Abstract
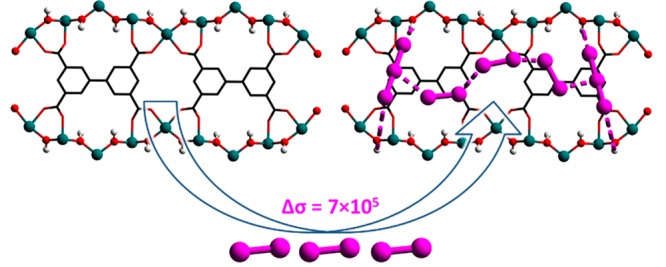
We report a comparative study of the binding of I2 (iodine) in a pair of redox-active metal–organic framework (MOF) materials, MFM-300(VIII) and its oxidized, deprotonated analogue, MFM-300(VIV). Adsorption of I2 in MFM-300(VIII) triggers a host-to-guest charge-transfer, accompanied by a partial (∼30%) oxidation of the VIII centers in the host framework and formation of I3– species residing in the MOF channels. Importantly, this charge-transfer induces a significant enhancement in the electrical conductivity (Δσ = 700000) of I2@MFM-300(VIII/IV) in comparison to MFM-300(VIII). In contrast, no host–guest charge-transfer or apparent change in the conductivity was observed upon adsorption of I2 in MFM-300(VIV). High-resolution synchrotron X-ray diffraction of I2@MFM-300(VIII/IV) confirms the first example of self-aggregation of adsorbed iodine species (I2 and I3–) into infinite helical chains within a MOF.
Short abstract
Adsorption of I2 in MFM-300(VIII) induces host-to-guest charge-transfer, resulting in the formation of a mixed-valence material I2@MFM-300(VIII/IV), which shows a significant enhancement in the electrical conductivity (Δσ = 700000) in comparison with MFM-300(VIII).
Introduction
Nuclear energy shows promise to bridge future gaps in the supply of electricity.1 However, the radionuclides generated from the nuclear power plant can pose significant risks on both human health and ecosystems if emitted into the environment.2 Radioactive iodine (primarily comprised of 129I and 131I) is a key volatile waste that can be spread through air and interferes with human metabolic processes.3 Various techniques and materials have been applied for I2 capture,4,5 and porous materials, because of their high porosity and fast adsorption kinetics, are considered to be emerging sorbents for the efficient removal of I2.
Porous solid-state sorbents with rigid structures such as zeolites, C atoms, and silica materials have been widely studied for I2 adsorption.6−8 Metal–organic framework (MOF) materials provide a unique platform to investigate their interaction with adsorbed I2 because of their crystalline nature and tunable structural properties.9 Various approaches, including linker functionalization10 and shaping of the porosity,11 have been reported to improve I2 adsorption in modified MOFs. However, the binding of I2 in MOFs with redox-active metal centers (e.g., FeII/III, CrII/III, VIII/IV, and NiII/III) remain largely unexplored, which can be attributed to the scarcity of reported stable redox-active MOFs.12−15 Furthermore, collapse or, to a lesser extent, degradation of the MOF upon inclusion of I2 can occur, thus restricting the investigation of the host–guest binding via a charge-transfer mechanism.
Herein we report the adsorption and structural study of binding domains for I2 in a pair of stable redox-active MOFs, MFM-300(VIII) and MFM-300(VIV).16 Host–guest charge-transfer has been unambiguously observed in MFM-300(VIII) by electron paramagnetic resonance (EPR) spectroscopy, promoting a 700000 times enhancement in the electrical conductivity of the I2-loaded MOF material in comparison to MFM-300(VIII). In contrast, there is an absence of host–guest charge-transfer or an apparent change in the conductivity for I2-adsorbed MFM-300(VIV). We also report the unusual self-aggregation of confined I2 and I3– molecules into a 1D helical chain within the channels of MFM-300(V).
Results and Discussion
Structure of MFM-300(VIII,IV)
MFM-300(VIII), [V2(OH)2(L)] (H4L = biphenyl-3,3′,5,5′-tetracarboxylic acid), crystallizes in a tetragonal system in which the VIII center is coordinated by six O donors, four from carboxylates and two from bridging hydroxyl groups μ2-OH. This affords an infinite chain of [V2(OH)2O4] moieties along the c axis (Figure 1), and these are further bridged by the deprotonated organic linkers to afford a rigid wine-rack-type open framework with square-shaped channels of ∼6.7 Å diameter. Oxidation of MFM-300(VIII) in air yields the analogue MFM-300(VIV), [V2(O)2(L)], where the VIII center is oxidized to VIV, and this is coupled with deprotonation of hydroxyl to oxy bridges. MFM-300(VIV) retains the same overall framework topology except for a small contraction on the V–O bond distances accompanied by a slight decrease in the pore volume from 0.49 to 0.48 cm3 g–1 upon oxidation.
Figure 1.
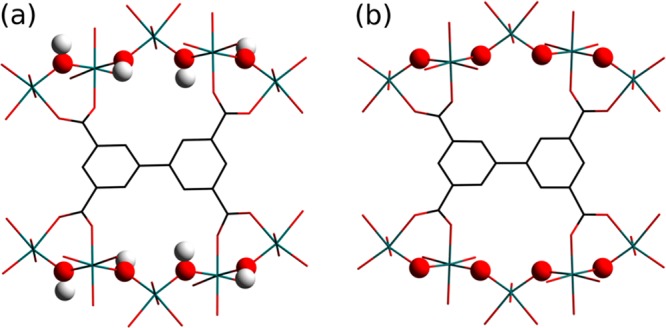
Views along the b axis of the infinite metal chains in (a) MFM-300(VIII) and (b) MFM-300(VIV). The hydroxyl groups (H atom, white; O atom, red) in MFM-300(VIII) are deprotonated to an O2– bridge (red) in MFM-300(VIV).
Iodine Adsorption in MFM-300(VIII,IV)
The as-synthesized MOFs were exchanged with acetone over a period of 1 week. The desolvated samples were prepared by heating the acetone-exchanged samples under vacuum for 1 day at 150 °C until no weight loss of solvent was observed by thermogravimetric analysis (TGA). The desolvated MOF was transferred into a vessel under dry N2 containing a vial of solid I2. The I2 vapor was allowed to diffuse into the desolvated MOF at 343 K for 2 days to allow full adsorption. The color of both MOF materials changed from pale green and brown for VIII and VIV materials, respectively, to dark brown. Scanning electron microscopy (SEM) images confirm the absence of surface-adsorbed I2 or changes in the crystal morphology upon adsorption of I2 (Figure S3). The maximum adsorption capacities of I2 in MFM-300(VIII) and MFM-300(VIV) have been determined by TGA–mass spectrometry (MS) to be 1.42 g g–1 and 1.25 g g–1, respectively (Figure 2). The difference in the adsorption uptake is due to a slight variation in the pore volumes and window sizes. These uptakes are higher than those previously reported for redox-active MOFs, such as BOF-117 (0.66 g g–1) and Cu[Ni(pdt)2]14 (H2pdt = pyrazine-2,3-dithiol; 0.18 g g–1), and are comparable with the robust ZIF-818 (1.25 g g–1) but lower than HKUST-119 (1.75 g g–1), which incorporates open metal sites and a larger pore volume (0.74 cm3 g–1). The adsorption of I2 in MFM-300(V) materials is fully reversible, and no apparent loss in capacity was observed for three cycles of sorption–desorption in both MOFs (Figures S4–S6). The densities of adsorbed I2 within the pores of MFM-300(VIII) and MFM-300(VIV) are calculated to be 2.90 and 2.60 g cm–3, respectively. The former is comparable to the best-behaving MOF to date [3.08 g cm–3 in MFM-300(Sc)].20
Figure 2.
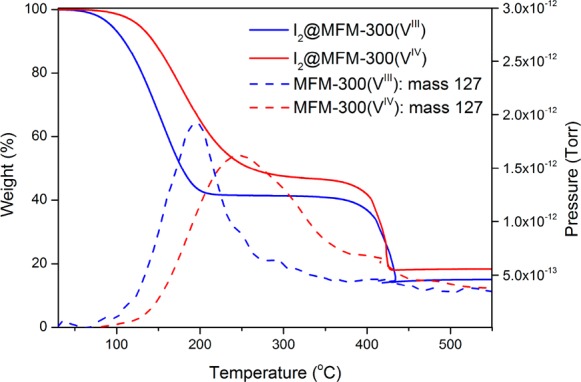
TGA–MS plots for I2-saturated MFM-300(VIII) and MFM-300(VIV).
Determination of I2 Binding Sites within MFM-300(VIII/IV)
The binding sites for adsorbed I2 molecules within MFM-300(V) have been elucidated by high-resolution synchrotron powder X-ray diffraction (PXRD). Structural analysis of I2-loaded MOF samples at approximately 1.0 I2/V loading via Rietveld refinement confirmed the absence of any structural phase changes and revealed formulae of [V2(OH)2(L)]·1.03I2·0.6I3– and [V2(O)2(L)]·2.2I2 for I2-loaded MFM-300(VIII) and MFM-300(VIV), respectively. Upon loading of I2 into MFM-300(VIII), changes are observed in the V–O bond lengths and angles, and three independent sites for neutral I2 molecules and one site for anionic I3– are observed within the channel (Figure 3a). Bond-valence-sum (BVS) calculations (Table 1) give an overall valence of 3.28 for the V center, consistent with its partial oxidation, and this is balanced by the presence of triiodide I3– anions. The I3– anion (occupancy = 0.3) is located near the hydroxyl group from the [VIIIO4(OH)2] moiety with a short distance [I3–···H–O = 2.94(1) Å], indicating the formation of a strong hydrogen bond between the triiodide and the −OH groups. It is worth noting that protons on the hydroxyl groups cannot be conclusively located from the PXRD data, and it is likely that these protons are partially delocalized to accompany the host–guest charge-transfer. III2 is located interstitially between two phenyl rings of neighboring ligand molecules [III2···phenyl ring = 4.68(1) and 5.15(1) Å] with an occupancy of 0.25. IIII2 and IIV2 adopt low occupancies (0.16 and 0.10, respectively), reside in the center of the channel, and are stabilized by intermolecular interactions (Figure S10). These results confirm partial oxidation of the framework by adsorbed I2 molecules to afford a mixed-valence I2@MFM-300(VIII/IV) material.
Figure 3.
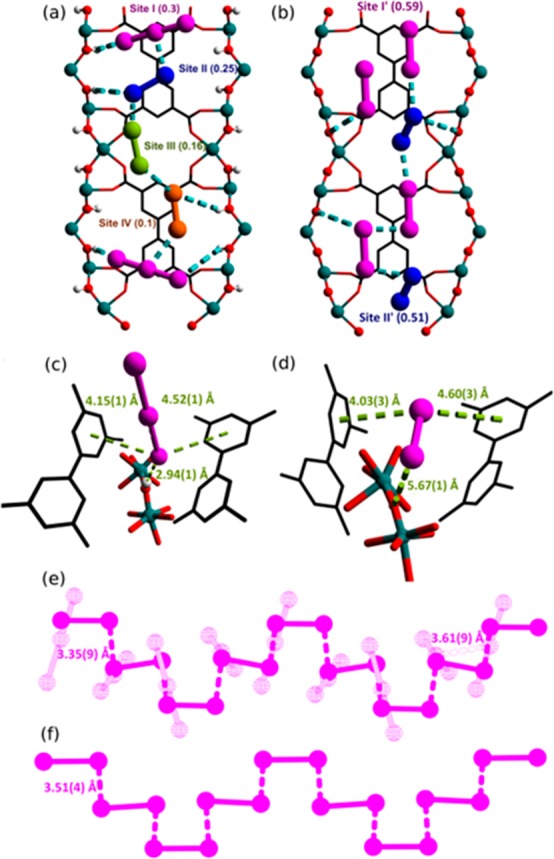
Views along the b axis of I2-loaded (a) MFM-300(VIII) and (b) MFM-300(VIV) obtained by high-resolution synchrotron PXRD. Views of binding sites for I3– and I2 in (c) MFM-300(VIII) and (d) MFM-300(VIV), respectively. Views of I2 (solid) and I3– (pale wire frame) in (e) I2@MFM-300(VIII/IV) and (f) I2@MFM-300(VIV).
Table 1. Bond-Length and BVS Calculations of MFM-300(VIII), MFM-300(VIV), and I2@MFM-300(VIII/IV).
| MFM-300(VIII) | MFM-300(VIV) | I2@MFM-300(VIII/IV) | |
|---|---|---|---|
| V–Obridging (Å) | 1.978(1) | 1.838(1) | 1.901(2) |
| V–Ocarboxylate (Å) | 2.004(2) | 1.971(2) | 2.014(1) |
| 2.007(2) | 2.031(2) | 2.070(9) | |
| ∠V---Obridging---V(O) | 125.6(1) | 134.7(2) | 130.1(1) |
| V---V distance (Å) | 3.519 | 3.392 | 3.447 |
| BVS calculation for V | 3.027 | 3.960 | 3.278 |
[V2(O)2(L)]·2.2I2 shows two primary binding domains, I′ and II′, with occupancies of 0.59 and 0.51, respectively, for adsorbed I2 molecules within the channel. There is little difference on the V–O bond distances in MFM-300(VIV) upon adsorption of I2, suggesting the absence of host–guest charge-transfer. Also, there is an absence of direct binding of adsorbed I2 molecules with the pore interior such as the oxy bridges [I2I′···Obridge = 5.67(1) Å; III′2···Obridge = 5.22(1) Å]. A detailed examination of [V2(O)2(L)]·2.2I2 confirmed that the confined I2 molecules within the pores aggregate to form an unusual helical chain running through the channel with a distance of 3.51(4) Å between adjacent I2 molecules. This intermolecular I2···I2 distance is comparable to that [3.35(9) Å] observed in the single helical chain of I2 and I3– in I2@MFM-300(VIII/IV) (Figure 3e,f). A more detailed structural analysis of I2@MFM-300(VIV) by single-crystal X-ray diffraction reveals a similar structural model of infinite helical I2 chains with an intermolecular I2···I2 distance of 3.53(4) Å (Figure S13). To date, the existence of one-fold helical chains of I2 has only been confirmed by theoretical studies,21 while the linear I2 chain has been observed crystallographically in Ln2Cu5(OH)2(pydc)6(H2O)8 (H2pydc = pyridine-2,5-dicarboxylic acid).22 Thus, the helical I2 chain observed in I2@MFM-300(V) at crystallographic resolution represents the first example of such a motif in porous materials. The formation of triple-helical I2 chains has been previously observed in MFM-300(Sc).
Spectroscopic Analysis of I2@MFM-300(VIII/IV)
X-ray photoelectron spectroscopy (XPS) was used to investigate the valence of adsorbed I2 species within MFM-300(V) (Figure 4a,b). For I2@MFM-300(VIV), one chemical species (one doublet for the spin–orbit splitting of the 3d level) of I2 was observed with the I 3d5/2 photoelectron peak at 620.7 eV, indicating that only one type of adsorbed I2 species is trapped inside the pore and all of the adsorbed I2 molecules remain neutral. For I2@MFM-300(VIII/IV), however, two characteristic chemical species (I 3d5/2 at 619.1 and 620.7 eV) were observed, corresponding to the I3– and I2 moieties, respectively,23 consistent with the structural models. Electron-accepting guest inclusion results in an increase in the electrical conductivity24,25 so measurements of the electrical conductivity of the bare and I2-loaded MFM-300(V) materials were performed to examine the effect of host–guest charge-transfer.
Figure 4.
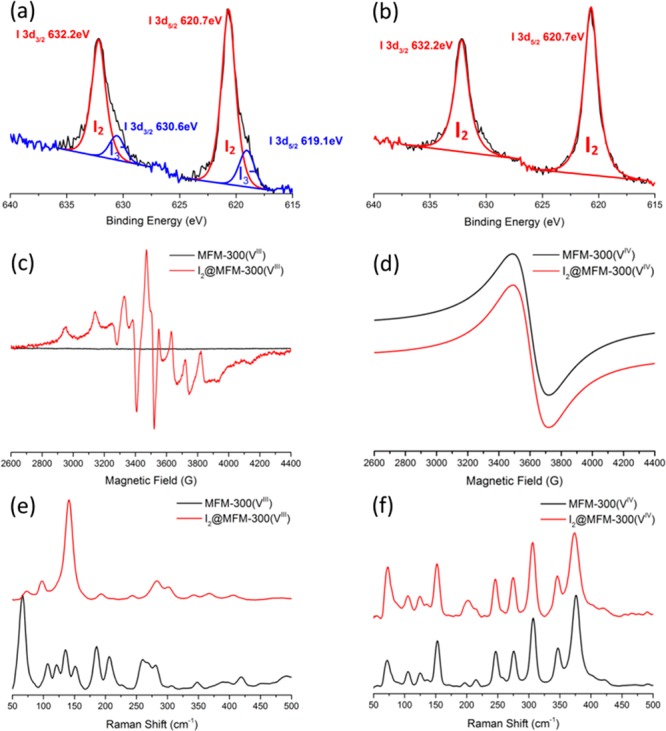
XPS spectra of I2-loaded (a) MFM-300(VIII) and (b) MFM-300(VIV). EPR spectra of desolvated and I2-loaded (c) MFM-300(VIII) and (d) MFM-300(VIV). Raman spectra of desolvated and I2-loaded (e) MFM-300(VIII) and (f) MFM-300(VIV).
The conductivity of MFM-300(VIII) was measured to be 1.7 × 10–10 S/cm, but I2@MFM-300(VIII/IV) shows a significant enhancement (Δσ = 700000) in conductivity in the dark to 1.2 × 10–4 S/cm (Figure S14). This can be attributed to both the oxidized V–O(H)–V skeletons and generated iodide chains that provide further transport pathways to facilitate electron transfer.26 The value is comparable to the state-of-the-art conductivity observed for I2-loaded MOFs [1 × 10–4 S/cm for I2@Cu[Ni(pdt)2]14 and 2.16 × 10–4 S/cm for I2@[Tb3(Cu4I4)3(ina)9]n27 (H2pdt = pyrazine-2,3-dithiol; Hina = isonicotinic acid)] (Table 2). Measurements on MFM-300(VIV) and I2@MFM-300(VIV) show very low conductivities (<1 × 10–10 S/cm) in both cases. Thus, the host–guest charge-transfer in I2-loaded MFM-300(VIII) enhances and promotes the electrical conductivity of the resultant mixed-valence I2@MFM-300(VIII/IV) material. Electrochemical impedance spectroscopy confirms (Figure S16) that I2@MFM-300 (VIII/IV) with a charge-transfer resistance (Rct) of 383 Ω shows higher electron conduction compared with bare MFM-300(VIII) with a Rct value of 1112 Ω.
Table 2. Summary of Electrical Conductivities for I2-Loaded MOFsa.
| MOF | conductivity for bare MOFs (S/cm) | conductivity for I2-loaded MOFs (S/cm) | conductivity enhancement (magnitudes) | ref |
|---|---|---|---|---|
| Cu[Ni(pdt)2]b | 1 × 10–8 | 1 × 10–4 | ∼104 | (14) |
| [Cu6(pybz)8(OH)2](I–)2c | 8.04 × 10–9 | 8.11 × 10–7 | ∼102 | (29) |
| [Co1.5(bdc)1.5(H2bpz)]d | 2.59 × 10–9 | 1.56 × 10–6 | ∼103 | (30) |
| [Co(ebic)2]nd | 2.46 × 10–9 | 2.21 × 10–7 | ∼102 | (31) |
| [Eu(L1)]d | 8.27 × 10–7 | 2.71 × 10–5 | ∼102 | (32) |
| IFMC-15d | 2.59 × 10–9 | 2.07 × 10–7 | ∼102 | (33) |
| {[(Me2NH2)2]·[Cd3(5-tbip)4]}nd | 1.71 × 10–8 | 1.30 × 10–6 | ∼102 | (34) |
| MET-3d | 0.77 × 10–4 | 1 × 10–3 | ∼101 | (35) |
| [Tb3(Cu4I4)3(ina)9]nc | 5.72 × 10–11 | 2.16 × 10–4 | ∼108 | (27) |
| [Zn3(dl-lac)2(pybz)2]nc | σ∥=3.4 × 10–3 | (9) | ||
| σ⊥ = 1.7 × 10–4 | ||||
| [Zn(ebic)2]nd | 4.33 × 10–9 | 3.47 × 10–7 | ∼102 | (31) |
| MFM-300(VIII)d | 1.7 × 10–10 | 1.16 × 10–4 | ∼106 | this work |
The value of the electrical conductivity for solid I2 is 1 × 10–9 S/cm. H2pdt = pyrazine- 2,3-dithiol, Hpybz = 4-pyridylbenzoic acid, H2bdc = benzene-1,4-dicarboxylic acid, bpz = 3,3′,5,5′-tetramethyl-4,4′-bipyrazole, Hebic = 2-ethyl-1H-benzo[d]imidazole-5-carboxylic acid, H3L1 = biphenyl-3,4′,5-tricarboxylate, H2-5-tbip = 5-tert-butylisophthalic acid, Hina = isonicotinic acid, H2-DL-lac = lactic acid, Hpybz = 4-pyridylbenzoic acid, and Hebic = 2-ethyl-1H-benzo[d]imidazole-5-carboxylic acid.
Films were used for testing of the electrical conductivity.
Single crystals were used for testing of the electrical conductivity.
Pressed pellets were used for testing of the electrical conductivity.
EPR spectroscopy confirms partial oxidation of the V centers in MFM-300(VIII) upon adsorption of I2 (Figure 4c). MFM-300(VIII) is EPR-silent at X band, as is common for VIII materials because of the typically very large zero-field splitting of a d2, S = 1 ion (up to tens of cm–1).16 In contrast, I2@MFM-300(VIII/IV) shows a typical VIV (d1, S = 1/2) EPR spectrum with resolution of the 51V (I = 7/2) hyperfine interaction (Figure S15 and Table S3 for simulation and parameters). Both MFM-300(VIV) and I2@MFM-300(VIV) give a broad, unresolved EPR signal (Figure 4d) consistent with VIV under magnetically non-dilute conditions. Thus, the EPR spectroscopic results are entirely consistent with partial oxidation of VIII to VIV in I2@MFM-300(VIII/IV) but a negligible effect of I2 on the metal ions in I2@MFM-300(VIV).
Raman spectroscopy was applied to examine the nature of the interaction between adsorbed I2 molecules and the MOF hosts. For MFM-300(VIII), two new peaks are observed at 150 and 185 cm–1 upon adsorption of I2; the former band is prominent in the spectrum and assigned to the asymmetric stretching mode of I3– ions within the pores, while the latter is attributed to the vibration of neutral I2 molecules.28 For MFM-300(VIV), in the low-energy region, a characteristic peak at 205 cm–1 is observed for I2-loaded MFM-300(VIV), which can be assigned to the intrinsic vibration of trapped I2 molecules. Compared to the intramolecular I–I vibration (ca. 180 cm–1 for solid I2), the blue shift (Δ = 25 cm–1) can be assigned to the stronger intermolecular interaction of confined I2 molecules within the helical chains, fully consistent with the structural models derived from synchrotron X-ray diffraction data.
Conclusion
In summary, we report the adsorption and binding domains of I2 in a pair of redox-active MOF materials, MFM-300(VIII) and MFM-300(VIV), which provide an excellent platform to examine the host–guest charge-transfer properties. Adsorption of I2 in MFM-300(VIII) induces host–guest charge-transfer via partial oxidation of the V centers and formation of I3– species in the pore to balance the overall charge. As a result, 7 × 105 enhancement of the electrical conductivity is observed for the I2-loaded mixed-valence I2@MFM-300(VIII/IV). In contrast, there is an absence of host–guest charge-transfer observed for MFM-300(VIV) upon loading of I2 with no change in the inherent low conductivity of the parent MOF. In both cases, unusual self-aggregation of confined I2 molecules into helical chains within the MOF hosts has been observed at crystallographic resolution, defining the molecular details for the underlying host–guest binding interactions and paving the way for the design and discovery of new functional materials with improved I2 adsorption properties.
Acknowledgments
We thank EPSRC (EP/I011870, EP/K038869, and EP/P001386), ERC (AdG 742041), and the Royal Society and University of Manchester for funding. We thank EPSRC for funding of the EPSRC National Service for EPR Spectroscopy at Manchester. We are especially grateful to Diamond Light Source for access to the Beamline I11. X.Z. and R.F. acknowledge financial support from China Scholarship Council and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001, respectively.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.inorgchem.9b02176.
Synthesis, iodine adsorption measurements, powder X-ray diffraction, SEM images, and Rietveld refinement results (PDF)
Accession Codes
CCDC 1915136–1915138 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Gralla F.; Abson D. J.; Møller A. P.; Lang D. J.; von Wehrden H. Energy Transitions and National Development Indicators: A Global Review of Nuclear Energy Production. Renewable Sustainable Energy Rev. 2017, 70, 1251–1265. 10.1016/j.rser.2016.12.026. [DOI] [Google Scholar]
- Deblonde G. J.-P.; Ricano A.; Abergel R. J. Ultra-Selective Ligand-Driven Separation of Strategic Actinides. Nat. Commun. 2019, 10, 2438–2447. 10.1038/s41467-019-10240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstadt A. E.; Nelson N. C.; Claude A. K.; Refsal K. R.; Scott-Moncrieff J. C.; Petroff B. K.; Langlois D. K. Radioactive Iodine Uptake in Hyperthyroid Cats after Administration of Recombinant Human Thyroid Stimulating Hormone. J. Vet. Intern. Med. 2018, 32, 1891–1896. 10.1111/jvim.15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’dowd C. D.; Jimenez J. L.; Bahreini R.; Flagan R. C.; Seinfeld J. H.; Hämeri K.; Pirjola L.; Kulmala M.; Jennings S. G.; Hoffmann T. Marine Aerosol Formation from Biogenic Iodine Emissions. Nature 2002, 417, 632–636. 10.1038/nature00775. [DOI] [PubMed] [Google Scholar]
- Sava D. F.; Garino T. J.; Nenoff T. M. Iodine Confinement into Metal–Organic Frameworks (MOFs): Low-Temperature Sintering Glasses To Form Novel Glass Composite Material (GCM) Alternative Waste Forms. Ind. Eng. Chem. Res. 2012, 51, 614–620. 10.1021/ie200248g. [DOI] [Google Scholar]
- Wang P.; Xu Q.; Li Z.; Jiang W.; Jiang Q.; Jiang D. Exceptional Iodine Capture in 2D Covalent Organic Frameworks. Adv. Mater. 2018, 30, 1801991. 10.1002/adma.201801991. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam K. S.; Sarma D.; Malliakas C. D.; Polychronopoulou K.; Riley B. J.; Pierce D. A.; Chun J.; Kanatzidis M. G. Chalcogenide Aerogels as Sorbents for Radioactive Iodine. Chem. Mater. 2015, 27, 2619–2626. 10.1021/acs.chemmater.5b00413. [DOI] [Google Scholar]
- Yan Z.; Yuan Y.; Tian Y.; Zhang D.; Zhu G. Highly Efficient Enrichment of Volatile Iodine by Charged Porous Aromatic Frameworks with Three Sorption Sites. Angew. Chem., Int. Ed. 2015, 54, 12733–12737. 10.1002/anie.201503362. [DOI] [PubMed] [Google Scholar]
- Zeng M.-H.; Wang Q.-X.; Tan Y.-X.; Hu S.; Zhao H.-X.; Long L.-S.; Kurmoo M. Rigid Pillars and Double Walls in a Porous Metal-Organic Framework: Single-Crystal to Single-Crystal, Controlled Uptake and Release of Iodine and Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 2561–2563. 10.1021/ja908293n. [DOI] [PubMed] [Google Scholar]
- Falaise C.; Volkringer C.; Facqueur J.; Bousquet T.; Gasnot L.; Loiseau T. Capture of Iodine in Highly Stable Metal-Organic Frameworks: A Systematic Study. Chem. Commun. 2013, 49, 10320–10322. 10.1039/c3cc43728k. [DOI] [PubMed] [Google Scholar]
- Marshall R. J.; Griffin S. L.; Wilson C.; Forgan R. S. Stereoselective Halogenation of Integral Unsaturated C-C Bonds in Chemically and Mechanically Robust Zr and Hf MOFs. Chem. - Eur. J. 2016, 22, 4870–4877. 10.1002/chem.201505185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozek C. K.; Dincǎ M. Ti3+-, V2+/3+-, Cr2+/3+-, Mn2+-, and Fe2+-Substituted MOF-5 and Redox Reactivity in Cr- and Fe-MOF-5. J. Am. Chem. Soc. 2013, 135, 12886–12891. 10.1021/ja4064475. [DOI] [PubMed] [Google Scholar]
- D’Alessandro D. M. Exploiting Redox Activity in Metal–organic Frameworks: Concepts, Trends and Perspectives. Chem. Commun. 2016, 52, 8957–8971. 10.1039/C6CC00805D. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y.; Jacobs B.; Allendorf M. D.; Long J. R. Conductivity, Doping, and Redox Chemistry of a Microporous Dithiolene-Based Metal-Organic Framework. Chem. Mater. 2010, 22, 4120–4122. 10.1021/cm101238m. [DOI] [Google Scholar]
- Zeng M. H.; Yin Z.; Tan Y. X.; Zhang W. X.; He Y. P.; Kurmoo M. Nanoporous cobalt(II) MOF Exhibiting Four Magnetic Ground States and Changes in Gas Sorption upon Post-Synthetic Modification. J. Am. Chem. Soc. 2014, 136, 4680–4688. 10.1021/ja500191r. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Godfrey H. G. W.; da Silva I.; Cheng Y.; Savage M.; Tuna F.; McInnes E. J. L.; Teat S. J.; Gagnon K. J.; Frogley M. D.; et al. Modulating Supramolecular Binding of Carbon Dioxide in a Redox-Active Porous Metal-Organic Framework. Nat. Commun. 2017, 8, 14212–14222. 10.1038/ncomms14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. J.; Suh M. P. Dynamic and Redox Active Pillared Bilayer Open Framework : Single-Crystal-to-Single-Crystal Transformations upon Guest Removal, Guest Exchange, and Framework Oxidation. J. Am. Chem. Soc. 2004, 126, 15844–15851. 10.1021/ja0466715. [DOI] [PubMed] [Google Scholar]
- Sava D. F.; Rodriguez M. A.; Chapman K. W.; Chupas P. J.; Greathouse J. A.; Crozier P. S.; Nenoff T. M. Capture of Volatile Iodine, a Gaseous Fission Product, by Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2011, 133, 12398–12401. 10.1021/ja204757x. [DOI] [PubMed] [Google Scholar]
- Sava D. F.; Chapman K. W.; Rodriguez M. A.; Greathouse J. A.; Crozier P. S.; Zhao H.; Chupas P. J.; Nenoff T. M. Competitive I2 Sorption by Cu-BTC from Humid Gas Streams. Chem. Mater. 2013, 25, 2591–2596. 10.1021/cm401762g. [DOI] [Google Scholar]
- Zhang X.; da Silva I.; Godfrey H. G. W.; Callear S. K.; Sapchenko S. A.; Cheng Y.; Vitorica-Yrezabal I. J.; Frogley M. D.; Cinque G.; Tang C. C.; et al. Confinement of Iodine Molecules into Triple-Helical Chains within Robust Metal–organic Frameworks. J. Am. Chem. Soc. 2017, 139, 16289–16296. 10.1021/jacs.7b08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z.; Liu C.-J.; Li Y.; Jing X.-D.; Yuan Q. Helicity Analysis of Single, Double, and Triple Helical Iodine Chains inside Single-Walled Silicon Carbide Nanotubes. Can. J. Phys. 2017, 95, 731–737. 10.1139/cjp-2016-0901. [DOI] [Google Scholar]
- Hu X. L.; Sun C. Y.; Qin C.; Wang X. L.; Wang H. N.; Zhou E. L.; Li W. E.; Su Z. M. Iodine-Templated Assembly of Unprecedented 3d-4f Metal-Organic Frameworks as Photocatalysts for Hydrogen Generation. Chem. Commun. 2013, 49, 3564–3566. 10.1039/c3cc39173f. [DOI] [PubMed] [Google Scholar]
- Hsu S. L.; Signorelli A. J.; Pez G. P.; Baughman R. H. Highly Conducting Iodine Derivatives of Polyacetylene: Raman, XPS, and X-Ray Diffraction Studies. J. Chem. Phys. 1978, 69, 106–111. 10.1063/1.436393. [DOI] [Google Scholar]
- Talin A. A.; Centrone A.; Ford A. C.; Foster M. E.; Stavila V.; Haney P.; Kinney R. A.; Szalai V.; El Gabaly F.; Yoon H. P.; Léonard F.; Allendorf M. D. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. 10.1126/science.1246738. [DOI] [PubMed] [Google Scholar]
- Schneider C.; Ukaj D.; Koerver R.; Talin A. A.; Kieslich G.; Pujari S. P.; Zuilhof H.; Janek J.; Allendorf M. D.; Fischer R. A. High Electrical Conductivity and High Porosity in a Guest@MOF Material: Evidence of TCNQ Ordering within Cu3BTC2micropores. Chem. Sci. 2018, 9, 7405–7412. 10.1039/C8SC02471E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Campbell M. G.; Dinca M. Electrically Conductive Porous Metal-Organic Frameworks. Angew. Chem., Int. Ed. 2016, 55, 3566–3579. 10.1002/anie.201506219. [DOI] [PubMed] [Google Scholar]
- Hu Y.-Q.; Li M.-Q.; Wang Y.; Zhang T.; Liao P.-Q.; Zheng Z.; Chen X.-M.; Zheng Y.-Z. Direct Observation of Confined I–...I2...I– Interactions in a Metal-Organic Framework: Iodine Capture and Sensing. Chem. - Eur. J. 2017, 23, 8409–8413. 10.1002/chem.201702087. [DOI] [PubMed] [Google Scholar]
- Blake A. J.; Li W.-S.; Lippolis V.; Parsons S.; Radek C.; Devillanova F. A.; Gould R. O.; Schroder M. Template Self-Assembly of Polyiodide Networks. Chem. Soc. Rev. 1998, 27, 195–205. 10.1039/a827195z. [DOI] [PubMed] [Google Scholar]
- Yin Z.; Wang Q. X.; Zeng M. H. Iodine Release and Recovery, Influence of Polyiodide Anions on Electrical Conductivity and Nonlinear Optical Activity in an Interdigitated and Interpenetrated Bipillared-Bilayer Metal-Organic Framework. J. Am. Chem. Soc. 2012, 134, 4857–4863. 10.1021/ja211381e. [DOI] [PubMed] [Google Scholar]
- Li G.-P.; Zhang K.; Zhao H.-Y.; Hou L.; Wang Y.-Y. Increased Electric Conductivity upon I2 Uptake and Gas Sorption in a Pillar-Layered Metal-Organic Framework. ChemPlusChem 2017, 82, 716–720. 10.1002/cplu.201700063. [DOI] [PubMed] [Google Scholar]
- Yu F.; Li D. D.; Cheng L.; Yin Z.; Zeng M. H.; Kurmoo M. Porous Supramolecular Networks Constructed of One-Dimensional Metal-Organic Chains: Carbon Dioxide and Iodine Capture. Inorg. Chem. 2015, 54, 1655–1660. 10.1021/ic502650z. [DOI] [PubMed] [Google Scholar]
- Hao Z.; Yang G.; Song X.; Zhu M.; Meng X.; Zhao S.; Song S.; Zhang H. A Europium(iii) Based Metal-Organic Framework: Bifunctional Properties Related to Sensing and Electronic Conductivity. J. Mater. Chem. A 2014, 2, 237–244. 10.1039/C3TA13179C. [DOI] [Google Scholar]
- He W. W.; Li S. L.; Yang G. S.; Lan Y. Q.; Su Z. M.; Fu Q. Controllable Synthesis of a Non-Interpenetrating Microporous Metal-Organic Framework Based on Octahedral Cage-like Building Units for Highly Efficient Reversible Adsorption of Iodine. Chem. Commun. 2012, 48, 10001–10003. 10.1039/c2cc34196d. [DOI] [PubMed] [Google Scholar]
- Chaudhari A. K.; Mukherjee S.; Nagarkar S. S.; Joarder B.; Ghosh S. K. Bi-Porous Metal–organic Framework with Hydrophilic and Hydrophobic Channels: Selective Gas Sorption and Reversible Iodine Uptake Studies. CrystEngComm 2013, 15, 9465–9471. 10.1039/c3ce40795k. [DOI] [Google Scholar]
- Gándara F.; Uribe-Romo F. J.; Britt D. K.; Furukawa H.; Lei L.; Cheng R.; Duan X.; O’Keeffe M.; Yaghi O. M. Porous, Conductive Metal-Triazolates and Their Structural Elucidation by the Charge-Flipping Method. Chem. - Eur. J. 2012, 18, 10595–10601. 10.1002/chem.201103433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


