen WH. 2016. Dental caries—not just holes in teeth! A perspective. Mol Oral Microbiol. 31(3):228–233. [DOI] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H, Koo H. 2018. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26(3):229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA. 2018. Getting to know “the known unknowns”: heterogeneity in the oral microbiome. Adv Dent Res. 29(1):66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers NI, Palmer RJ, Jr, Cisar JO, Kolenbrander PE. 2008. Characterization of a Streptococcus sp.–Veillonella sp. community micromanipulated from dental plaque. J Bacteriol. 190(24):8145–8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jia L, Zhang Q, Zhou X, Liu Z, Li B, Zhu Z, Wang F, Yu C, Zhang Q, et al. 2017. A novel antimicrobial peptide against dental-caries-associated bacteria. Anaerobe. 47:165–172. [DOI] [PubMed] [Google Scholar]
- Ding Y, Wang W, Fan M, Tong Z, Kuang R, Jiang W, Ni L. 2014. Antimicrobial and anti-biofilm effect of Bac8c on major bacteria associated with dental caries and Streptococcus mutans biofilms. Peptides. 52:61–67. [DOI] [PubMed] [Google Scholar]
- Eckert R, He J, Yarbrough DK, Qi F, Anderson MH, Shi W. 2006. Targeted killing of Streptococcus mutans by a pheromone-guided “smart” antimicrobial peptide. Antimicrob Agents Chemother. 50(11):3651–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R, Qi F, Yarbrough DK, He J, Anderson MH, Shi W. 2006. Adding selectivity to antimicrobial peptides: Rational design of a multidomain peptide against Pseudomonas spp. Antimicrob Agents Chemother. 50(4):1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SS, Blackledge MS, Michalek S, Su L, Ptacek T, Eipers P, Morrow C, Lefkowitz EJ, Melander C, Wu H. 2017. Targeting of Streptococcus mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. J Dent Res. 96(7):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner D, Paris S, Schwendicke F. 2016. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. J Dent. 48:16–25. [DOI] [PubMed] [Google Scholar]
- Guo L, McLean JS, Lux R, He X, Shi W. 2015. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep. 5:18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, McLean JS, Yang Y, Eckert R, Kaplan CW, Kyme P, Sheikh O, Varnum B, Lux R, Shi W, et al. 2015. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci U S A. 112(24):7569–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis E, Parsaei Y, Klein MI, Koo H. 2017. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol Oral Microbiol. 32(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, McLean JS, Guo L, Lux R, Shi W. 2014. The social structure of microbial community involved in colonization resistance. ISME J. 8(3):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare A, Marsh PD, Diaz PI. 2017. Ecological therapeutic opportunities for oral diseases. Microbiol Spectr. 5(4). doi: 10.1128/microbiolspec.BAD-0006-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Palmer SR, Ahn SJ, Richards VP, Williams ML, Nascimento MM, Burne RA. 2016. A highly arginolytic streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol. 82(7):2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature. 486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Hu Y, Yang M, Liu H, Jiang G. 2017. Enhanced immune response to a dual-promoter anti-caries DNA vaccine orally delivered by attenuated Salmonella typhimurium. Immunobiology. 222(5):730–737. [DOI] [PubMed] [Google Scholar]
- Kaplan CW, Sim JH, Shah KR, Kolesnikova-Kaplan A, Shi W, Eckert R. 2011. Selective membrane disruption: mode of action of C16G2, a specifically targeted antimicrobial peptide. Antimicrob Agents Chemother. 55(7):3446–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney KM, Yurist-Doutsch S, Arrieta MC, Finlay BB. 2014. Effects of antibiotics on human microbiota and subsequent disease. Annu Rev Microbiol. 68:217–235. [DOI] [PubMed] [Google Scholar]
- Liao Y, Brandt BW, Li J, Crielaard W, Van Loveren C, Deng DM. 2017. Fluoride resistance in Streptococcus mutans: a mini review. J Oral Microbiol. 9(1):1344509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 50(4):353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López A, Camelo-Castillo A, Ferrer MD, Simon-Soro Á, Mira A. 2017. Health-associated niche inhabitants as oral probiotics: the case of Streptococcus dentisani. Front Microbiol. 8:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WD. 1890. The micro-organisms of the human mouth. Philadelphia: S. S. White Dental Mfg. Co. [PMC free article] [PubMed] [Google Scholar]
- Nogueira RD, Alves AC, Napimoga MH, Smith DJ, Mattos-Graner RO. 2005. Characterization of salivary immunoglobulin A responses in children heavily exposed to the oral bacterium Streptococcus mutans: influence of specific antigen recognition in infection. Infect Immun. 73(9):5675–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N, Suneja B, Walsh L. 2018. a. Beyond Streptococcus mutans: clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br Dent J. 224(4):219–225. [DOI] [PubMed] [Google Scholar]
- Philip N, Suneja B, Walsh LJ. 2018. b. Ecological approaches to dental caries prevention: paradigm shift or shibboleth? Caries Res. 52(1–2):153–165. [DOI] [PubMed] [Google Scholar]
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, Tagami J, Twetman S, Tsakos G, Ismail A. 2017. Dental caries. Nat Rev Dis Primers. 3:17030. [DOI] [PubMed] [Google Scholar]
- Ren Z, Cui T, Zeng J, Chen L, Zhang W, Xu X, Cheng L, Li M, Li J, Zhou X, et al. 2016. Molecule targeting glucosyltransferase inhibits Streptococcus mutans biofilm formation and virulence. Antimicrob Agents Chemother. 60(1):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saputo S, Faustoferri RC, Quivey RG., Jr. 2018. Vitamin D compounds are bactericidal against Streptococcus mutans and target the bacitracin-associated efflux system. Antimicrob Agents Chemother. 62(1). pii: e01675-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Soro A, Mira A. 2015. Solving the etiology of dental caries. Trends Microbiol. 23(2):76–82. [DOI] [PubMed] [Google Scholar]
- St Michael F, Yang Q, Cairns C, Vinogradov E, Fleming P, Hayes AC, Aubry A, Cox AD. 2018. Investigating the candidacy of the serotype specific rhamnan polysaccharide based glycoconjugates to prevent disease caused by the dental pathogen Streptococcus mutans. Glycoconj J. 35(1):53–64. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Santarpia P, Lavender S, Gittins E, Liu Z, Anderson MH, He J, Shi W, Eckert R. 2011. Clinical efficacy of a specifically targeted antimicrobial peptide mouth rinse: targeted elimination of Streptococcus mutans and prevention of demineralization. Caries Res. 45(5):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafranski SP, Winkel A, Stiesch M. 2017. The use of bacteriophages to biocontrol oral biofilms. J Biotechnol. 250:29–44. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Smith DJ. 1993. Significance of salivary antibody in dental disease. Ann N Y Acad Sci. 694:202–215. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Nash DA. 2006. The scientific and public-health imperative for a vaccine against dental caries. Nat Rev Immunol. 6(7):555–563. [DOI] [PubMed] [Google Scholar]
- ten Cate JM, Featherstone JD. 1991. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 2(3):283–296. [DOI] [PubMed] [Google Scholar]
- Weintraub JA, Hilton JF, White JM, Hoover CI, Wycoff KL, Yu L, Larrick JW, Featherstone JD. 2005. Clinical trial of a plant-derived antibody on recolonization of mutans streptococci. Caries Res. 39(3):241–250. [DOI] [PubMed] [Google Scholar]
- Willey JM, Sherwood L, Woolverton CJ, Prescott LM, Willey JM. 2011. Prescott’s microbiology. New York: McGraw-Hill. [Google Scholar]