Abstract
Hepatitis C virus (HCV) accounts for 15%–20% of cases of acute infection, and chronic HCV infection is developed in about 50%–80% of HCV patients. Unfortunately, due to the lack of proper medical care, difficulty in screening for HCV infection, and lack of awareness resulted in chronic HCV infection in 71 million people on a global scale, and about 399,000 deaths in 2016. It is crucial to recognize that the effective use of antiviral medicines can cure more than 95% of HCV infected people. The Global Health Sector Strategy (GHSS) aim is to reduce the new HCV infections and the HCV associated mortality by 90% and 65%, respectively. Therefore, the methods that are simple, yet powerful enough to detect HCV infections with high sensitivity, specificity, and a shorter window period are crucial to restrain the global burden of HCV healthcare. This article focuses on the technologies used for the detection of HCV in clinical specimens.
Keywords: anti-HCV antibody, HCV core antigen, NS3, NS4, NS5, HCV detection, viruses, Flaviviridae
1. Introduction
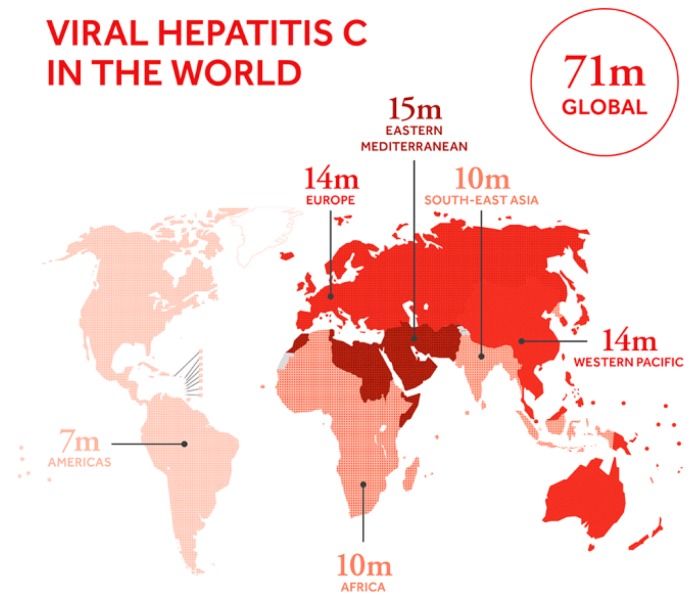
Hepatitis C virus (HCV) is an RNA virus of the family Flaviviridae that accounts for 15%–20% cases of acute infection, and chronic HCV infection is developed in about 50%–80% of HCV patients. About 15%–30% of patients with chronic HCV infection have a risk of cirrhosis within 20 years. There are 71 million people with chronic hepatitis C virus infection on a global scale, and about 399,000 people died due to hepatitis C in 2016 as shown in Figure 1 [1,2]. Most of the chronically infected patients are asymptomatic and can lead a healthy life. However, the disease develops into fibrosis, cirrhosis, and possibly hepatocellular carcinoma (HCC) in about 20% of chronically infected patients during the period of 10–30 years [3,4,5,6].
Figure 1.
Viral hepatitis C in the world (adopted from the global hepatitis report, 2017. https://www.who.int/hepatitis/news-events/global-hepatitis-report2017-infographic/en/).
The World Health Assembly endorsed the Global Health Sector Strategy (GHSS) on viral hepatitis 2016–2021 in 2016, which targets for the elimination of viral hepatitis as a public threat by 2030. The aim is to reduce the new infections and the associated mortality by 90% and 65%, respectively. The effective use of antiviral medicines can cure more than 95% of HCV infected people, thereby reducing the mortality associated with cirrhosis and liver cancer. However, the limited access to early and accurate screening, diagnosis, and treatment [7,8,9] is the most significant barrier to achieve the goal outlined in the GHSS. A method that allows, rapid, accurate, and highly efficient method for the detection of HCV is crucial for mass screening. Such a technique can prove vital to avoid the transmission of infection and to help physicians to begin the antiviral therapy [10,11].
The most common drawbacks including patient’s anxiety while waiting for test results, adverse effects of treatment and complications related to liver biopsy limit screening for hepatitis C. However, the benefits of HCV screening outweigh these drawbacks. The primary benefit of HCV screening is that the 90%–100% of patients can be treated with current drugs if the infected individuals are identified before the chronic HCV is developed into the advanced fibrosis, cirrhosis, or hepatocellular carcinoma [12,13]. It is vital to notice that the reduction in all-cause mortality is highly associated with the sustained virologic clearance for more than six months after the treatment [14]. Therefore, it is crucial to identify individuals with hepatitis C infection before they develop the symptoms of the disease.
It appears that HCV detection methods are based on molecular assays and serological assays. The molecular assays are RT-PCR based nucleic acid amplification tests (NAT’s) for detection HCV RNA in samples, including blood and other body fluids [15,16,17]. However, the NAT-based assays are not cost-effective, and usually they are not suitable for screening of HCV in a large population for rapid diagnosis [18]. On the contrary, the serological assays are often designed for the detection of HCV antigens, and anti-HCV antibodies in the serum or plasma are quickly taken from venipuncture. Hence, the serological tests are suitable for the mass screening of HCV in the general population. Further, the serological assays are highly applicable to the treatment monitoring and for the confirmation of the virologic clearance [19].
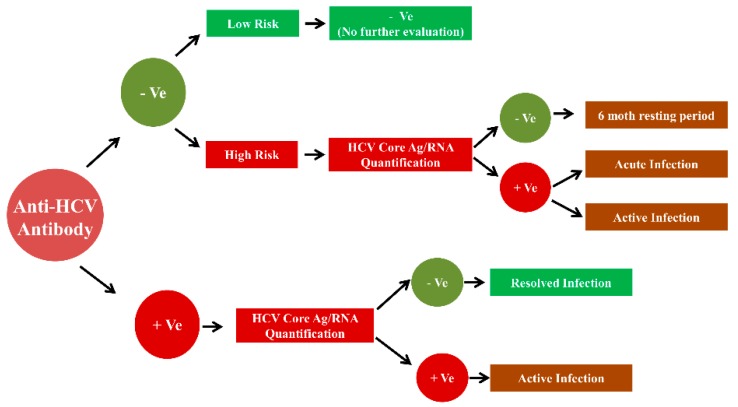
In general, the enzyme immunoassays are used to detect the anti-HCV antibodies, antigens in the HCV screening settings. The testing for HCV RNA is then used for the confirmation of HCV infection and identification of specific HCV genotype is standard practice for the diagnostic evaluation of HCV infection, as shown in Figure 2 [20].
Figure 2.
Algorithm for Hepatitis C screening.
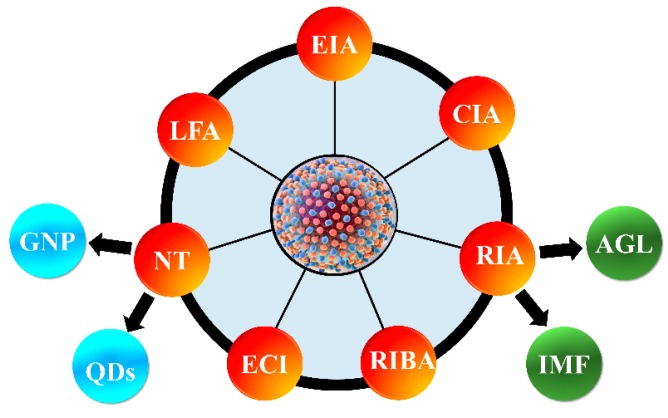
At present, various serological tests based on enzyme immunoassay (EIA), chemiluminescence immunoassay (CIA), rapid immunoassays (RA) including agglutination (AGL) and immune-filtration (IMF), recombinant immunoblot assay (RIBA), electrochemical immunosensor HCV detection (EI), nano-metal technology (NT) including gold nanoparticles (GNP) and quantum dots (QDs), and lateral flow assay (LFAs) are widely used for the HCV screening in clinical specimens. In many cases, the longer turn-around time, cost, size of the instrument, and necessity of highly trained technicians limits the use of currently available methods in the resource-limited settings [21,22]. Therefore, a low cost, rapid, simple, and accurate method are crucial for sizeable population-based HCV screening. The development of a highly sensitive point-of-care (POC) test for screening of HCV in resource-limited settings is crucial to provide a lower cost of healthcare in developing countries. In this critical review, we have discussed various technologies used for qualitative and quantitative detection of HCV, as shown in Figure 3. This article also elaborates the advantages and disadvantages of the protein-based HCV detection technologies, their efficacy, and future trend to improve their applications in resource-limited settings.
Figure 3.
Various methods used for the qualitative and quantitative detection of hepatitis C virus (HCV) antigens, and anti-HCV antibodies for HCV screening. EIA, enzyme immunoassay; CIA, chemiluminescence immunoassay; RIA, rapid immunoassays; AGL, agglutination; IMF, immune-filtration; RIBA, recombinant immunoblot assay; EI, electrochemical immunosensor; NT, nano-metal technology; GNP, gold nanoparticles; QDs, quantum dots; LFA, lateral flow assay.
2. HCV Proteins and Their Functions
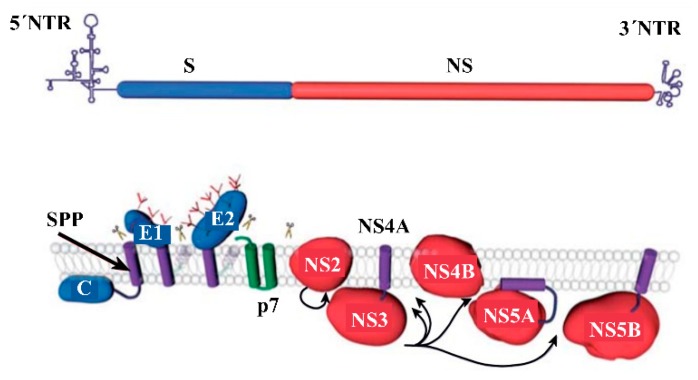
The genome of HCV encodes a single polyprotein, which is a characteristic of the members of the Flaviviridae family [23]. An HCV polyprotein containing 3010 amino acids is processed by cellular and viral proteases to generate ten polypeptides, as shown in Figure 4 [24].
Figure 4.
Organization of HCV genome (top) and the ten different sections of a polyprotein that are processed to generate HCV specific proteins (bottom). Scissors and arrows indicate cleavages by the peptidase to produce ten proteins with unique functions [24].
As shown in Figure 4, the HCV genome consists of four regions, including 5’UTR, structural proteins (S), nonstructural proteins (NS), and 3’UTR. A single polyprotein is representing ten different sections for structural proteins C (core), E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B. The processing of polyprotein by host endoplasmic reticulum (ER) signal peptidase(s) enzymes generates structure proteins. Whereas, the cleavage of polyprotein by HCV proteases generates nonstructural proteins [25]. The information on the structural and non-structural proteins with respect to their genetic stability, function, molar mass, and their applications in HCV screening by detecting them as antigens or their corresponding antibodies are summarized in Table 1.
Table 1.
HCV protein, their functions, molar mass, and their applications in HCV screening by detecting them as antigens or their corresponding antibodies.
| HCV Proteins | Genetic Stability | Function | Molar Mass | HCV Antigen Detection? | Anti-HCV Antibody Detection? |
|---|---|---|---|---|---|
| Structural Proteins | |||||
| Core (C): P22 | Stable | A significant component of viral nucleocapsid, Binds viral RNA during assembly | 20 KDa [26] | Yes [27] | Yes [28] |
| E: gp 35 envelope glycoproteins | A high degree of genetic diversity | Receptor binding and HCV entry into target cells | 31 kDa [26] | Yes [27] | - |
| E2: gp 70 envelope glycoproteins | 62 kDa [26] | Yes [29,30] | - | ||
| Non-Structural Proteins | |||||
| NS1: p7 small polypeptide | Stable | Ion channel localized to plasma membrane | 7 KDa [24] | - | - |
| NS2: p23 | - | Component of NS2-3 proteinase | 21 KDa [24] | - | - |
| NS3: p70 | - | Serine protease and RNA helicase | 69 KDa [24] | Yes [31] | Yes [7] |
| NS4A: p8 | Stable | Protease cofactor | 6 KDa [24] | - | Yes [32] |
| NS4B: p27 | Stable | Proteins | 27 KDa [24] | - | Yes [7] |
| Components of the viral replicase complex | |||||
| NS5A:p56/58 | Stable | Cofactor for NS5B | 56 KDa [24] | Yes [33] | Yes [7] |
| Regulate response to INF-α treatment | |||||
| NS5B: p68 | Stable | RNA dependent polymerase | 68 KDa [24] | - | Yes [7] |
The core protein is an RNA-binding protein that forms a viral nucleocapsid, and it is cleaved from the C-terminus of polyprotein by a host signal peptidase [34]. The HCV core antigen, a phosphoprotein containing 191 amino acids, is one of the potential diagnostic markers. HCV antibody-based technologies find it challenging to identify the individuals who have resolved their infection and individuals with active disease. However, HCV core antigen tests are designed to detect the circulating HCV core antigen, and thus, these test can diagnose an active infection in patients. Therefore, most HCV screening methods are directed towards the detection of the HCV core antigen [35,36,37,38].
The HCV glycoproteins, E1 and E2, are cleaved from the polyprotein by a host signal peptidase [39]. These glycoproteins are type-I transmembrane proteins with a large N-terminal ectodomain and a C-terminal transmembrane domain [40]. The primary function of E1 and E2 is to bind with the receptor and allowing the entry of HCV into the host cells. Thus these proteins are detected as antigens. The p7 polypeptide is at the junction between the region of the structural and nonstructural protein of the HCV polyprotein, and it acts as an ion channel by localizing on the plasma membrane [41]. The host signal peptidase cleaves the p7 from the HCV polyprotein [39]. The NS2 is an integral membrane protein that is not essential for the formation of the replication complex [42,43]. However, from the structure of NS2, it is revealed that it is a dimeric cysteine protease with two active sites [44]. The NS3 is co-expressed with NS4A, and it is found in association with ER or ER-like membranes. When NS3 is expressed alone, it is distributed in the cytoplasm and nucleus [45]. Further, it is found that the NS3 is a bifunctional protein with serine protease and RNA helicase activity. The NS4A acts as a protease cofactor and contributes one beta-strand to the N-terminal protease domain [46].
The NS4B is a highly hydrophobic nonstructural protein, possibly containing four transmembrane regions. The N-terminal and C-terminal of NS4B are known to be localized in the cytosol. Whereas, a fraction of the N-terminal is also being found in the ER lumen [61,62]. NS4B functions as a component of the viral replicase complex. The NS5A functions as a cofactor for NS5B, and it is found to regulate the response to INF-α treatment. The NS5A is a membrane-associated protein that contains an α-helical structure at its N-terminal, which serves as an anchor to attach on the cell membranes. Similar to most of the HCV proteins, the NS5A is detected in association with ER or ER-derived membranes [63,64]. NS5B is a membrane-associated protein. However, in contrast with the NS5A, the transmembrane region of the NS5B is on the C-terminal [65], and it plays a vital role during the RNA replication in cell cultures [66]. Analogous to NS5A and most HCV proteins, the NS5B is also detected in association with ER or ER-derived membranes [67].
Shown in Table 2 are various technologies that are used for the detection of HCV antigens and anti-HCV antibodies in clinical specimens. The advantages and disadvantages of these technologies are discussed in the following sections.
Table 2.
The technologies used for the detection of HCV proteins either as HCV antigen or the anti-HCV antibodies for the HCV screening.
| Technology | HCV Proteins | Core | E1 | E2 | NS1 | NS2 | NS3 | NS4A | NS4 | NS5A | NS5B | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EIA | Ab | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [31,47,48] | ||||
| Ag | ✓ | ✓ | ||||||||||
| CIA | Ab | ✓ | ✓ | ✓ | ✓ | ✓ | [28,35,49,50] | |||||
| Ag | ✓ | |||||||||||
| RA | Ab | ✓ | ✓ | ✓ | ✓ | [21,51] | ||||||
| Ag | ||||||||||||
| RIBA | Ab | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [30,52] | |||
| Ag | ||||||||||||
| EI | Ab | [52] | ||||||||||
| Ag | ✓ | ✓ | ||||||||||
| NT | Ab | ✓ | ✓ | ✓ | [53,54,55,56] | |||||||
| Ag | ✓ | ✓ | ✓ | |||||||||
| LFA | Ab | ✓ | ✓ | ✓ | [57,58] | |||||||
| Ag | ✓ | |||||||||||
| ABA | Ab | [29,59,60] | ||||||||||
| Ag | ✓ | ✓ |
Ag: HCV-antigen; Ab: Anti-HCV antibody.
3. Enzyme Immunoassay (EIA)
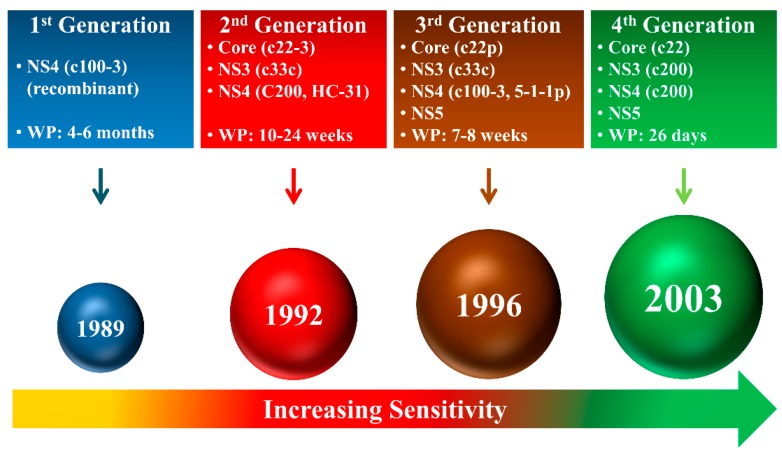
As of now, the assays for the detection of the HCV antigen and anti-HCV antibody have evolved through four generations of HCV immunoassays to identify HCV infected patients as shown in Figure 5 [7].
Figure 5.
Generations of the protein-based HCV assays (WP, window period).
Since 1989 with the beginning of first-generation immunoassays, wherein the recombinant c100-3 epitope from the NS4 region was used for the identification of HCV infected patients. However, the window period (WP) from the incident infection to the detection of the protein was 4–6 months. The first-generation assays lacked the sensitivity and the specificity. The second generation assays were developed to overcome the drawbacks of the first generation assays. The second generation assays developed in 1992 incorporated epitopes c22-3, c33c, c200, and HC-31 from the HCV core, NS3, NS4, and NS4 regions, respectively. The window period was successfully reduced to 10–24 weeks. The sensitivity of second-generation assays for HCV detection was significantly improved, making them applicable in clinically relevant settings [7,68,69,70]. The third-generation assays were developed in 1996 with a basic principle of detection of anti-HCV antibodies in plasma or serum against several HCV protein epitopes. The third-generation assays were multi-target format and included detection of antigens from the core (c22p), NS3 (c33c), NS4 (c100-3, 5-1-1p), and NS5 regions. The multi-target based assays demonstrated enhanced performance than the previous generations and were more effective with the ability to reduce the window period to 7–8 weeks [7,68,71,72]. However, the drawback of the assays in this generation was that the low positive predictive values in a low prevalence of HCV infection (<10%) [73,74].
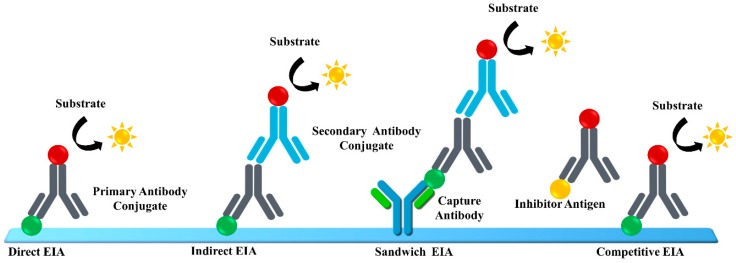
The fourth-generation assays, more commonly known as the antigen–antibody combo assay, simultaneously detect the HCV antigen and antibody. These assays are more convenient as two HCV markers are identified in the same test. The fourth-generation assays provide a single platform for the detection of antigen and antibodies in human sera. Hence, they are highly applicable in resource-constrained settings. Further, it is essential to notice that the fourth-generation assays are very sensitive as the window period is reduced to an all-time low of 26 days. Recently, a fourth-generation assay was reported to increase reactivity for the detection of antigens derived from the core, NS3, NS4A, NS4B, and NS5A regions for the detection of HCV genotypes 1a and 1b. Moreover, the improved detection of NS3 and NS4 antigens allows highly sensitive detection of HCV genotypes 2 and 3a [28]. There are several reports on the detection of anti-HCV antibodies using enzyme immunoassays for hepatitis C testing, as shown in Figure 6 [75,76,77,78].
Figure 6.
Methods for the detection of anti-HCV antibodies using enzyme immunoassays.
The HCV immunoassays can be divided into three categories, those targeting anti-HCV antibody detection and those targeting the HCV core antigen detection, and those targeting multiple HCV antigen detection.
3.1. Anti-HCV Antibody Detection
Lopes et al. reported the evaluation of an EIA for anti-HCV antibody detection using single antigen [79]. A recombinant c22 antigen was localized on the solid-phase and allowed to complex with anti-HCV antibodies in serum samples to detect anti-HCV IgG. The complexes were detected by using horseradish peroxidase goat anti-human IgG. This method demonstrated sensitivity and specificity of 95% and 97%, respectively, in a study of 145 healthy controls and 106 patients with confirmed HCV infection.
Tests for anti-HCV antibodies are usually qualitative with either a positive or a negative result. However, several studies have found that the specimen with low signal/cutoff (S/C) ratios commonly assigned false-positive results. Dufour et al. investigated specimens with low positive results (S/C ratios ≤ 3.7) with recombinant immunoblot assay and found that 86% of the samples were HCV negative. Therefore, they recommended that the laboratories should report the S/C ratio for anti-HCV EIA results. Moreover, supplemental RIBA testing should be performed for the specimens with low-positive values to avoid reporting false-positive results [48].
3.2. Core Antigen Detection
An immunoassay that detects and quantifies the total HCV core protein in serum was reported to detect the core antigen in the antibody-negative early phase of hepatitis C infection specimens. Hence, this assay is highly applicable for HCV screening blood banks for the screening of blood products [80,81]. Massaguer et al. reported on the application and the performance of HCV core antigen immunoassay for the monitoring of a viral load after liver transplantation [82]. It is known that the measurement of HCV-RNA concentration provides crucial information on viral load in the liver transplantation settings. However, besides being expensive, HCV-RNA testing is not routinely available in all laboratories. Massaguer et al. found that the quantification of HCV core antigen is appropriate for monitoring the viral load in HCV-infected patients undergoing liver transplantation. Alzahrani et al. also reported that the screening of blood donors by HCV core antigen assays has high potential in minimizing the risk of using HCV positive blood from a patient with the early phase of hepatitis C infection [83].
3.3. Multiple Antigen Detection
The current standard in diagnosing HCV infection is a two-step approach that requires screening with an anti-HCV antibody test followed by HCV-RNA detection to confirm the infection [84]. One of the reasons behind this two-step approach was the low specificity and sensitivity of currently available HCV core antigen assays [85,86]. Therefore, to improve the sensitivity and specificity of the HCV antigen assays so that they can be applied for the one stem detection of HCV infection, Hu et al. proposed the use of a test that simultaneously detects HCV core antigen and HCV nonstructural proteins [87,88]. The assay for the detection of multiple HCV antigens showed 98.9% specificity and 100% sensitivity compared to serum anti-HCV antibody assay and HCV-RNA detection assays.
4. Chemiluminescence Immunoassay (CIA)
The CIA uses a luminescent molecule, which serves as an indicator of the analytic reaction by emitting the visible or near-visible (λ = 300–800 nm) radiation. In many cases the enzymes used in CIA convert a substrate to a reaction product that emits photons. The CIA test is an epitope-specific antibody detection test and generally shows a very similar sensitivity and specificity to the third-generation EIA test [89].
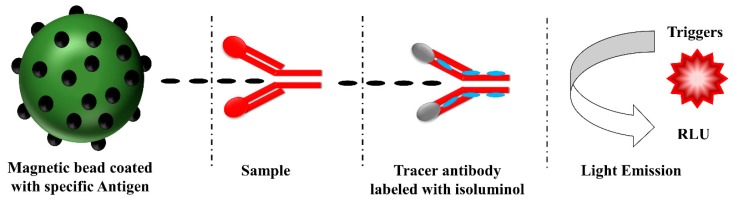
A principle behind the diagnostic testing of antibodies by using CIA is depicted in Figure 7. The antigens specific to the anti-HCV antibodies are coated on the magnetic beads. The addition of samples to the solution containing magnetic bead coated with antigens allows capturing the anti-HCV antibodies. The captured anti-HCV antibodies are then detected with the tracer antibody labeled with isoluminol. Upon enzymatic reactions catalyzed by peroxidase, the substrate is converted into a reaction product that emits photons, and then the emitted photons are detected by using a detector [90]. The CIA method is successfully applied for the screening of HCV infected patients by detecting anti-HCV antibodies. The automated CIA assays show high precision, high reliability, short turn-around time, and are technically simple due to full automation [89].
Figure 7.
CIA principle in the diagnostic testing of autoantibodies [90].
4.1. Anti-Hcv Antibody Detection
Currently, various commercial automatic CIA assays are available for the detection of anti-HCV antibodies in clinical laboratories [49]. These assays are replacing the conventional EIAs, particularly in high-volume clinical laboratories because of the automation and high sensitivities. The CLA exhibited considerably increased sensitivity and specificity, high positive predictive value compared to those of EIA for the detection of anti-HCV antibodies [91]. Various commercial assays available for the detection of the anti-HCV antibodies based on the CIA method are listed in Table 3.
Table 3.
Characteristics of automated anti-HCV antibody assays approved for in vitro diagnostics.
| Assay | Assay Principle | Solid Phase | HCV Antigen | Reaction Time (min) |
|---|---|---|---|---|
| Architect Anti-HCV, Abbott Laboratories | ECIA | Paramagnetic Particles | Core, NS3, NS4 | 29 |
| The LIAISON® XL murex HCV Ab, DiaSorin | CIA | Paramagnetic Particles | Core, NS3, NS4 | 46 |
| Vitros Anti-HCV, Ortho Clinical Diagnostics | CIA | Microwell | Core, NS3, NS4, NS5 | 55 |
| Elecsys Anti-HCV, Roche Diagnostics | ECA | Paramagnetic Particles | Core, NS3, NS4 | 18 |
| ADVIA Centaur HCV Assay, Siemens | CIA | Magnetic Particles | C22-3 (core), NS3, c200, NS5 | 58 |
| Access HCV Ab PLUS, Bio-Rad Laboratories | CIA | Paramagnetic Particles | Core, NS3, NS4, NS5 | 55 |
Kim et al. evaluated the performance comparison of four anti-HCV CIAs, including the Architect Anti-HCV assay, the Vitros Anti-HCV assay, Access HCV Ab PLUS assay, and the Elecsys Anti-HCV assay. According to their report, these assays showed good agreement for anti-HCV antibody detection with a range of 94.5% to 98.1%. The specificities of 98.8%, 96.5%, 98.2%, and 98.2% were found for Architect, Vitros, Access, and Elecsys assays, respectively [92]. Ismail et al. evaluated the performance of fully automated, enhanced CIA assay for the detection of anti-HCV antibody and found the sensitivity and specificity of 98.9% and 97.2%, respectively [91]. Tang et al. reported a systematic review and meta-analysis to evaluate the diagnostic accuracies of EIA based assays [93]. Feng et al. assessed the HISCL Anti-HCV assay, which is based on CIA technology and found that this assay showed 98.97% and 100% sensitivity and specificity, respectively, for the detection of HCV infections in clinical samples [94].
4.2. Core Antigen Detection
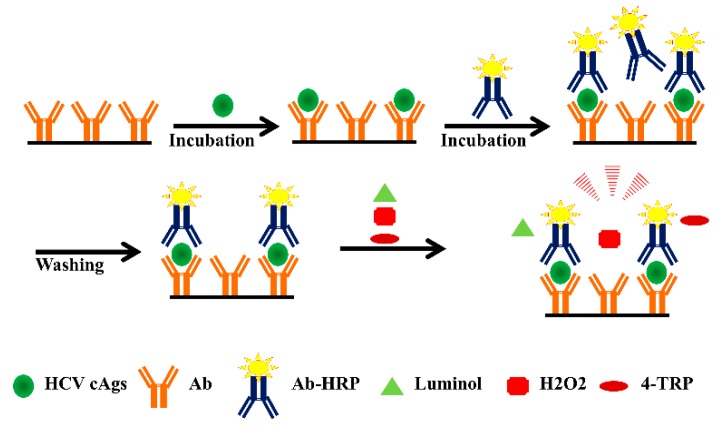
It is crucial to notice that the HCV core antigens can be detected in clinical samples during the window period with an automated CIA. Therefore, the development of highly sensitive CIA methods for the detection of HCV antigens is crucial for the identification of the HCV in the early stage of infection. A typical CIA based method used for the detection of HCV core antigen is depicted in Figure 8.
Figure 8.
Schematic illustration of a CIA used for the detection of the HCV core antigen.
There are limited research reports on the use of CIA for the detection of HCV antigens in order to identify the people with HCV infections. Muerhoff et al. detected HCV core antigen in human serum and plasma with an automated CIA and showed a 99.9% specificity and sensitivity [35]. Morota et al. reported the microparticle CIA for the quantitative determination of HCV core antigen and found the specificity of 99.8% by testing 5403 specimens [50]. Rockstroh et al. reported the results of HCV core antigen CIA assay in comparison with the HCV RNA test. They found the concordance of 99.5% and 99.24% between the HCV core antigen and HCV RNA in pre-treatment samples and post-treatment week 12 samples. The specificity in anti-HCV positive HCV RNA negative samples tested was 100% [95]. Liu et al. used the p-phenol derivative, 4-(1,2,4-triazol-1-yl)phenol (4-TRP) as an efficient enhancer of the luminol–hydrogen peroxide (H2O2)–horseradish peroxidase (HRP) in the CIA system for detection of the HCV core antigen. Their method showed a good linear relationship for the HCV core antigen concentration in the range of 0.6–3.6 pg/mL [96]. There are no reports on the detection of multiple HCV antigens using the CIA method.
5. Rapid Immunoassays (RIA)
Rapid immunoassays are defined as those that have the following characteristics, (i) turn-around time till the result is less than 30 minutes, (ii) moderately complex assays that do not need to follow the Clinical Laboratory Improvement Amendments of 1988 (CLIA’88), and (iii) an assay that does not require specialized equipment [97,98]. The majority of rapid immunoassays (RIA) assays are applicable in point-of-care settings, and they are manual single-use devices. The RIA designs that are used these days are latex agglutination, immunofiltration (flow-through), immunochromatographic (lateral flow), and optical immunoassays (OIA) [99]. The agglutination and immunofiltration assays will be briefly introduced in this article, and the lateral flow assays will be described in a separate section. The RIA tests are developed for the detection of anti-HCV antibodies associated with core, NS3, NS4, and NS5 regions as well as antigens of the virus [98]. The RIA is suitable where the infrastructure and the laboratory expertise are limited [100].
The agglutination (AGL) based method uses particles that are coated with an analyte-specific capture antibody. The analyte in a test sample triggers the formation of aggregates that can be visible to the naked eye. The lack of sensitivity and specificity are the major drawbacks of the agglutination-based assays. However, due to their rapid nature, low cost, and requirement of minimal reagents, they are used widely [101]. The agglutination based RIA are reported for the detection of antibodies only.
Immunofiltration (IMF) assays, commonly known as flow-through immunoassays, developed for the detection of anti-HCV antibodies. The antigens specific to the anti-HCV antibody are immobilized on the porous immunofiltration membrane. When a sample is applied to the membrane, it passes through the membrane, and the anti-HCV antibodies in the samples bind to the immobilized antigens. The captured antibodies are then detected with the anti-HCV antibody specific immunoglobulin G that are designed to produce distinct colors on the region.
5.1. Anti-HCV Antibody Detection
Daniel et al. reported the RIA that was intended for the detection of anti-HCV antibodies. In comparison to EIA, RIA demonstrated sensitivity and specificity of 99.3% and 99.0%, respectively [102]. Firdaus et al. evaluated the performance of RIA in clinical samples. Around 15.74% of these samples were HCV seropositive by ELISA, and 11.02% were RNA positive by nested RT-PCR. Therefore, the results of their study showed that the RIA alone could not be relied on as an absolute diagnostic tool for screening HCV [103].
5.2. Core Antigen Detection
Mikawa et al. reported a rapid one-step immunochromatographic assay for the detection of HCV core antigen detection. For this method, they expressed in the HCV core antigen in Escherichia coli a recombinant fusion protein with glutathione S-transferase (GST). The expression of a correct protein was confirmed by immunological detection with HCV positive serum. This method was capable of detecting 0.25–12.0 μg of the recombinant protein [57].
6. Recombinant Immunoblot Assay (RIBA)
The recombinant immunoblot assay can detect the anti-HCV antibodies present in the blood of HCV infected patients. RIBA was mainly used as a secondary confirmation test when the first-line screening test for HCV showed positive or indeterminate results. However, the use of RIBA based assays was discontinued because of the improved sensitivities of other methods used for HCV detection. However, before its discontinuation, RIBA detected anti-HCV antibodies using the recombinant antigens and synthetic peptides from a core, NS3, and NS5 proteins for immobilization onto a membrane [104,105]. Due to its robust specificity, this method was used as additional serological testing, and it became clinically obsolete with the availability of molecular tests [106]. Even though there are several reports on the application of RIBA for the detection of HCV infected patients, other immunoassays with higher sensitivity and specificity took precedence [107,108,109,110].
7. Electrochemical Immunosensors (EI)
Similar to any other immunoassay, the sensitivity and specificity of EI based assays depend on the highly specific molecular recognition between antigens and antibodies. The EI based assays have attracted massive attention from the scientific community for their fast and highly sensitive detection antigens [111,112]. The advantages of EI are high sensitivity, short turn-around time, and cost-effectiveness [113,114].
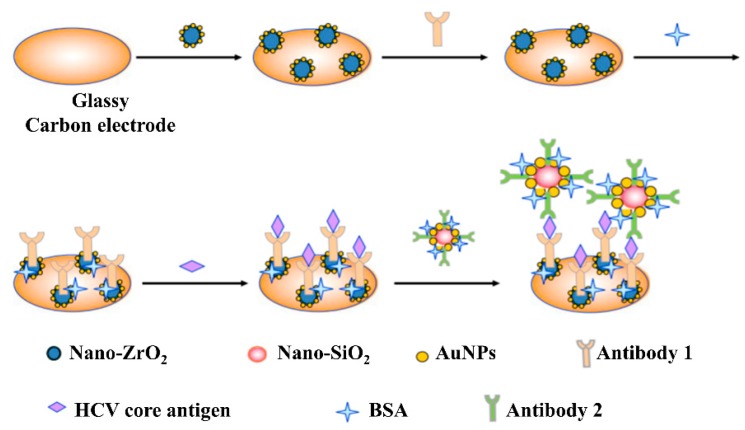
Figure 9 depicts a typical fabrication method of an EI and its application in the detection of an analyte. As shown in Figure 9, the nanocomposites of AuNPs/ZrO2-Chits and AuNPs/SiO2-Chits were used for the detection of the HCV core antigen. The primary HCV core antibodies are immobilized on the AuNPs/ZrO2-Chits nanocomposite modified on glassy carbon electrode (GCE). Whereas, the secondary antibodies are immobilized on the AuNPs/SiO2-Chits nanocomposite. Cyclic voltammetry is used to detect the formation of the sandwich-type complex upon addition of the sample. The EI based assays are known to exhibit high sensitivity, selectivity, and good reproducibility [115].
Figure 9.
Schematic illustration of the electrochemical immunosensor construction process and its application in the detection of HCV core antigen [115].
7.1. Anti-HCV Antibody Detection
Zhao et al. recently reported a portable EI assay for the multiplexed detection of antibodies against the HIV core antigen and HCV core antigen in serum [116]. The EI assay used microfluidic paper-based electrochemical immunosensor for detection of antibodies in eight samples at a time within 20 min. The portable, low-cost, easy to use, and high-throughput are the few advantages of this method.
7.2. Core Antigen Detection
Ma et al. reported an ultrasensitive and selective EI for the detection of the HCV core antigen. For the development of EI, they used graphitized mesoporous carbon–methylene blue (GMCs–MB) nanocomposite as an electrode. The horseradish peroxidase-DNA-coated carboxyl multi-wall carbon nanotubes (CMWNTs) were used as a secondary antibody layer. Under optimum conditions, the EI exhibited a detection limit of 0.01 pg mL−1 with high selectivity [117]. Valipour et al. reported a label-free EI for ultrasensitive detection of HCV core antigen in serum samples. With the help of a modified glassy carbon electrode, the linear detection range of 0.08–110 pg mL−1 with the detection limit of 10 fg mL−1 was achieved [118].
7.3. Detection of Other Antigens
Liang et al. developed a sandwich immunoassay for the detection of HCV NS5a protein using a glassy carbon electrode modified with an au-moo3/chitosan nanocomposite. The assay showed a wide detection range of 1 to 50 µg mL−1 with the detection limit of 1 ng mL−1 [119].
8. Nanotechnology
The remarkable progress in nanoscience and nanotechnology in the last decade has opened a new way of developing assays for the identification of HCV infection with high sensitivity and specificity [120]. The use of nanomaterials and nanoparticles such as gold nanoparticles (GNP), quantum dots (QDs), silver nanoparticles, carbon or silica nanoparticles, and magnetic beads had led the way for the development of highly sensitive immunosensors [121,122,123,124,125]. In this article, only the methods that use GNP or QDs for the detection of HCV were discussed.
8.1. Anti-HCV Antibody Detection
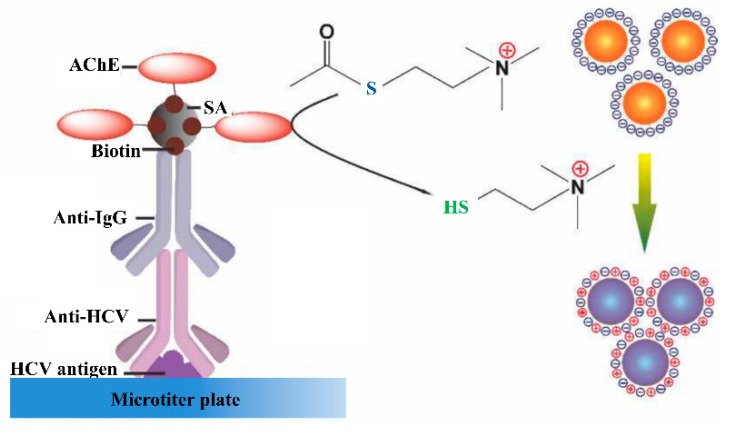
Recently, Cheng et al. developed a technique that allows the enzyme-mediated assembly of GNPs for the colorimetric detection of the anti-HCV antibody, as shown in Figure 10 [126].
Figure 10.
The GNPs in the solution phase can be triggered to form aggregates via an electrostatic interaction by thiocholine generated from AchE catalyzed hydrolysis reaction in the presence of anti-HCV antibody (adopted from Cheng Y, Tang H, Jiang J. Anal. Methods, 2017, 9, 3777–3781).
As shown in Figure 10, the aggregation of GNPs induced by the acetylcholinesterase-catalyzed reaction allows colorimetric detection of the anti-HCV antibody with the detection limit of 10−13 g mL−1 anti-HCV antibody. Duan et al. developed an assay for rapid and simultaneous detection of anti-HBV and anti-HCV antibodies on a protein chip using nano-gold immunological amplification and the silver staining method. The assay used a mixture of NS3, NS5, and HCV core antigens for the detection of anti-HCV antibodies with the detection limit of 3 ng mL−1 [53]. Liu et al. reported the protein array-based detection of HCV using QDs. The anti-HCV antibody in serum was detected by immobilizing the highly purified HCV NS3, NS4, NS5, and core antigens on the surface of encoded beads. A clinical study using this method found that the sensitivity, specificity, and accuracy were 97.5%, 96.0%, and 97.1%, respectively [127].
8.2. Core Antigen Detection
Yin et al. developed an assay for HV core antigen detection based on the GNP probes. In this method, anti-HCVcAg monoclonal antibodies were functionalized on the magnetic microparticles probes were with that recognize and bind HCV core antigen. The GNPs were modified with the polyclonal antibody and the barcode single-stranded DNA (ssDNA). The HCV core antigen in the samples allows the formation of GNP-HCV core antigen-MMP sandwich immuno-complex, which is then separated magnetically. The magnetically separated immuno-complex with the barcode ssDNA is characterized by real-time PCR for the quantification of HCV core antigen. The detection limit of this method was reported to be 1 fg mL−1 [54].
8.3. Detection of Other Antigens
Roh et al. proposed the QDs-supported RNA oligonucleotide method for the detection of HCV NS5B protein using a biochip with high sensitivity and specificity. Their approach allowed to detect the HCV NS5B viral protein in the range of 1 µg mL−1 to 1 ng mL−1 and a limit of detection of 1 ng mL−1 [56,128]. Roh et al. reported a technique that uses a nanoparticle-supported aptamer probe. The target HCV NS3 was detected visually by using the QDs based RNA aptamer with the detection limit of the 5 ng mL−1 level [55].
9. Lateral Flow Assay (LFA)
Lateral flow assay, also known as immuno-chromatographic assays (ICA), is gaining high interest recently because they are low-cost, simple, rapid, and allow the integration with portable detection devices. The LFAs are performed on a membrane strip with various parts including the application pad, conjugate pad, nitrocellulose membrane, and adsorption pad assembled on a plastic backing [129]. In general, the nitrocellulose membrane contains two lines, one each for an analyte and internal control, as shown in Figure 11.
Figure 11.
A typical structure of a lateral flow immunoassay test strip [129].
9.1. Anti-HCV Antibody Detection
Xiang et al. reported a double antibody sandwich-lateral flow immunoassay for the rapid and straightforward detection of HCV. They screened several recombinant proteins, including HCV core antigen, E1, E2, P7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B for the highly specific screening of HCV. In this study, they found that the full-length core and NS3 proteins have the dominant immunodominant epitopes of the HCV genome that were used for the development of LFA [58]. Kosack et al. evaluated the diagnostics accuracy of the ImmunoFlow HCV from Core Diagnostics for the detection of anti-HCV antibodies. The assay demonstrated 100% sensitivity and 100% specificity [130]. There are several reports on the OraQuick HCV rapid antibody test. Lee et Gao et al. reported the overall specificities of OraQuick HCV test to 99.6%–99.9% [131]. Cha et al. also evaluated the OraQuick HCV test for its performance in the detection of HCV and found the clinical sensitivity and specificity of 97.8% and 100%, respectively [132]. The sensitivity and specificity of this method were 94.1% and 99.5%, respectively [133].
9.2. Core Antigen Detection
Wang et al. developed the method for detection of the HCV core antigen by using a highly specific aptamer. The detection limits for their method were found to be 10 pg mL−1 and 100 pg mL−1 by using scanner detection and detection with naked eyes [134].
10. Conclusions
The development of technologies for the identification of HCV infected patients has been a hot topic of research for the last three decades. It is crucial to screen the blood donors for HCV infection to avoid the spread of HCV. Furthermore, it is crucial to screen organ donors in liver transplant surgeries. Beyond this, the screening of HCV in the general population is of paramount importance to control hepatitis associated illness including fibrosis, cirrhosis, and possibly hepatocellular carcinoma, because they are preventable. In many cases, it is crucial to detect HCV infection during the window period. It is evident from the presented articles that research on the HCV detection methods has reduced the window period from six months to 26 days. Therefore, the current study should be focused on the ultrafast HCV screening methods that not only demonstrate high sensitivity and specificity but also they should demonstrate the ability to detect the HCV as early as in less than a week after infection. It is known that the effective use of antiviral medicines can cure more than 95% of HCV infected people. Nonetheless, only screening of HCV is not enough for combating the HCV infection because, for the successful treatment of hepatitis, it is important to know the HCV genotype. Therefore, future efforts should be engaged towards the simplistic yet highly specific and sensitive methods that not only screen for HCV but also allow the HCV genotyping simultaneously.
Author Contributions
Conceptualization, S.D.W.; S.B.N., Methodology, Writing—original draft preparation S.D.W.; D.P.; K.S.-S.; and S.B.N.; Writing—review and editing, S.D.W.; D.P.; S.B.N.; Funding acquisition, S.B.N.
Funding
Hallym University Research Fund (HRF-201901-006) supported this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.WHO Global Hepatitis Report. [(accessed on 14 August 2019)];2017 Available online: https://afro.who.int/sites/default/files/2017-06/9789241565455-eng.pdf.
- 2.Li H.-C., Lo S.-Y. Hepatitis C virus: Virology, diagnosis and treatment. World J. Hepatol. 2015;7:1377–1389. doi: 10.4254/wjh.v7.i10.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravitz L. Introduction: A smouldering public-health crisis. Nature. 2011;474:S2–S4. doi: 10.1038/474S2a. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter H.J., Seeff L.B. Recovery, Persistence, and Sequelae in Hepatitis C Virus Infection: A Perspective on Long-Term Outcome. Semin. Liver Dis. 2000;20:17–36. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 6.Al Olaby R.R., Azzazy H.M. Hepatitis C virus RNA assays: Current and emerging technologies and their clinical applications. Expert Rev. Mol. Diagn. 2011;11:53–64. doi: 10.1586/erm.10.101. [DOI] [PubMed] [Google Scholar]
- 7.Bajpai M., Gupta E., Choudhary A. Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays. Asian J. Transfus. Sci. 2014;8:19–25. doi: 10.4103/0973-6247.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas L., Nguyen M.D., Ke Q. Clinical Monitoring of Chronic Hepatitis C Based on its Natural History and Therapy. Hum. Endocr. Pract. 2013;19:292–300. [Google Scholar]
- 9.Smith B.D., Morgan R.L., A Beckett G., Falck-Ytter Y., Holtzman D., Teo C.-G., Jewett A., Baack B., Rein D.B., Patel N., et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR. Recomm. Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 10.Schnuriger A., Dominguez S., Valantin M.-A., Tubiana R., Duvivier C., Ghosn J., Simon A., Katlama C., Thibault V. Early Detection of Hepatitis C Virus Infection by Use of a New Combined Antigen-Antibody Detection Assay: Potential Use for High-Risk Individuals. J. Clin. Microbiol. 2006;44:1561–1563. doi: 10.1128/JCM.44.4.1561-1563.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter S.S. Laboratory Assays for Diagnosis and Management of Hepatitis C Virus Infection. J. Clin. Microbiol. 2002;40:4407–4412. doi: 10.1128/JCM.40.12.4407-4412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeuzem S., Berg T., Gane E., Ferenci P., Foster G.R., Fried M.W., Hezode C., Hirschfield G.M., Jacobson I., Nikitin I., et al. Simeprevir Increases Rate of Sustained Virologic Response Among Treatment-Experienced Patients with HCV Genotype-1 Infection: A Phase IIb Trial. Gastroenterology. 2014;146:430–441. doi: 10.1053/j.gastro.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 13.Joshi S.N. Hepatitis C Screening. Ochsner J. 2014;14:664–668. [PMC free article] [PubMed] [Google Scholar]
- 14.Backus L.I., Boothroyd D.B., Phillips B.R., Belperio P., Halloran J., Mole L.A. A Sustained Virologic Response Reduces Risk of All-Cause Mortality in Patients with Hepatitis C. Clin. Gastroenterol. Hepatol. 2011;9:509–516.e1. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. WHO Library Cataloguing-in-Publication Data; Geneva, Switzerland: 2014. [PubMed] [Google Scholar]
- 16.Gavin C., Andrew T., Kelly C., Corklin S., John H., George D., Juergen R., Jordan F. Role of Serologic and Molecular Diagnostic Assays in Identification and Management of Hepatitis C Virus Infection. J. Clin. Microbiol. 2016;54:265–273. doi: 10.1128/JCM.02407-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amjad M., Moudgal V., Faisal M. Laboratory Methods for Diagnosis and Management of Hepatitis C Virus Infection. Lab. Med. 2013;44:292–299. doi: 10.1309/LMASROYD8BRS0GC9. [DOI] [Google Scholar]
- 18.Clerc O., Greub G. Routine use of point-of-care tests: Usefulness and application in clinical microbiology. Clin. Microbiol. Infect. 2010;16:1054–1061. doi: 10.1111/j.1469-0691.2010.03281.x. [DOI] [PubMed] [Google Scholar]
- 19.Loggi E., Galli S., Vitale G., Di Donato R., Vukotic R., Grandini E., Margotti M., Guarneri V., Furlini G., Galli C., et al. Monitoring the treatment of hepatitis C with directly acting antivirals by serological and molecular methods. PLoS ONE. 2017;12:e0187755. doi: 10.1371/journal.pone.0187755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Recomm. Rep. 2013;52:1–13. [PubMed] [Google Scholar]
- 21.Khuroo M.S., Khuroo N.S., Khuroo M.S. Diagnostic Accuracy of Point-of-Care Tests for Hepatitis C Virus Infection: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0121450. doi: 10.1371/journal.pone.0121450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freiman J.M., Tran T.M., Schumacher S.G., White L.F., Ongarello S., Cohn J., Philippa J.E., Benjamin P.L., Claudia M.D. HCV Core Antigen Testing for Diagnosis of HCV Infection: A systematic review and meta-analysis. Ann. Intern. Med. 2016;165:345–355. doi: 10.7326/M16-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penin F., Dubuisson J., Rey F.A., Moradpour D., Pawlotsky J.M. Structural biology of hepatitis C virus. Hepatology. 2004;39:5–19. doi: 10.1002/hep.20032. [DOI] [PubMed] [Google Scholar]
- 24.Dubuisson J. Hepatitis C virus proteins. World J. Gastroenterol. 2007;13:2406–2415. doi: 10.3748/wjg.v13.i17.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed K.E., Rice C.M. Overview of Hepatitis C Virus Genome Structure, Polyprotein Processing, and Protein Properties. Curr. Top. Microbiol. Immunol. 2000;242:55–84. doi: 10.1007/978-3-642-59605-6_4. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen S.U., Bassendine M.F., Burt A.D., Bevitt D.J., Toms G.L. Characterization of the genome and structural proteins of hepatitis C virus resolved from infected human liver. J. Gen. Virol. 2004;85:1497–1507. doi: 10.1099/vir.0.79967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Awady M.K., El Abd Y.S., Shoeb H.A., Tabll A.A., Hosny A.E.D.M.S., El Shenawy R.M., Atef K., El Din N.G.B., Bahgat M.M. Circulating viral core and E1 antigen levels as supplemental markers for HCV Chronic hepatitis. Virol. J. 2006;3:67. doi: 10.1186/1743-422X-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delphine D., Parissa V., Jeanine S., Elisabeth D., Anne-Marie R.A. Sensitivity of a rapid immuno-chromatographic test for Hepatitis C antibodies detection. J. Clin. Virol. 2008;41:129–133. doi: 10.1016/j.jcv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Park J.H., Jee M.H., Kwon O.S., Keum S.J., Jang S.K. Infectivity of hepatitis C virus correlates with the amount of envelope protein E2: Development of a new aptamer-based assay system suitable for measuring the infectious titer of HCV. Virology. 2013;439:13–22. doi: 10.1016/j.virol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Zaaijer H.L., Vallari D.S., Cunningham M., Lesniewski R., Reesink H.W., Der Van Poel C.L., Lelie P.N. E2 and NS5: New antigens for detection of hepatitis C virus antibodies. J. Med. Virol. 1994;44:395–397. doi: 10.1002/jmv.1890440415. [DOI] [PubMed] [Google Scholar]
- 31.Xie L., Wu X.-D., Huang D.-Z., Chen H.-L., He L.-X., Wang J., Han D.-K. Clinical application and analysis of hepatitis C virus NS3 antigen detection by ELISA in human serum. Chin. Med. J. 2007;120:294–299. doi: 10.1097/00029330-200702020-00008. [DOI] [PubMed] [Google Scholar]
- 32.Barth S., Blohm L., Holz S., Piechotta G., Dammers C., Kleines M., Melmer G., Nebling E., Puttman C., Albers J., et al. Rapid detection of different human anti-HCV immunoglobulins on electrical biochips. Antib. Technol. J. 2014;4:23. doi: 10.2147/ANTI.S54763. [DOI] [Google Scholar]
- 33.Chaudhary R., Burres E. Detection of Antibody to Envelope (E2) Antigen of Hepatitis C Virus. Can. J. Infect. Dis. 1997;8:229–231. doi: 10.1155/1997/386268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santolini E., Migliaccio G., La Monica N. Biosynthesis and biochemical properties of the hepatitis C virus core protein. J. Virol. 1994;68:3631–3641. doi: 10.1128/jvi.68.6.3631-3641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muerhoff A.S., Jiang L., Shah D.O., Gutierrez R.A., Patel J., Garolis C., Kyrk C.R., Leckie G., Frank A., Stewart J.L., et al. Detection of HCV core antigen in human serum and plasma with an automated chemiluminescent immunoassay. Transfusion. 2002;42:349–356. doi: 10.1046/j.1537-2995.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 36.Shah D.O., Chang C.D., Jiang L.X., Cheng K.Y., Muerhoff A.S., Gutierrez R.A., Leary T.P., Desai S.M., Batac-Herman I.V., Salbilla V.A., et al. Combination HCV core antigen and antibody assay on a fully automated chemiluminescence analyzer. Transfusion. 2003;43:1067–1074. doi: 10.1046/j.1537-2995.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 37.Piccoli P.L., Franchini M., Gandini G., Antonello O., Olzer D., Turrini A., Aprili G. HCV core antigen assay. Transfusion. 2001;41:1172. doi: 10.1046/j.1537-2995.2001.41091172.x. [DOI] [PubMed] [Google Scholar]
- 38.Laperche S., Elghouzzi M.-H., Morel P., Le Marrec N., Girault A., Bouchardeau F., Deschaseaux M., Piquet Y., Asso-Bonnet M., Servant-Delmas A., et al. Is an assay for simultaneous detection of hepatitis C virus core antigen and antibody a valuable alternative to nucleic acid testing? Transfusion. 2005;45:1965–1972. doi: 10.1111/j.1537-2995.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 39.Dubuisson J., Penin F., Moradpour D. Interaction of hepatitis C virus proteins with host cell membranes and lipids. Trends Cell Boil. 2002;12:517–523. doi: 10.1016/S0962-8924(02)02383-8. [DOI] [PubMed] [Google Scholar]
- 40.Deleersnyder V., Pillez A., Wychowski C., Blight K., Xu J., Hahn Y.S., Rice C.M., Dubuisson J. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 1997;71:697–704. doi: 10.1128/jvi.71.1.697-704.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin C., Lindenbach B.D., Prágai B.M., McCourt D.W., Rice C.M. Processing in the hepatitis C virus E2-NS2 region: Identification of p7 and two distinct E2-specific products with different C termini. J. Virol. 1994;68:5063–5073. doi: 10.1128/jvi.68.8.5063-5073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blight K.J., Kolykhalov A.A., Rice C.M. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 43.Lohmann V., Körner F., Koch J., Herian U., Theilmann L., Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 44.Lorenz I.C., Marcotrigiano J., Dentzer T.G., Rice C.M. Structure of the catalytic domain of the hepatitis C virus NS2-3 protease. Nature. 2006;442:831–835. doi: 10.1038/nature04975. [DOI] [PubMed] [Google Scholar]
- 45.Sansonno D.E., Dammacco F., Rice C.M., Blum H.E., Moradpour D., Wölk B., Kräusslich H.-G. Subcellular Localization, Stability, and trans-Cleavage Competence of the Hepatitis C Virus NS3-NS4A Complex Expressed in Tetracycline-Regulated Cell Lines. J. Virol. 2000;74:2293–2304. doi: 10.1128/jvi.74.5.2293-2304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J., Morgenstern K., Lin C., Fox T., Dwyer M., Landro J., Chambers S., Markland W., Lepre C., O’Malley E., et al. Crystal Structure of the Hepatitis C Virus NS3 Protease Domain Complexed with a Synthetic NS4A Cofactor Peptide. Cell. 1996;87:343–355. doi: 10.1016/S0092-8674(00)81351-3. [DOI] [PubMed] [Google Scholar]
- 47.Sillanpää M., Melén K., Porkka P., Fagerlund R., Nevalainen K., Lappalainen M., Julkunen I. Hepatitis C virus core, NS3, NS4B and NS5A are the major immunogenic proteins in humoral immunity in chronic HCV infection. Virol. J. 2009;6:84. doi: 10.1186/1743-422X-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dufour D.R., Talastas M., Fernandez M.D., Harris B., Strader D.B., Seeff L.B. Low-positive anti-hepatitis C virus enzyme immunoassay results: An important predictor of low likelihood of hepatitis C infection. Clin. Chem. 2003;49:479–486. doi: 10.1373/49.3.479. [DOI] [PubMed] [Google Scholar]
- 49.Saludes V., González V., Planas R., Matas L., Ausina V., Martró E. Tools for the diagnosis of hepatitis C virus infection and hepatic fibrosis staging. World J. Gastroenterol. 2014;20:3431–3442. doi: 10.3748/wjg.v20.i13.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morota K., Fujinami R., Kinukawa H., Machida T., Ohno K., Saegusa H., Takeda K. A new sensitive and automated chemiluminescent microparticle immunoassay for quantitative determination of hepatitis C virus core antigen. J. Virol. Methods. 2009;157:8–14. doi: 10.1016/j.jviromet.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Fondjo C.L.K., Ngoupo P.A.T., Ngono L., Plantier J.C., Njouom R. Performace evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus in Cameroon. BMC Res. Notes. 2018;11(1):352. doi: 10.1186/s13104-018-3465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin P., Fabrizi F., Dixit V., Quan S., Brezina M., Kaufman E., Dinello R., Polito A., Gitnick G. Automated RIBA Hepatitis C Virus (HCV) Strip Immunoblot Assay for Reproducible HCV Diagnosis. J. Clin. Microbiol. 1998;36:387–390. doi: 10.1128/jcm.36.2.387-390.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan L., Wang Y., Li S.S., Wan Z., Zhai J. Rapid and simultaneous detection of human hepatitis B virus and hepatitis C virus antibodies based on a protein chip assay using nano-gold immunological amplifi cation and silver staining method. BMC Infect. Dis. 2005;5:53. doi: 10.1186/1471-2334-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin H.-Q., Ji C.-F., Yang X.-Q., Wang R., Yang S., Zhang H.-Q., Zhang J.-G. An improved gold nanoparticle probe-based assay for HCV core antigen ultrasensitive detection. J. Virol. Methods. 2017;243:142–145. doi: 10.1016/j.jviromet.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Changhyun R., Ho-Young L., Sang-Eun K., Sung-Kee J. Quantum-dots-based detection of hepatitis C virus (HCV) NS3 using RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2010;85:1130–1134. [Google Scholar]
- 56.Roh C., Lee H.Y., Kim S.E., Jo S.K. A highly sensitive and selective viral protein detection method based on RNA oligonucleotide nanoparticle. Int. J. Nanomed. 2010;5:323. doi: 10.2147/IJN.S10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mikawa A.Y., Santos S.A.T., Kenfe F.R., Da Silva F.H., Da Costa P.I. Development of a rapid one-step immunochromatographic assay for HCV core antigen detection. J. Virol. Methods. 2009;158:160–164. doi: 10.1016/j.jviromet.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Xiang T., Jiang Z., Zheng J., Lo C., Tsou H., Ren G., Zhang J., Huang A., Lai G. A novel double antibody sandwich-lateral flow immunoassay for the rapid and simple detection of hepatitis C virus. Int. J. Mol. Med. 2012;30:1041–1047. doi: 10.3892/ijmm.2012.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S., Kim Y.S., Jo M., Jin M., Lee D.-K., Kim S. Chip-based detection of hepatitis C virus using RNA aptamers that specifically bind to HCV core antigen. Biochem. Biophys. Res. Commun. 2007;358:47–52. doi: 10.1016/j.bbrc.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 60.Pleshakova T.O., Kaysheva A.L., Shumov I.D., Ziborov V.S., Bayzyanova J.M., Konev V.A., Uchaikin V.F., Archakov A.I., Ivanov Y.D. Detection of Hepatitis C Virus Core Protein in Serum Using Aptamer-Functionalized AFM Chips. Micromachines. 2019;10:129. doi: 10.3390/mi10020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hügle T., Fehrmann F., Bieck E., Kohara M., Kräusslich H.-G., Rice C.M., Blum H.E., Moradpour D. The Hepatitis C Virus Nonstructural Protein 4B Is an Integral Endoplasmic Reticulum Membrane Protein. Virology. 2001;284:70–81. doi: 10.1006/viro.2001.0873. [DOI] [PubMed] [Google Scholar]
- 62.Lundin M., Monné M., Widell A., Von Heijne G., Persson M.A.A. Topology of the Membrane-Associated Hepatitis C Virus Protein NS4B. J. Virol. 2003;77:5428–5438. doi: 10.1128/JVI.77.9.5428-5438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brass V., Bieck E., Montserret R., Wölk B., Hellings J.A., Blum H.E., Penin F., Moradpour D. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 2002;277:8130–8139. doi: 10.1074/jbc.M111289200. [DOI] [PubMed] [Google Scholar]
- 64.Penin F., Ramboarina S., Montserret R., Ficheux D., Moradpour D., Brass V., Appel N., Blum H.E., Bartenschlager R. Structure and Function of the Membrane Anchor Domain of Hepatitis C Virus Nonstructural Protein 5A. J. Boil. Chem. 2004;279:40835–40843. doi: 10.1074/jbc.M404761200. [DOI] [PubMed] [Google Scholar]
- 65.Ivashkina N., Wölk B., Lohmann V., Bartenschlager R., Blum H.E., Penin F., Moradpour D. The Hepatitis C Virus RNA-Dependent RNA Polymerase Membrane Insertion Sequence Is a Transmembrane Segment. J. Virol. 2002;76:13088–13093. doi: 10.1128/JVI.76.24.13088-13093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moradpour D., Brass V., Bieck E., Friebe P., Gosert R., Blum H.E., Bartenschlager R., Penin F., Lohmann V. Membrane Association of the RNA-Dependent RNA Polymerase Is Essential for Hepatitis C Virus RNA Replication†. J. Virol. 2004;78:13278–13284. doi: 10.1128/JVI.78.23.13278-13284.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt-Mende J., Bieck E., Hügle T., Penin F., Rice C.M., Blum H.E., Moradpour D. Determinants for Membrane Association of the Hepatitis C Virus RNA-dependent RNA Polymerase. J. Boil. Chem. 2001;276:44052–44063. doi: 10.1074/jbc.M103358200. [DOI] [PubMed] [Google Scholar]
- 68.Barrera J.M., Franscis B., Ercilla G., Nelles M., Archod D., Darner J., Lee S.R. Improved detection of Anti-HCV in Post transfuson Hepatitis by third generation ELISA. Vox Sang. 1995;68:15–18. doi: 10.1111/j.1423-0410.1995.tb02538.x. [DOI] [PubMed] [Google Scholar]
- 69.Morishima C., Gretch D.R. Clinical use of Hepatitis C virus test for the diagnosis and monitoring during therapy. Clin. Liver Dis. 1999;3:717–740. doi: 10.1016/S1089-3261(05)70235-X. [DOI] [PubMed] [Google Scholar]
- 70.Lanoir D., Touzet S., Meyaud-Kraemer L., Trepo C., Colin C., Bailly F. The HEPATIS Group. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: An analysis of the literature. J. Viral Hepat. 2001;8:87–95. doi: 10.1046/j.1365-2893.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 71.Hamid M.S., Mitra R., Alizadeh A.H.M. Recommendations for Prevention and Control of Hepatitis C Virus (HCV) Infection and HCV-Related Chronic Disease. J. Liver. 2013;3:147. [Google Scholar]
- 72.Alter H.J. New kit on the block: Evaluation of second-generation assays for detection of antibody to the hepatitis C virus. Hepatology. 1992;15:350–353. doi: 10.1002/hep.1840150228. [DOI] [PubMed] [Google Scholar]
- 73.Araujo A.C., Hayden T.M., Kamili S., Drobeniuc J. Laboratory Diagnostics for Hepatitis C Virus Infection. Clin. Infect. Dis. 2012;55:S43–S48. doi: 10.1093/cid/cis368. [DOI] [PubMed] [Google Scholar]
- 74.Chen S.L. The Natural History of Hepatitis C Virus (HCV) Infection. Int. J. Med. Sci. 2006;3:47. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffin J.F.T., Spittle E., Rodgers C.R., Liggett S., Cooper M., Bakker D., Bannantine J.P. Immunoglobulin G1 Enzyme-Linked Immunosorbent Assay for Diagnosis of Johne’s Disease in Red Deer (Cervus elaphus) Clin. Diagn. Lab. Immunol. 2005;12:1401–1409. doi: 10.1128/CDLI.12.12.1401-1409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swellam M., Mahmoud M.S., Ali A.A.-F. Diagnosis of hepatitis C virus infection by enzyme-linked immunosorbent assay and reverse transcriptase-nested polymerase chain reaction: A comparative evaluation. IUBMB Life. 2011;63:430–434. doi: 10.1002/iub.469. [DOI] [PubMed] [Google Scholar]
- 77.Porcelli B., Ferretti F., Vindigni C., Terzuoli L. Assessment of a Test for the Screening and Diagnosis of Celiac Disease. J. Clin. Lab. Anal. 2014;30:65–70. doi: 10.1002/jcla.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cano H., Candela M.J., Lozano M.L., Vicente V. Application of a new enzyme-linked immunosorbent assay for detection of total hepatitis C virus core antigen in blood donors. Transfus. Med. 2003;13:259–266. doi: 10.1046/j.1365-3148.2003.00452.x. [DOI] [PubMed] [Google Scholar]
- 79.Lopes E., Granato C., Lanzoni V., Granero L., Paranhos-Baccalà G., Tomiyama H., Silva A., Ferraz M. Evaluation of an enzyme immunoassay for hepatitis C virus antibody detection using a recombinant protein derived from the core region of hepatitis C virus genome. Memórias do Instituto Oswaldo Cruz. 2000;95:717–720. doi: 10.1590/S0074-02762000000500019. [DOI] [PubMed] [Google Scholar]
- 80.Laperche S., Le Marrec N., Simon N., Bouchardeau F., Defer C., Maniez-Montreuil M., Levayer T., Zappitelli J.-P., Lefrère J.-J., Maniez-Montreuil M., et al. A new HCV core antigen assay based on disassociation of immune complexes: An alternative to molecular biology in the diagnosis of early HCV infection. Transfusion. 2003;43:958–962. doi: 10.1046/j.1537-2995.2003.00430.x. [DOI] [PubMed] [Google Scholar]
- 81.Icardi G., Ansaldi F., Bruzzone B.M., Durando P., Lee S., De Luigi C., Crovari P. Novel Approach to Reduce the Hepatitis C Virus (HCV) Window Period: Clinical Evaluation of a New Enzyme-Linked Immunosorbent Assay for HCV Core Antigen. J. Clin. Microbiol. 2001;39:3110–3114. doi: 10.1128/JCM.39.9.3110-3114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massaguer A., Forns X., Costa J., Feliu A., García-Retortillo M., Navasa M., Rimola A., García-Valdecasas J.C., Sánchez-Tapias J.M. Performance of Hepatitis C Virus Core Antigen Immunoassay in Monitoring Viral Load after Liver Transplantation. Transplantation. 2005;79:1441–1444. doi: 10.1097/01.TP.0000158356.81437.FA. [DOI] [PubMed] [Google Scholar]
- 83.Alzahrani A.J., Obeid O.E. Detection of hepatitis c virus core antigen in blood donors using a new enzyme immunoassay. J. Fam. Community Med. 2004;11:103–107. [PMC free article] [PubMed] [Google Scholar]
- 84.Garbuglia A.R., Monachetti A., Galli C., Sabatini R., Ferreri M.L., Capobianchi M.R., Bagnarelli P. HCV core antigen and HCV-RNA in HIV/HCV co-infected patients with different HCV genotypes. BMC Infect. Dis. 2014;14:222. doi: 10.1186/1471-2334-14-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuo Y.H., Chang K.C., Wang J.H., Tsai P.S., Hung S.F., Hung C.H., Chen C.H., Lu S.N. Is hepatitis C virus core antigen an adequate marker forcommunity screening? J. Clin. Microbiol. 2012;50:1989–1993. doi: 10.1128/JCM.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ottiger C., Gygli N., Huber A.R. Detection limit of architect hep-atitis C core antigen assay in correlation with HCV RNA, andrenewed confirmation algorithm for reactive anti-HCV samples. J. Clin. Virol. 2013;58:535–540. doi: 10.1016/j.jcv.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 87.Cui W., Hu K.-Q., Hu K. A highly specific and sensitive hepatitis C virus antigen enzyme immunoassay for One-step diagnosis of viremic hepatitis C virus infection. Hepatology. 2016;64:415–424. doi: 10.1002/hep.28663. [DOI] [PubMed] [Google Scholar]
- 88.Hu K.-Q., Cui W., Rouster S.D., Sherman K.E. Hepatitis C virus antigens enzyme immunoassay for one-step diagnosis of hepatitis C virus coinfection in human immunodeficiency virus infected individuals. World J. Hepatol. 2019;11:442–449. doi: 10.4254/wjh.v11.i5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dufour D.R., Talastas M., Fernandez M.D., Harris B. Chemiluminescence Assay Improves Specificity of Hepatitis C Antibody Detection. Clin. Chem. 2003;49:940–944. doi: 10.1373/49.6.940. [DOI] [PubMed] [Google Scholar]
- 90.Cinquanta L., Fontana D.E., Bizzaro N. Chemiluminescent immunoassay technology: What does it change in autoantibody detection? Auto Immun. Highlights. 2017;8:9. doi: 10.1007/s13317-017-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ismail N., Fish G.E., Smith M.B. Laboratory Evaluation of a Fully Automated Chemiluminescence Immunoassay for Rapid Detection of HBsAg, Antibodies to HBsAg, and Antibodies to Hepatitis C Virus. J. Clin. Microbiol. 2004;42:610–617. doi: 10.1128/JCM.42.2.610-617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sinyoung K., Jeong-Ho K., Seoyoung Y., Youn-Hee P., Hyon-Suk K. Clinical Performance Evaluation of Four Automated Chemiluminescence Immunoassays for Hepatitis C Virus Antibody Detection. J. Clin. Microbiol. 2008;46:3919–3923. doi: 10.1128/JCM.01603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang W., Chen W., Amini A., Boeras D., Falconer J., Kelly H., Peeling R., Varsaneux O., Tucker J.D., Easterbrook P. Diagnostic accuracy of tests to detect Hepatitis C antibody: A meta-analysis and review of the literature. BMC Infect. Dis. 2017;17:695. doi: 10.1186/s12879-017-2773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng S., Wei B., Liu Q., Wang T., Li D., Rao C., Tao C., Wang L. Evaluation of the Novel HISCL Chemiluminescence Enzyme Immunoassay for Laboratory Screening of Hepatitis C Virus. Clin. Vaccine Immunol. 2016;23:652–654. doi: 10.1128/CVI.00078-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rockstroh J.K., Feld J.J., Chevaliez S., Cheng K., Wedemeyer H., Sarrazin C., Maasoumy B., Herman C., Hackett J., Cohen D.E., et al. HCV core antigen as an alternate test to HCV RNA for assessment of virologic responses to all-oral, interferon-free treatment in HCV genotype 1 infected patients. J. Virol. Methods. 2017;245:14–18. doi: 10.1016/j.jviromet.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Liu J., Zhang L., Fu C., Wang Y., Sun S. Employment of 4-(1,2,4-triazol-1-yl)phenol as a signal enhancer of the chemiluminescent luminol-H2O2-horseradish peroxidase reaction for detection of hepatitis C virus in real samples. Luminescence. 2015;30:1297–1302. doi: 10.1002/bio.2896. [DOI] [PubMed] [Google Scholar]
- 97.Hawkins R.C. Laboratory Turnaround Time. Clin. Biochem. Rev. 2007;28:179–194. [PMC free article] [PubMed] [Google Scholar]
- 98.Smith B.D., Jewett A., Drobeniuc J., Kamili S. Rapid diagnostic HCV antibody assays. Antivir. Ther. 2012;17:1409–1413. doi: 10.3851/IMP2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vercauteren G. Hepatitis C Assays: Operational Characteristics (Phase i) Blood Safety and Clinical Technology, World Health Organization; Geneva, Switzerland: 2001. Report 1, 2001, WHO/BCT/BTS/01.2. [Google Scholar]
- 100.Chevaliez S., Poiteau L., Rosa I., Soulier A., Roudot-Thoraval F., Laperche S., Hezode C., Pawlotsky J.-M. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin. Microbiol. Infect. 2016;22:459.e1–459.e6. doi: 10.1016/j.cmi.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 101.Parker S.P., Khan H.I., Cubitt W.D. Detection of Antibodies to Hepatitis C Virus in Dried Blood Spot Samples from Mothers and Their Offspring in Lahore, Pakistan. J. Clin. Microbiol. 1999;37:2061–2063. doi: 10.1128/jcm.37.6.2061-2063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Daniel H.D.J., Abraham P., Raghuraman S., Vivekanandan P., Subramaniam T., Sridharan G. Evaluation of a Rapid Assay as an Alternative to Conventional Enzyme Immunoassays for Detection of Hepatitis C Virus-Specific Antibodies. J. Clin. Microbiol. 2005;43:1977–1978. doi: 10.1128/JCM.43.4.1977-1978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Firdaus R., Saha K., Sadhukhan P.C. Rapid immunoassay alone is insufficient for the detection of hepatitis C virus infection among high-risk population. J. Viral. Hepat. 2013;20:290–293. doi: 10.1111/jvh.12002. [DOI] [PubMed] [Google Scholar]
- 104.Skidmore S. Recombinant immunoblot assay for hepatitis C antibody. Lancet. 1990;335:1346. doi: 10.1016/0140-6736(90)91230-8. [DOI] [PubMed] [Google Scholar]
- 105.Van Der Poel C., Reesink H., Van Boven J., Cuypers H., Winkel I., Exel-Oehlers P., Schaasberg W., Lelie P., Weiner A., Polito A., et al. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet. 1991;337:317–319. doi: 10.1016/0140-6736(91)90942-I. [DOI] [PubMed] [Google Scholar]
- 106.Contreras A.M., Toribio J.G., Celis A., Rivera P.K., Méndez C., Olivares L., Alvarado M.A., Tornero-Romo C.M., Orozco-Hernández A., Hernández-Lugo M.I., et al. Very low hepatitis C antibody levels predict false-positive results and avoid supplemental testing. Transfusion. 2008;48:2540–2548. doi: 10.1111/j.1537-2995.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- 107.Feucht H.H., Zöllner B., Polywka S., Laufs R. Study on Reliability of Commercially Available Hepatitis C Virus Antibody Tests. J. Clin. Microbiol. 1995;33:620–624. doi: 10.1128/jcm.33.3.620-624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rafik M., Bakr S., Soliman D., Mohammed N., Ragab D., Elhady W.A., Samir N. Characterization of differential antibody production against hepatitis C virus in different HCV infection status. Virol. J. 2016;13:116. doi: 10.1186/s12985-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pereira F.M., Zarife M.A.S., Reis E.A.G., Reis M.G. Indeterminate RIBA results were associated with the absence of hepatitis C virus RNA (HCV-RNA) in blood donors. Rev. Soc. Bras. Med. Trop. 2014;47:12–17. doi: 10.1590/0037-8682-0222-2013. [DOI] [PubMed] [Google Scholar]
- 110.Moorman A.C., Drobenuic J., Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. J. Clin. Virol. 2017;89:1–4. doi: 10.1016/j.jcv.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang D., Yuan R., Chai Y. Ultrasensitive Electrochemical Immunosensor for Clinical Immunoassay Using Thionine-Doped Magnetic Gold Nanospheres as Labels and Horseradish Peroxidase as Enhancer. Anal. Chem. 2008;80:1582–1588. doi: 10.1021/ac702217m. [DOI] [PubMed] [Google Scholar]
- 112.Mani V., Chikkaveeraiah B.V., Patel V., Gutkind J.S., Rusling J.F. Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano. 2009;3:585–594. doi: 10.1021/nn800863w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu L., Liu B., Li S., Zhang W., Xie G. Improved electrochemical immunosensor for myeloperoxidase in human serum based on nanogold/cerium dioxide-BMIMPF6/l-Cysteine composite film. Colloids Surfaces B Biointerfaces. 2011;86:339–344. doi: 10.1016/j.colsurfb.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 114.Moraes M.L., Lima L.R., Silva R.R., Cavicchioli M., Ribeiro S.J.L. Immunosensor Based on Immobilization of Antigenic Peptide NS5A-1 from HCV and Silk Fibroin in Nanostructured Films. Langmuir. 2013;29:3829–3834. doi: 10.1021/la304404v. [DOI] [PubMed] [Google Scholar]
- 115.Ma C., Xie G., Zhang W., Liang M., Liu B., Xiang H. Label-free sandwich type of immunosensor for hepatitis C viruscore antigen based on the use of gold nanoparticles on a nanostructured metal oxide surface. Microchim. Acta. 2012;178:331–340. doi: 10.1007/s00604-012-0842-1. [DOI] [Google Scholar]
- 116.Zhao C., Liu X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Biomicrofluidics. 2016;10:024119. doi: 10.1063/1.4945311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma C., Liang M., Wang L., Xiang H., Jiang Y., Li Y., Xie G. MultisHRP-DNA-coated CMWNTs as signal labels for an ultrasensitive hepatitis C virus core antigen electrochemical immunosensor. Biosens. Bioelectron. 2013;47:467–474. doi: 10.1016/j.bios.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 118.Valipour A., Roushani M. Using Boehmite Nanoparticles as an Undercoat, and Riboflavin as a Redox Probe for Immunosensor Designing: Ultrasensitive Detection of Hepatitis C Virus Core Antigen. Anal. Bioanal. Chem. Res. 2018;5:353–361. [Google Scholar]
- 119.Liang M., Wang L., Ma C., Zhang M., Xie G. Sandwich Immunoassay for Hepatitis C Virus Non-Structural 5A Protein Using a Glassy Carbon Electrode Modified with an Au-MoO 3 /Chitosan Nanocomposite. Anal. Lett. 2013;46:1241–1254. doi: 10.1080/00032719.2012.755684. [DOI] [Google Scholar]
- 120.Abraham A.M., Kannangai R., Sridharan G. Nanotechnology: A new frontier in virus detection in clinical practice. Indian J. Med Microbiol. 2008;26:297. [PubMed] [Google Scholar]
- 121.Suni I.I. Impedance methods for electrochemical sensors using nanomaterials. TrAC Trends Anal. Chem. 2008;27:604–611. doi: 10.1016/j.trac.2008.03.012. [DOI] [Google Scholar]
- 122.Wang J., Lin Y. Functionalized carbon nanotubes and nanofibers for biosensing applications. TrAC Trends Anal. Chem. 2008;27:619–626. doi: 10.1016/j.trac.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cui R., Huang H., Yin Z., Gao D., Zhu J.-J. Horseradish peroxidase-functionalized gold nanoparticle label for amplified immunoanalysis based on gold nanoparticles/carbon nanotubes hybrids modified biosensor. Biosens. Bioelectron. 2008;23:1666–1673. doi: 10.1016/j.bios.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 124.Jie G., Zhang J., Wang D., Cheng C., Chen H.-Y., Zhu J.-J. Electrochemiluminescence Immunosensor Based on CdSe Nanocomposites. Anal. Chem. 2008;80:4033–4039. doi: 10.1021/ac800052g. [DOI] [PubMed] [Google Scholar]
- 125.Chen X., Zhang K., Zhou J., Xuan J., Yan W., Jiang L.-P., Zhu J.-J. Electrochemical immunosensor based on colloidal carbon sphere array. Biosens. Bioelectron. 2010;25:1130–1136. doi: 10.1016/j.bios.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 126.Cheng Y.-H., Tang H., Jiang J.-H. Enzyme mediated assembly of gold nanoparticles for ultrasensitive colorimetric detection of hepatitis C virus antibody. Anal. Methods. 2017;9:3777–3781. doi: 10.1039/C7AY01086A. [DOI] [Google Scholar]
- 127.Liu J., Zhang G.-X. [A protein array based on quantum dots (QDs) encoded microbeads for detection of hepatitis C virus] Zhonghua shi yan he lin chuang bing du xue za zhi Zhonghua shiyan he linchuang bingduxue zazhi Chin. J. Exp. Clin. Virol. 2013;27:67–69. [PubMed] [Google Scholar]
- 128.Roh C. A Facile Inhibitor Screening of Hepatitis C Virus NS3 Protein Using Nanoparticle-Based RNA. Biosensors. 2012;2:427–432. doi: 10.3390/bios2040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koczula K.M., Gallotta A. Lateral flow assays. Essays Biochem. 2016;30:111–120. doi: 10.1042/EBC20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kosack C.S., Nick S., Shanks L. Diagnostic accuracy evaluation of the ImmunoFlow HCV rapid immunochromatographic test for the detection of hepatitis C antibodies. J. Virol. Methods. 2014;204:6–10. doi: 10.1016/j.jviromet.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 131.Lee S.R., Kardos K.W., Schiff E., Berne C.A., Mounzer K., Banks A.T., Tatum H.A., Friel T.J., Demicco M.P., Lee W.M., et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J. Virol. Methods. 2011;172:27–31. doi: 10.1016/j.jviromet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 132.Cha Y.J., Park Q., Kang E.-S., Yoo B.C., Park K.U., Kim J.-W., Hwang Y.-S., Kim M.H. Performance Evaluation of the OraQuick Hepatitis C Virus Rapid Antibody Test. Ann. Lab. Med. 2013;33:184–189. doi: 10.3343/alm.2013.33.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gao F., Talbot E.A., Loring C.H., Power J.J., Dionne-Odom J., Alroy-Preis S., Jackson P., Bean C.L. Performance of the OraQuick HCV Rapid Antibody Test for Screening Exposed Patients in a Hepatitis C Outbreak Investigation. J. Clin. Microbiol. 2014;52:2650–2652. doi: 10.1128/JCM.00132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang C., Zhang L., Shen X. Development of a Nucleic Acid Lateral Flow Strip for Detection of Hepatitis C Virus (Hcv) Core Antigen. Nucleotides Nucleic Acids. 2013;32:59–68. doi: 10.1080/15257770.2013.763976. [DOI] [PubMed] [Google Scholar]