Abstract
Liver fibrosis is caused by excessive accumulation of extracellular matrix during chronic liver injuries. Although clinical evidence suggests that liver fibrosis can be reversed, there is no standard therapy for liver fibrosis. Moreover, there is a lack of diagnostic tools to detect early-stage liver fibrosis. Activation of hepatic stellate cells (HSCs) is the key step during liver fibrogenesis, and its mechanism has been extensively studied by various cell culture and animal models. Targeted delivery of therapeutic agents to activated HSCs is therefore critical for the successful treatment of liver fibrosis. A number of protein markers have been found to be overexpressed in activated HSCs, and their ligands have been used to specifically deliver various antifibrotic agents. In this review, we summarize these HSC-specific protein markers and their ligands for targeted delivery of antifibrotic agents.
Introduction
Liver fibrosis is a worldwide health problem and characterized by excessive accumulation of extracellular matrix (ECM) after chronic liver injuries. If detected early, liver fibrosis can be reversed by removing the underlying etiologies, followed by treatments to attenuate liver injuries. Otherwise, liver fibrosis will advance to liver cirrhosis, which is irreversible and one of the leading causes of mortality and morbidity in the world (Lozano et al., 2012; Yoon et al., 2016). Currently, there is no standard therapy for liver fibrosis, and there are no noninvasive diagnostic tools to detect early-stage liver fibrosis.
Activation of quiescent hepatic stellate cells (HSCs) in the liver is the key milestone during liver fibrogenesis. HSCs can be activated by various conditions including viral infection, nonalcoholic fatty liver disease, alcoholic steatohepatitis, toxins, and autoimmune and biliary diseases. After activation, quiescent HSCs migrate to the injury site, differentiate into myofibroblasts, and secrete large amounts of ECM as well as proinflammatory cytokines (Hernandez-Gea and Friedman, 2011). The composition of ECM in the liver is shifted from type IV collagen to type I and type III collagen during liver fibrogenesis.
Activated HSCs are the major cells in fibrotic liver to secrete excessive ECM (Kisseleva, 2017). As a result, activated HSCs are the target cells for antifibrotic agents. HSCs interact intensively with other cells in the liver, such as Kupffer cells, hepatocytes, endothelial cells, and immune cells by autocrine or paracrine functions of various cytokines and chemokines (Schuppan et al., 2018).
A number of the mediators, such as the transforming growth factor β1 (TGF-β1), insulin-like growth factor I (IGFI), platelet-derived growth factor (PDGF), reactive oxygen species, and endothelin-1 can activate quiescent HSCs. Among them, TGF-β1 is the most crucial cytokine for liver fibrosis. It binds to TGF-β receptors and regulates the synthesis and degradation of type I collagen, which contains two α1 (I) and one α2 (I) chains and is the major component of the ECM in fibrotic liver (Verrecchia and Mauviel, 2007).
PDGF, which is primarily secreted by Kupffer cells, is considered to be the second most important mitogen for the activation of HSCs. The PDGF family consists of PDGF-A, PDGF-B, PDGF-C, and PDGF-D. PDGF-D binds to PDGF receptor β (PGFGRβ) and induces autophosphorylation and activates the downstream signaling molecules such as protein kinase B (PKB/Akt), mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase p38 (JNK-p38), and extracellular signal–regulated kinases 1/2 (ERK1/2) (Borkham-Kamphorst et al., 2007).
A wide range of therapeutic agents including small molecules, proteins, small interfering RNA (siRNAs), microRNA (miRNAs), and plasmids have been investigated for the treatment of liver fibrosis (Tu et al., 2014; Wang et al., 2015; Yoon et al., 2016; Liu et al., 2017; Middleton et al., 2018). However, antifibrotic agents cannot easily reach HSCs in fibrotic liver because HSCs only account for 5% to 8% of total liver cells (Geerts, 2001). In addition, accumulated ECM and closure of endothelial fenestrae inhibit the delivery of antifibrotic agents to the liver (Garcia-Banuelos et al., 2002). Delivery of antifibrotic agents to HSCs is also reduced by the reduced space of Disse (Varin and Huet, 1985). Targeted delivery of therapeutic agents to HSCs is thus critical for the successful treatment of liver fibrosis.
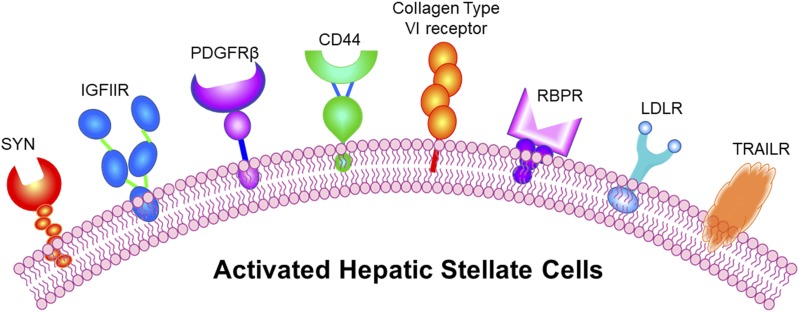
A number of receptors—including type VI collagen receptor, retinol-binding protein (RBP) receptor, platelet-derived growth factor receptor (PDGFR), synaptophysin, insulin-like growth factor-II receptor (IGFIIR), low-density lipoprotein receptor (LDLR), and cluster of differentiation 44 (CD44)—are overexpressed in activated HSCs (Fig. 1), and their ligands have been used to specifically deliver various antifibrotic agents (Table 1). Several articles have reviewed the mechanisms of liver fibrogenesis, therapeutic targets for liver fibrosis, nanoscale delivery systems for antifibrotic agents, and some targeting ligands for fibrotic liver (Li and Wang, 2009; Schon et al., 2016; Schuppan et al., 2018). In this review, we summarize all the possible HSC-specific markers that can be potentially exploited for targeted delivery of antifibrotic agents to fibrotic liver. We hope this review article will serve as a one-stop reference for scientists who are interested in improving the specificity of their antifibrotic agents to the liver.
Fig. 1.
Receptors overexpressed in activated HSCs: synaptaphysin (SYN), insulin growth factor receptor 2 receptor (IGFIIR), platelet-derived growth factor receptor (PDGFR), cluster of differentiation 44 (CD44), collagen type VI receptor, retinol-binding protein (BBP) receptor, low-density lipoprotein receptor (LDLR), and TNF-related apoptosis-inducing ligand receptor (TRAILR).
TABLE 1.
HSC-specific markers for targeted delivery of antifibrotic agents
| HSC-Specific Marker | Targeting Ligand | Carrier | Drug | Reference |
|---|---|---|---|---|
| Type VI collagen receptor | Cyclic RGD peptide (C*GRGDSPC*) | Liposome | IFN-α1b | Du et al., 2007 |
| Polymersome | Oxymatrine | Yang et al., 2014 | ||
| Conjugate | HSA | Beljaars et al., 2000 | ||
| RBP receptor | Vitamin A | Liposome | siRNA | Sato et al., 2008 |
| Nanoparticle | Oligonucleotide | Zhang et al., 2015 | ||
| PDGFR-β | Cyclic peptide (C*SRNLIDC*) | Conjugate | HSA | Beljaars et al., 2003 |
| Liposome | IFNγ | Li et al., 2012 | ||
| Synaptophysin | scAb | Conjugate | Tributyltin | Douglass et al., 2008a |
| IGFIIR | M6P | Conjugate | Y27632 | Bansal et al., 2011b |
| Peptide | Nanocomplex | siRNA | Zhao et al., 2018 | |
| Aptamer | Conjugate | siRNA | Chen et al., 2017 | |
| LDLR | Cholesterol | Conjugate | Oligonucleotide | Cheng et al., 2006 |
| Nanocomplex | siRNA | Shukla et al., 2013 | ||
| CD44 | Hyaluronic acid | Nanoparticle | Curcumin | Chen et al., 2016 |
| Micelle | Losartan | Thomas et al., 2015 | ||
| Nanocomplex | siRNA | Park et al., 2011 | ||
| Conjugate | TRAIL | Yang et al., 2015 |
Type VI Collagen Receptor
Type VI collagen is a minor but important matrix protein in the liver. In a normal liver, type VI collagen is mainly distributed in the portal areas and at the membranes of endothelial cells, hepatocytes, and HSCs. The expression of type VI collagen is markedly elevated during liver fibrogenesis (Schuppan et al., 1985; Loreal et al., 1992; Takahara et al., 1995; Beljaars et al., 2000). Type VI collagen is one of the most important matrix proteins involved in cell adhesion to the surrounding matrix. It also modulates the homeostasis of matrix by maintaining the interaction between matrix molecules and the cells (Li et al., 2015). Type VI collagen receptor contains three α chains and is mainly expressed on activated HSCs but not in normal liver cells (Popov and Schuppan, 2009). Type VI collage receptor has thus been exploited for targeted delivery of antifibrotic agents to HSCs (Beljaars et al., 2000; Du et al., 2007).
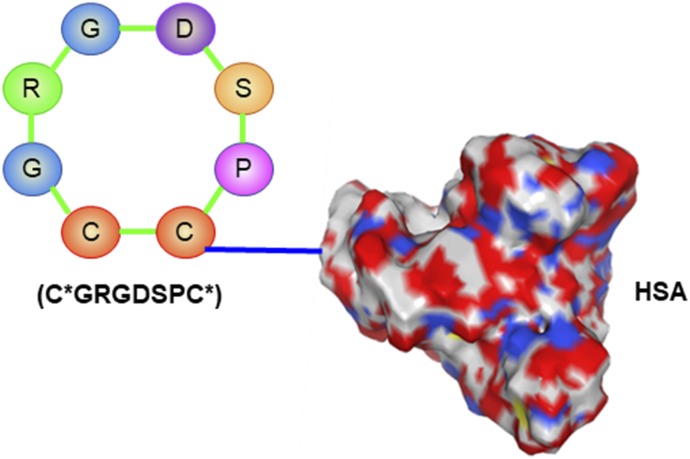
A cyclic arginylglycylaspartic acid (RGD) peptide C*GRGDSPC* (* denotes the cyclizing cysteine residue) was discovered as a ligand for the type VI collagen receptor (Marcelino and McDevitt, 1995). The cyclic peptide was conjugated to human serum albumin (HSA) in a 10:1 molar ratio (Fig. 2). In vitro studies showed that the cyclic peptide-modified HSA would specifically bind to activated rat HSCs and enter the cells via internalization. A biodistribution study in rats with induced liver fibrosis demonstrated high accumulation of the peptide-modified HSA in activated HSCs (Beljaars et al., 2000). The cyclic RGD peptide was also attached to a biodegradable polymersome encapsulating the antifibrotic agent oxymatrine. The polymersome significantly inhibited the proliferation of activated HSCs and reduced the expressions of α smooth muscle actin and collagen in the cells. The peptide-modified polymersome exhibited higher antifibrotic activity in bile duct-ligated (BDL) rats than did the unmodified polymersome (Yang et al., 2014).
Fig. 2.
Cyclic RGD peptide C*GRGDSPC* conjugated human serum albumin for type VI collagen receptor.
Another cyclic RGD peptide, C*GRGDSPK*, was also exploited as a targeting ligand for the type VI collagen receptor. The peptide preferentially binds to activated HSCs rather than hepatocytes. An interferon-α1b loaded liposome was coupled with the RGD peptide to achieve targeted delivery to fibrotic liver via recognition of type VI collagen receptors on HSCs. In rats with BDL-induced liver fibrosis, the RGD peptide-coupled liposome exhibited 10-fold higher accumulation in HSCs than the unmodified liposome. Moreover, the RGD peptide-modified liposome demonstrated significant higher antifibrotic activity in the rats with liver fibrosis (Du et al., 2007).
Retinol-Binding Protein (RBP) Receptor
A key role of quiescent HSCs in the liver is to store up to 80% of vitamin A as retinyl palmitate in lipid droplets. Vitamin A binds to RBP in the systematic circulation and is taken up by HSCs via RBP receptors (Higashi et al., 2005). In addition, vitamin A is taken up more efficiently by activated HSCs rather than quiescent HSCs (Senoo et al., 2007; Sato et al., 2008). As a result, vitamin A and RBP have been used as ligands for HSC-specific delivery.
In one study, the domain III of an albumin was fused to RBP for HSC-specific delivery. The fusion protein can be efficiently taken up by HSCs in vitro and in vivo. More importantly, the fusion protein ameliorated liver fibrosis in rats induced by carbon tetrachloride (CCl4) or BLD (Park et al., 2012; Lee et al., 2015). Using a similar concept, Zhang et al. (2015) developed a retinol-modified nanoparticle encapsulating an antifibrotic antisense oligonucleotide. The nanoparticle binds to RBP in the serum and is selectively taken up by HSCs via the RBP receptor. The nanoparticles successfully suppressed the expression of type I collagen in the liver and reversed BDL- and CCl4-induced liver fibrosis in mice (Zhang et al., 2015).
In a pioneering in vivo study using siRNA for liver fibrosis therapy, a gp46 siRNA was encapsulated in a liposome coupled with vitamin A. The functionalized liposome efficiently silenced gp46, inhibited collagen secretion in HSCs, and reversed CCl4- and BDL-induced liver fibrosis in rats. This was one of the first animal studies demonstrating the therapeutic potential of siRNAs in an animal model (Sato et al., 2008).
Similar strategies have been adopted by scientists to deliver various antifibrotic agents to HSCs (Sauvant et al., 2011; Duong et al., 2015; Lee et al., 2015; Pan et al., 2016). A phase 1b/2 clinical trial was recently conducted to evaluate the safety and tolerability of a vitamin A–coupled lipid nanoparticle containing siRNA targeting heat shocking protein 47 (HSP47) in patients with moderate to extensive liver fibrosis (ClinicalTrials.gov Identifier: NCT02227459).
Platelet-Derived Growth Factor Receptor-β (PDGFR-β)
Platelet-derived growth factor (PDGF) plays a critical role in the initiation and progression of liver fibrogenesis (Beljaars et al., 2003). It binds to PDGFR and regulates the migration, proliferation, and survival of HSCs. PDGFR is not only up-regulated in activated HSCs in animals but also is up-regulated in human fibrotic livers (Campbell et al., 2005; Borkham-Kamphorst and Weiskirchen, 2016). Particularly, PDGFR-β is dramatically overexpressed on activated HSCs, and its expression is much higher than that on other PDGFR-β-positive cells (Friedman, 2003). Therefore, several PDGFR-β-targeted delivery systems have been investigated for antifibrotic agents (Bansal et al., 2011a,b; Li et al., 2012).
The cyclic peptide C*SRNLIDC* (* denotes the cyclizing cysteine residue) was designed based on the receptor-binding residues of the PDGF B-chain. The cyclic peptide-conjugated HSA highly accumulated in the liver of the rats with BDL-induced liver fibrosis. Moreover, an intrahepatic distribution study showed that the majority of the peptide-conjugate HSA is located in HSCs, indicating selective binding of the cyclic peptide to PDGFR-β on activated HSCs (Beljaars et al., 2003).
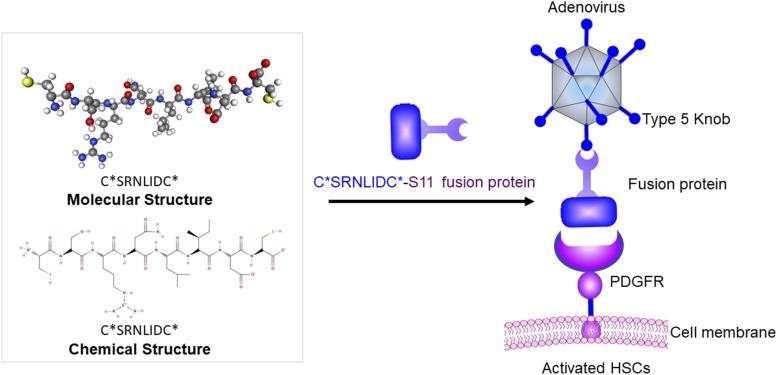
In another study, the cyclic peptide C*SRNLIDC* was fused to a single-chain antibody fragment targeting the knob of a recombinant adenovirus. After binding to the adenovirus, the fusion protein retargeted the adenovirus to activated HSCs and abolished the virus’s natural tropism for hepatocytes and Kupffer cells (Fig. 3). This assembly provides a platform to selectively deliver antifibrotic genes to activated HSCs without nonspecific uptake by neighboring cells, such as Kupffer cells and hepatocytes (Schoemaker et al., 2008).
Fig. 3.
A fusion protein to change the tropism of adenovirus to HSCs. The PDGFRβ-specific cyclic peptide C*SRNLIDC* was fused to a single-chain antibody fragment targeting the knob of a recombinant adenovirus. After binding to the adenovirus, the fusion protein retargets the adenovirus to activated HSCs and abolishes its natural tropism for hepatocytes and Kupffer cells.
The cyclic peptide C*SRNLIDC* was also conjugated to interferon-γ (IFNγ) using polyethylene glycol (PEG) as a linker. The peptide-conjugated IFNγ showed specific binding to PDGFR-β on cultured HSCs and inhibited their activation. It also significantly inhibited fibrogenesis in acute liver injury and in CCl4-induced liver fibrosis animal models without inducing IFNγ-related side effects. By contrast, unmodified IFNγ failed to inhibit fibrogenesis in both animal models (Bansal et al., 2011b).
Instead of direct conjugation to IFNγ, the cyclic peptide C*SRNLIDC* was also coupled to a liposome encapsulating IFNγ, leading to higher uptake in activated HSCs. Compared with free IFNγ and unmodified liposome encapsulating IFNγ, the cyclic peptide-coupled liposome demonstrated higher inhibitory effect on the proliferation of HSCs. In vivo studies in rats with thioacetamide-induced liver fibrosis showed a high accumulation of the peptide-coupled liposome in activated HSCs. Moreover, an enhanced antifibrotic effect of the peptide-coupled liposome was observed (Li et al., 2012).
Synaptophysin
Synaptophysin (SYN) is a membrane glycoprotein present in the presynaptic vesicles of neurons, adrenal medulla, and other neuroendocrine epithelial cells (Wiedenmann et al., 1986). Its main function involves exocytosis of neurotransmitter. HSCs also express several neuroectodermal differentiation markers such as glial fibrillary acidic protein (GFAP) and nestin (Cassiman et al., 1999). SYN was found present in HSCs in both human and rat liver biopsies. After only 36 hours of galactosamine intoxication, rat liver biopsies revealed significant SYN expression in comparison with normal rat liver biopsies. This result suggests that SYN is a potential marker for HSCs (Cassiman et al., 1999).
Researchers have used SYN as a marker to differentiate portal fibroblasts from HSCs of fibrotic livers of humans, rats, and mice (Iwaisako et al., 2014). However, studies have found that canine HSCs (quiescent and activated) are negative for SYN.
Ijzer et al. (2006) investigated the morphologic characteristics of canine HSCs and myofibroblasts. They reported that muscle-specific actin clone HHF35 (HHF35) and α smooth muscle actin were potent markers for canine HSCs and myofibroblasts, but liver sections were consistently negative for SYN and GFAP. SYN has been used as a diagnostic marker because of its variable expressions with respect to different liver stresses and injuries. In a study, rats with nonalcoholic fatty liver disease (NAFLD) showed high hepatic inflammation, necrosis and fatty infiltration. Apart from Sirius Red staining, which is a primary staining method for collagen fibers, SYN staining was used to evaluate HSC activation and the fibrogenesis. Xiao et al. (2014) reported distinct increase in SYN positive areas in a rat model of NAFLD and subsequent decline in SYN expression after therapeutic intervention with Epigallocatechin gallate.
Despite its unique expression patterns during liver fibrosis and NAFLD, SYN has not been thoroughly investigated as a ligand for targeted drug delivery. However, SYN is advantageous over other markers for targeted delivery of antifibrotic agents because it forms part of endocytosing vesicles, leading to increased chance of endocytosis of its ligand and associated cargos (Douglass et al., 2008a). A group of researchers discovered a single-chain antibody (scAb), C1-3, that binds to SYN on activated HSCs in fibrotic mouse liver. C1-3 scAb was generated by phage display biopanning against the peptide sequence that is part of the extracellular domain of SYN (Elrick et al., 2005). Studies have shown that C1-3 scAb binds specifically to activated HSCs but not hepatocytes. The C1-3 scAb-conjugated tributyl tin was delivered into HSCs and exert its activity (Douglass et al., 2008a). This demonstrated the feasibility of using C1-3 scAb as a targeting ligand for HSC-specific delivery. One potential limitation of this strategy is the nonspecific delivery to neuroendocrine and neural cells. However, there are no evidence showing that scAb can cross the blood–brain barrier, which addresses the nonspecific issue to some degree (Douglass et al., 2008b).
Insulin-Like Growth Factor II Receptor (IGFIIR)
Also known as the cation-independent mannose-6-phosphate receptor (M6PR), the 300 kDa IGFIIR is a member of the IGF signaling family (Morgan et al., 1987). The major functions of IGFIIR include regulating the activity of insulin like growth factor II (IGFII); transportation of M6P-tagged lysosomal enzymes from Golgi network and outside of cells to lysosomes; and activation of TGF-β. M6P-tagged proteins are transported to lysosomes for degradation by lysosomal acid hydrolases (Puxbaum et al., 2012). It is noteworthy that IGFIIR is unrelated to tyrosine kinase activity and proliferation (Sedlaczek et al., 2003). As a result, IGFIIR can be exploited as a surface maker for targeted drug delivery without inducing the proliferation of HSCs.
While IGFIIR is expressed in quiescent HSCs, its expression is strongly upregulated (∼20 folds) in activated HSCs relative to quiescent HSCs (Beljaars et al., 1999; Mousavi et al., 2013). The expression of IGFIIR is often positively correlated with chronic liver injury (Hellemans et al., 2004). Moreover, IGFIIR-mediated ligand internalization is 3 times faster than cellular endocytosis in activated HSCs (York et al., 1999). Therefore, IGFIIR is considered as a promising receptor for HSC-targeted drug delivery (Ye et al., 2005; Gary-Bobo et al., 2007; Mousavi et al., 2013). Additionally, upregulation of IGFIIR is also a signature feature of nephroblastoma, hepatocellular carcinoma and rhabdomyosarcoma (Sedlaczek et al., 2003).
Mannose 6 phosphate (M6P) is the natural ligand for IGFIIR. IGFIIR consists of two M6P binding extracellular domains. Generally, the affinity of M6P toward IGFIIR is low, but it can be enhanced by oligomerization of the receptor (Byrd and MacDonald, 2000). Dimerization of a ligand has also proven to increase its binding affinity toward IGFIIR (Chen et al., 2015). Researchers have conjugated multiple M6Ps to HSA to deliver triplex-forming oligonucleotide (TFO) to activated HSCs in rats. This delivery system demonstrated high specificity toward HSCs and efficiently delivered the therapeutic load to target cells. Moreover, in vivo studies also revealed that TFO was selectively distributed in HSCs of fibrotic rats (Ye et al., 2006).
Similarly, the Rho-kinase inhibitor (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride (Y27632) was conjugated with M6P/HSA, and its in vivo biodistribution was studied (van Beuge et al., 2011a,b). In comparison with free Y27632, the Y27632-M6P/HSA conjugate demonstrated exclusive distribution in HSCs. The conjugated Y27632 significantly reduced the collagen deposition in the extracellular matrix in contrast to the free Y27632. M6P-GlcNAc is another such conjugate, which has shown high binding affinity toward IGFIIR (Liu et al., 2013). Despite the high binding affinity of the M6P conjugate toward IGFIIR, the greatest limitations remain the difficulty in conjugation and relatively low binding affinity (approximately KD 23 µM) of M6P (Jeanjean et al., 2006).
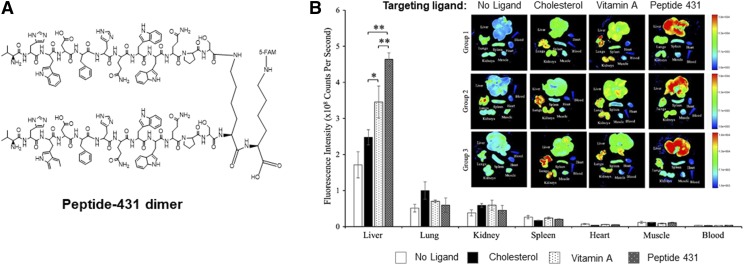
Using a novel combinatorial biopanning strategy, Chen et al. (2015) discovered an IGFIIR-specific peptide, peptide-431. Peptide-431 demonstrated high specificity toward IGFIIR expressed on human and rat HSCs. It has been used as a promising ligand for antifibrotic siRNA nanocomplexes (Fig. 4).(Zhao et al., 2018) This novel peptide is a promising ligand for the delivery of a therapeutic payload to activated HSCs.
Fig. 4.
Peptide-431, an IGF2R-specific peptide, enhances the accumulation of a siRNA nanocomplex in fibrotic liver. (A) Structure of the peptide-431 dimer. (B) Biodistribution of the siRNA nanocomplexes modified with different ligands including cholesterol, vitamin A, and peptide-431. Adapted from Zhao et al. (2018).
Chen et al. (2017) also discovered IGFIIR-specific aptamer using systematic evolution of ligands exponential enrichment (SELEX). The aptamer was annealed to the 3′ end of an siRNA and effectively delivered the siRNA to rat and human HSCs. The aptamer-siRNA chimera also exhibited high accumulation in the liver of the rats with CCl4-induced liver fibrosis.
Low-Density Lipoprotein Receptor (LDLR)
The liver is the major organ for cholesterol homeostasis by controlling lipid synthesis and uptake in the body (van de Sluis et al., 2017). The expressions of LDLR and high-density lipoprotein receptor-scavenger receptor class B type1 (SR-B1) are high in the liver (Gao and Brigstock, 2003; Wolfrum et al., 2007). Clearance of plasma lipids in the liver is mediated by LDLR and LDLR-related protein 1 (van de Sluis et al., 2017).
LDLR plays a critical role in cholesterol metabolism via recognizing the apolipoprotein B100 of cholesterol-rich LDL and mediating a rapid endocytosis recycling process (Rudenko et al., 2002). LDLR binds to cholesterol-rich LDL at neutral pH through the “ligand-binding domain” with the help of Ca2+ but rapidly dissociates from these ligands at acidic pH in the endosome (Rudenko et al., 2002; Rudenko and Deisenhofer, 2003). LDLR also induces receptor-mediated endocytosis for very-low-density lipoprotein and chylomicron remnants through binding with apolipoprotein E (Rudenko et al., 2002).
Cholesterol was found accumulated in HSCs of the mice with CCl4- and BDL-induced liver fibrosis. Accumulation of cholesterol in HSCs leads to sensitization of the cells to TGF-β and subsequently aggravates fibrogenesis (Teratani et al., 2012). Furthermore, the exacerbation of liver fibrosis and activation of HSCs also enhance the accumulation of cholesterol in HSCs, which is partially correlated with the increased expression of LDLR (Tomita et al., 2014).
A triplex-forming oligonucleotide targeting the genomic DNA of Type I collagen was conjugated with cholesterol and showed higher uptake in the liver of the rats with dimethylnirosamine-induced liver fibrosis (Cheng et al., 2006). In another study, cholesterol was used as a targeting ligand and was coupled to a streptavidin-based siRNA nanocomplex. The cholesterol-modified nanocomplex showed a higher uptake in HSC-T6 (LDLR+) cells rather than in Caco-2 (LDLR-) cells. Meanwhile, incubation of HSC-T6 cells with puromycin, an LDLR inhibitor, suppressed the cellular uptake of the cholesterol-modified nanocomplex (Shukla et al., 2013).
Cluster of Differentiation 44 (CD44)
CD44 is the major receptor for hyaluronic acid (HA), which is an important component of the extracellular matrix. CD44 was initially found overexpressed in liver biopsies from patients with alcoholic liver disease (Urashima et al., 2000). Similar results were observed in rats with dimethylnitrosamine-induced liver fibrosis. CD44 was highly expressed in fibrotic liver but only weakly expressed in normal liver. Although HA was localized in HSCs and endothelial cells, CD44 was mainly detected in infiltrating lymphocytes, Kupffer cells, and some endothelial cells (Satoh et al., 2000). In another study, researchers specifically investigated the expression of CD44 in HSCs from normal and fibrotic rats. The CD44 splice variant, CD44v6, is highly expressed in activated HSCs and facilitates the migration of the HSCs (Kikuchi et al., 2005).
HA is a well-established ligand for CD44 and has been used for HSC-targeted delivery of antifibrotic agents. In one study, curcumin was encapsulated in HA-modified polylactide nanoparticles. The nanoparticle induced cell death of activated HSCs without affecting quiescent HSCs and hepatocytes (Chen et al., 2016). In another study, HA was conjugated to 5β-cholanic acid to form micelles and encapsulate losartan. The HA micelles showed specific accumulation in fibrotic liver and ameliorated liver fibrosis in mice (Thomas et al., 2015).
HA was also used for targeted delivery to cirrhotic livers in mice. Quantum dots (QDots) were conjugated to HA and demonstrated higher uptake by HSCs than by normal hepatocytes. Biodistribution study showed specific delivery of the QDots to HSCs in mice with liver cirrhosis (Kim et al., 2010). The same group then developed HA-coupled polyethyleneimine to deliver a TGF-β siRNA for the treatment of liver cirrhosis. The siRNA/HA-polyethyleneimine nanocomplex entered HSCs via CD44-mediated endocytosis and significantly attenuated liver cirrhosis (Park et al., 2011).
Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Receptor (TRAILR)
The tumor necrosis factor receptor superfamily (TNFRSF) are cytokine receptors, including TRAILR1 (death receptor 4), TRAILR2 (death receptor 5), TRAILR3 (decoy receptor 1), and TRAILR4 (decoy receptor 1) (Locksley et al., 2001). TNF-related apoptosis-inducing ligand (TRAIL) is a known anticancer agent that induces apoptosis of cancer cells by specifically targeting TRAILRs (Singh et al., 2017). It was recently found that some immune cells, such as natural killer cells, play important roles in preventing liver fibrosis by killing activated HSCs in a TRAIL-dependent manner. Once activated, HSCs are more susceptible to apoptotic factors, such as TRAIL.
TRAILR1/2 contain a death domain, which plays an essential role in the apoptosis of HSCs (Ashkenazi and Dixit, 1998; Singh et al., 2017). Moreover, TRAILR1/2 are highly expressed in activated HSCs (Taimr et al., 2003; Yang et al., 2015). For example, during the activation of human HSCs (LX-2), the mRNA expression of TRAILR1 and TRAILR2 was increased by 18-fold and 17.6 fold, respectively. Similar results were observed in murine HSCs (Taimr et al., 2003).
In addition to binding to TRAILR1/2 to induce apoptosis of HSCs, TRAIL also binds to TRAILR3/4, which are also expressed on human HSCs (Singh et al., 2017). Because they lack a death domain, TRAILR3/4 act as a decoy receptors to limit the engagement of TRAIL with TRAILR1/2 (Falschlehner et al., 2007). A recent study has shown that silencing the expression of TRAILR3/4 in human HSCs sensitizes the cells to apoptosis (Singh et al., 2017).
Despite its therapeutic potential, TRAIL’s clinical applications are limited because of its very short half-life in the body. HA was conjugated to the N-terminal amino group of TRAIL to achieve targeted delivery to HSCs because CD44 is overexpressed in fibrotic livers. The TRAIL-HA conjugate showed similar biologic activity as native TRAIL but a higher accumulation in the liver. Moreover, the conjugate exhibited long circulation times in the blood for more than 4 days and reversed liver fibrosis in a rat model (Yang et al., 2015). In another study, PEG was conjugated to TRAIL to prolong its half-life. The PEG-conjugated TRAIL showed an extended half-life not only in rodents but also in nonhuman primates. PEG-TRAIL also attenuated CCl4-induced liver fibrosis in rats (Oh et al., 2016).
Although TRAILR has yet to be exploited as a marker for HSC-specific drug delivery, it is possible to discover artificial ligands of TRAILR using in vitro selection procedures, such as phage display biopanning or SELEX. The artificial ligands can then be conjugated to antifibrotic agents to increase their uptake by HSCs.
Conclusion and Perspective
Scientists have made good progress in understanding the mechanisms of liver fibrogenesis and have discovered numerous potential targets for antifibrotic therapy. The major hurdle in developing effective antifibrotic therapies is that antifibrotic agents cannot easily reach activated HSCs, which are the major players in liver fibrogenesis. HSCs only account for a very small percentage of total liver cells (Geerts, 2001). In addition, accumulated ECM and closure of endothelial fenestrae inhibit the delivery of antifibrotic agents to the fibrotic liver (Garcia-Banuelos et al., 2002).
Targeted delivery of antifibrotic agents to activated HSCs is therefore critical for the successful treatment of liver fibrosis. Despite great efforts in developing targeted delivery systems for liver fibrosis in preclinical studies, the importance of targeted drug delivery has not been fully realized in clinical trials. A number of clinical trials have been conducted for the treatment of liver fibrosis using small molecules, proteins, monoclonal antibodies, and nucleic acids. Among them, only one clinical trial (NCT02227459) used vitamin A as a targeting ligand for the antifibrotic siRNA.
A number of protein markers, including type VI collagen receptor, RBP receptor, PDGFR, synaptophysin, IGFIIR, LDLR, and CD44, are overexpressed in activated HSCs, and their ligands have been used to specifically deliver various antifibrotic agents in preclinical studies. Currently, most investigators focus on natural ligands, which are very limited and generally have low affinity for these receptors. In addition, some of the natural ligands may activate downstream signaling pathways after binding to their receptors on HSCs.
A potential solution for this dilemma is to use affinity selection technology such as phage display biopanning to discover peptide- or antibody-based ligands, or SELEX to discover aptamer-based ligands. It is noteworthy that the Nobel Prize in chemistry 2018 was awarded to two scientists for “the phage display of peptides and antibodies,” supporting the great promise of affinity selection technology.
Compared with natural ligands, artificial ligands including peptides, antibody fragments, antibodies, and aptamers have higher affinity and are more flexible for chemical modification and coupling to antifibrotic agents or delivery systems. In particular, peptides and aptamers are attractive ligands because of their small size, ease of production, and nonimmunogenicity.
Considering the fact that targeted delivery of antifibrotic agents has shown promising results in numerous animal studies, we believe that incorporating HSC-specific ligands in antifibrotic agents could dramatically increase their success rate in clinical studies.
Abbreviations
- BDL
bile duct ligated
- CCl4
carbon tetrachloride
- CD44
cluster of differentiation 44
- ECM
extracellular matrix
- GFAP
glial fibrillary acidic protein
- HA
hyaluronic acid
- HSA
human serum albumin
- HSCs
hepatic stellate cells
- IFNγ
interferon-gamma
- IGFI
insulin-like growth factor I
- IGFIIR
insulin-like growth factor II receptor
- LDLR
low-density lipoprotein receptor
- M6P
mannose-6-phosphate
- M6PR
mannose-6-phosphate receptor
- NAFLD
nonalcoholic fatty liver disease
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PEG
polyethylene glycol
- RBP
retinol-binding protein
- RGD
arginylglycylaspartic acid
- TFO
triplex-forming oligonucleotide
- TGF
transforming growth factor
- TRAIL
TNF-related apoptosis-inducing ligand
- TRAILR
TNF-related apoptosis-inducing ligand receptor
- scAb
single-chain antibody
- SELEX
systematic evolution of ligands by exponential enrichment
- siRNA
small interfering RNA
- SYN
synaptophysin
- Y27632
4-[(1R)-1-aminoethyl]-N-pyridin-4-ylcyclohexane-1-carboxamide
Authorship Contributions
Participated in research design: Chen, Cheng.
Wrote or contributed to the writing of the manuscript: Chen, Jain, Liu, Zhao, Cheng.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism (2R01AA021510), and partly supported by the National Institute of General Medical Sciences (R01GM121798) and the National Cancer Institute (R01CA231099). K.C. was also supported by an American Cancer Society-Lee National Deim Day Research Scholar Grant (RSG-15-132-01-CDD).
References
- Ashkenazi A, Dixit VM. (1998) Death receptors: signaling and modulation. Science 281:1305–1308. [DOI] [PubMed] [Google Scholar]
- Bansal R, Prakash J, de Ruijter M, Beljaars L, Poelstra K. (2011a) Peptide-modified albumin carrier explored as a novel strategy for a cell-specific delivery of interferon gamma to treat liver fibrosis. Mol Pharm 8:1899–1909. [DOI] [PubMed] [Google Scholar]
- Bansal R, Prakash J, Post E, Beljaars L, Schuppan D, Poelstra K. (2011b) Novel engineered targeted interferon-gamma blocks hepatic fibrogenesis in mice. Hepatology 54:586–596. [DOI] [PubMed] [Google Scholar]
- Beljaars L, Molema G, Schuppan D, Geerts A, De Bleser PJ, Weert B, Meijer DK, Poelstra K. (2000) Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. J Biol Chem 275:12743–12751. [DOI] [PubMed] [Google Scholar]
- Beljaars L, Molema G, Weert B, Bonnema H, Olinga P, Groothuis GM, Meijer DK, Poelstra K. (1999) Albumin modified with mannose 6-phosphate: a potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology 29:1486–1493. [DOI] [PubMed] [Google Scholar]
- Beljaars L, Weert B, Geerts A, Meijer DK, Poelstra K. (2003) The preferential homing of a platelet derived growth factor receptor-recognizing macromolecule to fibroblast-like cells in fibrotic tissue. Biochem Pharmacol 66:1307–1317. [DOI] [PubMed] [Google Scholar]
- Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. (2007) Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol 46:1064–1074. [DOI] [PubMed] [Google Scholar]
- Borkham-Kamphorst E, Weiskirchen R. (2016) The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev 28:53–61. [DOI] [PubMed] [Google Scholar]
- Byrd JC, MacDonald RG. (2000) Mechanisms for high affinity mannose 6-phosphate ligand binding to the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem 275:18638–18646. [DOI] [PubMed] [Google Scholar]
- Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, Odell MM, Bauer RL, Ren HP, Haugen HS, et al. (2005) Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci USA 102:3389–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiman D, van Pelt J, De Vos R, Van Lommel F, Desmet V, Yap SH, Roskams T. (1999) Synaptophysin: a novel marker for human and rat hepatic stellate cells. Am J Pathol 155:1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YN, Hsu SL, Liao MY, Liu YT, Lai CH, Chen JF, Nguyen MT, Su YH, Chen ST, Wu LC. (2016) Ameliorative effect of curcumin-encapsulated hyaluronic acid-PLA nanoparticles on thioacetamide-induced murine hepatic fibrosis. Int J Environ Res Public Health 14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jin W, Liu H, Zhao Z, Cheng K. (2015) Discovery of peptide ligands for hepatic stellate cells using phage display. Mol Pharm 12:2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Liu H, Jain A, Zhang L, Liu C, Cheng K. (2017) Discovery of aptamer ligands for hepatic stellate cells using SELEX. Theranostics 7:2982–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Ye Z, Guntaka RV, Mahato RI. (2006) Enhanced hepatic uptake and bioactivity of type alpha1(I) collagen gene promoter-specific triplex-forming oligonucleotides after conjugation with cholesterol. J Pharmacol Exp Ther 317:797–805. [DOI] [PubMed] [Google Scholar]
- Douglass A, Wallace K, Koruth M, Barelle C, Porter AJ, Wright MC. (2008a) Targeting liver myofibroblasts: a novel approach in anti-fibrogenic therapy. Hepatol Int 2:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass A, Wallace K, Parr R, Park J, Durward E, Broadbent I, Barelle C, Porter AJ, Wright MC. (2008b) Antibody-targeted myofibroblast apoptosis reduces fibrosis during sustained liver injury. J Hepatol 49:88–98. [DOI] [PubMed] [Google Scholar]
- Du SL, Pan H, Lu WY, Wang J, Wu J, Wang JY. (2007) Cyclic Arg-Gly-Asp peptide-labeled liposomes for targeting drug therapy of hepatic fibrosis in rats. J Pharmacol Exp Ther 322:560–568. [DOI] [PubMed] [Google Scholar]
- Duong HT, Dong Z, Su L, Boyer C, George J, Davis TP, Wang J. (2015) The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension. Small 11:2291–2304. [DOI] [PubMed] [Google Scholar]
- Elrick LJ, Leel V, Blaylock MG, Duncan L, Drever MR, Strachan G, Charlton KA, Koruth M, Porter AJ, Wright MC. (2005) Generation of a monoclonal human single chain antibody fragment to hepatic stellate cells—a potential mechanism for targeting liver anti-fibrotic therapeutics. J Hepatol 42:888–896. [DOI] [PubMed] [Google Scholar]
- Falschlehner C, Emmerich CH, Gerlach B, Walczak H. (2007) TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol 39:1462–1475. [DOI] [PubMed] [Google Scholar]
- Friedman SL. (2003) Liver fibrosis—from bench to bedside. J Hepatol 38 (Suppl 1):S38–S53. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. (2003) Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res 27:214–220. [DOI] [PubMed] [Google Scholar]
- Garcia-Bañuelos J, Siller-Lopez F, Miranda A, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. (2002) Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther 9:127–134. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo M, Nirdé P, Jeanjean A, Morère A, Garcia M. (2007) Mannose 6-phosphate receptor targeting and its applications in human diseases. Curr Med Chem 14:2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts A. (2001) History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis 21:311–335. [DOI] [PubMed] [Google Scholar]
- Hellemans K, Verbuyst P, Quartier E, Schuit F, Rombouts K, Chandraratna RA, Schuppan D, Geerts A. (2004) Differential modulation of rat hepatic stellate phenotype by natural and synthetic retinoids. Hepatology 39:97–108. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gea V, Friedman SL. (2011) Pathogenesis of liver fibrosis. Annu Rev Pathol 6:425–456. [DOI] [PubMed] [Google Scholar]
- Higashi N, Sato M, Kojima N, Irie T, Kawamura K, Mabuchi A, Senoo H. (2005) Vitamin A storage in hepatic stellate cells in the regenerating rat liver: with special reference to zonal heterogeneity. Anat Rec A Discov Mol Cell Evol Biol 286:899–907. [DOI] [PubMed] [Google Scholar]
- Ijzer J, Roskams T, Molenbeek RF, Ultee T, Penning LC, Rothuizen J, van den Ingh TS. (2006) Morphological characterisation of portal myofibroblasts and hepatic stellate cells in the normal dog liver. Comp Hepatol 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, et al. (2014) Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA 111:E3297–E3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanjean A, Garcia M, Leydet A, Montero JL, Morère A. (2006) Synthesis and receptor binding affinity of carboxylate analogues of the mannose 6-phosphate recognition marker. Bioorg Med Chem 14:3575–3582. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Griffin CT, Wang SS, Bissell DM. (2005) Role of CD44 in epithelial wound repair: migration of rat hepatic stellate cells utilizes hyaluronic acid and CD44v6. J Biol Chem 280:15398–15404. [DOI] [PubMed] [Google Scholar]
- Kim KS, Hur W, Park SJ, Hong SW, Choi JE, Goh EJ, Yoon SK, Hahn SK. (2010) Bioimaging for targeted delivery of hyaluronic Acid derivatives to the livers in cirrhotic mice using quantum dots. ACS Nano 4:3005–3014. [DOI] [PubMed] [Google Scholar]
- Kisseleva T. (2017) The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 65:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Jeong H, Park S, Yoo W, Choi S, Choi K, Lee MG, Lee M, Cha D, Kim YS, et al. (2015) Fusion protein of retinol-binding protein and albumin domain III reduces liver fibrosis. EMBO Mol Med 7:819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, He L, Guo H, Chen H, Shan H. (2015) Targeting activated hepatic stellate cells (aHSCs) for liver fibrosis imaging. EJNMMI Res 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Li QH, Wang JY, Zhan CY, Xie C, Lu WY. (2012) Effects of interferon-gamma liposomes targeted to platelet-derived growth factor receptor-beta on hepatic fibrosis in rats. J Control Release 159:261–270. [DOI] [PubMed] [Google Scholar]
- Li F, Wang JY. (2009) Targeted delivery of drugs for liver fibrosis. Expert Opin Drug Deliv 6:531–541. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen Z, Jin W, Barve A, Wan YY, Cheng K. (2017) Silencing of α-complex protein-2 reverses alcohol- and cytokine-induced fibrogenesis in hepatic stellate cells. Liver Res 1:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Marshall J, Li Q, Edwards N, Chen G. (2013) Synthesis of novel bivalent mimetic ligands for mannose-6-phosphate receptors. Bioorg Med Chem Lett 23:2328–2331. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501. [DOI] [PubMed] [Google Scholar]
- Loréal O, Clément B, Schuppan D, Rescan PY, Rissel M, Guillouzo A. (1992) Distribution and cellular origin of collagen VI during development and in cirrhosis. Gastroenterology 102:980–987. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet (2013) 381:628]. Lancet 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino J, McDevitt CA. (1995) Attachment of articular cartilage chondrocytes to the tissue form of type VI collagen. Biochim Biophys Acta 1249:180–188. [DOI] [PubMed] [Google Scholar]
- Middleton SA, Rajpal N, Cutler L, Mander P, Rioja I, Prinjha RK, Rajpal D, Agarwal P, Kumar V. (2018) BET inhibition improves NASH and liver fibrosis. Sci Rep 8:17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO, Edman JC, Standring DN, Fried VA, Smith MC, Roth RA, Rutter WJ. (1987) Insulin-like growth factor II receptor as a multifunctional binding protein. Nature 329:301–307. [DOI] [PubMed] [Google Scholar]
- Mousavi SA, Fønhus MS, Kindberg GM, Tolleshaug H, Berg T. (2013) Enhanced activity of lysosomal proteases in activated rat hepatic stellate cells is associated with a concomitant increase in the number of the mannose-6-phosphate/insulin-like growth factor II receptor. Cell Biol Int 37:703–712. [DOI] [PubMed] [Google Scholar]
- Oh Y, Park O, Swierczewska M, Hamilton JP, Park JS, Kim TH, Lim SM, Eom H, Jo DG, Lee CE, et al. (2016) Systemic PEGylated TRAIL treatment ameliorates liver cirrhosis in rats by eliminating activated hepatic stellate cells. Hepatology 64:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan TL, Wang PW, Hung CF, Aljuffali IA, Dai YS, Fang JY. (2016) The impact of retinol loading and surface charge on the hepatic delivery of lipid nanoparticles. Colloids Surf B Biointerfaces 141:584–594. [DOI] [PubMed] [Google Scholar]
- Park K, Hong SW, Hur W, Lee MY, Yang JA, Kim SW, Yoon SK, Hahn SK. (2011) Target specific systemic delivery of TGF-β siRNA/(PEI-SS)-g-HA complex for the treatment of liver cirrhosis. Biomaterials 32:4951–4958. [DOI] [PubMed] [Google Scholar]
- Park S, Choi S, Lee MG, Lim C, Oh J. (2012) Retinol binding protein-albumin domain III fusion protein deactivates hepatic stellate cells. Mol Cells 34:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov Y, Schuppan D. (2009) Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology 50:1294–1306. [DOI] [PubMed] [Google Scholar]
- Puxbaum V, Nimmerfall E, Bäuerl C, Taub N, Blaas PM, Wieser J, Mikula M, Mikulits W, Ng KM, Yeoh GC, et al. (2012) M6P/IGF2R modulates the invasiveness of liver cells via its capacity to bind mannose 6-phosphate residues. J Hepatol 57:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G, Deisenhofer J. (2003) The low-density lipoprotein receptor: ligands, debates and lore. Curr Opin Struct Biol 13:683–689. [DOI] [PubMed] [Google Scholar]
- Rudenko G, Henry L, Henderson K, Ichtchenko K, Brown MS, Goldstein JL, Deisenhofer J. (2002) Structure of the LDL receptor extracellular domain at endosomal pH. Science 298:2353–2358. [DOI] [PubMed] [Google Scholar]
- Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, et al. (2008) Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol 26:431–442. [DOI] [PubMed] [Google Scholar]
- Satoh T, Ichida T, Matsuda Y, Sugiyama M, Yonekura K, Ishikawa T, Asakura H. (2000) Interaction between hyaluronan and CD44 in the development of dimethylnitrosamine-induced liver cirrhosis. J Gastroenterol Hepatol 15:402–411. [DOI] [PubMed] [Google Scholar]
- Sauvant P, Cansell M, Atgié C. (2011) Vitamin A and lipid metabolism: relationship between hepatic stellate cells (HSCs) and adipocytes. J Physiol Biochem 67:487–496. [DOI] [PubMed] [Google Scholar]
- Schoemaker MH, Rots MG, Beljaars L, Ypma AY, Jansen PLM, Poelstra K, Moshage H, Haisma HJ. (2008) PDGF-receptor β-targeted adenovirus redirects gene transfer from hepatocytes to activated stellate cells. Mol Pharm 5:399–406. [DOI] [PubMed] [Google Scholar]
- Schon HT, Bartneck M, Borkham-Kamphorst E, Nattermann J, Lammers T, Tacke F, Weiskirchen R. (2016) Pharmacological intervention in hepatic stellate cell activation and hepatic fibrosis. Front Pharmacol 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. (2018) Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol 68–69:435–451. [DOI] [PubMed] [Google Scholar]
- Schuppan D, Rühlmann T, Hahn EG. (1985) Radioimmunoassay for human type VI collagen and its application to tissue and body fluids. Anal Biochem 149:238–247. [DOI] [PubMed] [Google Scholar]
- Sedlaczek N, Hasilik A, Neuhaus P, Schuppan D, Herbst H. (2003) Focal overexpression of insulin-like growth factor 2 by hepatocytes and cholangiocytes in viral liver cirrhosis. Br J Cancer 88:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo H, Kojima N, Sato M. (2007) Vitamin A-storing cells (stellate cells). Vitam Horm 75:131–159. [DOI] [PubMed] [Google Scholar]
- Shukla RS, Tai W, Mahato R, Jin W, Cheng K. (2013) Development of streptavidin-based nanocomplex for siRNA delivery. Mol Pharm 10:4534–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh HD, Otano I, Rombouts K, Singh KP, Peppa D, Gill US, Böttcher K, Kennedy PTF, Oben J, Pinzani M, et al. (2017) TRAIL regulatory receptors constrain human hepatic stellate cell apoptosis. Sci Rep 7:5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. (2003) Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology 37:87–95. [DOI] [PubMed] [Google Scholar]
- Takahara T, Sollberg S, Muona P, Uitto J. (1995) Type VI collagen gene expression in experimental liver fibrosis: quantitation and spatial distribution of mRNAs, and immunodetection of the protein. Liver 15:78–86. [DOI] [PubMed] [Google Scholar]
- Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, Tominaga S, Hiroi S, Irie R, Okada Y, et al. (2012) A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 142:152–164e.10. [DOI] [PubMed] [Google Scholar]
- Thomas RG, Moon MJ, Kim JH, Lee JH, Jeong YY. (2015) Effectiveness of losartan-loaded hyaluronic acid (HA) micelles for the reduction of advanced hepatic fibrosis in C3H/HeN mice model. PLoS One 10:e0145512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, Okada Y, Kurihara C, Irie R, Yokoyama H, et al. (2014) Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 59:154–169. [DOI] [PubMed] [Google Scholar]
- Tu X, Zhang H, Zhang J, Zhao S, Zheng X, Zhang Z, Zhu J, Chen J, Dong L, Zang Y, et al. (2014) MicroRNA-101 suppresses liver fibrosis by targeting the TGFβ signalling pathway. J Pathol 234:46–59. [DOI] [PubMed] [Google Scholar]
- Urashima S, Tsutsumi M, Ozaki K, Tsuchishima M, Shimanaka K, Ueshima Y, Takase S. (2000) Immunohistochemical study of hyaluronate receptor (CD44) in alcoholic liver disease. Alcohol Clin Exp Res 24 (4 Suppl):34S–38S. [PubMed] [Google Scholar]
- van Beuge MM, Prakash J, Lacombe M, Gosens R, Post E, Reker-Smit C, Beljaars L, Poelstra K. (2011a) Reduction of fibrogenesis by selective delivery of a Rho kinase inhibitor to hepatic stellate cells in mice. J Pharmacol Exp Ther 337:628–635. [DOI] [PubMed] [Google Scholar]
- van Beuge MM, Prakash J, Lacombe M, Post E, Reker-Smit C, Beljaars L, Poelstra K. (2011b) Increased liver uptake and reduced hepatic stellate cell activation with a cell-specific conjugate of the Rho-kinase inhibitor Y27632. Pharm Res 28:2045–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sluis B, Wijers M, Herz J. (2017) News on the molecular regulation and function of hepatic low-density lipoprotein receptor and LDLR-related protein 1. Curr Opin Lipidol 28:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin F, Huet PM. (1985) Hepatic microcirculation in the perfused cirrhotic rat liver. J Clin Invest 76:1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. (2007) Transforming growth factor-beta and fibrosis. World J Gastroenterol 13:3056–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chu ES, Chen HY, Man K, Go MY, Huang XR, Lan HY, Sung JJ, Yu J. (2015) MicroRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget 6:7325–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE. (1986) Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA 83:3500–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al. (2007) Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol 25:1149–1157. [DOI] [PubMed] [Google Scholar]
- Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TY, Fung ML, Tipoe GL. (2014) Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr 53:187–199. [DOI] [PubMed] [Google Scholar]
- Yang J, Hou Y, Ji G, Song Z, Liu Y, Dai G, Zhang Y, Chen J. (2014) Targeted delivery of the RGD-labeled biodegradable polymersomes loaded with the hydrophilic drug oxymatrine on cultured hepatic stellate cells and liver fibrosis in rats. Eur J Pharm Sci 52:180–190. [DOI] [PubMed] [Google Scholar]
- Yang JA, Kong WH, Sung DK, Kim H, Kim TH, Lee KC, Hahn SK. (2015) Hyaluronic acid-tumor necrosis factor-related apoptosis-inducing ligand conjugate for targeted treatment of liver fibrosis. Acta Biomater 12:174–182. [DOI] [PubMed] [Google Scholar]
- Ye Z, Cheng K, Guntaka RV, Mahato RI. (2005) Targeted delivery of a triplex-forming oligonucleotide to hepatic stellate cells. Biochemistry 44:4466–4476. [DOI] [PubMed] [Google Scholar]
- Ye Z, Cheng K, Guntaka RV, Mahato RI. (2006) Receptor-mediated hepatic uptake of M6P-BSA-conjugated triplex-forming oligonucleotides in rats. Bioconjug Chem 17:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YJ, Friedman SL, Lee YA. (2016) Antifibrotic therapies: where are we now? Semin Liver Dis 36:87–98. [DOI] [PubMed] [Google Scholar]
- York SJ, Arneson LS, Gregory WT, Dahms NM, Kornfeld S. (1999) The rate of internalization of the mannose 6-phosphate/insulin-like growth factor II receptor is enhanced by multivalent ligand binding. J Biol Chem 274:1164–1171. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang C, Zha Y, Hu W, Gao Z, Zang Y, Chen J, Zhang J, Dong L. (2015) Corona-directed nucleic acid delivery into hepatic stellate cells for liver fibrosis therapy. ACS Nano 9:2405–2419. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Li Y, Jain A, Chen Z, Liu H, Jin W, Cheng K. (2018) Development of a peptide-modified siRNA nanocomplex for hepatic stellate cells. Nanomedicine (Lond) 14:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]