Abstract
Aims
It is thought that the majority of cardiovascular (CV) events are caused by vulnerable plaque. Such lesions are rupture prone, in part due to neovascularization. It is postulated that plaque vulnerability may be a systemic process and that vulnerable lesions may co-exist at multiple sites in the vascular bed. This study sought to examine whether carotid plaque vulnerability, characterized by contrast-enhanced ultrasound (CEUS)-assessed intraplaque neovascularization (IPN), was associated with significant coronary artery disease (CAD) and future CV events.
Methods and results
We investigated carotid IPN using carotid CEUS in 459 consecutive stable patients referred for coronary angiography. IPN was graded based on the presence and location of microbubbles within each plaque (0, not visible; 1, peri-adventitial; and 2, plaque core). The grades of each plaque were averaged to obtain an overall score per patient. Coronary plaque severity and complexity was also determined angiographically. Patients were followed for 30 days following their angiogram. This study found that a higher CEUS-assessed carotid IPN score was associated with significant CAD (≥50% stenosis) (1.8 ± 0.4 vs. 0.5 ± 0.6, P < 0.0001) and greater complexity of coronary lesions (1.7 ± 0.5 vs. 1.3 ± 0.8, P < 0.0001). Furthermore, an IPN score ≥1.25 could predict significant CAD with a high sensitivity (92%) and specificity (89%). The Kaplan–Meier analysis demonstrated a significantly higher proportion of participants having CV events with an IPN score ≥1.25 (P = 0.004).
Conclusion
Carotid plaque neovascularization was found to be predictive of significant and complex CAD and future CV events. CEUS-assessed carotid IPN is a clinically useful tool for CV risk stratification in high-risk cardiac patients.
Keywords: contrast-enhanced ultrasound, neovascularization, coronary artery disease, vulnerable plaque, carotid
Introduction
Atherosclerotic plaque rupture accounts for about 70% of all fatal cardiovascular (CV) events.1 Plaque progression and instability are associated with extensive intraplaque neovascularization (IPN), which increases susceptibility of the plaque to haemorrhage and rupture.1 Contrast-enhanced ultrasound (CEUS) is a simple and minimally invasive technique that allows for the visualization of IPN in large arteries, such as the carotid.2,3 In CEUS, IPN is identified by the presence of contrast microbubbles within the plaque moving from the adventitial side or plaque shoulder towards the plaque core.4,5 The reliability of CEUS for detecting IPN has been validated in previous studies, which showed that IPN assessed by CEUS correlated strongly with histological density of neovessels.6–8
Studies have shown that plaque instability often co-exists at multiple sites in the systemic vascular bed.1,9 IPN in the carotid artery has been associated with coronary lesion complexity and extent in patients with severe coronary lesions (≥70% stenosis).10 However, it has yet to be determined whether carotid IPN is able to predict significant coronary artery disease (CAD) and future CV events for the purpose of risk stratification. In this study, we investigated whether assessment of IPN using semi-quantitative analysis of CEUS of the carotid artery could predict significant CAD and future events in patients referred for coronary angiography.
Methods
Study design and population
This prospective study enrolled 459 consecutive patients who underwent coronary angiography from December 2016 to June 2018. The inclusion criteria were (i) ≥18 years of age; (ii) referred for clinically indicated angiography for assessment of CAD; and (iii) absence of clinical contraindication to angiography. Exclusion criteria were (i) previous carotid endarterectomy; (ii) allergy to perflutren; (iii) known or suspected cardiac shunt; (iv) previous percutaneous coronary intervention (≥1 week) or coronary artery bypass graft surgery; and (v) prior myocardial infarction (MI), stroke, or transient ischaemic attack (≥1 week). None of the patients enrolled in the study had known significant CAD (≥50% stenosis). Written informed consent was obtained from all participants prior to the study. This study conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board.
Evaluation of coronary lesions
Angiograms were scored by researchers blinded to the clinical features of the participants, as previously described.11,12 Briefly, stenosis in the left main, left anterior descending, circumflex, and right coronary arteries were graded as follows: 0 = no or minimal disease (0–19% narrowing in any segment), 1 = mild disease (20–49% narrowing in any segment), 2 = moderate disease (50–69% narrowing in any segment), and 3 = severe disease (≥70% narrowing within any segment of the main branches of the coronary artery or ≥50% in the left main coronary artery). Significant CAD was classified as ≥50% stenosis in any of the main coronary arteries. In addition, coronary plaque lesions were assessed for complexity. Lesions were considered to be complex if they possessed any one of the following characteristics: presence of a filling defect consistent with a thrombus, presence of diffuse disease, or a chronic total occlusion >3 months old.13
Focused ultrasound of the carotid artery
A focused B-mode carotid ultrasound was performed on all study participants as previously described,12 using a vascular ultrasound device equipped with a 9L-D linear-array transducer (2.4–10.0 MHz) (Vivid E9 Ultrasound System, GE Healthcare). Briefly, longitudinal images of each carotid artery were used to measure average carotid intima-media thickness (CIMT), maximum plaque height (MPH), defined as the maximum distance from the intima-lumen interface to the media-adventitia interface after comparing both left and right sides, and total plaque area (TPA), defined as the sum of the areas of all plaque lesions located in the carotid bifurcation and the proximal 1 cm of the internal and external carotid arteries of both sides.12,14 Atherosclerotic plaques were defined as focal structures encroaching into the arterial lumen with a height >1.5 mm or 50% intima-media thickness.15
CEUS of the carotid artery
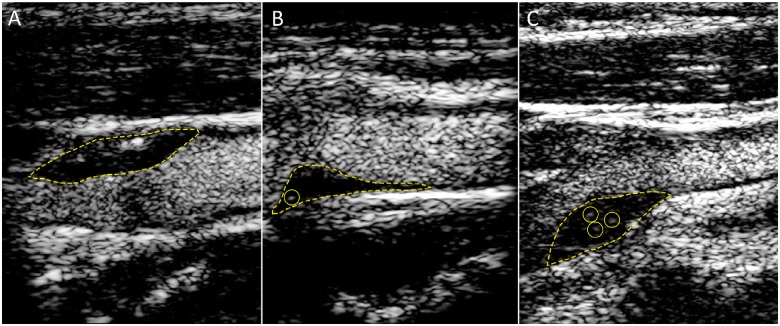
Carotid CEUS studies were performed and analysed by a researcher blinded to the history and characteristics of the patients. CEUS was performed using an ultrasound contrast agent, DEFINITY® (Lantheus Medical Imaging, Billerica, MA, USA). The mechanical index was lowered to 0.18, the dynamic range to 36, and the gain and compression adjusted to provide the highest contrast effect. After an intravenous injection of 0.2 mL of DEFINITY® diluted in 2.8 mL of 0.9% saline, ultrasound cineloops were recorded. IPN was identified by rapid movement of the echogenic reflectors of microbubbles within the plaque and scored as: 0, no visible microbubbles within the plaque; 1, minimal microbubbles confined to the shoulder or adventitial side of the plaque; or 2, microbubbles throughout the plaque, as previously described10 (Figure 1). The plaque scores on both sides were averaged to obtain a single overall neovascularization score per patient.
Figure 1.
Carotid intraplaque neovascularization scoring method. Representative contrast-enhanced ultrasound images of carotid plaques. 0, no visible microbubbles within the plaque; 1, minimal microbubbles confined to peri-adventitial areal; 2, microbubbles present throughout the plaque core. The yellow dotted line outlines the plaque lesion. Yellow circles depict intraplaque contrast microbubbles.
Follow-up study
Participants were followed for a period of 30 days or until the occurrence of one of the following CV events: coronary revascularization (i.e. percutaneous coronary intervention, coronary artery bypass surgery), heart failure, non-fatal MI, stroke, or cardiac death. Participants with acute coronary syndrome at the time of their original coronary angiogram were excluded from the follow-up analysis to reduce bias. In addition, coronary revascularization within 7 days of the original angiogram was excluded from analysis to prevent planned revascularization therapy from being assessed.
Statistical analysis
All descriptive data are expressed as the mean value ± standard deviation. Differences between two groups were compared with the independent t-test. The frequencies of binary variables were compared between two groups using the χ2 analysis. A receiver operating characteristics curve was used to determine the optimal threshold values of each predictive test. Contingency tables were used to determine the positive predictive value, negative predictive value, sensitivity, and specificity of each plaque analysis method relative to CAD. Univariate and multivariate logistic regression analyses were applied to determine risk factors associated with CAD and to adjust the model for significant risk factors, respectively. Survival analysis was carried out using the Kaplan–Meier method. The risk ratio for CV events during the follow-up period was assessed by Cox proportional hazards analysis. Interobserver and intraobserver reliability were assessed using the intraclass correlation coefficient (ICC) in a subset of 30 participants. All statistical analyses were carried out using JMP® Version 13 (SAS Institute Inc., Cary, NC, USA). A P-value of <0.05 was considered to be significant.
Results
Sample population characteristics in participants with and without significant CAD
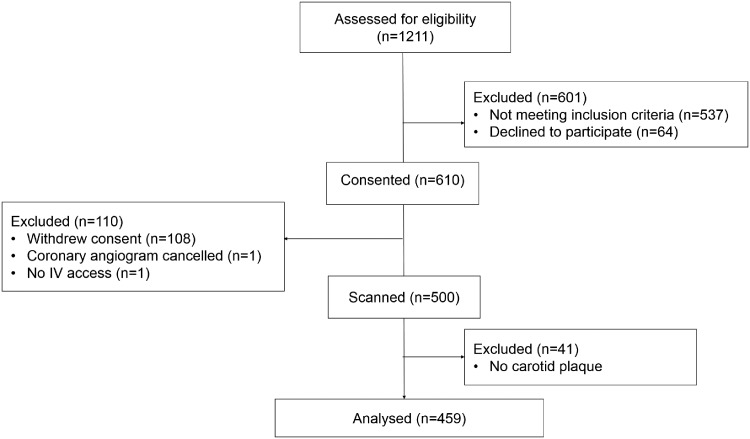
Of 1211 participants screened, 610 met the inclusion and exclusion criteria of the study. Scans were completed on 500 participants. The contrast agent was well tolerated in all patients and no adverse events reported. Loss of consented participants was mainly due to withdrawn consent (Figure 2). Upon image analysis, 41 participants were excluded due to the absence of carotid artery plaque. Overall, 459 participants were included in the study. There was a significant difference in age (66 ± 10 vs. 64 ± 11 years, P = 0.02) and sex (77% vs. 61% male, P = 0.0001) between participants with significant and non-significant CAD as well as in the number of participants with pre-existing diabetes, hyperlipidaemia, and smoking history (27% vs. 19%, P = 0.04; 64% vs. 50%, P = 0.003; and 71% vs. 61%, P = 0.02, respectively). There were also a significantly higher percentage of participants on statins, beta-blockers, and anti-platelets/coagulants in participant with significant CAD (all P < 0.05). Reasons for referral for coronary angiography included MI, chest pain, shortness of breath, positive stress test, or pre-operative coronary angiogram (Table 1).
Figure 2.
Enrolment and analysis of study participants. In total, 1211 were assessed for eligibility, 610 participants were consented, 500 were scanned, and 459 were included in the analysis. Loss of participants were largely due to unmet inclusion/exclusion criteria, declining to participate, withdrawal of consent, or lacking carotid plaque.
Table 1.
Sample population baseline characteristics
| Variables | Overall (n = 459) | Significant CAD (n = 284) | Non-significant CAD (n = 175) | P-value |
|---|---|---|---|---|
| Age (years) | 65.1 ± 10 | 66 ± 10 | 64 ± 11 | 0.02 |
| Male sex, n (%) | 326 (71) | 220 (77) | 106 (61) | 0.0001 |
| BMI (kg/m2) | 30.1 ± 5.9 | 29.9 ± 5.8 | 30.4 ± 6.2 | 0.34 |
| eGFR (mL/min*1.73 m2) | 78.3 ± 18 | 77.3 ± 18 | 80.0 ± 18 | 0.12 |
| Hypertension, n (%) | 317 (69) | 204 (15) | 113 (65) | 0.12 |
| Hyperlipidaemia, n (%) | 269 (59) | 182 (64) | 87 (50) | 0.003 |
| Diabetes mellitus, n (%) | 111 (24) | 78 (27) | 33 (19) | 0.04 |
| Smoking history, n (%) | 309 (67) | 203 (71) | 106 (61) | 0.02 |
| Family history of CVD, n (%) | 297 (65) | 189 (67) | 108 (62) | 0.32 |
| Medication use, n (%) | ||||
| Statin | 209 (46) | 167 (59) | 83 (47) | 0.02 |
| ACEI | 179 (39) | 113 (40) | 66 (38) | 0.69 |
| ARB | 44 (10) | 32 (11) | 12 (7) | 0.14 |
| β-blocker | 221 (48) | 153 (54) | 68 (39) | 0.002 |
| Ca-blocker | 87 (19) | 53 (19) | 34 (19) | 0.90 |
| Antiplatelet/coagulants | 339 (74) | 225 (79) | 114 (65) | 0.001 |
| Diuretics | 92 (20) | 54 (19) | 38 (22) | 0.55 |
| Reasons for referral, n (%) | ||||
| Myocardial infarction | 154 (34) | 142 (50) | 12 (7) | <0.0001 |
| Chest pain | 191 (42) | 130 (46) | 61 (35) | 0.59 |
| Shortness of breath | 108 (24) | 67 (24) | 41 (23) | 0.051 |
| Positive stress test | 124 (27) | 74 (26) | 50 (29) | 0.005 |
| Pre-operative | 49 (11) | 27 (10) | 22 (13) | 0.19 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate.
Plaque characteristics of participants with significant CAD
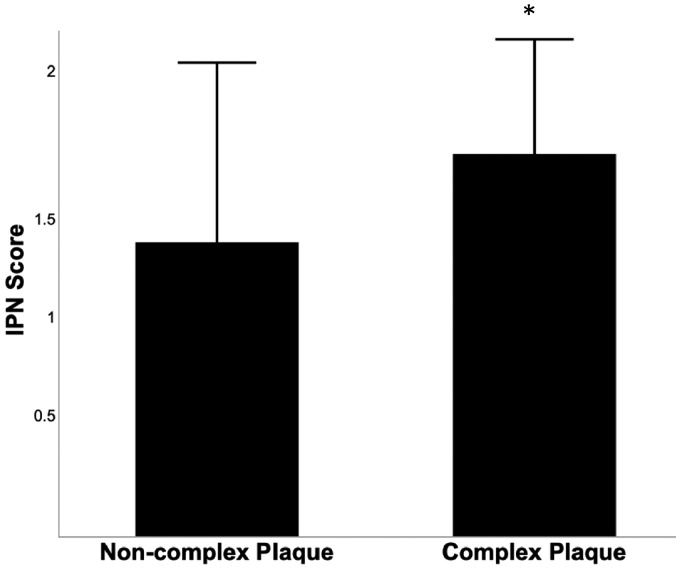
The sample population mean for CIMT, MPH, and TPA were found to be 0.76 ± 0.2 mm, 2.9 ± 1.2 mm, and 52 ± 44 mm2, respectively (Table 2). CIMT, MPH, and TPA were significantly higher in participants with significant CAD (all P < 0.001) (Table 2). CEUS cineloops were taken in order to analyse IPN and resultant plaque vulnerability. The mean IPN score in all participants was 1.3 ± 0.8. Participants with significant CAD had a greater carotid IPN score than those with no or mild CAD (1.8 ± 0.4 vs. 0.5 ± 0.6, P < 0.0001) (Table 2). In addition, 408 participants in whom coronary plaque was present were assessed for lesion complexity. We found that IPN score was significantly increased in participants who possessed at least one complex coronary lesion (1.3 ± 0.8 vs. 1.7 ± 0.5, P < 0.0001) (Figure 3).
Table 2.
Relationship between plaque characteristics and angiographic coronary artery disease
| Variables | Overall, mean ± SD (n = 459) | Significant CAD, mean ± SD (n = 284) | Non-significant CAD, mean ± SD (n = 175) | P-value |
|---|---|---|---|---|
| CIMT (mm) | 0.76 ± 0.2 | 0.78 ± 0.2 | 0.72 ± 0.1 | <0.0001 |
| MPH (mm) | 2.85 ± 1.2 | 3.1 ± 1.3 | 2.43 ± 1 | <0.0001 |
| TPA (mm2) | 51.8 ± 44 | 59.7 ± 45.9 | 38.9 ± 37.3 | <0.0001 |
| aIPN | 1.26 ± 0.8 | 1.76 ± 0.4 | 0.45 ± 0.6 | <0.0001 |
aIPN, average intraplaque neovascularization; CAD, coronary artery disease; CIMT, carotid intima-media thickness; MPH, maximum plaque height; SD, standard deviation; TPA, total plaque area.
Figure 3.
Carotid IPN is associated with complexity of coronary disease. Carotid IPN assessed using contrast-enhanced ultrasound is significantly increased in patients with angiographically assessed complex coronary lesions. *P < 0.05.
Univariate and multivariate analysis for participants with significant CAD
In total, 284 participants were found to have significant CAD after their angiogram. Of these patients, 147 received a coronary stent during their procedure. In the univariate logistic regression analysis, participants with significant CAD had a significant association with age [odds ratio per year (OR) 1.02], male sex (OR 2.24), smoking history (OR 1.63), hyperlipidaemia (OR 1.80), diabetes (OR 1.63), CIMT (OR 1.90), MPH (OR 2.32), TPA (OR 1.92), and IPN (OR 2.41) (Table 3). Multivariate logistic regression analysis including all factors found to be significant on univariate analysis also showed that B-mode ultrasound assessed CIMT, MPH, and TPA, as well as CEUS-assessed IPN score remained a significant independent risk factor for significant CAD (Table 3).
Table 3.
Univariate and multivariate logistic regression analyses for association between CAD and various parameters
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Age (per year) | 1.02 | 1.00–1.04 | 0.02 | 1.03 | 1.01–1.05 | 0.002 |
| Sex (male) | 2.24 | 1.48–3.38 | 0.0001 | 2.13 | 1.38–3.30 | 0.001 |
| Smoking | 1.63 | 1.10–2.43 | 0.02 | 1.59 | 1.05–2.43 | 0.03 |
| Hypertension | 1.40 | 0.93–2.09 | 0.10 | |||
| Hyperlipidaemia | 1.80 | 1.23–2.65 | 0.003 | 1.59 | 1.05–2.42 | 0.03 |
| Diabetes mellitus | 1.63 | 1.03–2.58 | 0.04 | 1.4 | 0.85–2.29 | 0.19 |
| BMI (kg/m2) | 0.98 | 0.95–1.02 | 0.05 | |||
| eGFR (mL/min*1.73 m2) | 0.99 | 0.98–1.00 | 0.12 | |||
| CIMT (per IQR) | 1.90 | 1.42–2.55 | <0.0001 | 1.59 | 1.17–2.16 | 0.003 |
| MPH (per IQR) | 2.32 | 1.72–3.14 | <0.0001 | 1.9 | 1.38–2.63 | <0.0001 |
| TPA (per IQR) | 1.92 | 1.47–2.51 | <0.0001 | 1.56 | 1.17–2.08 | 0.003 |
| aIPN (per score 0.25) | 2.41 | 2.08–2.79 | <0.0001 | 2.43 | 2.09–2.83 | <0.0001 |
aIPN, average intraplaque neovascularization; BMI, body mass index; CI, confidence interval; CIMT, carotid intima-media thickness; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MPH, maximum plaque height; TPA, total plaque area.
Value of IPN as a risk-stratification tool for significant CAD
The receiver operating characteristics curves and c-statistics indicated that carotid IPN was a better predictor of significant CAD (≥50% stenosis) [area under the curve (AUC) = 0.940] than MPH (AUC = 0.661), TPA (AUC = 0.665), and CIMT (0.625). Using a cut-off score of 1.25, carotid IPN was able to predict significant CAD with a higher sensitivity (92%) and specificity (89%) than CIMT, MPH, or TPA (Table 4A). The positive and negative predictive values of carotid IPN were 93% and 87%, respectively (Table 4A). It is noteworthy that when the threshold for significant CAD was increased to 70% stenosis, the predictive values of IPN decreased slightly, but remained higher than MPH, TPA, and CIMT (Table 4B).
Table 4.
Predictive values for CIMT, MPH, TPA, and IPN when used to predict CAD
| Variables (N = 459) | AUC | Cut-off | Significant CAD | Non-significant CAD | PPV (%) | NPV (%) | Sens (%) | Spec (%) | LR+ (%) | LR− (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. For predicting (≥50% stenosis) | ||||||||||||
| CIMT | 0.625 | ≥0.80 mm | TP | 132 | FP | 43 | 75 | 46 | 46 | 75 | 189 | 71 |
| <0.80 mm | FN | 152 | TN | 132 | ||||||||
| MPH | 0.661 | ≥2.41 mm | TP | 186 | FP | 72 | 72 | 51 | 65 | 59 | 159 | 59 |
| <2.41 mm | FN | 98 | TN | 103 | ||||||||
| TPA | 0.665 | ≥27.6 mm2 | TP | 216 | FP | 83 | 72 | 58 | 76 | 53 | 160 | 46 |
| <27.6 mm2 | FN | 68 | TN | 92 | ||||||||
| aIPN | 0.94 | ≥1.25 | TP | 260 | FP | 20 | 93 | 87 | 92 | 89 | 801 | 10 |
| <1.25 | FN | 24 | TN | 155 | ||||||||
| B. For predicting severe CAD (≥70% stenosis) | ||||||||||||
| CIMT | 0.603 | ≥0.75 mm | TP | 137 | FP | 85 | 62 | 55 | 56 | 61 | 143 | 72 |
| <0.75 mm | FN | 106 | TN | 131 | ||||||||
| MPH | 0.643 | ≥2.42 mm | TP | 161 | FP | 94 | 63 | 60 | 66 | 56 | 152 | 60 |
| <2.42 mm | FN | 82 | TN | 122 | ||||||||
| TPA | 0.667 | ≥24.8 mm2 | TP | 201 | FP | 117 | 63 | 70 | 83 | 46 | 153 | 38 |
| <24.8 mm2 | FN | 42 | TN | 99 | ||||||||
| aIPN | 0.853 | ≥1.25 | TP | 224 | FP | 56 | 80 | 89 | 92 | 74 | 356 | 11 |
| <1.25 | FN | 19 | TN | 160 | ||||||||
aIPN, average intraplaque neovascularization; AUC, area under the curve; CIMT, carotid intima-media thickness; FN, false negatives; FP, false positives; LR−, negative likelihood ratio; LR+, positive likelihood ratio; MPH, maximum plaque height; NPV, negative predictive value; PPV, positive predictive value; sens, sensitivity; spec, specificity; TN, true negatives; TP, true positives; TPA, total plaque area.
Value of IPN in predicting future CV events
All participants that were stable at the time of admission for their angiogram (n = 309) were followed for 30 days. Participants with an IPN score of 1.25 or greater (n = 159) had 25 CV events during the follow-up period, whereas participants with an IPN score of less than 1.25 (n = 150) had four CV events. A Kaplan–Meier analysis demonstrated that an IPN score ≥1.25 was associated with a significantly higher occurrence of CV events (P < 0.0001) (Figure 4). Univariate Cox proportional hazards model analysis showed that IPN and MPH were both significant risk contributors of future CV events (Table 5). Multivariate Cox proportional hazards model analysis demonstrated that IPN (risk ratio: 1.34; 95% confidence interval: 1.13–1.63; P = 0.001) was a significant and independent contributor of future CV events in patients with stable CAD.
Figure 4.
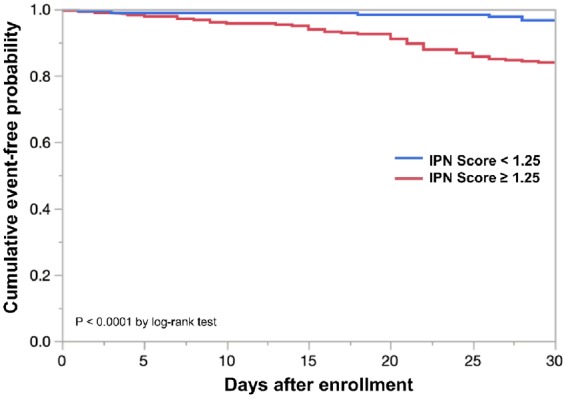
Survival analysis of stable patients based on carotid IPN. Kaplan–Meier curves show cumulative cardiovascular event-free survival according to an IPN score threshold of 1.25. Cumulative event-free survival in patients with significant IPN was significantly worse than that of patients without significant IPN.
Table 5.
Univariate and multivariate Cox proportional hazards analyses of risk factors for cardiovascular events
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variables | Risk ratio | 95% CI | P-value | Risk ratio | 95% CI | P-value |
| Age (per year) | 1.01 | 0.97–1.07 | 0.46 | |||
| Sex (male) | 1.95 | 1.76–6.08 | 0.20 | |||
| Smoking | 0.64 | 0.29–1.56 | 0.30 | |||
| Hypertension | 0.66 | 0.27–1.67 | 0.36 | |||
| Hyperlipidaemia | 1.13 | 0.50–2.71 | 0.77 | |||
| Diabetes mellitus | 0.89 | 0.33–2.23 | 0.82 | |||
| BMI (kg/m2) | 0.99 | 0.91–1.06 | 0.72 | |||
| eGFR (mL/min*1.73 m2) | 1.01 | 0.98–1.03 | 0.54 | |||
| CIMT (per IQR) | 1.43 | 0.83–2.44 | 0.19 | |||
| MPH (per IQR) | 1.62 | 1.00–2.53 | <0.05 | 1.18 | 0.75–1.83 | 0.46 |
| TPA (per IQR) | 1.11 | 0.72–1.50 | 0.61 | |||
| aIPN (per score 0.25) | 1.35 | 1.15–1.64 | 0.0007 | 1.34 | 1.13–1.63 | 0.001 |
aIPN, average intraplaque neovascularization; BMI, body mass index; CI, confidence interval; CIMT, carotid intima-media thickness; eGFR, estimated glomerular filtration rate; IPN, Intraplaque neovascularization; IQR, interquartile range; MPH, Maximum plaque height; TPA, total plaque area.
Reproducibility of CEUS analysis
To establish the reproducibility of a qualitative CEUS assessment, intraobserver and interobserver reliability analyses were conducted. For intraobserver reliability, 30 participants were reassessed by the same reader 30 days after the initial assessment. Interobserver reliability was assessed in two ways: first by having two different readers assess the same cineloops, and second, by having two operators perform the CEUS scans and then assessing their own cineloops. The ICC for intraobserver reliability was 0.88, which corresponded to good reliability.16 The ICC for two-reader reliability also showed good reliability at 0.87, whereas the ICC for two-operator reliability was 0.70, which corresponded to moderate reliability.
Discussion
The present study demonstrated that a higher grade of carotid IPN assessed using CEUS was associated with the presence of significant CAD (≥50% stenosis) in participants referred for coronary angiography. Furthermore, this study showed that after adjustment for traditional cardiac risk factors, carotid IPN was an independent predictor of CAD and demonstrated a greater sensitivity and specificity for predicting CAD than carotid CIMT, MPH, or TPA. In a 30-day follow-up period, participants with an IPN score meeting our pre-determined cut-off value of 1.25 for predicting CAD, had a higher probability of experiencing a CV event. Overall, this study outlines the potential utility of CEUS-evaluated carotid IPN as a clinically and practically informative CV risk stratification tool and a minimally invasive vascular imaging biomarker for CAD.
It is crucial that biomarkers that aid in stratifying participants based on their risk of future CV events are also able to predict the presence of significant CAD, since this is often a precursor of acute ischaemic cardiac events. Previously, we determined that MPH and TPA in the carotid artery were able to predict CAD.11,12 Furthermore, it has been shown that carotid plaque is a better predictor of CAD than CIMT.17 This is consistent with our finding that both MPH and TPA had higher sensitivities for predicting CAD than did CIMT in this cohort. However, the specificities of these tests are often low, partly because the testing population is higher risk, and partly because atherosclerosis is known to progress over time.18 Many individuals referred for coronary angiography have age-related carotid plaque development, limiting the use of MPH and TPA as an imaging biomarker for CAD. This outlines the importance of assessing both quantity and quality of carotid plaque when establishing risk-stratification tools for CAD.
CEUS of the carotid artery is a useful non-invasive tool that allows for the visualization of IPN. This technique can detect even the smallest microvasculature within a plaque lesion. In this study, we found that carotid IPN score had an even higher sensitivity for predicting CAD than CIMT, MPH, or TPA. Of note, the c-statistics presented in this study for MPH and TPA were lower than previously recorded.11,12 This is largely due to the fact that this study included only participants with carotid plaque that could be assessed for neovascularization. In addition, this study concentrated on a patient population with a slightly lower pre-test probability of CAD than previously reported, as we excluded all participants with previous MI, stroke, transient ischaemic attack, coronary stenting, and coronary artery bypass surgery. However, in this high-risk cardiac population, our data demonstrate the importance of assessing plaque quality rather than quantity.
A limitation of assessing carotid IPN is the presumption that there is a high correlation between two vascular beds. However, many studies have shown that plaque instability is not a local characteristic, but rather it occurs systemically.9,19 In support of this notion, we found that IPN score was significantly increased in participants with complex coronary disease. This aligns with the work done by Deyama et al.,10 which demonstrated that in patients with severe CAD (≥70% stenosis), carotid IPN was associated with complexity and extent of the coronary lesions, as well as the number of diseased vessels. Taken together, this data support the theory that plaque vulnerability is a systemic process, and higher degrees of neovascularization in one vascular bed may be representative of others.
Past studies have identified the value of carotid plaque detection in the prediction of future CV events. In 2015, Baber et al.20 demonstrated in roughly 6000 patients that carotid plaque burden detected with 3D ultrasound was associated with major adverse cardiac events. Furthermore, Sillesen et al.21 built upon this study by determining that, like carotid plaque burden, increasing maximum carotid plaque thickness was associated with an increased risk of future major adverse cardiac events compared with participants without carotid atherosclerosis. Thus, it follows that a high degree of carotid IPN, which signifies a high probability for intraplaque haemorrhage and rupture, would be associated with an increased risk for future CV events.1 This study demonstrated that a higher IPN score correlated to total 30-day CV events, including coronary revascularization, heart failutre, MI, stroke, and cardiac death, indicating that carotid IPN by CEUS assessment reflects systemic plaque vulnerability. Other studies have also demonstrated similar findings in slightly different populations. For example, in patients with known stable CAD, Zhu et al.22 found that the presence of contrast material enhancement of carotid plaque was a significant and independent predictor of future coronary events. In addition, using quantitative region of interest detection software, Nakamura et al.23 showed that plaque enhanced intensity in the carotid artery was an independent predictor of secondary cardiac events in patients with severe CAD (≥70% stenosis). This suggests that neovascularized carotid plaque lesions are vulnerable and can indicate angiographically unstable coronary plaques and future CV events. Non-invasive, accessible, cost-effective, and accurate methods that detect the instability of coronary lesions are crucial for the management of high-risk patients. This need outlines the utility of CEUS assessment of carotid plaque in providing clinically relevant information to aid in risk stratification.
Certain imaging modalities such as coronary computed tomography angiography and myocardial perfusion imaging are not practical for the screening of all high-risk patients.24 Thus, there is a call for minimally invasive and inexpensive screening methods with good predictive ability for CAD. Current commonly used risk-stratification tools include exercise and dobutamine stress tests and myocardial perfusion imaging. CEUS-assessed carotid IPN should not replace these tools, but rather be used alongside, in order to add to the information provided by traditional risk-factor assessment and enable reclassification of patients into more accurate risk categories.
Limitations
Our analysis concentrates on a patient population of mostly symptomatic participants that are not representative of the entire population that would be targeted for the use of CEUS in a primary prevention setting. However, many publications assessing CV risk stratification tools are conducted in patients referred for clinical angiography so that a comparison to the clinical standard diagnosis can be made. Secondly, there is high subjectivity due to the use of semi-quantitative image analysis. In order to mitigate this bias, we performed interobserver and intraobserver reliability studies, which each demonstrated good reliability. In addition, by using a semi-quantitative grading system, this technique becomes more accessible for use in a primary care setting. However, a larger study with a lower-risk population is required to confirm these observations. It is also noteworthy that our follow-up time of 30 days was quite short. Future studies will involve an analysis of long-term outcomes of patients with carotid IPN. Lastly, CEUS is a technique that is heavily dependent on the plane of the image. Thus, neovascularization in certain plaques may have been overlooked. Plaque lesions may also go unseen due to acoustic shadowing. To account for this, we completed extensive reproducibility studies, assessing interoperator reproducibility of both scanning and offline IPN analysis.
Conclusion
Carotid IPN is a useful non-invasive tool that can predict the presence and complexity of CAD. Furthermore, it is able to predict the development of future cardiac events in stable patients. This procedure can provide expedited, safe, accurate, and cost-effective patient care through information that will aid in CV risk stratification.
Acknowledgements
The authors thank the KGH catheterization laboratory staff for their support in this study.
Funding
This work was supported by a Canada Foundation for Innovation and Ontario Research Fund (CFI#29051), an Investigator Sponsored Research Grant from Lantheus Medical Imaging, a Ministry of Research, Innovation and Science Early Research Award (#ER15-11-029), a Garfield Kelly Cardiovascular Research and Development Fund Award, the South Eastern Ontario Academic Medical Organization, the Canadian Institutes of Health Research (Doctoral Research Award to L.E.M.), and the Heart and Stroke Foundation of Canada (Phase I Career Award to A.M.J.), Kingston, Canada.
Conflict of interest: This work was funded in part by Lantheus Medical Imaging Research Grant. Lantheus Medical Imaging did not have a role in the design or conduct of this study, the collection, analysis, or interpretation of the data or the preparation of the manuscript. All authors have no conflict of interest to declare.
References
- 1. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J. et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108:1664–72. [DOI] [PubMed] [Google Scholar]
- 2. Feinstein SB. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol 2006;48:236–43. [DOI] [PubMed] [Google Scholar]
- 3. Staub D, Schinkel AF, Coll B, Coli S, van der Steen AF, Reed JD. et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging 2010;3:761–71. [DOI] [PubMed] [Google Scholar]
- 4. Rajaram V, Pandhya S, Patel S, Meyer PM, Goldin M, Feinstein MJ. et al. Role of surrogate markers in assessing patients with diabetes mellitus and the metabolic syndrome and in evaluating lipid-lowering therapy. Am J Cardiol 2004;93:32–48C. [DOI] [PubMed] [Google Scholar]
- 5. Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P. et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 2010;41:41–7. [DOI] [PubMed] [Google Scholar]
- 6. Coli S, Magnoni M, Sangiorgi G, Marrocco-Trischitta MM, Melisurgo G, Mauriello A. et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol 2008;52:223–30. [DOI] [PubMed] [Google Scholar]
- 7. Giannarelli C, Ibanez B, Cimmino G, Garcia Ruiz JM, Faita F, Bianchini E. et al. Contrast-enhanced ultrasound imaging detects intraplaque neovascularization in an experimental model of atherosclerosis. JACC Cardiovasc Imaging 2010;3:1256–64. [DOI] [PubMed] [Google Scholar]
- 8. Hoogi A, Adam D, Hoffman A, Kerner H, Reisner S, Gaitini D.. Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am J Roentgenol 2011;196:431–6. [DOI] [PubMed] [Google Scholar]
- 9. Rothwell PM, Villagra R, Gibson R, Donders RC, Warlow CP.. Evidence of a chronic systemic cause of instability of atherosclerotic plaques. Lancet 2000;355:19–24. [DOI] [PubMed] [Google Scholar]
- 10. Deyama J, Nakamura T, Takishima I, Fujioka D, Kawabata K, Obata JE. et al. Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ J 2013;77:1499–507. [DOI] [PubMed] [Google Scholar]
- 11. Johri AM, Behl P, Hetu MF, Haqqi M, Ewart P, Day AG. et al. Carotid ultrasound maximum plaque height—a sensitive imaging biomarker for the assessment of significant coronary artery disease. Echocardiography 2016;33:281–9. [DOI] [PubMed] [Google Scholar]
- 12. Johri AM, Calnan CM, Matangi MF, MacHaalany J, Hetu MF.. Focused vascular ultrasound for the assessment of atherosclerosis: a proof-of-concept study. J Am Soc Echocardiogr 2016;29:842–9. [DOI] [PubMed] [Google Scholar]
- 13. Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB 3rd, Loop FD. et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation 1988;78:486–502. [DOI] [PubMed] [Google Scholar]
- 14. Johri AM, Chitty DW, Matangi M, Malik P, Mousavi P, Day A. et al. Can carotid bulb plaque assessment rule out significant coronary artery disease? A comparison of plaque quantification by two- and three-dimensional ultrasound. J Am Soc Echocardiogr 2013;26:86–95. [DOI] [PubMed] [Google Scholar]
- 15. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N. et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012;34:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koo TK, Li MY.. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inaba Y, Chen JA, Bergmann SR.. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 2012;220:128–33. [DOI] [PubMed] [Google Scholar]
- 18. Tabas I. 2016 Russell Ross memorial lecture in vascular biology: molecular-cellular mechanisms in the progression of atherosclerosis. Arterioscler Thromb Vasc Biol 2017;37:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O'Neill WW.. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med 2000;343:915–22. [DOI] [PubMed] [Google Scholar]
- 20. Baber U, Mehran R, Sartori S, Schoos MM, Sillesen H, Muntendam P. et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol 2015;65:1065–74. [DOI] [PubMed] [Google Scholar]
- 21. Sillesen H, Sartori S, Sandholt B, Baber U, Mehran R, Fuster V.. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging 2018;19:1042–50. [DOI] [PubMed] [Google Scholar]
- 22. Zhu Y, Deng YB, Liu YN, Bi XJ, Sun J, Tang QY. et al. Use of carotid plaque neovascularization at contrast-enhanced US to predict coronary events in patients with coronary artery disease. Radiology 2013;268:54–60. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura J, Nakamura T, Deyama J, Fujioka D, Kawabata K, Obata JE. et al. Assessment of carotid plaque neovascularization using quantitative analysis of contrast-enhanced ultrasound imaging is useful for risk stratification in patients with coronary artery disease. Int J Cardiol 2015;195:113–9. [DOI] [PubMed] [Google Scholar]
- 24. Katakami N, Kaneto H, Shimomura I.. Carotid ultrasonography: a potent tool for better clinical practice in diagnosis of atherosclerosis in diabetic patients. J Diabetes Invest 2014;5:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]