Abstract
Herpes zoster (HZ) has high morbidity in people living with HIV (PLHIV). We investigated immunological factors that correlated with the development of HZ in PLHIV with controlled HIV replication on antiretroviral therapy (ART). PLHIV who developed HZ on ART (cases), with undetectable plasma HIV RNA, and CD4 counts ≥200 cells/μL were matched 1:1 to controls by CD4 count, age, gender, race, and duration of ART. Varicella-zoster virus (VZV)-specific T cells and circulating regulatory T cells (Treg) were measured by flow cytometry before and after HZ. Differences between cases and controls were assessed by paired t-tests and longitudinal changes by Wilcoxon signed rank test. HZ cases (N = 31) had higher CD4+FOXP3+CD25+% Treg before HZ compared with 31 controls. After VZV ex vivo restimulation, cases had lower T cell responses, including CD8+perforin+% cytotoxic T lymphocytes (CTLs), CD4+IL10+%, and CD4+TGFβ+% compared with controls. Overall, Treg negatively correlated with VZV-specific Th1 responses. Moreover, Treg decreased over time on ART in HZ cases, VZV-CTLs were stable and did not increase even after HZ. Increased circulating Treg and decreased VZV-specific T cell immune responses were associated with the risk of HZ in PLHIV. The kinetics of Treg over time, but not of VZV-CTLs, paralleled the natural history of HZ, whose incidence decreases over time on effective ART.
Keywords: human immunodeficiency virus, varicella zoster virus, herpes zoster, regulatory T cells, cytotoxic T cells, immune correlates with risk of herpes zoster
Introduction
Herpes zoster (HZ) was a very common complication of HIV infection before the advent of effective antiretroviral therapy (ART)1,2 and remains more frequent in people living with HIV (PLHIV) than in the general population despite the widespread utilization of ART.3,4 This indicates that cell-mediated immune (CMI) reconstitution is incomplete. The importance of CMI in preventing HZ is well established, but its mechanism of action is not well defined.5–7 Varicella-zoster virus (VZV) reactivates in neurons of the dorsal root ganglia (DRG), travels through axons to the dermal/epidermal junction, and replicates in the basal epidermal cell layer to cause HZ.8 Clinical observations provide evidence that CMI sufficient to prevent HZ may allow VZV to reactivate, and replicate in a limited manner: VZV DNA can be found in the blood and oropharynx of asymptomatic healthy adults, suggesting that the virus replicates in the DRG and/or skin, and enters the blood stream to seed distant sites without causing symptoms.9–12 The factors that allow reactivated VZV to progress to HZ or not are not known.
In the general population, the risk of HZ increases with age ≥50 years. Aging is accelerated in PLHIV, which is an additional factor that may contribute to the increased incidence of HZ in PLHIV on ART. PLHIV and older individuals share decreased viral-specific Th1 responses and increased exhausted, senescent, and regulatory T cells (Treg).13–16 CD8+ T cells have increased expression of inhibitory immunological checkpoints.17–19 We have previously shown that 47 children perinatally infected with HIV (PHIV) that developed HZ had lower VZV-specific CD8+CD107a+% cytotoxic T lymphocytes (CTLs) compared with 141 PHIV controls matched ∼3:1 to the HZ cases by age at enrollment and at primary varicella infection.20 These differences were independent of CD4+% or plasma HIV viral load, suggesting that VZV-specific CTLs were protective against HZ. In contrast, high Treg and activated T cells were associated with increased risk of HZ in PHIV.
Our goals in this study were to advance the validation of VZV-specific CTL as a surrogate marker of protection against HZ21,22 and to identify additional factors that may predict the risk of HZ in PLHIV on stable and effective ART, with undetectable plasma RNA and CD4+ counts ≥200 cells/dL.
Study Design and Methods
Study design
This was a matched case–control study that used plasma and peripheral blood mononuclear cells (PBMCs) cryopreserved and stored in AIDS clinical trials group longitudinal linked randomized trials (ALLRT), a prospective cohort study that enrolled subjects from AIDS clinical trials group (ACTG) antiretroviral studies and followed them beyond the duration of the parent study.23 Inclusion criteria were ≥200 CD4+ cells/dL and HIV plasma RNA <200 copies/mL. Main exclusion criteria were the presence of immune-suppressant conditions or medications other than those for HIV infection, recent opportunistic infections, and antiviral medication with activity against VZV. Cases were identified by incident HZ while in ALLRT and availability of PBMCs collected within 6 months before diagnosis. Controls without HZ were matched to the cases by age (±10 years), gender, race, duration of ART (±48 weeks), parent study and CD4+ cell numbers (±250 cells/dL) at the time of PBMC collection.
Flow cytometry
Cryopreserved PBMCs were thawed and surface stained with the following markers: CD3 Alexa Fluor 700 (clone UCHT-1; BD Biosciences), CD8 PC5.5 (clone A99019; Beckman Coulter), PD1 PE efluor610 (clone eBioJ105; eBioscience™), CD28 PE Cy7 (clone CD28.2; eBioscience), KLRG1 APC (clone 13-F12F2; eBioscience), CD127 PE Cy7 (clone HIL-7RM21; BD Biosciences), CD25 APC H7 (clone M-A251; BD Biosciences), TIM3 PE (clone 7D3; BD Biosciences), LAG3 PE (clone T47-530; BD Biosciences), CD57 BV421 (clone NK1; BD Biosciences), and CD39 APC Cy7 (clone A1; Biolegend). Cells were subsequently permeabilized using eBioscience FoxP3 transcription buffer and stained with FoxP3 APC (clone PCH101; eBioscience). Freshly thawed cells were also rested overnight in culture media containing RPMI 1640 (Corning), penicillin/streptomycin 100 U/mL/100 μg/mL (Gemini), 10 mM HEPES (Corning), and 10% FBS (Gibco). Rested PBMCs were stimulated with VZV OKA-virus at 104 pfu or mock stimulated for 48 h. At the end of stimulation 2 μg/mL of Brefeldin A (Sigma-Aldrich) was added to VZV- and mock-stimulated cultures for 4 h before surface staining with the following markers: CD3 Alexa Fluor 700 (clone UCHT-1; BD Biosciences), CD8 PC5.5 (clone A99019; Beckman Coulter), CD39 FITC (clone A1; Biolegend), and CTLA-4 PE CF594 (clone BN13; BD Biosciences). After permeabilization, cells were stained with perforin PE (clone dG9; eBioscience), IL-10 PE Cy7 (clone JES3-9D7; Biolegend), TGFβ APC (clone TW4-6H10; Biolegend), TNFα APC Cy7 (clone MAb11; Biolegend), and IFNγ BV421 (clone 4S.B3; Biolegend). Flow cytometry acquisition used a 10-color 3-laser Gallios instrument (Beckman-Coulter) and analysis used Kaluza (Beckman-Coulter) and FlowJo software (BD Biosciences). Results were expressed as percentages of the CD3+CD8−(CD4+) and CD3+CD8+ parent populations. In the case of stimulated cells, final results were obtained by subtracting mock- from VZV-stimulated results.
Statistical analysis
A sample size of 35 case–control sets with evaluable data was estimated as providing ∼90% power to detect an effect size of 0.56 for a specific biomarker between cases and controls (two-sided paired t-test with a significance level of .05).
For each prespecified marker, the differences between cases and controls were assessed by paired t-tests. Wilcoxon signed-rank tests were used to assess marker change over time. Results were not adjusted for multiple comparisons. Correlations between markers were assessed using Pearson correlations.
Results
Characteristics of the study population
Out of 145 ALLRT participants with HZ, only 31 fulfilled the inclusion/exclusion criteria for this study. The demographic and HIV disease characteristics of cases and controls at PBMC collection were similar by design (Table 1). Cases had significantly lower time on ART (mean ± SD of 117 ± 130 weeks vs. 125 ± 130 weeks; p = .046), had lower nadir CD4+ cells/dL (p < .001) and included a lower proportion of individuals with chronic hepatitis C virus infection (6% vs. 23%; p = .06). PBMCs were collected from cases at mean ± SD of 2.7 ± 2.4 months before the defining episode of HZ. None of the participants had received zoster vaccine live (ZVL) before the last sample of PBMCs was collected.
Table 1.
Participant Characteristics
| Variable | Cases (N = 31) | Controls (N = 31) | p |
|---|---|---|---|
| Mean years of age (SD) | 41.3 (10.0) | 40.1 (9.4) | |
| Males, n (%) | 23 (74) | 23 (74) | |
| Race/ethnicity, n (%) | |||
| White non-Hispanic | 14 (45) | 14 (45) | |
| Black non-Hispanic | 11 (35) | 11 (35) | |
| Hispanic | 6 (19) | 6 (19) | |
| Year of index PBMC sample, n (%) | |||
| 2001–2005 | 7 (23) | 7 (23) | |
| 2006–2009 | 12 (39) | 10 (32) | |
| 2010–2013 | 12 (39) | 14 (45) | |
| Mean CD4+ cells/dL (SD) | 489 (233) | 512 (214) | .24a |
| Mean CD8+ cells/dL (SD) | 892 (528) | 849 (453) | .75a |
| Mean CD4/CD8 ratio (SD) | 0.63 (0.34) | 0.79 (0.60) | .13a |
| Plasma HIV RNA <50 copies/mL, n (%) | 29 (94) | 27 (87) | .41b |
| Mean weeks on ART (SD) | 117 (130) | 125 (130) | .046a |
| Mean nadir CD4+ cells/dL (SD) | 156 (159) | 298 (190) | <.001a |
| Hepatitis C virus positive, n (%) | 2 (6) | 7 (23) | .06b |
Paired t-test.
McNemar's test.
ART, antiretroviral therapy; PBMCs, peripheral blood mononuclear cells.
Phenotypic T cell profiles in HZ cases and controls
CD4+ and CD8+ T cell subsets in PBMCs were analyzed for expression of regulatory, senescence, and exhaustion markers, including FOXP3, CD25, CD127, CD39, CD28, CD57, TIM3, LAG3, and KLRG1 at the matching point, corresponding to the closest blood draw before HZ in cases. HZ cases had higher CD4+FOXP3+CD25+% Treg compared with controls, but the difference did not reach statistical significance (2.93 vs. 2.36; p = .08). There were no other appreciable T cell phenotypic differences.
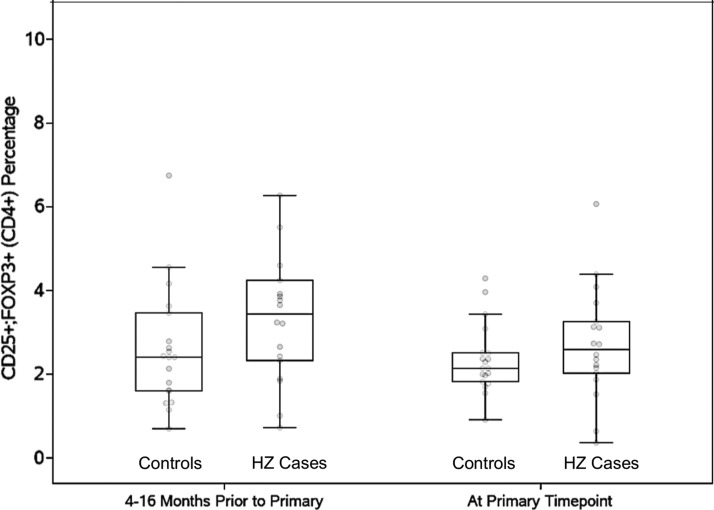
To determine the stability over time of the differences in CD4+FOXP3+CD25+% Treg between HZ cases and controls, we analyzed this subset in PBMCs collected before the matching point. Samples at 4–16 months before the matching point available from 18 HZ cases and 19 controls showed that Treg frequencies were stable over time in controls [mean difference (SD) = 0.24 (1.43); p = .94], but a significant decrease was observed in HZ cases [mean difference (SD) = 0.98 (1.75); p = .02; Fig. 1]. The data also showed that cases had higher CD4+FOXP3+CD25+% Treg compared with controls up to 16 months before development of HZ.
FIG. 1.
Longitudinal analysis of CD4+FOXP3+CD25+% Treg in PBMCs from HZ cases and controls. Data were derived from 18 HZ cases and 19 controls. The primary time point indicates the closest time before development of HZ in cases and also corresponds to the matching time point between cases and controls. The data show that the higher CD4+FOXP3+CD25+% Treg in cases compared with controls could be detected up to 16 months before development of HZ. HZ, herpes zoster; PBMCs, peripheral blood mononuclear cells.
VZV-specific functional T cell profiles in HZ cases and controls
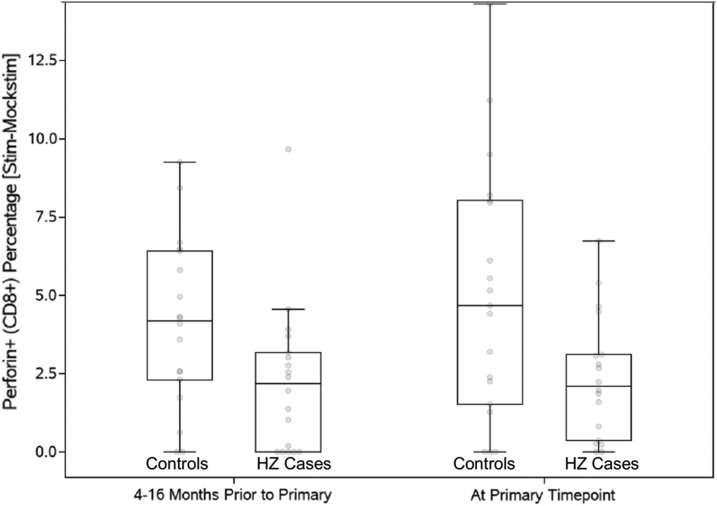
VZV-specific effector and regulatory T cell subsets were measured by ex vivo restimulation with VZV live virus, which is expressed in the context of MHC Class I and II, and has the ability to elicit both CD4+ and CD8+ T cell responses. HZ cases had lower VZV-specific CD8+perforin+% CTL (mean 2.61 vs. 4.14, p = .07), CD4+TGFβ+% Treg (0.96 vs. 1.88, p = .08), and CD4+IL10+% Treg (0.19 vs. 0.40, p = .04). Differences reached statistical significance only for IL10+ Treg (p = .04). We also investigated the stability of the VZV-specific CD8+perforin+% CTL over time and determined that these Th1 subsets did not significantly change over time in HZ cases or controls for up to 16 months before development of HZ (Fig. 2).
FIG. 2.
Longitudinal analysis of CD8+perforin+% CTL in HZ cases and controls. Data were derived from 18 HZ cases and 19 controls. The primary time point indicates the closest time before development of HZ in cases and also corresponds to the matching time point between cases and controls. The data show that the lower CD8+perforin+% CTL in cases compared with controls could be detected up to 16 months before development of HZ. CTLs, cytotoxic T lymphocytes.
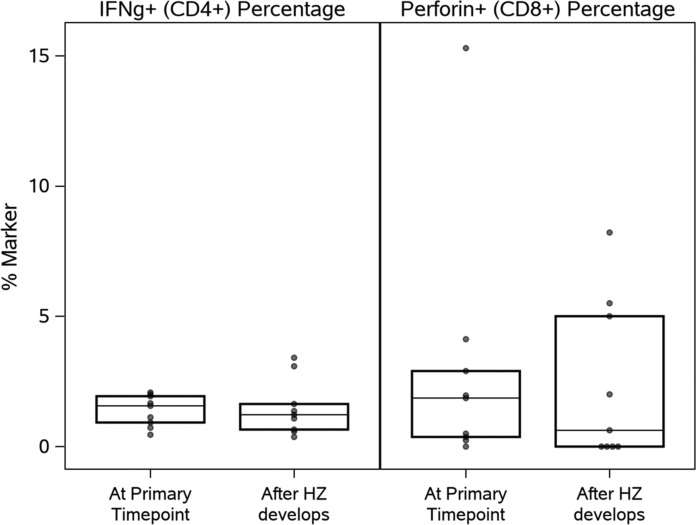
In nine HZ cases, PBMCs were available from 2 to 9 months after HZ. There was no significant increase in VZV-specific CTL or other markers after HZ (Fig. 3).
FIG. 3.
VZV-specific CD8+perforin+% CTL and CD4+IFNγ+% Th1 before and after HZ. Data were derived from 9 HZ cases. Boxes depict medians, upper, and lower quartiles. The primary time point indicates the closest time before development of HZ. The data show the lack of VZV CTL reconstitution after HZ (p > .1). VZV, varicella-zoster virus.
Correlation analyses of VZV-specific effectors and Treg in HZ cases and controls
To investigate a potential relationship between circulating Treg and VZV-specific Th1 and CTL responses, we performed correlation analyses (Table 2). The data showed significant positive correlations between VZV-specific CD8+perforin+% CTL and CD4+IFNg+% Th1 cells (r = 0.28; p = .03). CD4+FOXP3+CD25+% Treg were weakly negatively correlated with VZV-specific Th1 responses without reaching statistical significance (r = −0.23, p = .08), but not with VZV-CTL (r = −0.08, p = .57).
Table 2.
Correlation Analyses of Circulating Treg with Th1 and Cytotoxic T Lymphocyte Responses
| Marker | Circulating Treg | VZV-specific Th1 | VZV-specific CTL |
|---|---|---|---|
| Circulating Treg | |||
| Correlation | 1 | −0.23 | −0.08 |
| p | — | .08 | .57 |
| N | 61 | 60 | 60 |
| VZV-specific Th1 | |||
| Correlation | −0.23 | 1 | 0.28 |
| p | .08 | — | .03 |
| N | 60 | 60 | 60 |
| VZV-specific CTL | |||
| Correlation | −0.08 | 0.28 | 1 |
| p | .57 | .03 | — |
| N | 60 | 60 | 60 |
Bold indicates significant or strong trend assocaitions.
CTLs, cytotoxic T lymphocytes; VZV, varicella-zoster virus.
Discussion
This study showed a lack or at least a significant delay of VZV-specific T cell immune reconstitution in PLHIV who developed HZ. Interestingly, these individuals reached very low nadir CD4+ cell numbers with an average of ∼150 cells/dL, in contrast to the non-HZ controls, whose CD4+ cell numbers typically remained above ∼300 cells/dL. It is conceivable that many VZV-specific T cell clones were deleted when nadir CD4+ cell numbers were reached in HZ cases, which might have contributed to their difficulty in mounting a robust VZV-specific T cell response when the virus reactivated.
The fact that both VZV-specific CTL and VZV-specific Treg responses were lower in HZ cases compared with their matched controls supports the notion of global delay in VZV immune reconstitution as opposed to an imbalance between Th1 and Treg responses to VZV. The development of HZ in PLHIV after initiation of ART has been the object of controversy with respect to its relationship with immune reconstitution inflammatory syndrome (IRIS). The controversy arose from the epidemiological observations that the incidence of HZ is highest in the first few months after ART initiation followed by a gradual decline.24–26 However, the incidence of HZ was also similar in the 3 months before and after ART initiation, arguing in favor of a lag in VZV-specific immune reconstitution and against IRIS.4 The strongest pathophysiological argument against HZ as a manifestation of IRIS is that VZV is a lytic herpes virus with tissue-destructive capacity. Furthermore, the incidence of HZ is highest in individuals with decreased Th1 inflammatory responses against VZV, including older adults and hosts with CMI suppressive conditions,5–7 which is in accordance with our data showing lower VZV-specific Th1 responses in PLHIV who developed HZ compared with matched controls.
Furthermore, HZ cases showed marginally higher proportions of circulating Treg compared with controls. The association of increased circulating Treg with development of HZ was in accordance with our previous findings in PHIV.20 The mechanism whereby high proportion of nonspecific Treg may increase the likelihood of HZ is incompletely understood. The correlation analyses in this study showed an association of high circulating Treg and low VZV-specific Th1 responses before HZ. This finding is in accordance with previous reports showing that high proportions of Treg were associated with decreased Th1 responses to the live attenuated ZVL in older adults.27,28 It is important to note that Th1 responses to ZVL and HZ are very similar in composition and kinetics.29 Collectively, these observations suggest that Treg may dampen the generation of Th1 responses to VZV both in vitro and in vivo. Decreased Th1 responses to VZV in the context of viral reactivation may allow the virus to reach the critical mass necessary for symptomatic HZ.
The absence of an increase in VZV-specific Th1 responses after HZ in PLHIV was in contrast to previous studies in older adults and other immune-compromised hosts who showed robust increases in VZV-specific Th1 in response to VZV reactivation.9 The significance of our observation was limited by the small number of participants (N = 9) with pre- and post-HZ samples. However, our finding was in accordance with previous reports showing that the administration of the varicella vaccine or ZVL to PLHIV did not uniformly or significantly increase their Th1 responses at 6 weeks after vaccination compared with baseline.30,31 In contrast, an adjuvanted recombinant zoster vaccine (RZV) generated robust increases in VZV-specific CD4+ Th1 responses in PLHIV.32 Efficacy studies of ZVL or RZV have not been performed in PLHIV.
Despite successful ART, PLHIV did not increase VZV-CTL or VZV-Th1 in response to VZV reactivation manifested by HZ. This may explain the stability of VZV-CTL over time of ART in cases and controls. In contrast, Treg decreased with time on ART. Interestingly, the incidence of HZ also decreases over time after ART initiation. Based on the similar kinetics of HZ incidence and proportions of Treg, it is tempting to speculate that the proportion of Treg might have a larger effect on the risk of HZ in PLHIV than the proportion of VZV-CTL.
Our study was limited by the small sample size that fell below our a priori estimate of the number of participants necessary to generate significant results. Nevertheless, we were able to show marginally higher Treg in PLHIV who developed HZ compared with matched controls.
Treg may play a role in the development of HZ, most likely by inhibiting the VZV-specific immune responses in the context of VZV reactivation.
Acknowledgments
We thank the ACTG sites and study participants for their time and effort, and Frontier Science Foundation for data management. This study was supported by the Merck Investigator Initiated Program, Grant ID No. 51856 (A.W.) and by the National Institutes of Health through the following grants: AI 68636 and AI 38858 (ACTG); AI 68634 and AI 38855 (SDAC).
Author Disclosure Statement
A.W. receives research grants from Merck (moneys to the University of Colorado). The other authors do not have any conflicts to declare.
References
- 1. Buchbinder SP, Katz MH, Hessol NA, et al. : Herpes zoster and human immunodeficiency virus infection. J Infect Dis 1992;166:1153–1156 [DOI] [PubMed] [Google Scholar]
- 2. Veenstra J, Krol A, van Praag RM, et al. : Herpes zoster, immunological deterioration and disease progression in HIV-1 infection. AIDS 1995;9:1153–1158 [DOI] [PubMed] [Google Scholar]
- 3. Vanhems P, Voisin L, Gayet-Ageron A, et al. : The incidence of herpes zoster is less likely than other opportunistic infections to be reduced by highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2005;38:111–113 [DOI] [PubMed] [Google Scholar]
- 4. Levin MJ, Anderson JP, Seage GR, 3rd, Williams PL: Short-term and long-term effects of highly active antiretroviral therapy on the incidence of herpes zoster in HIV-infected children. J Acquir Immune Defic Syndr 2009;50:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gershon AA, Gershon MD: Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev 2013;26:728–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arvin AM: Humoral and cellular immunity to varicella-zoster virus: An overview. J Infect Dis 2008;197 Suppl 2:S58–S60 [DOI] [PubMed] [Google Scholar]
- 7. Weinberg A, Levin MJ: VZV T cell-mediated immunity. Curr Top Microbiol Immunol 2010;342:341–357 [DOI] [PubMed] [Google Scholar]
- 8. Arvin AM, Gilden D: Varicella-zoster virus. In: Fields Virology, Vol. 2 6th ed (Knipe D, Howley P., eds.) Lippincott, Williams and Wilkins, Philadelphia, PA, 2013, pp. 2015–2057 [Google Scholar]
- 9. Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM: Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J Infect Dis 1992;165:119–126 [DOI] [PubMed] [Google Scholar]
- 10. Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL: Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol 2008;80:1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL: Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol 2004;72:174–179 [DOI] [PubMed] [Google Scholar]
- 12. Mehta SK, Laudenslager ML, Stowe RP, Crucian BE, Sams CF, Pierson DL: Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav Immun 2014;41:210–217 [DOI] [PubMed] [Google Scholar]
- 13. Tenorio AR, Martinson J, Pollard D, Baum L, Landay A: The relationship of T-regulatory cell subsets to disease stage, immune activation, and pathogen-specific immunity in HIV infection. J Acquir Immune Defic Syndr 2008;48:577–580 [DOI] [PubMed] [Google Scholar]
- 14. Nilsson J, Boasso A, Velilla PA, et al. : HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 2006;108:3808–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vukmanovic-Stejic M, Sandhu D, Sobande TO, et al. : Varicella zoster-specific CD4+Foxp3+ T cells accumulate after cutaneous antigen challenge in humans. J Immunol 2013;190:977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou PF, Zhu LJ, Chen XY, Qiu ZQ: Age-related changes in CD4+CD25+FOXP3+ regulatory T cells and their relationship with lung cancer. PLoS One 2017;12:e0173048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duraiswamy J, Ibegbu CC, Masopust D, et al. : Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol 2011;186:4200–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khoury SJ, Sayegh MH: The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity 2004;20:529–538 [DOI] [PubMed] [Google Scholar]
- 19. Petrovas C, Casazza JP, Brenchley JM, et al. : PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 2006;203:2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinberg A, Huang S, Song LY, et al. : Immune correlates of herpes zoster in HIV-infected children and youth. J Virol 2012;86:2878–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosch RJ, Zhang X, Sandler NG: Study design issues in evaluating immune biomarkers. Curr Opin HIV AIDS 2013;8:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mildvan D, Landay A, De Gruttola V, Machado SG, Kagan J: An approach to the validation of markers for use in AIDS clinical trials. Clin Infect Dis 1997;24:764–774 [DOI] [PubMed] [Google Scholar]
- 23. Smurzynski M, Collier AC, Koletar SL, et al. : AIDS clinical trials group longitudinal linked randomized trials (ALLRT): Rationale, design, and baseline characteristics. HIV Clin Trials 2008;9:269–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haddow LJ, Moosa MY, Mosam A, Moodley P, Parboosing R, Easterbrook PJ: Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS One 2012;7:e40623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M: Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: A systematic review and meta-analysis. Lancet Infect Dis 2010;10:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tangsinmankong N, Kamchaisatian W, Lujan-Zilbermann J, Brown CL, Sleasman JW, Emmanuel PJ: Varicella zoster as a manifestation of immune restoration disease in HIV-infected children. J Allergy Clin Immunol 2004;113:742–746 [DOI] [PubMed] [Google Scholar]
- 27. Lelic A, Verschoor CP, Lau VW, et al. : Immunogenicity of varicella vaccine and immunologic predictors of response in a cohort of elderly nursing home residents. J Infect Dis 2016;214:1905–1910 [DOI] [PubMed] [Google Scholar]
- 28. Weinberg A, Levin M, Schmader K, et al. : Varicella zoster virus (VZV) cell-mediated immunity 3 years after the administration of zoster vaccine to septuagenarians immunized 10 years previously. Open Forum Infect Dis 2016;3:715 [Google Scholar]
- 29. Levin MJ, Cai GY, Lee K, et al. : Varicella-zoster virus DNA in blood after administration of herpes zoster vaccine. J Infect Dis 2018;217:1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benson CA, Andersen JW, Macatangay BJC, et al. : Safety and immunogenicity of zoster vaccine live in human immunodeficiency virus—Infected adults with CD4+ cell counts >200 cells/mL virologically suppressed on antiretroviral therapy. Clin Infect Dis 2018;67:1712–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weinberg A, Levin MJ, Macgregor RR: Safety and immunogenicity of a live attenuated varicella vaccine in VZV-seropositive HIV-infected adults. Hum Vaccin 2010;6:318–321 [DOI] [PubMed] [Google Scholar]
- 32. Berkowitz EM, Moyle G, Stellbrink HJ, et al. : Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: A phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015;211:1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]