Abstract
Aims
Coronary artery calcium score (CACS) is a strong predictor of major adverse cardiac events (MACE). Conversely, statins, which markedly reduce MACE risk, increase CACS. We explored whether CACS progression represents compositional plaque volume (PV) progression differently according to statin use.
Methods and results
From a prospective multinational registry of consecutive patients (n = 2252) who underwent serial coronary computed tomography angiography (CCTA) at a ≥ 2-year interval, 654 patients (61 ± 10 years, 56% men, inter-scan interval 3.9 ± 1.5 years) with information regarding the use of statins and having a serial CACS were included. Patients were divided into non-statin (n = 246) and statin-taking (n = 408) groups. Coronary PVs (total, calcified, and non-calcified; sum of fibrous, fibro-fatty, and lipid-rich) were quantitatively analysed, and CACS was measured from both CCTAs. Multivariate linear regression models were constructed for both statin-taking and non-statin group to assess the association between compositional PV change and change in CACS. In multivariate linear regression analysis, in the non-statin group, CACS increase was positively associated with both non-calcified (β = 0.369, P = 0.004) and calcified PV increase (β = 1.579, P < 0.001). However, in the statin-taking group, CACS increase was positively associated with calcified PV change (β = 0.756, P < 0.001) but was negatively associated with non-calcified PV change (β = −0.194, P = 0.026).
Conclusion
In the non-statin group, CACS progression indicates the progression of both non-calcified and calcified PV progression. However, under the effect of statins, CACS progression indicates only calcified PV progression, but not non-calcified PV progression. Thus, the result of serial CACS should be differently interpreted according to the use of statins.
Keywords: coronary artery atherosclerosis, statins, coronary computed tomography angiography, coronary artery calcium score, coronary artery calcification, Agatston score
Introduction
Coronary artery calcification (CAC) is one of the strongest predictors of major adverse cardiac events (MACE).1 Consequently, it has been hypothesized that a rapid increase in CAC indicates increased MACE risk, and attempts have been made to implement the monitoring of coronary artery calcium score (CACS) progression into a risk stratification tool to improve the identification of patients at higher risk.2–6 However, these attempts have yielded conflicting results, especially in patients who use statins.2–5
Statins, which markedly reduce MACE as proven in previous randomized clinical trials,7,8 are also able to alter coronary plaque characteristics.9,10 Importantly, emerging evidence suggests that statins induce the calcification of coronary artery plaques and, therefore, increase CAC.10,11 Thus, increase in CAC may not indicate an increased total burden of coronary artery disease (CAD) in patients treated with statins.12
To explain this discrepancy between CAC progression and statins, a direct comparison of the changes in CACS with changes in total coronary atherosclerotic burden, as assessed by plaque volume (PV), is required. However, the association between CACS progression and quantitative, compositional PV changes has only recently been evaluated,13 and the independent impact of statins on this association has not been directly explored. This is mainly because most CAC scan studies have been conducted in low-risk screening populations, whereas studies that analysed PV changes with respect to statin use employ invasive imaging techniques, allowing a focus on high-risk patients who have not undergone CAC scans.
Therefore, we explored whether the association between CAC progression and compositional PV progression differed between non-statin and statin-taking individuals, in a subset of patients who underwent serial coronary computed tomography angiography (CCTA).
Methods
Study design and population
The Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging (PARADIGM) study is a dynamic multinational observational registry that prospectively collected clinical, procedural, and follow-up data on patients who underwent clinically indicated serial CCTAs at an inter-scan interval of ≥2 years between 2003 and 2015.14 Clinical and laboratory results were collected within 1 month from both baseline and follow-up CCTA scans and clinical outcomes were followed. Hyperlipidaemia was defined using clinical guidelines at the time of enrolment of each patient.15,16 The study protocol complies with the Declaration of Helsinki and was approved by the institutional review boards of all participating centres.
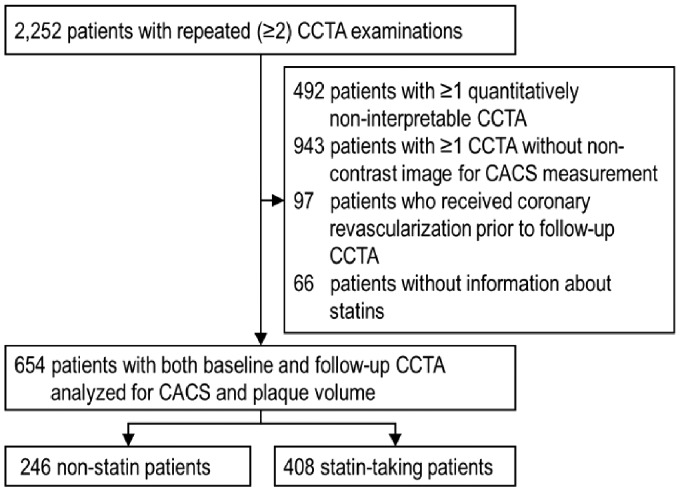
For the current analysis, patients who (i) had ≥1 CCTA uninterpretable for quantitative analysis (n = 492), (ii) had ≥1 CCTA not containing non-contrast images for CACS measurement (n = 943), (iii) underwent received coronary revascularization (either surgical or percutaneous) before follow-up CCTA (n = 97), and (iv) had no information on statins (n = 66) were excluded from the total PARADIGM population (n = 2252), leaving 654 patients (Figure 1).10 Patients were divided into non-statin and statin-taking groups.
Figure 1.
Flow chart of the study. CACS, coronary artery calcium score; CCTA, coronary computed tomography angiography.
CACS and quantitative CCTA analysis protocol
All acquisition and analysis of CCTAs were performed in direct accordance with guidelines.17,18 Datasets from each participating site were transferred to a core laboratory for blinded image analysis by level-III experienced readers.
Agatston CACS at both baseline (CCTA-1) and follow-up CCTAs (CCTA-2) were calculated on non-contrast images from each CCTA using a dedicated workstation (Vitrea v7.6; Vital Images, Inc., Minnetonka, MN, USA).19,20
For quantitative CCTA analysis to determine the total and compositional PVs, coronary atherosclerosis was evaluated on multiplanar and cross-sectional CCTA images using semi-automated plaque analysis software (QAngio CT Research Edition v2.1.9.1; Medis Medical Imaging Systems, Leiden, the Netherlands) with manual correction (Supplementary material Part III).21 Briefly, all coronary segments with a diameter of ≥2 mm were evaluated for every coronary artery and its branches using a modified 17-segment American Heart Association model.18,22 A coronary atherosclerotic plaque was defined as any tissue ≥1 mm3 within or adjacent to the lumen that could be discriminated from the surrounding structures and identified in ≥2 planes.18,22 Total PVs (mm3) of all analysed segments were added up to generate a per-patient level PV.23
PV was further sub-classified by composition using pre-defined Hounsfield unit (HU) cut-off values: (i) non-calcified (−30 to 350 HU) PV encompassing lipid-rich (−30 to 30 HU), fibro-fatty (30 to 130 HU), and fibrous (131 to 350 HU) PV; and (ii) calcified PV (≥351 HU).24,25 For longitudinal comparisons of CCTAs, coronary segments were co-registered between the CCTA-1 and CCTA-2 evaluations using branches and the distance from the ostium as landmarks.
Statistical analysis
Categorical variables are presented as absolute counts and percentages, and continuous variables are expressed as means±SD. Differences between continuous variables were analysed using Student’s t-test, and chi-square test or Fisher’s exact test was employed, as appropriate, for categorical variables.
To account for the difference in the analysed vessel length between patients and to provide equal weighting of each patient in the calculation of PV, normalized PVs were calculated as [(absolute PV/the total length of analysed coronary arteries) multiplied by the mean total analysed vessel length of the study population].13,23,26,27 CACS and PV progressions were defined as the difference of each value between CCTA-1 and CCTA-2 annualized by dividing with the inter-scan interval.13
We first confirmed whether statins have an impact on the progression of CAC in the whole study population by multivariate linear regression analysis. The correlation of annual CACS change with annual PV change in both statin-taking and non-statin groups was then analysed using Spearman’s correlation test.
To explore whether the association between CACS change and PV change differs based on statin treatment, multivariate linear regression models adjusted for age, male sex, ethnicity, hypertension, diabetes mellitus, hyperlipidaemia, family history of CAD, smoking history, body mass index, PV and CACS at baseline, changes in low-density lipoprotein level, and difference in vendors and tube voltages between the baseline and follow-up CCTA scans were constructed for both the non-statin and statin-taking groups. The differences between the beta coefficients (β) of models for each group were tested.
A two-tailed P-value of <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Study population and baseline characteristics
In the 654 patients included in the study, there were 246 in the non-statin and 408 in the statin-taking group (61 ± 10 years old, 55.8% male, inter-scan interval 3.9 ± 1.5 years, Table 1). In the statin-taking group, the information about statin therapy intensity was available in 398 patients (97.5%). Most of the patients were treated with moderate-intensity statins (n = 323, 81.2%), and others were treated with either high-intensity (n = 36, 9.0%) or low-intensity statins (n = 39, 9.8%) according to the current guidelines.15
Table 1.
Baseline clinical characteristics and lipid profiles
| Total (n = 654) | Non-statin group (n = 246) | Statin-taking group (n = 408) | P between groups | |
|---|---|---|---|---|
| CCTA inter-scan interval, years | 3.9 ± 1.5 | 3.8 ± 1.4 | 3.9 ± 1.5 | 0.557 |
| Age, years | 61.0 ± 9.9 | 60.2 ± 10.4 | 61.4 ± 9.4 | 0.150 |
| Male gender, n (%) | 365 (55.8) | 147 (59.8) | 218 (53.4) | 0.115 |
| Body mass index, kg/m2 | 24.8 ± 3.1 | 24.8 ± 3.2 | 24.8 ± 3.1 | 0.919 |
| Hypertension, n (%) | 371 (56.9) | 124 (50.4) | 247 (60.8) | 0.009 |
| Diabetes mellitus, n (%) | 157 (24.0) | 47 (19.1) | 110 (27.0) | 0.022 |
| Hyperlipidaemia, n (%) | 157 (24.0) | 24 (9.8) | 133 (32.6) | <0.001 |
| Family history of CAD, n (%) | 162 (24.8) | 62 (25.2) | 100 (24.5) | 0.842 |
| Smoking history, n (%) | 238 (36.4) | 94 (38.2) | 144 (35.3) | 0.467 |
| Typical chest pain, n (%) | 23 (3.5) | 5 (2.0) | 18 (4.4) | 0.128 |
| Atypical chest pain, n (%) | 527 (80.6) | 192 (78.0) | 335 (82.1) | 0.221 |
| Asymptomatic, n (%) | 8 (1.2) | 3 (1.2) | 5 (1.2) | >0.999 |
| Lipid profile at baseline (mg/dL) | ||||
| Total cholesterol | 183.9 ± 37.3 | 177.7 ± 30.2 | 187.7 ± 40.6 | <0.001 |
| Low-density lipoprotein | 114.7 ± 34.5 | 111.2 ± 27.5 | 116.8 ± 38.0 | 0.033 |
| High-density lipoprotein | 48.4 ± 13.4 | 48.6 ± 14.4 | 48.2 ± 12.7 | 0.716 |
| Triglycerides | 143.7 ± 82.2 | 136.3 ± 90.2 | 148.1 ± 76.8 | 0.093 |
| Lipid profile at follow-up (mg/dL) | ||||
| Total cholesterol | 168.3 ± 35.8 | 178.2 ± 30.3 | 162.3 ± 37.6 | <0.001 |
| Low-density lipoprotein | 94.5 ± 30.2 | 104.0 ± 25.7 | 88.8 ± 31.3 | <0.001 |
| High-density lipoprotein | 48.0 ± 11.7 | 47.9 ± 11.2 | 48.1 ± 12.1 | 0.799 |
| Triglycerides | 128.8 ± 73.1 | 133.2 ± 80.6 | 126.2 ± 68.2 | 0.246 |
CAD, coronary artery disease; CCTA, coronary computed tomography angiography; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The referral reason for CCTA for both CCTA was cardiac symptoms in 98.8% and 84.2% of patients in non-statin and statin-taking groups, respectively; however, the difference was not statistically significant (all P > 0.05). There were no differences in age and male sex, but statin-taking patients possessed more clinical risk factors for CAD including hypertension, diabetes mellitus, and hyperlipidaemia (all P < 0.05). The total cholesterol and low-density lipoprotein levels were all higher in statin-taking patients than in non-statin patients at baseline (all P < 0.05) but became lower in statin-taking patients at follow-up (all P > 0.05). During the mean 4.1 ± 2.0 years of follow-up after CCTA-2, 67 patients (10.3%) experienced MACE, mostly revascularization (n = 56, 8.6%), which more frequently occurred in the statin-taking group than in the non-statin group (13.3% vs. 5.3%, P = 0.001).
In the multivariate linear regression analysis, adjusted for age, male sex, ethnicity, hypertension, diabetes mellitus, hyperlipidaemia, family history of CAD, smoking history, body mass index, and changes in low-density lipoprotein levels, statins were positively associated with the progression of CACS {β [95% confidence interval (CI)]: 8.891 [1.039–16.743], P < 0.05}.
CCTA and CAC scan findings at baseline and follow-up according to statin use
At baseline, CACS was greater in the statin-taking group than in the non-statin group (146.2 ± 381.7 vs. 58.8 ± 231.4, P < 0.001; Table 2). In qualitative CCTA analysis, the total number of lesions per patient and stenosis involvement score were all greater in the statin-taking than in the non-statin group at both baseline and follow-up (all P < 0.001, see Supplementary data online, Table S3), and most patients had non-obstructive CAD in both groups.
Table 2.
Coronary computed tomography angiography findings at baseline and follow-up
| Non-statin group (n = 246) |
Statin-taking group (n = 408) |
P between groups |
||||
|---|---|---|---|---|---|---|
| Baseline | FU | Baseline | FU | Baseline | FU | |
| Coronary artery calcium scan | ||||||
| Total Agatston CACS | 58.8 ± 231.4 | 115.2 ± 426.2 | 146.2 ± 381.7 | 252.1 ± 530.5 | <0.001 | <0.001 |
| 0 (0, 30) | 6 (0, 72) | 29 (0, 128) | 62 (0, 254) | |||
| Agatston CACS category, n (%) | ||||||
| 0 | 132 (53.7) | 106 (43.1) | 138 (33.8) | 104 (25.5) | <0.001 | <0.001 |
| 1–99 | 82 (33.3) | 92 (37.4) | 153 (37.5) | 122 (29.9) | ||
| 100–399 | 26 (10.6) | 33 (13.4) | 79 (19.4) | 111 (27.2) | ||
| ≥400 | 6 (2.4) | 15 (6.1) | 38 (9.3) | 71 (17.4) | ||
| Quantitative CCTA analysis—normalized PVs (mm3) | ||||||
| Total PV | 70.0 ± 141.6 | 115.4 ± 206.4 | 142.3 ± 237.1 | 218.2 ± 319.2 | <0.001 | <0.001 |
| Calcified PV | 20.2 ± 68.6 | 42.5 ± 109.4 | 51.0 ± 131.6 | 103.7 ± 210.5 | <0.001 | <0.001 |
| Non-calcified PV | 49.8 ± 89.2 | 73.0 ± 122.7 | 91.3 ± 139.4 | 114.6 ± 157.0 | <0.001 | <0.001 |
| Fibrous PV | 31.7 ± 61.9 | 50.1 ± 84.6 | 62.5 ± 105.3 | 85.6 ± 118.9 | <0.001 | <0.001 |
| Fibrous-fatty PV | 16.2 ± 32.2 | 20.3 ± 42.8 | 25.3 ± 40.8 | 25.8 ± 44.9 | 0.002 | 0.122 |
| Lipid-rich PV | 1.9 ± 4.9 | 2.6 ± 7.5 | 3.6 ± 8.3 | 3.2 ± 8.8 | 0.001 | 0.324 |
| Annualized change in coronary artery calcium scan | ||||||
| Agatston CACS, /year | 14.6 ± 44.0 | 27.5 ± 50.8 | <0.001 | |||
| Annualized change in normalized PVs (mm3/year) | ||||||
| Total PV | 13.0 ± 21.7 | 20.2 ± 29.1 | <0.001 | |||
| Calcified PV | 6.0 ± 12.9 | 13.8 ± 25.7 | <0.001 | |||
| Non-calcified PV | 7.0 ± 17.3 | 6.4 ± 22.8 | 0.702 | |||
| Fibrous PV | 5.5 ± 11.9 | 6.2 ± 16.6 | 0.558 | |||
| Fibrous-fatty PV | 1.3 ± 8.4 | 0.3 ± 9.5 | 0.168 | |||
| Lipid-rich PV | 0.2 ± 1.8 | −0.06 ± 2.06 | 0.073 | |||
CACS, coronary artery calcium score; CCTA, coronary computed tomography angiography; FU, follow-up; PV, plaque volume.
In quantitative CCTA analysis, total PV and PVs by compositions were all greater in the statin-taking than in the non-statin group at baseline (all P < 0.001, Table 2, see Supplementary data online, Table S3). When annualized, the increase in CACS was greater in the statin-taking group than in the non-statin group (see Supplementary data online, Figure S5). The annualized change in total PV was also greater in the statin-taking than in the non-statin group, driven by the faster increase in calcified PV progression. However, there was no difference in the progression of non-calcified PV and all its components between both groups.
Spearman correlation analysis between CAC and PV progression
In the Spearman correlation analysis, annual calcified PV change was significantly associated with CACS change in both non-statin and statin-taking patients (R = 0.813 and 0.823, respectively, all P < 0.001, see Supplementary data online, Figure S6). Non-calcified PV progression had no correlation with CACS changes in both groups (all P > 0.05).
Impact of statins on the association between CAC and compositional PV changes
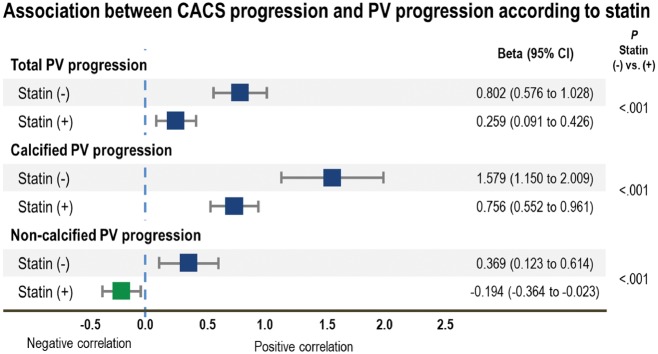
In multivariate linear regression analysis, the increase in CACS was associated with total PV increase in both the non-statin group [β (95% CI) 0.802 (0.576–1.028), P < 0.001] and statin-taking group [β (95% CI): 0.259 (0.091–0.426), P = 0.003] groups (Table 3 and Figure 2).
Table 3.
Multivariate linear regression analysis of the association between annual changes in CACS and annual PV changes according to statin treatment
| Non-statin group (n = 246) |
Statin-taking group (n = 408) |
P vs. groups | |||||
|---|---|---|---|---|---|---|---|
| β (95% CI) | SE | P | β (95% CI) | SE | P | ||
| Association with annual change in Agatston CACSa | |||||||
| Total PV | 0.802 | 0.115 | <0.001 | 0.259 | 0.085 | 0.003 | <0.001 |
| (0.576, 1.028) | (0.091, 0.426) | ||||||
| Calcified PV | 1.579 | 0.219 | <0.001 | 0.756 | 0.104 | <0.001 | <0.001 |
| (1.150, 2.009) | (0.552, 0.961) | ||||||
| Non-calcified PV | 0.369 | 0.125 | 0.004 | −0.194 | 0.087 | 0.026 | <0.001 |
| (0.123, 0.614) | (−0.364, −0.023) | ||||||
| Fibrous PV | 0.315 | 0.195 | 0.108 | −0.304 | 0.118 | 0.010 | 0.001 |
| (-0.067, 0.696) | (−0.535, −0.073) | ||||||
| Fibro-fatty PV | 0.697 | 0.234 | 0.003 | −0.131 | 0.203 | 0.520 | 0.010 |
| (0.238, 1.156) | (−0.530, 0.268) | ||||||
| Lipid-rich PV | 2.754 | 1.098 | 0.013 | −0.546 | 0.948 | 0.565 | 0.013 |
| (0.601, 4.907) | (−2.405, 1.313) | ||||||
PV progression was normalized and annualized. Adjusted for age, male sex, ethnicity, hypertension, diabetes mellitus, hyperlipidaemia, family history of CAD, smoking history, body mass index, PV and CACS at baseline, changes in low-density lipoprotein level, and difference in vendors and tube voltages between the baseline and follow-up CCTAs.
Changes in CACS were annualized.
CACS, coronary artery calcium score; CI, confidence interval; PV, plaque volume; SE, standard error.
Figure 2.
Progression in CACS represented coronary atherosclerotic PV progression differently depending on statin use in multivariate linear regression analysis adjusted for age, sex, race, hypertension, diabetes mellitus, dyslipidaemia, smoking history, family history of CAD, body mass index at baseline, PV and CACS at baseline, changes in low-density lipoprotein level, and difference in vendors and tube voltages between the baseline and follow-up CCTAs. CACS, coronary artery calcium score; CI, confidence interval; PV, plaque volume; Statin (−), non-statin group; Statin (+), statin-taking group.
When stratified by plaque compositions, the increase in CACS was positively associated with both calcified PV increase and non-calcified PV increase in the non-statin group [β (95% CI): 1.579 (1.150–2.009) and 0.369 (0.123–0.614), respectively, all P < 0.05]. Among the non-calcified PV constituents, only fibro-fatty and lipid-rich PV increases were independently associated with the increase in CACS [β (95% CI): 0.697 (0.238–1.156) and 2.754 (0.601–4.907), respectively, all P < 0.05], but not fibrous PV change (P > 0.05).
In the statin-taking group, although the increase in CACS was positively associated with increased calcified PV [β (95% CI): 0.756 (0.552–0.961), P < 0.001], non-calcified PV change showed a negative association [β (95% CI): −0.194 (−0.364 to −0.023), P = 0.026). Fibrous PV change was also independently and negatively associated with the increase in CACS [β (95% CI): −0.304 (−0.535 to −0.073), P = 0.010]. Although the β values for both fibro-fatty and lipid-rich PV were negative, they were not statistically significant.
Discussion
In the analysis of this large prospective observational CCTA registry, the association between CACS progression and compositional PV changes differed according to statin use. In non-statin patients, CACS progression reflected the progression of the overall coronary atherosclerotic burden, as observed in the multivariate analysis where CACS progression was associated with both calcified and non-calcified PV progression. In contrast, in statin-taking patients, CACS progression was associated only with calcified PV progression. Accordingly, the interpretation of CACS progression should differ according to statin use, as CAC progression in statin-taking patients does not entirely reflect the overall progression of the coronary atherosclerotic burden.
CACS has been employed for the risk stratification of the primary preventive population as a screening modality because of its relative simplicity, both in acquisition and interpretation, and the relatively low exposure to radiation.16 Elevated CACS portends a worse prognosis and has been widely used as an effective tool for the prognostication of future MACE.1 Accumulating evidences for CACS supporting its use in cardiovascular risk assessment also suggest that monitoring the increase in CACS would improve the prognostic power as rapid CACS progression has been shown to indicate increased risk of MACE.3,5,28,29
However, statins, one of the cornerstones in both the primary and secondary prevention of cardiovascular diseases based on its effect on reducing future MACE,7,8 have failed to attenuate the increase in CACS in previous studies.2,30 This discrepancy might be explained by recent observations where the pro-calcific effect of statins on coronary atherosclerotic plaques has been demonstrated in both invasive and non-invasive studies.31,32
A plausible hypothesis would be that the increase in CACS represents changes in coronary atherosclerotic plaques differently, depending on the presence of statins. The progression of CACS in patients undergoing statin treatment, at least in part, may reflect the stabilization or even the attenuation of CAD, rather than the progression of the atherosclerotic burden.12 To prove this hypothesis, evaluating the direct correlation between the changes in CACS and the PV of whole coronary arteries, instead of a specific target lesion, is mandatory as the first step, as the CAC scan reflects the global coronary atherosclerotic burden on a per-patient basis. In this regard, CCTA, which enables the concurrent determination of CACS and PV of the entire coronary vasculature, would be the most suitable imaging modality. This attempt was made only recently in a study that demonstrated a strong correlation between increases in CACS and PV.13 Although a significant increase in total and calcified PV was observed in statin users in this study,13 an independent impact of statins on the association between CACS and compositional PV changes was not explored and remained uncertain whether the correlation between the changes in CACS and plaque compositions would differ according to statin use.
In this study, we have expanded the observation by demonstrating that the correlation between CACS progression and the compositional changes in PV progression differs according to the use of statins. In the absence of statins, CACS elevation was associated with both annual calcified and non-calcified PV progression and its constituents including fibrous and lipid-rich PV. In contrast, in the presence of statins, CACS progression was associated only with calcified PV progression, but not with non-calcified PV progression. In the statin-taking group, non-calcified and fibro-fatty PV progression was negatively associated with increase in CACS. These results suggest that CACS progression in statin-taking patients does not necessarily imply the pure progression of the coronary atherosclerotic burden, especially the progression of the lipid component of a plaque—the determinants of plaque instability.9 These results are also in line with previous observations where statins were significantly associated only with increased total and calcified PV progression,13 and where statins failed to attenuate the increase in CACS.2,33
In all, these results suggest that the presence of statins during each CAC scan should be considered when interpreting changes in CACS. Whether this differential association between CACS progression and compositional PV progression according to statin use will also have a different impact on clinical outcomes and whether the cut-off values for defining clinically meaningful CACS progression should differ according to the use of statins are beyond the scope of the current analysis and remain to be proven. Based on current observations, future large-scale event-driven trials concurrently evaluating the impact of statins on changes in both CACS and coronary atherosclerotic characteristics now seem warranted to answer the above-mentioned questions.
The present study is not without limitations. First, because of the observational design of the study, selection bias seems unavoidable with respect to the enrolment of patients who underwent serial CCTAs. This resulted in the inclusion of only 654 patients of the total 2252 patients enrolled in the registry. As described above, the PARADIGM registry was specifically designed to describe the natural course of CAD in a low-risk population using non-invasive imaging. Based on the study design, it is plausible to assume that either high-risk patients subjected to invasive studies or revascularizations or patients with normal coronaries were omitted from the registry. The study population of PARADIGM generally represents low-risk patients as reflected by the low incidence of hard events and the high percentage of cardiac symptoms for referral reasons. There were also marked differences in comorbidities, baseline CACS, and PVs in the statin-taking and non-statin groups. Further, the difference in vendors and scan parameters used in baseline and follow-up CCTA scans (see Supplementary data online, Table S2) may have influenced the result.34,35 However, we adjusted the baseline CACS and PV and the difference in vendors and tube voltages between two CCTA scans in the multivariate analysis and tested the consistency of CCTA in quantitative compositional PV assessment to ensure the reliability and reproducibility of CCTA parameters (see Supplementary data online, Figures S2 and S3).36 Moreover, because no consensus on the use of serial CCTA for CAD monitoring currently exists,6 an observational study such as the PARADIGM study provides a unique opportunity to evaluate the correlation between CACS progression assessed by CAC scans and the change in plaque burden. Second, we could not stratify the association between CACS and PV according to the intensity of statin treatment. However, most patients (81.2% patients in the statin-taking group) were treated with moderate-intensity statins; we also adjusted the changes in low-density lipoprotein levels in the multivariate analysis to compensate for this limitation. Further, the impact of statins on the calcification of coronary atherosclerotic plaques is in line with that reported in previous studies and the coherence with prior observations supports the validity of our findings.9,11,31
In conclusion, an increase in CACS indicates changes in the coronary plaque burden and its composition differently in patients with statin treatment and those without. While CACS progression is associated with an increase in both calcified and non-calcified PV in non-statin patients, it is associated only with calcified PV progression, but not with non-calcified PV progression, in statin-taking patients. This result suggests that the presence of statins needs to be considered when interpreting the results of serial CACS and highlights the necessity of future prospective studies evaluating the effect of statins on CACS progression and its impact on clinical outcomes.
Funding
This work was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (Grant No. 2012027176). The study was also funded in part by the Dalio Institute of Cardiovascular Imaging and the Michael Wolk Foundation. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author has full access to all the data in the study and made the final decision to submit the manuscript for publication. H.-J.C. receives funding from by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (Grant No. 2012027176). J.K.M. receives funding from the National Institutes of Health (Grant Nos. R01 HL111141, R01 HL115150, R01 118019, and U01 HL 105907), the Qatar National Priorities Research Program (Grant No. 09-370-3-089), and GE Healthcare. J.K.M. served as a consultant at HeartFlow, is placed on the scientific advisory board of Arineta, and has an equity interest in MDDX. J.J.B. receives unrestricted research grants from Biotronik, Medtronic, Boston Scientific, and Edwards Lifesciences. E.J.C. receives funding from a National Research Foundation grant funded by the Korean Government (Ministry of Education, Science and Technology, NRF-2015R1D1A1A01059717). J.A.L. serves as a consultant and has stock options in HeartFlow and Circle Cardiovascular Imaging and receives speaking fees from GE Healthcare. M.J.B. receives grant support from the National Institutes of Health and GE Healthcare. H.S. receives grant support from Phillips/Volcano and St. Jude Abbott/Medtronic/Gilead. D.A. is on the Speakers Bureau for GE Healthcare and receives grant support from GE Healthcare and Bracco. G.P. receives institutional research grants from GE Healthcare, HeartFlow, Medtronic, Bracco, and Bayer. D.S.B. receives software royalties from Cedars-Sinai. R.V. has received institutional research support from 480 Biomedical, Abbott Vascular, Arterial Remodeling Technologies, BioSensors International, Biotronik, Boston Scientific, Celonova, Claret Medical, Cook Medical, Cordis, Edwards Lifesciences, Medtronic, MicroVention, OrbusNeich, ReCord, SINO Medical Technology, Spectranetics, Surmodics, Terumo Corporation, W.L. Gore, and Xeltis. R.V. also receives honoraria from 480 Biomedical, Abbott Vascular, Boston Scientific, Cook Medical, Lutonix, Medtronic, Terumo Corporation, and W.L. Gore, and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore.
Conflict of interest: S.-E.L, J.M.S., F.C., K.C., J.H.C., E.C., I.G., M.H., Y.J.K., A.K., B.K.L., E.M., H.M., G.R., S.S., P.H.S., J.N., L.J.S., and F.Y.L. have no conflicts of interests to disclose.
Supplementary Material
Other PARADIGM investigators: U.S. Coordinating Centre: Patricia Dunham, BA; Kimberly Elmore, MHA; Dan Gebow, PhD; Alexander van Rosendael, MD; and Wijnand Stuijfzand, MD.
PARADIGM Sites: Ralph Gentry; Taekyeong Kim, MD; Hanna Nieberler, Mark Pica.
References
- 1. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR. et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 2. Raggi P, Callister TQ, Shaw LJ.. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. ATVB 2004;24:1272.. [DOI] [PubMed] [Google Scholar]
- 3. Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS. et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong ND, Kawakubo M, LaBree L, Azen SP, Xiang M, Detrano R.. Relation of coronary calcium progression and control of lipids according to National Cholesterol Education Program guidelines. Am J Cardiol 2004;94:431–6. [DOI] [PubMed] [Google Scholar]
- 5. Lehmann N, Erbel R, Mahabadi AA, Rauwolf M, Mohlenkamp S, Moebus S. et al. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR study (Heinz Nixdorf Recall). Circulation 2018;137:665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 7.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- 8. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 9. Hattori K, Ozaki Y, Ismail TF, Okumura M, Naruse H, Kan S. et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter–IVUS. JACC Cardiovasc Imaging 2012;5:169–77. [DOI] [PubMed] [Google Scholar]
- 10. Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A. et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM Study. JACC Cardiovasc Imaging 2018;11:1475–84. [DOI] [PubMed] [Google Scholar]
- 11. Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR. et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273–82. [DOI] [PubMed] [Google Scholar]
- 12. Shaw LJ, Narula J, Chandrashekhar Y.. The never-ending story on coronary calcium: is it predictive, punitive, or protective? J Am Coll Cardiol 2015;65:1283.. [DOI] [PubMed] [Google Scholar]
- 13. Ceponiene I, Nakanishi R, Osawa K, Kanisawa M, Nezarat N, Rahmani S. et al. Coronary artery calcium progression is associated with coronary plaque volume progression: results from a quantitative semiautomated coronary artery plaque analysis. JACC Cardiovasc Imaging 2018;11:1785–94. [DOI] [PubMed] [Google Scholar]
- 14. Lee SE, Chang HJ, Rizvi A, Hadamitzky M, Kim YJ, Conte E. et al. Rationale and design of the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry: a comprehensive exploration of plaque progression and its impact on clinical outcomes from a multicenter serial coronary computed tomographic angiography study. Am Heart J 2016;182:72–9. [DOI] [PubMed] [Google Scholar]
- 15. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 16. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H. et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Rev Esp Cardiol (Engl Ed) 2017;70:115.. [DOI] [PubMed] [Google Scholar]
- 17. Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK. et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee: endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435–49. [DOI] [PubMed] [Google Scholar]
- 18. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ. et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 19. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R.. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 20. Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M. et al. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility–MESA study. Radiology 2005;236:477–84. [DOI] [PubMed] [Google Scholar]
- 21. Park HB, Lee BK, Shin S, Heo R, Arsanjani R, Kitslaar PH. et al. Clinical feasibility of 3D automated coronary atherosclerotic plaque quantification algorithm on coronary computed tomography angiography: comparison with intravascular ultrasound. Eur Radiol 2015;25:3073–83. [DOI] [PubMed] [Google Scholar]
- 22. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y. et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015;66:337–46. [DOI] [PubMed] [Google Scholar]
- 23. Papadopoulou SL, Neefjes LA, Garcia-Garcia HM, Flu WJ, Rossi A, Dharampal AS. et al. Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging 2012;5:S28–37. [DOI] [PubMed] [Google Scholar]
- 24. de Graaf MA, Broersen A, Kitslaar PH, Roos CJ, Dijkstra J, Lelieveldt BP. et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging 2013;29:1177–90. [DOI] [PubMed] [Google Scholar]
- 25. Pundziute G, Schuijf JD, Jukema JW, Decramer I, Sarno G, Vanhoenacker PK. et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. European Heart Journal 2008;29:2373–81. [DOI] [PubMed] [Google Scholar]
- 26. Mintz GS, Garcia-Garcia HM, Nicholls SJ, Weissman NJ, Bruining N, Crowe T. et al. Clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound regression/progression studies. EuroIntervention 2011;6:1123–30. [DOI] [PubMed] [Google Scholar]
- 27. Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D. et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 2004;292:2217–25. [DOI] [PubMed] [Google Scholar]
- 28. Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE. et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014;311:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D. et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229–36. [DOI] [PubMed] [Google Scholar]
- 30. Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ.. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med 1998;339:1972–8. [DOI] [PubMed] [Google Scholar]
- 31. Puri R, Libby P, Nissen SE, Wolski K, Ballantyne CM, Barter PJ. et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging 2014;15:380–8. [DOI] [PubMed] [Google Scholar]
- 32. Libby P. How does lipid lowering prevent coronary events? New insights from human imaging trials. Eur Heart J 2015;36:472–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raggi P, Davidson M, Callister TQ, Welty FK, Bachmann GA, Hecht H. et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women beyond endorsed lipid lowering with EBT scanning (BELLES). Circulation 2005;112:563–71. [DOI] [PubMed] [Google Scholar]
- 34. Symons R, Morris JZ, Wu CO, Pourmorteza A, Ahlman MA, Lima JA. et al. Coronary CT angiography: variability of CT scanners and readers in measurement of plaque volume. Radiology 2016;281:737–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dalager MG, Bottcher M, Dalager S, Andersen G, Thygesen J, Pedersen EM. et al. Imaging atherosclerotic plaques by cardiac computed tomography in vitro: impact of contrast type and acquisition protocol. Invest Radiol 2011;46:790–5. [DOI] [PubMed] [Google Scholar]
- 36. Lee SE, Park HB, Xuan D, Lee BK, Hong MK, Jang Y. et al. Consistency of quantitative analysis of coronary computed tomography angiography. J Cardiovasc Comput Tomogr 2019;13:48–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.