Abstract
Background
Hypertensive disorders of pregnancy (HDPs) are among the leading causes of maternal and perinatal morbidity and mortality worldwide and have been suggested to increase long-term cardiovascular disease risk in the offspring.
Objective
The objective of this study was to investigate whether HDPs are associated with cardiometabolic markers in childhood.
Search strategy
PubMed, The Cochrane Library and reference lists of included studies up to January 2019.
Selection criteria
Studies comparing cardiometabolic markers in 2–18-year-old children of mothers with HDP in utero, to children of mothers without HDP.
Data collection and analysis
Sixteen studies reported in 25 publications were included in this systematic review, of which three were considered as having high risk of bias. Thus 13 studies were included in the evidence synthesis: respectively two and eight reported pregnancy induced hypertension and preeclampsia, and three studies reported on both HDPs.
Main results
Most studies (n = 4/5) found a higher blood pressure in children exposed to pregnancy induced hypertension. Most studies (n = 7/10) found no statistically significantly higher blood pressure in children exposed to preeclampsia. No association was found between exposure to HDP and levels of cholesterol, triglycerides or glucose (n = 5/5). No studies investigated an association with (carotid) intima-media thickness, glycated haemoglobin or diabetes mellitus type 2.
Conclusions
Most studies showed that exposure to pregnancy induced hypertension is associated with a higher offspring blood pressure. There is no convincing evidence for an association between exposure to preeclampsia and blood pressure in childhood. Based on current evidence, exposure to HDP is not associated with blood levels of cholesterol, triglycerides and glucose in childhood.
Keywords: Hypertension, pregnancy-induced, pre-eclampsia, eclampsia, HELLP Syndrome, child, cardiovascular diseases, blood pressure, blood glucose, cholesterol, triglycerides
Introduction
Hypertensive disorders of pregnancy (HDPs) affect circa 10% of pregnancies.1 Both in lower–middle and in high income countries, the incidence of HDPs has increased throughout the last decades.2–6 HDPs are among the leading causes of maternal and perinatal morbidity and mortality worldwide.7 Exposure to HDP has been suggested to increase long-term cardiovascular disease (CVD) risk in the offspring.
A previous systematic review by Davis et al. reported that children of mothers with preeclampsia had increased blood pressure (BP).8 Pregnancy induced hypertension (PIH) was not addressed in the review, but there are indications that PIH is also associated with BP in childhood.9–11 The consistency of evidence has not been assessed systematically so far. Depending on the HDP phenotype, different pathophysiological pathways are involved in the development and clinical course of the disease12 and hence associations with cardiometabolic health in the offspring may be different as well.
We hypothesized that intra-uterine mechanisms underlie a possible association between HDP and cardiometabolic markers in childhood. HDP would affect the development of organs and vascular structures in the foetus, thereby programming the child towards adverse cardiometabolic health.13 Besides intra-uterine mechanisms, certain factors which lead to HDP as well as to adverse cardiometabolic outcomes in the offspring may explain an association between HDP and cardiometabolic health in childhood. For instance, a woman's predisposition to develop high BP may be inherited by her child, and HDP is merely an early reflection of this predisposition.14,15 Also, shared environment and lifestyle on the one hand may lead to the development of HDP and on the other hand may increase the risk of adverse cardiometabolic outcomes in the offspring.
We performed a systematic review to investigate whether in utero exposure to HDP – preeclampsia but also PIH, eclampsia and Haemolysis Elevated Liver enzymes and Low Platelets (HELLP) syndrome – is associated with adverse levels of cardiometabolic markers (BP, (carotid) intima-media thickness, cholesterol, triglycerides, fasting glucose, glycated haemoglobin (HbA1c), risk of diabetes mellitus type 2) in children up to 18 years of age.
Methods
Search strategy
This systematic review is reported in accordance with the recommendations as stated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Supplementary Material S.1 online).16 On 13 March 2017 electronic searches were performed in PubMed using the search fields of title/abstract in combination with MEdical Subject Headings (MESH), and in The Cochrane Library. The search was updated on 15 January 2019. The search strategy was developed in collaboration with an information specialist at our department and is described in the Supplementary Material (S.2). Two authors (LPMP and MACJ) independently screened studies based on title, followed by independent screening of abstracts and full-text articles. An abstract and full-text screening form was used to ensure systematic screening (Supplementary S.3). Disagreements in the study selection process were discussed and in the case of no consensus being reached, a third author was consulted (LvR). References of included studies and previous systematic reviews were manually screened to identify studies that were not found in PubMed.
This review is aimed at reviewing the evidence for an association of HDP with cardiometabolic outcomes in childhood. Since this review is part of a project that also aims to systematically review the evidence for an association of gestational diabetes mellitus with cardiometabolic outcomes in childhood, the search strategy was designed to include both pregnancy conditions.
Inclusion and exclusion criteria
The inclusion and exclusion criteria are summarized in Supplementary Material S.4. Studies were included if they compared cardiometabolic outcomes in 2–18 year old children of a mother with diagnosed HDP with children of a mother without HDP. Diagnosed HDPs were (as defined by the International Society for the Study of Hypertension in Pregnancy at the start of this review1): PIH, preeclampsia, eclampsia and HELLP syndrome. PIH was defined as systolic BP (SBP) ≥ 140 mmHg or diastolic BP (DBP) ≥ 90 mmHg without proteinuria that occurs after 20 weeks of gestation in a woman with previously normal BP. Preeclampsia was defined as PIH with proteinuria (≥0.3 g protein in a 24-h urine specimen). Eclampsia was defined as preeclampsia with grand mal seizures. HELLP syndrome was defined as severe preeclampsia with haemolysis, elevated liver enzymes and low platelet counts.1 We excluded infants of mothers with pre-existing hypertension since we aimed to investigate the effects of pregnancy complications as such. We hypothesized that hypertensive disorders developed during pregnancy stimulate intra-uterine mechanisms that affect the development of organs and vascular structures in the foetus, thereby programming the child towards adverse cardiometabolic health. Cardiometabolic outcomes of interest were: SBP and DBP, (carotid) intima-media thickness, serum cholesterol, triglycerides, fasting glucose, HbA1c and diabetes mellitus type 2. We excluded studies with self-reported outcomes, outcome diabetes mellitus type 1, and non-original studies such as expert views, editorials or comments. All studies were published in peer-reviewed journals in the English language, and performed in human participants.
Data extraction and critical appraisal
Data of included studies were extracted by two authors (LPMP and MACJ) using a structured data collection form (Supplementary Material S.5), including the key characteristics of the studies' design and population, exposure, outcome measure(s), as well as measures of association between exposure and outcome. A third author (HAS) checked the data extraction for accuracy.
The methodological quality of each included study was assessed by one author (MACJ) and checked by another author (LPMP), using the Newcastle–Ottawa Quality Assessment Scale for cohort studies.17 The scale consists of three categories for which a study can be awarded a maximum of two to four stars: selection (four stars), comparability (two stars) and outcome (three stars). More stars reflect better quality and thus lower risk of bias. Since the scale does not provide thresholds for the number of stars to identify studies with a high risk of bias, we defined our own criteria based on the results of the critical appraisal (Supplementary Material S.6). Studies were rated as having a high risk of bias when selection of exposed and non-exposed was not adequately reported or when loss to follow-up was high (>60%) and no reasons for this high loss were reported.
Evidence synthesis
Studies that were perceived as having a high risk of bias were excluded from evidence synthesis. Per HDP, the evidence was reviewed for each outcome separately. For continuous outcome measures, we compared mean levels between exposed and unexposed children, and if able to we compared regression coefficients with 95% confidence intervals (CIs) and/or p-values for the observed differences. Consideration was given to whether results varied between sexes and in the presence of confounding or mediating factors.
When multiple publications originated from the same study, we reported all those publications in the evidence synthesis section if these contained any novel result. In the case of duplicate results from one study, we reported only the publication with the most comprehensive data in the evidence synthesis section and reported the results of the overlapping publication(s) in the tables only.
Results
Study overview
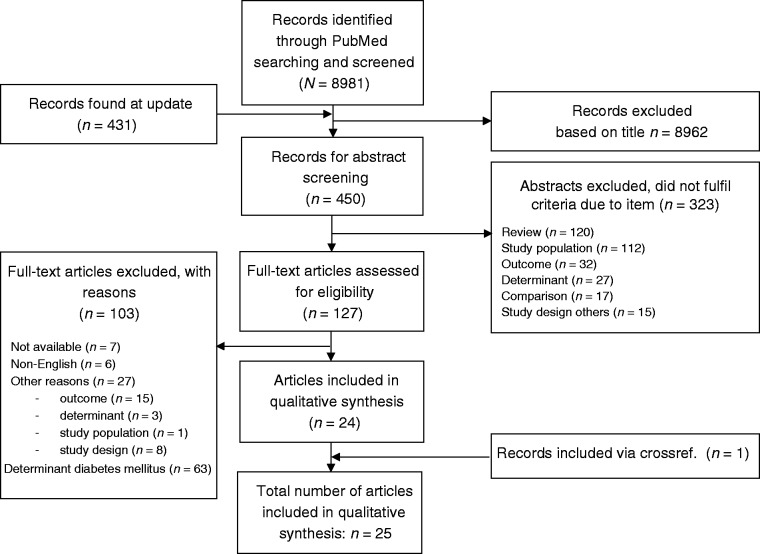
A total of 8981 articles were identified, of which we assessed 127 full texts for eligibility (Figure 1). Twenty-four publications satisfied the eligibility criteria and were selected for data extraction and consecutively critical appraisal and synthesis. One publication was additionally identified after screening reference lists of included studies and previous systematic reviews. This study was not found with our search strategy because either the exposure or the outcome was not explicitly studied and hence no related MESH terms have been assigned to this publication.
Figure 1.
Flowchart of study selection.
With our search update in 2019, we identified 431 additional articles, of which three papers fulfilled full text assessment. However, none of these articles satisfied the eligibility criteria.
Thus in total, 25 publications were included in this systematic review.9,10,18–40 These 25 publications originated from 16 population based studies. Eleven studies had one publication and five studies had multiple publications. The results of the included studies were reviewed per study instead of per publication.
Characteristics of the included studies
The characteristics of the 16 included studies are described in Table 1. Three studies included children of mothers with PIH, nine studies included children of mothers with preeclampsia and four studies reported on both HDPs separately. None of the studies investigated the association of eclampsia or HELLP syndrome with one of the outcomes of interest. Regarding the definition of PIH, there were no differences in BP threshold (BP ≥ 140 mmHg; DBP ≥ 90 mmHg, in absence of proteinuria), but in one study PIH could be defined at any time during pregnancy9 while in other studies women had to be at least 20 weeks pregnant. Regarding preeclampsia, there were no differences in BP threshold (BP ≥ 140 mmHg; DBP ≥ 90 mmHg), nor in proteinuria threshold (≥300 mg/24 h) between studies. One study in which preeclampsia was grouped into mild, moderate and severe preeclampsia defined hypertension by an increase in DBP only30 (Supplementary Table S.7).
Table 1.
Characteristics of the 25 included publications originating from 16 population studies.
| Study number | Reference number | First author | Year of publication | Study design | Location (name of study) | Recruitment period: year of birth | Source population Children from pregnant women recruited from… | Eligible population Children from mothers with and without HDP who were supposed to participate n (no. exposed/ unexposed) | Population for analysis: N (no. exposed/ unexposed) | Outcome | Age at outcome measurement | Child confounders | Maternal confounders |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertensive disorders: pregnancy induced hypertension | |||||||||||||
| 1 | 18 | Kotchena | 1979 | PC | Lexington, Kentucky, USA | 1971–1974 | Hospital (N = 409; n exposed=74/ n unexposed=335) (46% White and 54% Black) | 129 (74/random sample of 55 out of 335) | 100 (53/47) | SBP and DBP | 3–6 years | – | – |
| 19 | Kotchena | 1982 | Idem | Idem | Idem | Idem | Idem | 112 (62/50) 107 (59/48) | Idem | 3–6 and 6–9 years | – | – | |
| 2 | 20 | Bergel | 2000 | PC | Rosario, Argentina | 1987–1990 | Prenatal clinics (n = 614) | 614 (not reported) | 518 (not reported) | SBP | 5–9 years | Sex, age, height, BMI at outcome measurement, treatment status (calcium vs. placebo) | – |
| 3 | 21 | Svenssona,b | 1986 | PC | Göteborg, Sweden (Hypertension in Pregnancy Offspring Study) | 1969–1973 | General population (n = 17,000 pregnancies) | 521 (261/260)e | 54 (39/15)f | SBP and DBP | 10–15 yearsg | – | – |
| 22 | Himmelmanna,b | 1993 | Idem | Idem | Idem | Idem | Idem | 59 (42/17)f | Idem | 10.6–16.4 years | Sex, birth weight, age, weight, height, heart rate at outcome measurement | – | |
| 9 | Himmelmanna,b | 1994 | Idem | Idem | Idem | Idem | Idem | Idem | Idem | 10.6–16.4 years and 18.2 years | Idem | – | |
| 23 | Himmelmanna,b | 1997 | Idem | Idem | Idem | Idem | Idem | Idem | Idem and glucose at follow-up | 10.6–16.4 years and 18.2 years | Idem | – | |
| Hypertensive disorders: preeclampsia | |||||||||||||
| 4 | 24 | Kvehaugena | 2010 | PC | Ullevål, Oslo, Norway (CHASE Study) | 2001–2004 | Hospital (n = 149) | 149 (not reported) | 40 (23/17) | SBP and DBP | 5–8 years | – | – |
| 25 | Kvehaugena | 2011 | Idem | Idem | Idem | Idem | 149 (not reported) | 34 (20/14) | SBP, DBP, triglycerides, cholesterol (total, HDL, LDL) | Idem | – | – | |
| 5 | 26 | Palti | 1989 | PC | Rehovot, Israel | 1980 | Hospital | Not reported | 188 (94/94 matched on sex, birth date, birth order, maternal age, marital status, ethnicity) | SBP and DBP | 6 years | – | – |
| 6 | 27 | Lazdamb | 2012 | PC | Oxford, United Kingdom | 1998–2003 | Hospital (N = 964; n exposed =428/n unexposed =536) | 618 (309/309) | 47 (33/14; their mothers were matched on age, parity and year of delivery) | SBP, cholesterol (LDL, total, HDL), triglycerides and glucose | 6–13 years | – | – |
| 7 | 28 | Langford | 1980 | PC | Hinds County, Mississippi, USA | 1965–1967 | Schools (Black females) | 586 (186/400) | 413 (115/298) | SBP and DBP | 7–11 years | – | – |
| 8 | 29 | Alsnes | 2014 | PC | Stavanger, Norway | 1993–1995 | Hospital (N = 12,804; n exposed =307/n unexposed = 12,497) | 926 (307/619) | 601 (218/383) | Cholesterol (total, HDL, non-HDL) and glucose | 10–11 years | – | – |
| 9 | 30 | Øglaend | 2009 | PC | Stavanger, Norway | 1993–1995 | General population (N = 239,000) | 890 (276/614) | 537 (181/356) | SBP and DBP | 11–12 years | BMI at outcome measurement | BMI, BP |
| 10 | 31 | Tenholaa | 2003 | PC | Kuopio, Finland | 1984–1986 | Hospital | Not reported (84/not reported) | 120 (60/ 60) For BP: 119 (59/60) (unexposed children were matched on sex, gestational age and birth size) | SBP, DBP, cholesterol (total, HDL, LDL), triglycerides and glucose | 12 years | Weight and height at outcome measurement | – |
| 32 | Tenholaa | 2006 | Idem | Idem | Idem | Idem | Idem | 114 (57/57 matched on sex, gestational age and birth size) | SBP and DBP | Idem | – | – | |
| 11 | 33 | Jayet | 2010 | PC | La Paz, Bolivia | 1990–1996c | La Paz | 146 (56/90) | 138 (48/90) | SBP and DBP | 13–14 years | – | – |
| 12 | 34 | Vatten | 2003 | CS | Nord Trøndelag, Norway (The Young-HUNT Study) | 1979–1984d | General population (N = 4980) | 4980 (not reported) | 4096 (243/3853) | SBP and DBP | 13–19 years | Birth weight, gestational age, age and BMI at outcome measurement. Birthweight adjusted for length of gestation as effect modifier | – |
| Hypertensive disorders: pregnancy induced hypertension and preeclampsia | |||||||||||||
| 13 | 35 | Hillerb | 2007 | PC | South Australia (Australian Calcium Trial) | 1992–1998 | General population (not reported) | 414 (65*/28#/321§) *Gestational hypertension #Preeclampsia §Normotensive pregnancy | 179 (31*/136§) (12#/136§) *Gestational hypertension #Preeclampsia §Normotensive pregnancy | SBP and DBP | 4–7 years | – | – |
| 14 | 36 | Belfort | 2012 | PC | Seven medical centres, USA (Infant Health and Development Program (IHDP)) | 1984 | Hospital (N = 1080) | 931 (not reported) | 694 (112*/582§) (22#/672§) *Preeclampsia #Other hypertensive disorders §Normotensive pregnancy | SBP | 6.5 years | Sex, age, height, behavioural state at outcome measurement, and blood pressure measurement method (gestational age as effect modifier) | Age, education, ethnicity, annual household income |
| 15 | 37 | Staleya | 2015 | Idem | Avon, UK (ALSPAC) | 1991–1992 | General population (N = 14,273) | Not reported | 6619 (253*/5295¶) (954#/5295¶) (117§/5295¶) *Existing hypertension #Gestational hypertension §Preeclampsia ¶Normotensive pregnancy | SBP and DBP | 7–18 years | Sex, height, BMI at outcome measurement (gestational age, birth weight, breastfeeding as mediators) | Parity, age, parental BMI, education, smoking during pregnancy, occupational social class (mode of delivery as mediator) |
| 38 | Geelhoeda | 2010 | PC | Idem | Idem | Idem | 13,678 (not reported) | 6668 (1118*/5345§) (205#/5345§) *Gestational hypertension #Preeclampsia §Normotensive pregnancy | SBP and DBP | 9 years | Sex, age, weight, height at outcome measurement (gestational age, birth weight as mediators) | Parity, age, parental BMI, education, smoking during pregnancy, occupational social class (mode of delivery as mediator) | |
| 10 | Lawlora | 2012 | Idem | Idem | Idem | Idem | 11,443 (not reported) for outcome BP 11,719 (not reported) for outcomes cholesterol and triglycerides | BP: 4654 (771*/3781§) (102#/3781§) Cholesterol and triglycerides: 3537 (598*/2869) (70#/2869) *Gestational hypertension #Preeclampsia §Normotensive pregnancy | SBP, DBP, cholesterol (HDL, non-HDL) and triglycerides | 9–12 years | Sex, age, weight, height at outcome measurement, dietary sodium intake (gestational age, birth weight as mediators) | Idem | |
| 39 | Fraser | 2013 | Idem | Idem | Idem | Idem | 13,617 (not reported) | 2888 (431*/2404§) (53#/2404§) *Gestational hypertension #Preeclampsia §Normotensive pregnancy | SBP, DBP, cholesterol (total, HDL, LDL) triglycerides and glucose | 17 years | Sex, age (gestational age, birth weight, BMI at outcome measurement as mediators) | Parity, age, maternal pre-pregnancy BMI, household social class, smoking during pregnancy (mode of delivery as mediator) | |
| 16 | 40 | Miettola | 2013 | PC | Oulu and Lapland, Finland (Northern Finland Birth Cohort 1986 (NFB1986)) | 1985–1986 | General population (not reported) | 9432 (not reported) | BP: 5573 (331*/5045§) (197#/5045§) Cholesterol and triglycerides: 3.7–8.2% missing Glucose: 3.7–14.1% missing *Gestational hypertension #Preeclampsia §Normotensive pregnancy | SBP, DBP, cholesterol (total, HDL, LDL), triglycerides and glucose | 16 years | Sex, birth weight, gestational age, BMI at outcome measurement | Parity, socioeconomic status, prepregnancy BMI |
These publications have used the same population study: Kotchen et al. (1979, 1982); Svensson et al., Himmelmann et al. (1993, 1994, 1997); Tenhola et al. (2003, 2006); Kvehaugen et al. (2010 and 2011); Geelhoed et al., Lawlor et al., Fraser et al., Staley et al. (2010, 2012, 2013, 2015).
Defined as a high risk of bias study in the risk of bias assessment.
Year of birth was estimated by earliest year of recruitment (2004) minus oldest age (14 years) at participation and latest year of recruitment (2009) minus youngest age (13 years) at participation.
Year of birth was estimated by earliest year of recruitment (1995) minus oldest age (16 years) at participation and latest year of recruitment (1997) minus youngest age (13 years) at participation.
Reported in Svensson A, Andersch B and Hansson L. Prediction in later hypertension following a hypertensive pregnancy. J Hypertens 1983; 03: 391–398.
Hypertensive pregnancies consists of two groups: children of mothers who had sustained hypertension after hypertensive pregnancy, and children of mothers who were normotensive after hypertensive pregnancy.
This age is reported in the paper, but study population is the same as the study population in papers by Himmelman et al. (1993, 1994, 1997).
HDP: hypertensive disorders of pregnancy; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein; BMI: body mass index; PC: prospective cohort study; CS: cross-sectional study.
Fifteen studies were prospective cohort studies and one study was a cross-sectional study. Studies were performed in European countries, the USA, Australia, Argentina, Bolivia and Israel. The children of mothers with and without HDP were born between 1969 and 2004 and were mainly recruited from the general population or from hospitals. Cardiometabolic outcome measures were: SBP (n = 15 studies), DBP (n = 12 studies), serum cholesterol (n = 6 studies), triglycerides (n = 5 studies) and glucose (n = 6 studies). Measurement methods of the outcome measures were comparable (Supplementary Material S.8). None of the studies investigated the association of HDP with the other outcomes of interest in this review, that is, (carotid) intima-media thickness, HbA1c and diabetes mellitus type 2.
Risk of bias assessment
Based on our predefined criteria, we identified three studies (one with preeclampsia and PIH, one with only PIH and one with only preeclampsia as the determinant) with a high risk of selection and information bias: the publications from the Hypertension in Pregnancy Offspring Study9,21–23 and the studies by Lazdam et al.27 and Hiller et al.35 These studies were excluded from the evidence synthesis. Results of these studies are shown in Supplementary Material S.9 and S.10. Thus, evidence synthesis was performed for 13 studies (two with PIH, eight with preeclampsia and three studies reported on both hypertensive disorders separately) reported in 19 publications.
Evidence synthesis
Pregnancy induced hypertension
Associations with offspring blood pressure
BP was reported as an outcome in five studies (Table 2). Four of these five studies observed a higher BP in children of mothers with PIH than in children of mothers without PIH.10,18,36–40 The study by Kotchen et al.18 observed a 4.5 mmHg higher SBP and no different DBP at 3–6 years in PIH-exposed children. At 6–8 years, results were stratified for sex; PIH-exposed boys had a 4.8 mmHg higher SBP and no different DBP from unexposed boys, while PIH-exposed girls had no different BP from unexposed girls.19 Belfort et al.36 observed a 3.5 mmHg higher SBP at 6.5 years, and Miettola et al.40 observed a 2.5% higher SBP and a 3.3% higher DBP at 16 years in PIH-exposed versus unexposed children. In the ALSPAC study, PIH-exposed children had a higher BP than unexposed children, with a mean difference in respectively SBP and DBP that remained similar during childhood: 1.98 and 0.97 mmHg at seven years,37 2.04 and 1.07 mmHg at nine years38 2.04 and 1.10 mmHg at 10–11 years,10 and 2.06 and 1.11 mmHg at 17 years.39 These associations were not mediated by birth weight, gestational age, method of delivery, or breastfeeding. One study20 observed no association between exposure to PIH and SBP at 5–8 years of age. Thus, most studies observed a higher BP in children who were exposed to PIH.
Table 2.
Studies on the association between pregnancy induced hypertension and systolic blood pressure or diastolic blood pressure (mmHg) in childhood.
| PIH |
Normotensive pregnancy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study number | Reference number | First author (year) | Age at outcome measurement (range in years) | (Sub)group | Mean (SD) | N | Mean (SD) | N | Mean difference | 95% CI or p-valuea |
| Systolic blood pressure | ||||||||||
| 1 | 18 | Kotchen (1979) | 3–6 | All children | 97.6 (1.3) | 53 | 93.1 (1.5) | 47 | 4.5b | p < 0.03 |
| 19 | Kotchen (1982) | 3–6 | Boys | 99.2 (1.8 SE) | 29 | 98.5 (1.9 SE) | 26 | 0.7b | p=n.s. | |
| Girls | 101.5 (1.4 SE) | 33 | 98.7 (1.5 SE) | 24 | 2.88b | p=n.s. | ||||
| 6–9 | Boys | 104.3 (1.8 SE) | 28 | 99.5 (1.3 SE) | 26 | 4.8b | p < 0.05 | |||
| Girls | 99.3 (1.3 SE) | 31 | 100.5 (2.1 SE) | 22 | –1.2b | p=n.s. | ||||
| 2 | 20 | Bergel (2000) | 5–9 | All children | Not reported | Not reported | Not reported | Not reported | Crude: 0.0 | –0.9, 0.9 |
| Adjustedc: 0.2 | –0.6, 1.1 | |||||||||
| 14 | 36 | Belfort (2012) | 6.5 | All children | Not reported | 22 | Not reported | 672 | Crude: not reported | Not reported |
| Adjustedd: 3.5 | 0.0, 7.0 | |||||||||
| 15 | 37 | Staley (2015) | 7 | All children | Not reported | 954 | Not reported | 5.295 | Crude: 2.51 | 1.82, 3.20 |
| Adjustede: 1.98 | 1.32, 2.65 | |||||||||
| 38 | Geelhoed (2010) | 9 | All children | 105.2 (10.1) | 1.118 | 102.2 (9.1) | 5.345 | Crude: 3.06 | 2.46, 3.66 | |
| Adjustedf: 2.04 | 1.42, 2.67 | |||||||||
| 10 | Lawlor (2012) | 10–11 | All children | 106 (9) | 1.039 | 104 (9) | 5.367 | Crude: 2b | p < 0.001 | |
| Adjustedg: geometric mean: 2.04 | 1.33, 2.76 | |||||||||
| 39 | Fraser (2013) | 17 | All children | 120.5 (11.3) | 431 | 117.6 (10.4) | 2.404 | Crude: not reported | Not reported | |
| Adjustedh: 2.06 | 1.28, 2.84 | |||||||||
| 16 | 40 | Miettola (2013) | 16 | All children | Geometric mean (IQR): | 331 | Geometric mean (IQR): | 5.045 | Crude: 3b | p < 0.001 |
| 117 (107, 128) | 114 (106, 123) | Adjustedi: % difference: 2.5 | 1.4, 3.6 | |||||||
| Boys | Geometric mean (IQR): | Not reported | Geometric mean (IQR): | Not reported | Crude: 4.0b | p < 0.001 | ||||
| 124 (117, 133) | 120 (113, 128) | |||||||||
| Girls | Geometric mean (IQR): | Not reported | Geometric mean (IQR): | Not reported | Crude: 1.0b | p = 0.138 | ||||
| 110 (104, 117) | 109 (103, 117) | |||||||||
| Diastolic blood pressure | ||||||||||
| 1 | 18 | Kotchen (1979) | 3–6 | All children | 40.9 (1.9) | 53 | 40.8 (3.3) | 47 | 0.1b | p=n.s. |
| 19 | Kotchen (1982) | 3–6 | Boys | 57.0 (1.7 SE) | 29 | 60.3 (2.5 SE) | 26 | –3.3b | p=n.s. | |
| Girls | 60.3 (1.6 SE) | 33 | 59.8 (2.5 SE) | 24 | 0.5b | p=n.s. | ||||
| 6–9 | Boys | 59.4 (2.0 SE) | 28 | 56.9 (2.1 SE) | 26 | 2.5b | p=n.s. | |||
| Girls | 54.4 (1.9 SE) | 31 | 56.5 (3.0 SE) | 22 | –2.1b | p=n.s. | ||||
| 15 | 37 | Staley (2015) | 7 | All children | Not reported | 954 | Not reported | 5.295 | Crude: 1.07 | 0.57, 1.57 |
| Adjustede: 0.97 | 0.46, 1.48 | |||||||||
| 38 | Geelhoed (2010) | 9 | All children | 58.2 (6.0) | 1.118 | 57.2 (6.4) | 5.345 | Crude: 1.44 | 1.03, 1.86 | |
| Adjustedc: 1.07 | 0.60, 1.54 | |||||||||
| 10 | Lawlor (2012) | 10–11 | All children | 61 (8) | 1.039 | 60 (8) | 5.367 | Crude: not reported | Not reported | |
| Adjustedg: geometric mean: 1.10 | 0.47, 1.73 | |||||||||
| 39 | Fraser (2013) | 17 | All children | 66 (7.2) | 431 | 64.5 (6.8) | 2.404 | Crude: 1.5b | p < 0.001 | |
| Adjustedh: 1.11 | 0.54, 1.69 | |||||||||
| 16 | 40 | Miettola (2013) | 16 | All children | Geometric mean (IQR): | 331 | Geometric mean (IQR): | 5.045 | Crude: 2b | p < 0.001 |
| 69 (65, 74) | 67 (62, 72) | Adjustedi: % difference: 3.3 | 2.0, 4.6 | |||||||
| Boys | Geometric mean (IQR): | Not reported | Geometric mean (IQR): | Not reported | Crude: 2.0b | p = 0.006 | ||||
| 70 (65, 75) | 68 (63, 73) | |||||||||
| Girls | Geometric mean (IQR): | Not reported | Geometric mean (IQR): | Not reported | Crude: 3.0b | p < 0.001 | ||||
| 69 (64, 74) | 66 (62, 71) | |||||||||
p=n.s., not statistically significant.
We calculated the mean difference if this was not reported by the authors.
Adjusted for offspring sex, body mass index, height, age at outcome measurement, and calcium supplement status during pregnancy.
Adjusted for offspring sex, height z-score, age, blood pressure measurement method, child behavioural state at outcome measurement; maternal age, maternal education, annual household income and ethnicity.
Adjusted for offspring sex, body mass index, height at outcome measurement; maternal age, prepregnancy body mass index (BMI), parity, smoking during pregnancy, education and head of household social class.
Adjusted for offspring weight, height, and height squared at outcome measurement; maternal age, parental prepregnancy BMI, parity, maternal smoking during pregnancy, and social class.
Adjusted for offspring sex, body mass index, height, height-squared, age and dietary sodium at outcome measurement; maternal age, pre-pregnancy BMI, nulliparity, smoking during pregnancy, education and head of household social class.
Adjusted for offspring sex and age; maternal age, prepregnancy BMI, parity, maternal smoking during pregnancy, and household social class.
Adjusted for offspring sex; nulliparity, maternal prepregnancy BMI and socioeconomic position.
PIH: pregnancy induced hypertension; CI: confidence interval; SE: standard error; IQR: interquartile range
Associations with cholesterol and triglycerides
Two studies included blood cholesterol and triglycerides as outcome (Table 3). In one study,40 a 2.1 mmol/L higher total cholesterol level was observed at 16 years of age in children exposed to PIH. No association was found between exposure to PIH and high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides. In the other study, also no association was found between exposure to PIH and HDL cholesterol, non-HDL cholesterol and triglycerides at 9–10 years,10 nor with total, HDL- and LDL-cholesterol and triglycerides at 17 years.39
Table 3.
Studies on the associations between pregnancy induced hypertension and cholesterol, triglycerides and glucose (mmol/L).
| PIH |
Normotensive pregnancy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study number | Reference number | First author (year) | Age at outcome measurement (range in years) | Outcome | (Sub)group | Mean (SD) | N | Mean (SD) | N | Mean difference | 95% CI or p-value |
| Cholesterol | |||||||||||
| 15 | 10 | Lawlor (2012) | 9–10 | HDL cholesterol | All children | 1.38 (0.29) | 598 | 1.40 (0.31) | 2.869 | Crude: not reported | Not reported |
| Adjusteda: –0.01 | –0.03, 0.02 | ||||||||||
| Non-HDL cholesterol | All children | 2.88 (0.64) | 598 | 2.87 (0.65) | 2.869 | Crude: not reported | Not reported | ||||
| Adjusteda: 0.01 | –0.05, 0.07 | ||||||||||
| 39 | Fraser (2013) | 17 | Total cholesterol | All children | 3.8 (0.6) | 431 | 3.8 (0.7) | 2.404 | Crude: not reported | p = 0.76 | |
| Adjustedb: 0.01 | –0.06, 0.08 | ||||||||||
| HDL cholesterol | All children | 1.3 (0.3) | 431 | 1.3 (0.3) | 2.404 | Crude: not reported | p = 0.18 | ||||
| Adjustedb: –0.01 | −0.05, 0.02 | ||||||||||
| LDL cholesterol | All children | 2.1 (0.6) | 431 | 2.1 (0.6) | 2.404 | Crude: not reported | p = 0.25 | ||||
| Adjustedb: 0.03 | −0.03, 0.09 | ||||||||||
| 16 | 40 | Miettola (2013) | 16 | Total cholesterol | All children | Geometric mean (IQR): | 316 | Geometric mean (IQR): | 4.518 | Crude: 0.09a | p = 0.088 |
| 4.27 (3.80, 4.70) | 4.18 (3.70, 4.70) | Adjustedc: % difference: 2.1 | 0.05, 4.2 | ||||||||
| Boys | Geometric mean (IQR): 4.13 (3.70, 4.85) | Not reported | Geometric mean (IQR): 4.02 (3.60, 4.50) | Not reported | Crude: 0.11a | p = 0.113 | |||||
| Girls | Geometric mean (IQR): 4.43 (4.00, 4.90) | Not reported | Geometric mean (IQR): 4.35 (3.90, 4.80) | Not reported | Crude: 0.08a | p = 0.393 | |||||
| HDL cholesterol | All children | Geometric mean (IQR): | 316 | Geometric mean (IQR): | 4.518 | Crude: 0.02a | p = 0.349 | ||||
| 1.40 (1.24, 1.61) | 1.38 (1.20, 1.59) | Adjustedc: % difference: 2.4 | −0.03, 4.8 | ||||||||
| Boys | Geometric mean (IQR): 1.31 (1.17, 1.50) | Not reported | Geometric mean (IQR): 1.29 (1.14, 1.48) | Not reported | Crude: 0.02a | p = 0.575 | |||||
| Girls | Geometric mean (IQR): 1.49 (1.30, 1.72) | Not reported | Geometric mean (IQR): 1.46 (1.29, 1.66) | Not reported | Crude: 0.03a | p = 0.344 | |||||
| LDL cholesterol | All children | Geometric mean (IQR): | 316 | Geometric mean (IQR): | 4.518 | Crude: 0.05a | p = 0.288 | ||||
| 2.22 (1.90, 2.60) | 2.17 (1.90, 2.60) | Adjustedc: % difference: 1.8 | −1.2, 4.8 | ||||||||
| Boys | Geometric mean (IQR): 2.19 (1.80, 2.60) | Not reported | Geometric mean (IQR): 2.12 (1.80, 2.50) | Not reported | Crude: 0.07a | p = 0.166 | |||||
| Girls | Geometric mean (IQR): 2.25 (1.90, 2.60) | Not reported | Geometric mean (IQR): 2.23 (1.90, 2.60) | Not reported | Crude: 0.02a | p = 0.907 | |||||
| Triglycerides | |||||||||||
| 15 | 10 | Lawlor (2012) | 9–10 | Triglycerides | All children | Geometric mean (95% CI): 1.03 (1.00, 1.06) | 598 | Geometric mean (95% CI): 1.03 (1.01, 1.04) | 2.869 | Crude: not reported | Not reported |
| Adjusteda: ratio of geometric mean: 0.98 | 0.94, 1.04 | ||||||||||
| 39 | Fraser (2013) | 17 | Triglycerides | All children | Median (IQR): 0.8 (0.6, 1.0) | 431 | Median (IQR): 0.8 (0.6, 1.0) | 2.404 | Crude: not reported | p = 0.985 | |
| Adjustedb: per cent difference in means: –1.1 | −4.9, 2.9 | ||||||||||
| 16 | 40 | Miettola (2013) | 16 | Triglycerides | All children | Geometric mean (IQR): | 316 | Geometric mean (IQR): | 4.518 | Crude: −0.03a | p = 0.219 |
| 0.72 (0.55, 0.95) | 0.75 (0.57, 0.97) | Adjustedc: % difference: -4.0 | −8.6, 0.8 | ||||||||
| Boys | Geometric mean (IQR): 0.69 (0.50, 0.93) | Not reported | Geometric mean (IQR): 0.74 (0.55, 0.95) | Not reported | Crude: −0.05a | p = 0.109 | |||||
| Girls | Geometric mean (IQR): 0.76 (0.58, 0.99) | Not reported | Geometric mean (IQR): 0.77 (0.59, 0.98) | Not reported | Crude: −0.01a | p = 0.985 | |||||
| Glucose | |||||||||||
| 15 | 39 | Fraser (2013) | 17 | Glucose | All children | 5.0 (0.4) | 431 | 5.1 (0.6) | 2.404 | Crude: −0.1a | p = 0.25 |
| Adjustedb: –0.04 | −0.10, 0.02 | ||||||||||
| 16 | 40 | Miettola (2013) | 16 | Glucose | All children | Geometric mean (IQR): | 316 | Geometric mean (IQR): | 4.518 | Crude: 0a | p = 0.946 |
| 5.14 (4.90, 5.40) | 5.14 (4.90, 5.40) | Adjustedc: % difference: −0.1 | −1.2, 1.0 | ||||||||
| Boys | Geometric mean (IQR): 5.28 (5.05, 5.50) | Not reported | Geometric mean (IQR): 5.28 (5.00, 5.50) | Not reported | Crude: 0.00a | p = 0.998 | |||||
| Girls | Geometric mean (IQR): 4.99 (4.80, 5.20) | Not reported | Geometric mean (IQR): 5.02 (4.80, 5.30) | Not reported | Crude: −0.03a | p = 0.813 | |||||
Adjusted for offspring sex and age, body mass index (BMI), height and height-squared at outcome measurement; maternal age, nulliparity, smoking during pregnancy, prepregnancy BMI, education and head of household social class.
Adjusted for offspring sex and age; maternal age, parity, smoking during pregnancy, prepregnancy BMI and household social class.
Adjusted for offspring sex; nulliparity, maternal prepregnancy BMI and socioeconomic position.
PIH: pregnancy induced hypertension; CI: confidence interval; LDL: low-density lipoprotein; HDL: high-density lipoprotein; IQR: interquartile range
Associations with glucose
Two studies included blood glucose as outcome and observed no association between exposure to PIH and glucose at 1640 and 17 years39 (Table 3).
Preeclampsia
Associations with offspring blood pressure
BP was reported as an outcome in ten studies (Table 4). Three studies observed a higher BP in children of mothers with preeclampsia, with increases in SBP ranging from 2.9 mmHg to 3.2 mmHg and increases in DBP ranging from 1.7 mmHg to 3.6 mmHg.26,31,34 Five studies observed no different BP between children of mothers with and without preeclampsia.24–26,30,33,36,40 In the ALSPAC study, the association of exposure to preeclampsia with BP was not consistently observed throughout childhood; children of mothers with preeclampsia had no statistically significantly higher BP at 737 and 10–11 years,10 but they had a 2.05 mmHg higher SBP at nine years38 and a 1.71 mmHg higher DBP at 17 years.39 This association was mediated by birth weight, gestational age, mode of delivery and body mass index (BMI) at outcome assessment. Langford and Watson28 stratified results for sex; preeclampsia-exposed boys and girls had no different SBP at 7–11 years, but preeclampsia-exposed girls had a 5.8 mmHg higher DBP than unexposed girls. Thus, most studies observed no consistent association between exposure to preeclampsia and BP in childhood.
Table 4.
Studies on the association between preeclampsia and systolic blood pressure or diastolic blood pressure (mmHg).
| PE |
Normotensive pregnancy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study number | Reference number | First author (year) | Age at outcome measurement (range in years) | (Sub)group | Mean (SD) | N | Mean (SD) | N | Mean difference | 95% CI or p-valuea |
| Systolic blood pressure | ||||||||||
| 4 | 24 | Kvehaugen (2010) | 5–8 | All children | Median (25,75 pct): 100.0 (95.0, 105.0) | 23 | Median (25,75 pct): 100.0 (92.5, 103.0) | 17 | Median: 0b | p = 0.210 |
| 25 | Kvehaugen (2011) | 5–8 | All children | 99.8 (6.7) | 26 | 98.2 (5.7) | 15 | 1.6b | p = 0.4 | |
| 5 | 26 | Palti (1989) | 6 | All children | 101.3 (10.2) | 94 | 99.8 (9.5) | 94 | 1.5 | p = n.s. |
| Boys | 103.8 (9.9) | 45 | 99.8 (9.3) | 45 | 4.0 | p = 0.05 | ||||
| Girls | 99.1 (10.0) | 49 | 99.8 (9.8) | 49 | 0.7 | p = n.s. | ||||
| 14 | 36 | Belfort (2012) | 6.5 | All children | Not reported | 112 | Not reported | 582 | Crude: not reported | Not reported |
| Adjustedc: −0.7 | −2.4, 1.0 | |||||||||
| 7 | 28 | Langford (1980) | 7–11 | Boys | 100.9 (11.3) | 59 | 100.0 (12.6) | 164 | 0.9b | p = n.s |
| Girls | 103.3 (13.5) | 56 | 100.4 (12) | 134 | 2.9b | p = 0.08 | ||||
| 15 | 37 | Staley (2015) | 7 | All children | Not reported | 117 | Not reported | 5.295 | Crude: 1.45 | −0.39, 3.29 |
| Adjustedd: 1.22 | −0.52, 2.97 | |||||||||
| 38 | Geelhoed (2010) | 9 | All children | 104.5 (8.8) | 205 | 102.2 (9.1) | 5.345 | Crude: 2.36 | 1.09, 3.64 | |
| Adjustede: 2.05 | 0.72, 3.38 | |||||||||
| 10 | Lawlor (2012) | 10–11 | All children | 107 (11) | 143 | 104 (9) | 5.367 | Crude: not reported | Not reported | |
| Adjustedf: geometric mean: 1.82 | 0.03, 3.62 | |||||||||
| 39 | Fraser (2013) | 17 | All children | 120.2 (10.1) | 53 | 117.6 (40.4) | 2.404 | Crude: 2.6b | p = 0.03 | |
| Adjustedg: 1.12 | −0.89, 3.12 | |||||||||
| 9 | 30 | Øglaend (2009) | 11–12 | All children | 115.3 (9.8) | 181 | 113.5 (8.5) | 356 | Crude: 1.8 | 0.2, 3.5 |
| Adjusted: 0.4 | −1.2, 2.0 | |||||||||
| 10 | 31 | Tenhola (2003) | 12 | All children | 116.4 (95% CI 114.1, 118.7) | 59 | 113.2 (95% CI 110.9, 115.5) | 60 | 3.2b | Adjusted: p = 0.021 |
| 32 | Tenhola (2006) | 12 | All children | 116.8 | 57 | 113.0 | 57 | 3.8b | Not reported | |
| 11 | 33 | Jayet (2010) | 13–14 | All children | 108 (9) | 48 | 110 (11) | 90 | Crude: −2.00 | −5.41, 1.41 |
| 12 | 34 | Vatten (2003) | 13–19 | All children | 122.4 (95% CI 121.1, 123.8) | 220 | 119.5 (95% CI 119.2, 119.9) | 3.479 | 2.9b | Adjusted: p=<0.001 |
| 16 | 40 | Miettola (2013) | 16 | All children | Geometric mean: (IQR): 116 (108, 125) | 197 | Geometric mean: (IQR): 114 (106, 123) | 5.045 | Crude: 2b | p = 0.145 |
| Adjustedh: % difference: 0.7 | −0.8, 2.1 | |||||||||
| Boys | Geometric mean: (IQR): 121 (114, 131) | Not reported | Geometric mean: (IQR): 120 (113, 128) | Not reported | Crude: 1.0b | p = 0.496 | ||||
| Girls | Geometric mean: (IQR): 110 (104, 116) | Not reported | Geometric mean: (IQR): 109 (103, 117) | Not reported | Crude: 1.0b | p = 0.534 | ||||
| Diastolic blood pressure | ||||||||||
| 4 | 24 | Kvehaugen (2010) | 5–8 | All children | Median (25,75 pct): 60.0 (55.0, 65.0) | 23 | Median (25,75 pct): 60.0 (55.0, 60.0) | 17 | Median: 0b | p = 0.604 |
| 25 | Kvehaugen (2011) | 5–8 | All children | 60.0 (55.0 to 64.0) | 26 | 60.0 (55.0 to 60.0) | 17 | 0.0b | p = 0.7 | |
| 5 | 26 | Palti (1989) | 6 | All children | 66.2 (8.3) | 94 | 63.9 (8.0) | 94 | 2.3 | p = 0.03 |
| Boys | 68.3 (8.5) | 45 | 64.6 (7.8) | 45 | 3.6 | p = 0.04 | ||||
| Girls | 64.3 (7.8) | 49 | 63.2 (8.2) | 49 | 0.8 | p=n.s. | ||||
| 7 | 28 | Langford (1980) | 7–11 | Boys | 58.6 (16.5) | 59 | 58.4 (13.7) | 164 | 0.2b | p=n.s |
| Girls | 63.7 (12.5) | 56 | 57.9 (13.0) | 134 | 5.8b | p = 0.05 | ||||
| 15 | 37 | Staley (2015) | 7 | All children | Not reported | 117 | Not reported | 5.295 | Crude: 0.84 | −0.50, 2.18 |
| Adjustedd: 0.80 | −0.53, 2.13 | |||||||||
| 38 | Geelhoed (2010) | 9 | All children | 58.6 (6.6) | 205 | 57.2 (6.4) | 5.345 | Crude: 0.99 | 0.10, 1.89 | |
| Adjustede: 1.00 | −0.01, 2.01 | |||||||||
| 10 | Lawlor (2012) | 10–11 | All children | 62 (8) | 143 | 60 (8) | 5.367 | Crude: not reported | Not reported | |
| Adjustedf: geometric mean: 1.40 | −0.17, 2.98 | |||||||||
| 39 | Fraser (2013) | 17 | All children | 66.6 (7) | 53 | 64.5 (6.8) | 2.404 | Crude: 2.1b | p = 0.006 | |
| Adjustedg: 1.71 | 0.23, 3.17 | |||||||||
| 9 | 30 | Øglaend (2009) | 11–12 | All children | 66.4 (6.8) | 181 | 65.3 (7.0) | 356 | Crude: 1.0 | −0.2, 2.3 |
| Adjusted: 0.0 | −1.3, 1.4 | |||||||||
| 10 | 31 | Tenhola (2003) | 12 | All children | 73.9 (95% CI 72.1, 75.7) | 59 | 70.3 (95% CI 68.2, 72.4) | 60 | 3.6b | Adjusted: p = 0.022 |
| 32 | Tenhola (2006) | 12 | All children | 74.3 | 57 | 70.5 | 57 | 3.8b | Not reported | |
| 11 | 33 | Jayet (2010) | 13–14 | All children | 73 (7) | 48 | 73 (7) | 90 | Crude: 0.0 | −3.0 to 4.0 |
| 12 | 34 | Vatten (2003) | 13–19 | All children | 65.3 (95% CI 64.3, 66.3) | 220 | 63.6 (95% CI 63.4, 63.9) | 3.479 | 1.7b | Adjusted: p = 0.001 |
| 16 | 40 | Miettola (2013) | 16 | All children | Geometric mean (IQR): 68 (64, 74) | 197 | Geometric mean (IQR): 67 (62, 72) | 5.045 | Crude: 1b Adjustedh: % difference: 1.6 | p = 0.020 p=−0.01, 3.3 |
| Boys | Geometric mean (IQR): 69 (65, 74) | Not reported | Geometric mean (IQR): 68 (63, 73) | Not reported | Crude: 1.0b | p = 0.066 | ||||
| Girls | Geometric mean (IQR): 67 (62, 72) | Not reported | Geometric mean (IQR): 66 (62, 71) | Not reported | Crude: 1.0b | p = 0.347 | ||||
p=n.s., not statistically significant.
We calculated the mean difference if this was not reported by the authors.
Adjusted for offspring sex, height z-score, age, blood pressure measurement method, child behavioural state at outcome measurement; maternal age, maternal education, annual household income and ethnicity.
Adjusted for offspring sex, body mass index, height at outcome measurement; maternal age, prepregnancy body mass index (BMI), parity, smoking during pregnancy, education and head of household social class.
Adjusted for offspring sex, BMI, height, age at outcome measurement; and calcium supplement status during pregnancy.
Adjusted for offspring sex body mass index, height, height-squared, age and dietary sodium at outcome measurement; maternal age, prepregnancy BMI, nulliparity, smoking during pregnancy, education and head of household social class.
Adjusted for offspring sex and age; maternal age, prepregnancy BMI, parity, smoking during pregnancy and household social class.
Adjusted for offspring sex; nulliparity, maternal prepregnancy BMI and socioeconomic position.
PE: preeclampsia; pct: percentiles; CI: confidence interval; IQR: interquartile range
Associations with cholesterol and triglycerides
Five studies included blood cholesterol as outcome, of which four studies also included triglycerides (Table 5). In the study by Kvehaugen et al.25 a 0.58 mmol/L higher median level of total cholesterol was observed at 5–8 years in children of mothers with preeclampsia. Four studies observed no association between exposure to preeclampsia and total cholesterol in childhood.29,31,39,40 All five studies observed no association between exposure to preeclampsia and the level of HDL, non-HDL and LDL cholesterol in childhood.25,29,31,39,40 Similarly, no association was found between preeclampsia and the level of triglycerides in childhood. Thus, most studies observed no association between exposure to preeclampsia and cholesterol or triglycerides in childhood.
Table 5.
Studies on the association between preeclampsia and cholesterol, triglycerides and glucose (mmol/L).
| PE |
Normotensive pregnancy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study number | Reference number | First author (year) | Age at outcome measurement (range in years) | Outcome | (Sub)group | Mean (SD) | N | Mean (SD) | N | Mean difference | 95% CI or p-value |
| Cholesterol | |||||||||||
| 4 | 24 | Kvehaugen (2011) | 5–8 | Total cholesterol | All children | Median (IQR): 5.01 (4.44, 5.39) | 20 | Median (IQR): 4.43 (4.00, 5.00) | 14 | Median difference: 0.58a | p = 0.04 |
| HDL cholesterol | All children | 1.60 (0.31) | 1.41 (0.34) | 0.19a | p = 0.1 | ||||||
| LDL cholesterol | All children | Median (IQR): 1.81 (1.20, 2.42) | Median (IQR): 1.53 (1.12, 2.26) | Median difference: 0.28a | p = 0.3 | ||||||
| 15 | 10 | Lawlor (2012) | 9–10 | HDL cholesterol | All children | 1.37 (0.30) | 88 | 1.40 (0.31) | 3.369 | Crude: not reported | Not reported |
| Adjustedb: −0.03 | −0.11, 0.04 | ||||||||||
| Non-HDL cholesterol | All children | 2.87 (0.59) | 88 | 2.87 (0.65) | 3.369 | Crude: not reported | Not reported | ||||
| Adjustedb: −0.01 | −0.16, 0.14 | ||||||||||
| 15 | 39 | Fraser (2013) | 17 | Total cholesterol | All children | 3.6 (0.6) | 53 | 3.8 (0.7) | 2.404 | Crude: −0.2a | p = 0.19 |
| Adjustedc: −0.11 | −0.29, 0.07 | ||||||||||
| HDL cholesterol | All children | 1.2 (0.2) | 53 | 1.3 (0.3) | 2.404 | Crude: −0.1a | p = 0.17 | ||||
| Adjustedc: −0.03 | −0.11, 0.05 | ||||||||||
| LDL cholesterol | All children | 2.0 (0.6) | 53 | 2.1 (0.6) | 2.404 | Crude: −0.1a | p = 0.34 | ||||
| Adjustedc: −0.08 | −0.24, 0.08 | ||||||||||
| 8 | 29 | Alnes (2014) | 10–11 | Total cholesterol | Boys, mild preeclampsia | 4.45 (0.12) | Not reported | 4.35 (0.06) | 383 | Not reported | Boys, p = 0.29 for normotensive vs. all types of preeclampsia |
| Boys, moderate preeclampsia | 4.41 (0.15) | Not reported | |||||||||
| Boys, severe preeclampsia | 4.65 (0.15) | Not reported | |||||||||
| Girls, mild preeclampsia | 4.45 (0.13) | Not reported | 4.42 (0.05) | 383 | Not reported | Girls, p = 0.18 for normotensive vs. all types of preeclampsia | |||||
| Girls, moderate preeclampsia | 4.58 (0.09) | Not reported | |||||||||
| Girls, severe preeclampsia | 4.20 (0.15) | Not reported | |||||||||
| HDL cholesterol | Boys, mild preeclampsia | 1.70 (0.05) | Not reported | 1.74 (0.03) | 383 | Not reported | Boys, p = 0.26 for normotensive vs. all types of preeclampsia | ||||
| Boys, moderate preeclampsia | 1.66 (0.07) | Not reported | |||||||||
| Boys, severe preeclampsia | 1.84 (0.07) | Not reported | |||||||||
| Girls, mild preeclampsia | 1.57 (0.07) | Not reported | 1.64 (0.03) | 383 | Not reported | Girls, p = 0.40 for normotensive vs. all types of preeclampsia | |||||
| Girls, moderate preeclampsia | 1.69 (0.05) | Not reported | |||||||||
| Girls, severe preeclampsia | 1.71 (0.08) | Not reported | |||||||||
| Non-HDL cholesterol | Boys, mild preeclampsia | 2.76 (1.12) | Not reported | 2.60 (0.06) | 383 | Not reported | Boys, p = 0.31 for normotensive vs. all types of preeclampsia | ||||
| Boys, moderate preeclampsia | 2.77 (0.14) | Not reported | |||||||||
| Boys, severe preeclampsia | 2.81 (0.14) | Not reported | |||||||||
| Girls, mild preeclampsia | 2.88 (0.13) | Not reported | 2.78 (0.05) | 383 | Not reported | Girls, p = 0.11 for normotensive vs. all types of preeclampsia | |||||
| Girls, moderate preeclampsia | 2.89 (0.09) | Not reported | |||||||||
| Girls, severe preeclampsia | 2.49 (0.14) | Not reported | |||||||||
| 10 | 31 | Tenhola (2003) | 12 | Total cholesterol | All children | 4.54 (95% CI 4.32, 4.76) | 60 | 4.50 (95% CI 4.29, 4.71) | 60 | 0.04a | Adjusted: p = 0.618 |
| HDL cholesterol | All children | 1.31 (95% CI 1.24, 1.38) | 1.36 (95% CI 1.29, 1.43) | −0.05a | Adjusted: p = 0.468 | ||||||
| LDL cholesterol | All children | 2.82 (95% CI 2.63, 3.01) | 2.75 (95% CI 2.57, 2.93) | 0.07a | Adjusted: p = 0.342 | ||||||
| 16 | 40 | Miettola (2013) | 16 | Total cholesterol | All children | Geometric mean (IQR): | 174 | Geometric mean (IQR): | 4.518 | Crude: 0.01a | p = 0.979 |
| 4.19 (3.70, 4.70) | 4.18 (3.70, 4.70) | Adjustedd: % difference: 0.4 | −2.3, 3.3 | ||||||||
| Boys | Geometric mean (IQR): 4.01 (3.50, 4.40) | Not reported | Geometric mean (IQR): 4.02 (3.60, 4.50) | Not reported | Crude: −0.01a | p = 0.998 | |||||
| Girls | Geometric mean (IQR): 4.41 (3.85, 5.05) | Not reported | Geometric mean (IQR): 4.35 (3.90, 4.80) | Not reported | Crude: 0.06a | p = 0.755 | |||||
| HDL cholesterol | All children | Geometric mean (IQR): | 174 | Geometric mean (IQR): | 4.518 | Crude: −0.02a | p = 0.777 | ||||
| 1.36 (1.17, 1.62) | 1.38 (1.20, 1.59) | Adjustedd: % difference: -0.1 | −3.2, 3.1 | ||||||||
| Boys | Geometric mean (IQR): 1.27 (1.12, 1.47) | Not reported | Geometric mean (IQR): 1.29 (1.14, 1.48) | Not reported | Crude: −0.02a | p = 0.706 | |||||
| Girls | Geometric mean (IQR): 1.47 (1.25, 1.72) | Not reported | Geometric mean (IQR): 1.46 (1.29, 1.66) | Not reported | Crude: 0.01a | p = 0.942 | |||||
| LDL cholesterol | All children | Geometric mean (IQR): | 174 | Geometric mean (IQR): | 4.518 | Crude: 0.01a | p = 0.965 | ||||
| 2.18 (1.80, 2.60) | 2.17 (1.90, 2.60) | Adjustedd: % difference: 0.4 | −3.5, 4.4 | ||||||||
| Boys | Geometric mean (IQR): 2.12 (1.80, 2.50) | Not reported | Geometric mean (IQR): 2.12 (1.80, 2.50) | Not reported | Crude: 0.00a | p = 0.965 | |||||
| Girls | Geometric mean (IQR): 2.26 (1.95, 2.60) | Not reported | Geometric mean (IQR): 2.23 (1.90, 2.60) | Not reported | Crude: 0.03a | p = 0.895 | |||||
| Triglycerides | |||||||||||
| 4 | 25 | Kvehaugen (2011) | 5–8 | Triglycerides | All children | Median (IQR): 0.60 (0.53, 0.73) | 20 | Median (IQR): 0.58 (0.53, 0.82) | 14 | 0.02a | p = 0.9 |
| 15 | 10 | Lawlor (2012) | 9–10 | Triglycerides | All children | Geometric mean (95%CI): 0.99 (0.91, 1.09) | 70 | Geometric mean (95%CI): 1.03 (1.01, 1.04) | 2.869 | Crude: not reported Adjustedb: ratio of geometric mean: 0.96 | Not reported 0.85, 1.07 |
| 15 | 39 | Fraser (2013) | 17 | Triglycerides | All children | Median (IQR): 0.7 (0.6, 1.1) | 53 | Median (IQR): 0.8 (0.6, 1.0) | 2.404 | Crude: −0.1a | p = 0.56 |
| Adjustedc: % difference: −0.01 | −10.9, 10.8 | ||||||||||
| 10 | 31 | Tenhola (2003) | 12 | Triglycerides | All children | 0.90 (95%CI 0.80, 1.00) | 60 | 0.86 (95%CI 0.78, 0.94) | 60 | 0.04a | p = 0.617 |
| 16 | 40 | Miettola (2013) | 16 | Triglycerides | All children | Geometric mean (IQR): | 174 | Geometric mean (IQR): | 4.518 | Crude: 0.0a | p = 0.997 |
| 0.75 (0.56, 1.02) | 0.75 (0.57, 0.97) | Adjustedd: % difference: −0.2 | −6.5, 6.6 | ||||||||
| Boys | Geometric mean (IQR): 0.76 (0.57, 0.99) | Not reported | Geometric mean (IQR): 0.74 (0.55, 0.95) | Not reported | Crude: 0.02a | p = 0.809 | |||||
| Girls | Geometric mean (IQR): 0.74 (0.56, 1.05) | Not reported | Geometric mean (IQR): 0.77 (0.59, 0.98) | Not reported | Crude: −0.03a | p = 0.739 | |||||
| Glucose | |||||||||||
| 8 | 29 | Alnes (2014) | 10–11 | Glucose | Boys, mild preeclampsia | 4.88 (0.05) | Not reported | 4.88 (0.03) | 383 | Not reported | Boys, p = 0.51 for normotensive vs. all types of preeclampsia |
| Boys, moderate preeclampsia | 4.92 (0.06) | Not reported | |||||||||
| Boys, severe preeclampsia | 4.79 (0.06) | Not reported | |||||||||
| Girls, mild preeclampsia | 4.81 (0.07) | Not reported | 4.74 (0.03) | 383 | Not reported | Girls, p = 0.45 for normotensive vs. all types of preeclampsia | |||||
| Girls, moderate preeclampsia | 4.83 (0.05) | Not reported | |||||||||
| Girls, severe preeclampsia | 4.76 (0.08) | Not reported | |||||||||
| 10 | 31 | Tenhola (2003) | 12 | Glucose | All children | 4.3 (95% CI 4.2, 4.4) | 60 | 4.4 (95% CI 4.3, 4.5) | 60 | −0.1a | p = 0.371 |
| 16 | 40 | Miettola (2013) | 16 | Glucose | All children | Geometric mean (IQR): | 174 | Geometric mean (IQR): | 4.518 | Crude: 0.0a | p = 0.993 |
| 5.14 (4.90, 5.50) | 5.14 (4.90, 5.40) | Adjustedd: % difference: −0.3 | −1.8, 1.2 | ||||||||
| Boys | Geometric mean (IQR): 5.31 (5.10, 5.60) | Not reported | Geometric mean (IQR): 5.28 (5.00, 5.50) | Not reported | 0.03a | p = 0.793 | |||||
| Girls | Geometric mean (IQR): 4.95 (4.80, 5.30) | Not reported | Geometric mean (IQR): 5.02 (4.80, 5.30) | Not reported | −0.07a | p = 0.509 | |||||
| 15 | 39 | Fraser (2013) | 17 | Glucose | All children | 5.1 (0.4) | 53 | 5.1 (0.6) | 2.404 | Crude: 0.0a | p = 0.65 |
| Adjustedc: 0.001 | −0.15, 0.16 | ||||||||||
We calculated the mean difference if this was not reported by the authors.
Adjusted for offspring sex, body mass index, height, height-squared, and age at outcome measurement; maternal age, prepregnancy body mass index (BMI), nulliparity, smoking during pregnancy, education and head of household social class.
Adjusted for offspring sex and age; maternal age, prepregnancy BMI, parity, smoking during pregnancy and household social class.
Adjusted for offspring sex; nulliparity, maternal prepregnancy BMI and socioeconomic position.
PE: preeclampsia; CI: confidence interval; LDL: low-density lipoprotein; HDL: high-density lipoprotein; IQR: interquartile range
Associations with glucose
Four studies included blood glucose as outcome and observed no association between exposure to preeclampsia and the level of glucose in childhood29,31,39,40 (Table 5).
Discussion
Summary of findings
This systematic review of 16 studies scopes the association between HDP and cardiometabolic markers in childhood. Most studies showed that exposure to PIH was associated with a higher BP in childhood. There was no convincing evidence that preeclampsia is also associated with higher BP in childhood. No association was observed between exposure to PIH or preeclampsia and cholesterol, triglycerides and glucose. There were no studies that investigated the association between HDP and (carotid) intima-media thickness, HbA1c and diabetes mellitus type 2. None of the studies investigated the association of eclampsia or HELLP syndrome with one of the outcomes of interest.
Comparison of findings with existing evidence
This is the first systematic review of the association between PIH and cardiovascular risk factors in childhood. In 2012, Davis et al.8 systematically reviewed the association between preeclampsia and cardiovascular risk factors in childhood and early adulthood. In their meta-analysis, exposure to preeclampsia was associated with a 2.39 mmHg (95% CI 1.74, 3.05) higher SBP and a 1.35 mm Hg (95% CI 0.90, 1.80) higher DBP. In contrast, most studies in our review observed no higher SBP or DBP in children exposed to preeclampsia compared with those unexposed. The discrepancy between our findings and those by Davis et al. can be explained by more recently published studies in which no association was found between exposure to preeclampsia and SBP or DBP.36,40 In addition, the study by Lazdam et al.,41 in which a strong association between preeclampsia and BP in adulthood was observed, was not included in our evidence synthesis since we investigated an association only in childhood.
In line with the results of Davis et al., we observed no association between in utero exposure to preeclampsia and levels of cholesterol and glucose in childhood.
We did not perform a quantitative meta-analysis because the ages at which cardiometabolic outcomes were investigated varied strongly between studies. In general, BP levels increase from childhood into adolescence due to growth.42 A mean difference in BP between HDP exposed and unexposed children observed in childhood can be similar to a mean difference in BP observed in adolescence, but the relative difference in BP would be larger in childhood due to the lower baseline BP at this age.
Possible underlying mechanisms
First, the higher BP in offspring exposed to PIH may be programmed via an intra-uterine mechanism. Miettola et al.40 suggested a mechanism in which irregulation of maternal and foetal glucocorticoids is involved, but their hypothesis was based on evidence from animal studies investigating prenatal stress rather than PIH specifically. To our knowledge, there are no other studies in which intra-uterine mechanisms are described.
Second, HDPs are associated with adverse perinatal outcomes such as small for gestational age and preterm birth,43 which in turn are associated with higher BP in children.44 Only few studies in this review investigated the potential mediating effects of these perinatal factors in the association of PIH and offspring BP. In the ALSPAC study,10,37,38 however, the association of PIH with offspring BP was not explained by birth weight, gestational age, method of delivery, breastfeeding or offspring BMI at outcome measurement.
Third, the higher BP in offspring exposed to PIH may reflect genetic susceptibility to develop high BP. Women who are genetically predisposed to develop hypertension are more likely to respond more extremely to physiological changes due to pregnancy, which may lead to endothelial dysfunction and PIH.14 Pregnancy can thus be seen as a stress-test in which a genetic predisposition to CVD will be unmasked by an indication of HDP.14 This genetic predisposition may be inherited by the mothers' offspring, independent of PIH-related conditions in utero.15
Last, shared environment and lifestyle, which on the one hand leads to the development of HDP and on the other hand increases the risk of adverse cardiometabolic outcomes in the offspring, may explain the association of PIH with BP. For instance, maternal obesity is an important risk factor for HDP,43 but is also related to offspring BMI and BP.45–47 Two studies in this review investigated whether maternal obesity amongst other potential confounders explained the association of PIH with offspring BP, and found that the association between PIH and higher SBP in childhood remained statistically significant after adjustment.10,37–40 Nevertheless, obesity is known to interact with both environmental factors and a genetic component.46 This well-known concept that offspring BP depends on both genetic and shared (familial) environmental factors is called familial aggregation of BP.48 This is also supported by results from Miliku et al.49 in which both higher maternal and higher paternal BP were associated with higher childhood BP.
In this systematic review most studies observed no association between preeclampsia and offspring BP. Exposure to preeclampsia would affect the development of organs and vascular structures in the foetus, thereby programming the child towards adverse cardiometabolic health. For example, microvascular adaptations,50,51 endothelial dysfunction25,33 and myocardial dysfunction52 have been observed in the offspring of mothers with preeclampsia. A possible explanation for the lack of association in most of the studies is that exposure to preeclampsia in itself does not lead to higher BP in childhood. Preeclampsia is accompanied by an immunological response which induces different pathophysiological pathways in utero. It has been suggested that interaction between this in utero effect and adverse environmental factors (e.g. unhealthy lifestyle) during pregnancy leads to an increase in offspring BP.53 Apart from data on smoking during pregnancy in the ALSPAC study,10,36–38 data on adverse factors during pregnancy were lacking in the studies in this review, and thus we could not investigate this hypothesis.
Limitations
Our review has some limitations. First, due to the large variation in the children's ages at outcome measurement, we were not able to perform a meta-analysis and thus we could not provide a pooled estimate for the association between HDP and offspring BP. Instead, we counted the number of studies which did and did not observe a statistically significant association.
Second, there were few studies that investigated cardiometabolic outcomes other than BP in relation to HDP. We selected cardiometabolic outcomes which we expected to be available in epidemiological studies performed in children. For example, (carotid) intima-media thickness is increasingly studied as an endpoint in children. However, we found no study that investigated an association of exposure to HDP and (carotid) intima-media thickness. Possibly we have missed studies that selected other cardiometabolic outcomes.
Last, the studies in our review poorly reported on factors that might shed light on the possible underlying mechanisms. As mentioned earlier, data on perinatal factors were scarce, as well as data on adverse factors during pregnancy. In addition, we were not able to investigate whether BP lowering medication or severity of the HDP influence the association of HDP with offspring BP.
Relevance of findings and perspectives
HDP can be harmful for both the mother54 and unborn child.55 This systematic review shows that HDPs, in particular PIH, also have long term consequences for offspring BP. This is in line with the results of Tapp et al.;56 they demonstrated an adverse cardiometabolic health (abnormalities of the retinal microvasculature, cardiac structure and increased BP) in adult offspring exposed to HDP in utero. It is known that BP tracks from childhood into adulthood.57 Even small increases in BP, as observed in most of the studies in this review, may have a large impact on the cardiovascular health of the general population if those increases are widespread in the population.58
Perspectives
The exact underlying mechanisms – genetic susceptibility, shared familial environment, intra-uterine effects – of the association between PIH and offspring BP are puzzling. However, exposure to HDP, in particular PIH, leads to higher BP values in the offspring. Modifiable factors which could induce the development of high BP in the offspring should therefore be tackled. This stresses the importance of guiding (future) parents toward a healthier lifestyle before and during pregnancy, but also a healthy lifestyle of the whole family after pregnancy contributes to healthier BP levels in the offspring.
A higher BP was also found amongst women with a history of HDP: trajectories of classical CVD risk factors are altered and hypertension already occurs significantly more in the fourth decade.59,60 Blood pressure seems to be the main driver of increased CVD risk both among women with a history of HDP and their offspring. Based on our findings and those of Groenhof et al., it could be argued that CVD prevention should begin earlier than currently practised in women with a history of HDP,61 and should also be accessible to HDP exposed offspring.
Conclusions
Most studies in this systematic review showed that children exposed to PIH in utero have a higher BP than children who were not exposed to PIH. Most studies found no association between exposure to preeclampsia and BP in childhood. The studies in this review did not observe an association between HDP and blood cholesterol, triglycerides and glucose. We found no studies that investigated an association between HDP and HbA1c, diabetes mellitus type 2 or (carotid) intima media thickness.
Supplemental Material
Supplemental material, Supplemental Material for Hypertensive disorders of pregnancy and cardiometabolic outcomes in childhood: A systematic review by Maria AC Jansen, Linda PM Pluymen, Geertje W Dalmeijer, T Katrien J Groenhof, Cuno SPM Uiterwaal, Henriëtte A Smit and Lenie van Rossem in European Journal of Preventive Cardiology
Acknowledgement
We gratefully acknowledge René Spijker for his help in setting up the search strategy. Systematic review registration in Prospero: CRD42017070509.
Author contribution
MACJ and LPMP contributed equally to this work. MACJ, LPMP, HAS, GWD and LvR had the main role in research protocol design. MACJ and LPMP did the literature search, performed title and abstract screening, data extraction and drafted the manuscript. HAS additionally contributed to the screening and data extraction process. GWD, TKJG, CSPMU, HAS and LvR contributed to interpretation and participated in the critical revision of the article. All authors (MACJ, LPMP, GWD, TKJG, CSPMU, HAS and LvR) approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: LvR was supported by a grant from the Netherlands Heart Foundation, no. 2013T025. The funding source(s) had no role in the collection, analysis and interpretation of the data, writing of the report, or in the decision to submit the article for publication.
References
- 1.Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 2014; 4: 97–104. [DOI] [PubMed] [Google Scholar]
- 2.Bateman BT, Bansil P, Hernandez-Diaz S, et al. Prevalence, trends, and outcomes of chronic hypertension: A nationwide sample of delivery admissions. Am J Obstet Gynecol 2012; 206: 134.e131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klungsoyr K, Morken NH, Irgens L, et al. Secular trends in the epidemiology of pre-eclampsia throughout 40 years in Norway: prevalence, risk factors and perinatal survival. Paediatr Perinat Epidemiol 2012; 26: 190–198. [DOI] [PubMed] [Google Scholar]
- 4.Lisonkova S, Joseph KS. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013; 209: 544.e541–544.e512. [DOI] [PubMed] [Google Scholar]
- 5.Sebastian T, Yadav B, Jeyaseelan L, et al. Small for gestational age births among South Indian women: Temporal trend and risk factors from 1996 to 2010. BMC Pregnancy Childbirth 2015; 15: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallis AB, Saftlas AF, Hsia J, et al. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008; 21: 521–526. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia, Geneva: World Health Organization, 2011. . [PubMed] [Google Scholar]
- 8.Davis EF, Lazdam M, Lewandowski AJ, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics 2012; 129: e1552–1561. [DOI] [PubMed] [Google Scholar]
- 9.Himmelmann A, Svensson A, Hansson L. Five-year follow-up of blood pressure and left ventricular mass in children with different maternal histories of hypertension: The Hypertension in Pregnancy Offspring Study. J Hypertens 1994; 12: 89–95. [PubMed] [Google Scholar]
- 10.Lawlor DA, Macdonald-Wallis C, Fraser A, et al. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: Findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J 2012; 33: 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice MM, Landon MB, Varner MW, et al. Pregnancy-associated hypertension and offspring cardiometabolic health. Obstet Gynecol 2018; 131: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts JM, Pearson G, Cutler J, et al. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 2003; 41: 437–445. [DOI] [PubMed] [Google Scholar]
- 13.Davis EF, Newton L, Lewandowski AJ, et al. Pre-eclampsia and offspring cardiovascular health: Mechanistic insights from experimental studies. Clin Sci (Lond) 2012; 123: 53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening?. BMJ 2002; 325: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrap SB. Hypertension: Genes versus environment. Lancet 1994; 344: 169–171. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surgery 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 18.Kotchen JM, Kotchen TA, Cottrill CM, et al. Blood pressures of young mothers and their first children 3–6 years following hypertension during pregnancy. J Chronic Dis 1979; 32: 653–659. 1. [DOI] [PubMed] [Google Scholar]
- 19.Kotchen JM, McKean HE, Kotchen TA. Blood pressure of young mothers and their children after hypertension in adolescent pregnancy: Six- to nine-year follow-up. Am J Epidemiol 1982; 115: 861–867. [DOI] [PubMed] [Google Scholar]
- 20.Bergel E, Haelterman E, Belizan J, et al. Perinatal factors associated with blood pressure during childhood. Am J Epidemiol 2000; 151: 594–601. [DOI] [PubMed] [Google Scholar]
- 21.Svensson A, Sigstrom L. Blood pressure, erythrocyte sodium and potassium concentrations and Na+K+ATPase activity in children with hypertensive mothers. J Hypertens 1986; 4: 269–272. [DOI] [PubMed] [Google Scholar]
- 22.Himmelmann A, Svensson A, Hansson L. Blood pressure and left ventricular mass in children with different maternal histories of hypertension: The Hypertension in Pregnancy Offspring Study. J Hypertens 1993; 11: 263–268. [DOI] [PubMed] [Google Scholar]
- 23.Himmelmann A, Himmelmann K, Svensson A, et al. Glucose and insulin levels in young subjects with different maternal histories of hypertension: The Hypertension in Pregnancy Offspring Study. J Intern Medic 1997; 241: 19–22. [DOI] [PubMed] [Google Scholar]
- 24.Kvehaugen AS, Andersen LF, Staff AC. Anthropometry and cardiovascular risk factors in women and offspring after pregnancies complicated by preeclampsia or diabetes mellitus. Acta Obstet Gynecol Scand 2010; 89: 1478–1485. [DOI] [PubMed] [Google Scholar]
- 25.Kvehaugen AS, Dechend R, Ramstad HB, et al. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension 2011; 58: 63–69. [DOI] [PubMed] [Google Scholar]
- 26.Palti H, Rothschild E. Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum Dev 1989; 19: 263–269. [DOI] [PubMed] [Google Scholar]
- 27.Lazdam M, de la Horra A, Diesch J, et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension 2012; 60: 1338–1345. [DOI] [PubMed] [Google Scholar]
- 28.Langford HG, Watson RL. Prepregnant blood pressure, hypertension during pregnancy, and later blood pressure of mothers and offspring. Hypertension 1980; 2: 130–133. [PubMed] [Google Scholar]
- 29.Alsnes IV, Janszky I, Forman MR, et al. A population-based study of associations between preeclampsia and later cardiovascular risk factors. Am J Obstet Gynecol 2014; 211: 657.e1–7. [DOI] [PubMed] [Google Scholar]
- 30.Oglaend B, Forman MR, Romundstad PR, et al. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens 2009; 27: 2051–2054. [DOI] [PubMed] [Google Scholar]
- 31.Tenhola S, Rahiala E, Martikainen A, et al. Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. J Clin Endocrinol Metab 2003; 88: 1217–1222. [DOI] [PubMed] [Google Scholar]
- 32.Tenhola S, Rahiala E, Halonen P, et al. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: Evaluation by ambulatory blood pressure monitoring. Pediatr Res 2006; 59: 320–324. [DOI] [PubMed] [Google Scholar]
- 33.Jayet PY, Rimoldi SF, Stuber T, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 2010; 122: 488–494. [DOI] [PubMed] [Google Scholar]
- 34.Vatten LJ, Romundstad PR, Holmen TL, et al. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol 2003; 101: 529–533. [DOI] [PubMed] [Google Scholar]
- 35.Hiller JE, Crowther CA, Moore VA, et al. Calcium supplementation in pregnancy and its impact on blood pressure in children and women: Follow up of a randomised controlled trial. Aust N Z J Obstet Gynaecol 2007; 47: 115–121. [DOI] [PubMed] [Google Scholar]
- 36.Belfort MB, Gillman MW, McCormick MC. Prenatal and perinatal predictors of blood pressure at school age in former preterm, low birth weight infants. J Perinatol 2012; 32: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: Findings from a prospective study. J Am Heart Assoc 2015; 4: e001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geelhoed JJ, Fraser A, Tilling K, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: The Avon Longitudinal Study of Parents and Children. Circulation 2010; 122: 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension 2013; 62: 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miettola S, Hartikainen AL, Vaarasmaki M, et al. Offspring's blood pressure and metabolic phenotype after exposure to gestational hypertension in utero. Eur J Epidemiol 2013; 28: 87–98. [DOI] [PubMed] [Google Scholar]
- 41.Lazdam M, de la Horra A, Pitcher A, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: Is endothelial dysfunction the underlying vascular mechanism?. Hypertension 2010; 56: 159–165. [DOI] [PubMed] [Google Scholar]
- 42.Falkner B, Daniels SR. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension 2004; 44: 387–388. [DOI] [PubMed] [Google Scholar]
- 43.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25: 391–403. [DOI] [PubMed] [Google Scholar]
- 44.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: A systematic review of the literature. J Hypertens 2000; 18: 815–831. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig-Walz H, Schmidt M, Gunther ALB, et al. Maternal prepregnancy BMI or weight and offspring's blood pressure: Systematic review. Matern Child Nutr 2018; 14: e12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen LA, Nielsen TR, Holm JC. The impact of familial predisposition to obesity and cardiovascular disease on childhood obesity. Obes Facts 2015; 8: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolton P, Xiao M. The intergenerational transmission of body mass index across countries. Econ Hum Biol 2017; 24: 140–152. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Xu X, Su S, et al. Familial aggregation and childhood blood pressure. Curr Hypertens Rep 2015; 17: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miliku K, Bergen NE, Bakker H, et al. Associations of maternal and paternal blood pressure patterns and hypertensive disorders during pregnancy with childhood blood pressure. J Am Heart Assoc 2016; 5: e003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yesil GD, Gishti O, Felix JF, et al. Influence of maternal gestational hypertensive disorders on microvasculature in school-age children: The Generation R Study. Am J Epidemiol 2016; 184: 605–615. [DOI] [PubMed] [Google Scholar]
- 51.Islam M, Jafar TH, Bux R, et al. Association of parental blood pressure with retinal microcirculatory abnormalities indicative of endothelial dysfunction in children. J Hypertens 2014; 32: 598–605. [DOI] [PubMed] [Google Scholar]
- 52.Fugelseth D, Ramstad HB, Kvehaugen AS, et al. Myocardial function in offspring 5–8 years after pregnancy complicated by preeclampsia. Early Hum Dev 2011; 87: 531–535. [DOI] [PubMed] [Google Scholar]
- 53.Stojanovska V, Scherjon SA, Plosch T. Preeclampsia as modulator of offspring health. Biol Reprod 2016; 94: 53. [DOI] [PubMed] [Google Scholar]
- 54.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: A systematic review. Lancet 2006; 367: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 55.Basso O, Rasmussen S, Weinberg CR, et al. Trends in fetal and infant survival following preeclampsia. JAMA 2006; 296: 1357–1362. [DOI] [PubMed] [Google Scholar]
- 56.Tapp RJ, Hughes AD, Kahonen M, et al. Cardiometabolic health among adult offspring of hypertensive pregnancies: The Cardiovascular Risk in Young Finns Study. J Am Heart Assoc 2018; 7: e006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation 2008; 117: 3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rose G. Strategy of prevention: Lessons from cardiovascular disease. Br Med J (Clin Res Ed) 1981; 282: 1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groenhof TKJ, Zoet GA, Franx A, et al. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension 2019; 73: 171–178. [DOI] [PubMed] [Google Scholar]
- 60.Groenhof TKJ, van Rijn BB, Franx A, et al. Preventing cardiovascular disease after hypertensive disorders of pregnancy: Searching for the how and when. Eur J Prev Cardiol 2017; 24: 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heida KY, Bots ML, de Groot CJ, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: A Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol 2016; 23: 1863–1879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material for Hypertensive disorders of pregnancy and cardiometabolic outcomes in childhood: A systematic review by Maria AC Jansen, Linda PM Pluymen, Geertje W Dalmeijer, T Katrien J Groenhof, Cuno SPM Uiterwaal, Henriëtte A Smit and Lenie van Rossem in European Journal of Preventive Cardiology