Abstract
A coated microneedle array comprises sharp micrometer-sized needle shafts attached to a base substrate and coated with a drug on their surfaces. Coated microneedles are under investigation for drug delivery into the skin and other tissues, and a broad assortment of active materials, including small molecules, peptides, proteins, deoxyribonucleic acids, and viruses, have been coated onto microneedles. To coat the microneedles, different methods have been developed. Some coating methods achieve selective coating of just the microneedle shafts, whereas other methods coat not only microneedle shafts but also the array base substrate. Selective coating of just the microneedle shafts is more desirable since it provides control over drug dosage, prevents drug waste, and offers high delivery efficiency. Different excipients are added to the coating liquid to modulate its viscosity and surface tension in order to achieve uniform coatings on microneedles. Coated microneedles have been used in a broad range of biomedical applications. To highlight these different applications, a table summarizing the different active materials and the amounts coated on microneedles is provided. We also discuss factors that should be considered when deciding suitability of coated microneedles for new-drug delivery applications. In recent years, many coated microneedles have been investigated in human clinical trials, and there is now a strong effort to bring the first coated microneedle-based product to market.
Introduction
Microneedles are sharp protrusions measuring less than a millimeter in length. Microneedles were originally invented for drug and vaccine delivery through the skin (Henry et al., 1998). Microneedles achieve this objective by piercing the top layer of the skin, the stratum corneum, which is the principal barrier to transdermal delivery of drugs (Bouwstra and Ponec, 2006). Microneedles are attractive because they are minimally painful (Gill et al., 2008; Haq et al., 2009; Nguyen and Park, 2018) and can be applied by patients in the comfort of their homes (Birchall et al., 2011). A microneedle array (also known as a microneedle patch) comprises one or more individual microneedles situated on a flat base (also called a substrate). When the individual microneedles rise perpendiclular to the flat base, it is called a two-dimensional array, and when the individual microneedle run parallel to the flat base mimicking a “fork”, it is called a one-dimensional array. On the basis of their design and mode of use, microneedles can be categorized into four basic types: hollow microneedles, dissolvable microneedles, poke-and-patch microneedles, and coated microneedles. These classes have been reviewed before (Prausnitz, 2004; Quinn et al., 2014), and we only briefly introduce them in this work. A hollow microneedle is similar to a conventional hypodermic needle but is notably much shorter. It has a hollow bore and liquid can be injected through it. A dissolvable microneedle on the other hand is made entirely of either drug or of a matrix in which the drug is dispersed. The dissolvable microneedle upon insertion into the skin can dissolve within minutes or can degrade over a longer time. In the “poke-and-patch” approach, a solid microneedle is used to pretreat the skin to increase its permeability by creating a micrometer-sized perforation, after which the drug formulation or a transdermal patch i applied over the pretreated skin. Finally, with a coated microneedle, the drug is coated as a solid film on the microneedle surface. When the coated microneedle is inserted into the skin it carries the coated payload with it. Once in the skin, within minutes the coating dissolves, after which the microneedles can be removed and safely discarded.
A coated microneedle is a versatile delivery system. The same microneedle patch can be used for the delivery of a broad spectrum of materials ranging from small molecules, to proteins, to DNA, to viruses, and even to microparticles (Gill and Prausnitz, 2007a). In contrast to the dissolvable microneedle, whose mechanical properties can change when the encapsulated drug fraction is altered or a different drug is dispersed in its matrix, the mechanical properties of a solid microneedle are not impacted when a different drug is coated on it surface. Furthermore, the coated microneedle system has been evaluated as a delivery system for not just the skin (Prausnitz, 2017) but also the eye (Jiang et al., 2007; Kim et al., 2014; Song et al., 2015), vascular tissue (Lee et al., 2014, 2017b), and the oral cavity (McNeilly et al., 2014; Ma et al., 2015). A recent article by Rzhevskiy et al. (2018) has reviewed the use of microneedles for drug delivery into different tissues and organs.
To use coated microneedles for different applications, it is important to consider the quality of coatings, reproducibility of the coating process, and the delivery efficiency. In the first publication on coated microneedles (Matriano et al., 2002), it was reported that the microneedle patches were coated by immersion in a coating solution. This resulted in coating of the entire microneedle array, including the base substrate. A few years later, to improve delivery efficiencies and to reduce drug wastage, more precise coating methods were developed to restrict coatings to just the microneedle shafts; one such method was published in 2004 (Cormier et al., 2004) and another in 2007 (Gill and Prausnitz, 2007a). Early studies also described the influence of liquid properties on the uniformity of the coating of microneedles and demonstrated that a broad range of materials could be coated onto microneedles with precision (Gill and Prausnitz, 2007b). Since the publication of these early studies, many microneedle coating methods have been developed. The objective of our review is to highlight the important design criteria and parameters that should be considered in the development of coated microneedles and to review the different microneedle coating methods. A comprehensive analysis of in vivo and in vitro results from the application of coated microneedles is outside the scope of this review. We refer the readers to the recent microneedle review articles for this information (Prausnitz, 2017; Shin et al., 2017; Nguyen and Park, 2018; Rodgers et al., 2018).
The Concept of Coated Microneedles
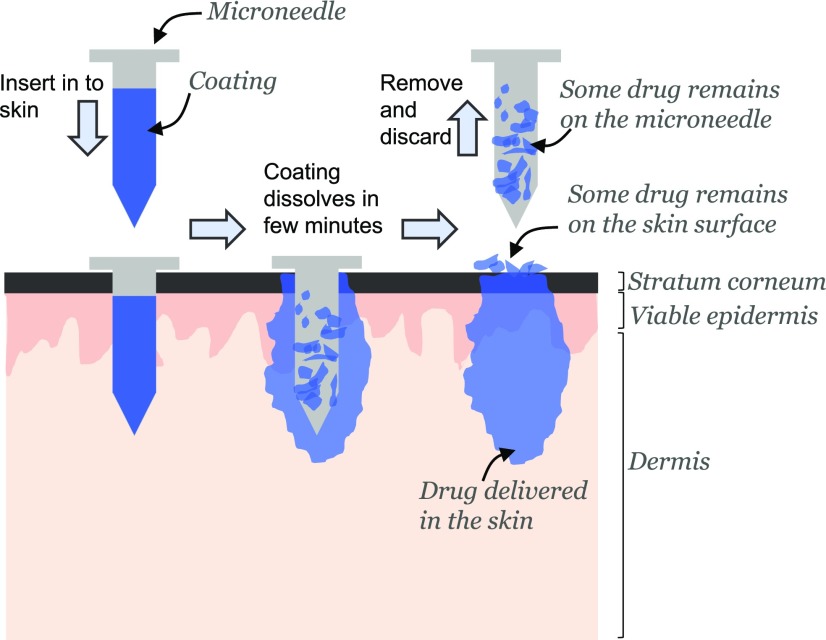
A coated microneedle comprises a sharp, solid-core microneedle structure on which is coated a solid film containing the active compound and water-soluble inactive excipients. The water-soluble excipients not only aid in the microneedle coating process but also catalyze the detachment of the film from the microneedle surface. The process by which a coated microneedle delivers its payload into the skin is schematically shown in Fig. 1. When a drug-coated microneedle is inserted into the skin, the coating encounters the interstitial fluid present in the tissue. Contact with this aqueous medium helps to dissolve the water-soluble excipients in the microneedle coating, which in turn initiates the detachment of the coating from the microneedle surface. Depending on the aqueous solubility of the coating excipients, the detachment of the coating can finish within seconds to minutes. It is only necessary that the coating detach from the microneedle surface before microneedles are removed from the skin; the complete dissolution of the material left behind can occur in due time. Coated microneedles can be used to deliver their coated payload not only to skin but also other tissues.
Fig. 1.
Schematic describing how coated microneedles facilitate delivery of active materials into the skin. A microneedle shaft is first coated with the active material. When the coated microneedle is inserted into the skin, the coating encounters the interstitial fluid that bathes the cells. This causes the coated material to start dissolving. After a certain duration the microneedle is removed. A portion of the material has been delivered into the skin, but some material remains on the skin surface and some continues to stick to the microneedle shaft after it has been removed. The ratio of the material delivered into the skin to the total amount coated on a microneedle before insertion is called the delivery efficiency of the microneedle.
Desirable Attributes in Microneedle Coatings
Uniform Coatings.
To ensure reproducible delivery of active molecules, it is important to produce uniform coatings on microneedle surfaces. If as depicted in Fig. 2-i, coatings are irregular, random, and patchy in nature, poor consistency in the mass of material being coated is achieved. Therefore, a primary attribute required in microneedle coatings is that they should form a uniform coverage on the microneedle shafts without uncoated spots.
Fig. 2.
Possible outcomes of a microneedle-coating process. After a certain coating methodology has been employed to coat microneedles, different outcomes are possible with respect to placement of the coatings on the microneedle patch. (i) Coatings are randomly produced and do not uniformly cover the surfaces, (ii) coatings uniformly cover the microneedle shaft but also cover a small region of the substrate near the base of the microneedles, (iii) coatings uniformly cover the entire surface of the patch including the microneedle shafts and the base substrate, (iv) coatings cover just the shaft of the microneedles and do not contaminate the base substrate.
No Deposition of Coatings on the Base Substrate of the Microneedle Patch.
Only microneedle shafts penetrate the skin. As such, drug that is not coated on the microneedle shafts but is instead situated on the base substrate of the microneedle patch (Fig. 2-ii and 2-iii) is not deliverable into the skin. This wasted coating located between the microneedles can be quite significant. For example, a 10 × 10 mm patch with 10,000 microneedles/cm2, each measuring 250 μm in length and 40 μm in base diameter and tapering into a sharp point of less than 2 μm (Griffin et al., 2017; Fernando et al., 2018) has a total surface area of about 245 mm2, of which about 65% (158 mm2, assuming conical geometry) is useful since it is located on microneedle surfaces, whereas 87 mm2 (35%) constitutes the wasted coating on the base substrate. The most preferred scenario for producing coatings on microneedles is shown in Fig. 2-iv, wherein the coatings are not only uniform but are also situated only on the microneedle shafts. This can allow for a reproducible and a highly efficient coated microneedle delivery system.
Coating Methods
To coat microneedle surfaces with drug molecules, the two must be brought in physical contact with each other. Depending on how this contact is achieved, the coating methods can be classified into dip coating, inkjet coating, immersion coating, drop coating, and spray coating. These methods vary greatly in their ability to selectively coat the microneedles and can be further categorized into two groups: The first group of methods can generate coatings selectively on the microneedle shafts without coating the base substrate of the microneedle array, and the second group of coating methods result in coating of both the microneedle shafts and the base substrate of the microneedle array.
Coating Methods That Selectively Coat the Microneedle Shafts without Contaminating the Base Substrate of the Microneedle Array
Dip Coating.
In this method, a microneedle is dipped into the coating liquid. The microneedle, upon exiting the coating liquid entrains a liquid film on its surface. When the solvent in the liquid film evaporates, the dissolved or suspended solids that are present in the liquid film get deposited as a coating on the microneedle surface. However, at the micrometer length scale of the microneedles, contribution of the surface tension of the coating liquid becomes significant. Owing to surface tension–induced capillary action, the coating liquid rises along the microneedle length and the meniscus can touch the flat substrate of the microneedle shafts leading to contamination of the base substrate with the coating liquid. As discussed in the previous section, this is an undesirable outcome. To overcome this issue, two general approaches have been developed to selectively coat just the microneedle shafts. In the first approach a masking plate is used to prevent the base substrate from touching the coating liquid and to allow the microneedles to contact only the coating liquid. In the second approach a thin film of the coating liquid is created into which the microneedles are dipped. In particular, the thickness (depth) of the coating liquid is kept lower than the height of the microneedles. As a result, when the microneedles are dipped into the thin liquid film, the coating liquid is unable to rise significantly and thus is unable to touch the base substrate of the microneedle array. These two techniques are elaborated below.
Masked dip coating.
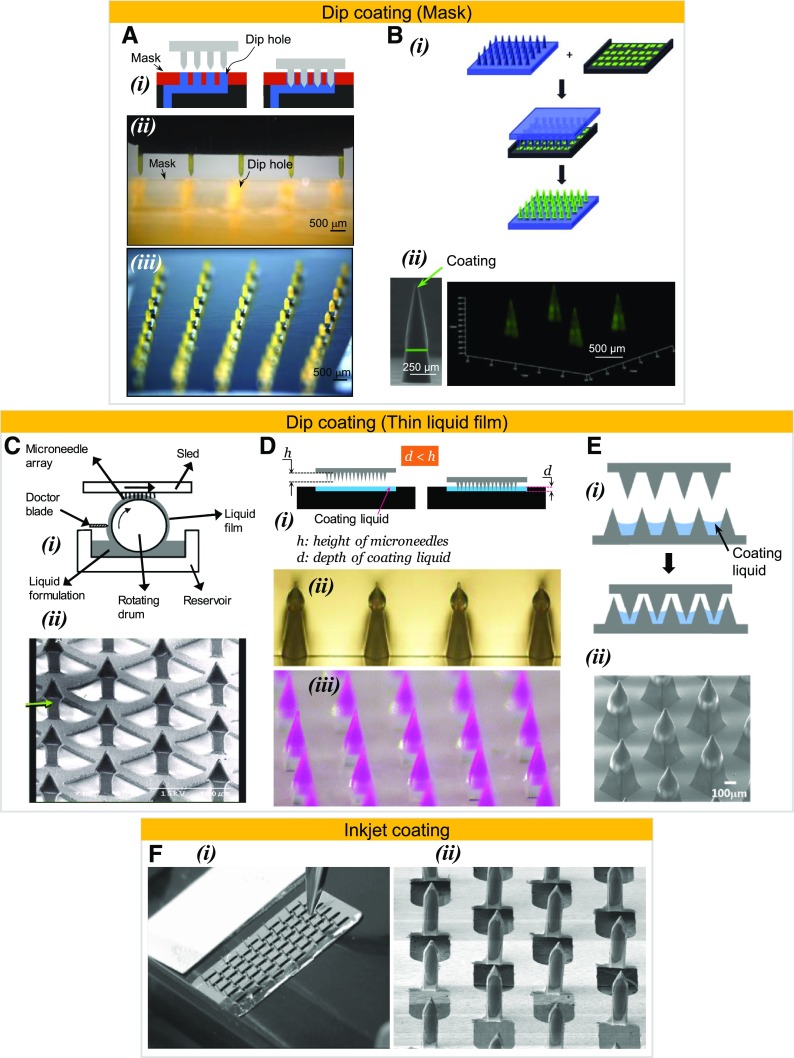
A physical mask can be introduced between the microneedle base substrate and the coating liquid interface. The mask is designed to allow contact of microneedles with the coating liquid but to physically keep the flat substrate of the microneedle patch from touching the liquid. The mask concept was first used by Gill and Prausnitz (2007a) (Fig. 3A-i), wherein the dip-holes in the mask were produced via laser machining, and later produced using microfabrication techniques by Caudill et al. (2018). Gill et al. laser machined a channel to hold the coating liquid and covered it with a masking plate with laser-drilled micrometer-sized holes (Fig. 3A-ii). The holes were made to precisely correspond to the locations of the microneedles in the array. After proper alignment, the microneedle arrays were successfully dip-coated without contaminating the base substrate. Figure 3A-ii shows a one-dimensional array being dip-coated, and Fig. 3A-iii shows a two-dimensional microneedle array coated with this approach. Instead of laser machining, Caudill et al., used the continuous additive manufacturing technique called continuous liquid interface production (CLIP) to fabricate both the microneedles and the masked dip-coating system (Caudill et al., 2018) (Fig. 3B-i), and successfully coated just the microneedle shafts (Fig. 3B-ii). An advantage of the masked dip-coating process is the fine control over the length of the microneedle that can be coated. Both Gill and Prausnitz (2007a) and Caudill et al. (2018) demonstrated this fine control by coating from as little as 25% to almost 100% of the microneedle shaft without contaminating the base substrate of the microneedle patch. The dip-coating technique with a mask has been used by other investigators (Andrianov et al., 2009). In a variation of the masked dip-coating method, a single orifice, which is often a small-diameter pipette tip (Chong et al., 2013) or a hypodermic needle (Witting et al., 2015) has also been used to dip coat the microneedles. Conceptually, the single orifice of the pipette or the hypodermic needle can be considered equivalent to a single masked hole that allows selective contact of the microneedle with the coating solution.
Fig. 3.
Microneedle-coating methods that have been shown to selectively coat the microneedle shafts without contaminating the base substrate of the microneedle array. (A-i) Concept of dip coating microneedle shafts via a protective mask, (A-ii) mask with laser-drilled dip holes placed on top of an underlying coating channel, (A-iii) stereomicrograph of a stainless steel microneedle array coated with riboflavin using a mask that contained dip holes affixed over a solution reservoir (Gill and Prausnitz, 2007b), (B-i) concept of dip-coating microneedles via an additively manufactured solution-filled coating mask device (Caudill et al., 2018), (B-ii) polyethylene glycol dimethacrylate microneedles coated with fluorescently labeled bovine serum albumin (Caudill et al., 2018; on the left is a scanning electron micrograph and on the right is a confocal micrograph), (C-i) concept of generating a thin coating liquid film on a roller using a doctor blade (Ameri et al., 2010), (C-ii) scanning electron micrograph of titanium microneedles coated with parathyroid hormone peptide using the drum roller thin-film method (Ameri et al., 2010), (D-i) concept of generating a thin liquid film using a reservoir that has a shallow depth, (D-ii) micrographs of liquid crystal polymer microneedles coated with lidocaine (Zhang et al., 2012a), (D-iii) stereomicrograph of poly(l-lactide) microneedles coated with lidocaine containing sulforhodamine B as a colored dopant (Baek et al., 2017), (E-i) concept of using a microstructure– well to create a thin liquid film and dipping microneedles into it (Lee et al., 2017a), (E-ii) scanning electron micrograph of poly(l-lactide) microneedles coated with bleomycin after dipping into a liquid film created in a microstructure– well (Lee et al., 2017a), (F-i) a piezoelectric nozzle coating onto a microneedle array (Uddin et al., 2015), (F-ii) scanning electron micrograph of a stainless steel microneedle array coated with 5-fluororacil via piezoelectric-based inkjet printing (Uddin et al., 2015). Figures used with permission from publishers.
Thin-film dip coating.
Besides using a mask, capillary rise can also be countered by reducing the thickness (depth) of the coating liquid. Dip coating of microneedles into a thin film of the coating liquid whose thickness measures less than the height of the microneedle shaft diminishes the capillary rise. Two main approaches have been used to generate the thin liquid film. In one approach a drum is partially submerged in the coating liquid and rotated (Fig. 3C-i). As the drum rotates a thin film of liquid is entrained on the drum circumference and its thickness is controlled with the help of a doctor blade. Microneedles are moved tangential to this liquid film and dipped into the film to achieve coatings (Cormier et al., 2004; Ameri et al., 2010). Ameri et al. placed a roller drum in a drug formulation reservoir (2 ml in volume) and rotated the roller at 50 rpm to generate a thin film of the coating liquid (approx. 100 μm) and dipped 100-μm long microneedles into this thin film to selectively coat just the microneedle shafts (Fig. 3C-ii) (Ameri et al., 2010). In the second approach, a thin film is prepared by using a shallow reservoir whose depth is less than the length of the microneedle shaft, and microneedles are subsequently dipped into this liquid film for coating (Fig. 3D-i). Different studies have used this coating approach (Zhang et al., 2012a,b; Kommareddy et al., 2013; Chen et al., 2015; Nair et al., 2015; Baek et al., 2017), and Fig. 3, D-ii and D-iii, are just two examples of microneedles coated in this manner. In yet another method, the approach -distance of microneedles and coating liquid is restricted by placing a physical barrier between them in the form of a wall called the dam, which stops the microneedle base from fully approaching the surface of the coating solution (Chen et al., 2017; Caudill et al., 2018). Using a different approach for creating a thin film, Lee et al. (2017a) used a microneedle array to itself to hold the coating solution such that the liquid filled the valleys in between the microneedles, and then dipped a second microneedle array into these valleys (Fig. 3E-i) (Lee et al., 2017a). An example of a microneedle array coated using this method is shown in Fig. 3E-ii.
Factors affecting dip coating.
Thickness of the coating deposited on microneedle shafts is an important determinant of the mass of drug coated on a microneedle patch; the greater the coating thickness, greater will be the mass coated. Different parameters can be tuned to control the coating thickness, one is the speed at which the microneedle exits the coating liquid A higher microneedle withdrawal speed from the coating liquid will lead to entrainment of a thicker liquid film on the microneedle surface, which will in turn lead to deposition of a thicker coating compared with a coating produced from lower microneedle withdrawal speeds (Gutfinger and Tallmadge, 1965; Scriven, 1988). However, studies investigating the effect of microneedle withdrawal speed are lacking, and most studies have neither controlled nor reported the measure of this parameter. The second parameter that can be tuned to control coating thickness is the solution viscosity (Gutfinger and Tallmadge, 1965). An increase in viscosity of the coating liquid can increase the thickness of the liquid film that is entrained, which can in turn lead to deposition of thicker coatings. For this reason, a viscosity enhancer is often added into the coating solution to increase the viscosity. The third parameter that can be used to control coating thickness is the number of times a microneedle is dipped into the coating solution, the greater the number of dips, the greater will be the coating thickness (Gill and Prausnitz, 2007b; Kim et al., 2010b, 2014; Pearton et al., 2012; Chen et al., 2015; Jain et al., 2016). Additionally, to achieve uniform and nonpatchy coatings, a surfactant is added into the coating solution. The surfactant helps to reduce the surface tension of the coating solution, which in turn permits proper wetting of the microneedle surface (Bierwagen, 1991). This ensures that the entire dipped portion of the microneedle gets coated without formation of uncoated spots. Another factor that can affect the amount coated is the drying time between dips. Pearton et al. (2012) found that increasing the drying time from 5 to 30 seconds significantly increased the mass of DNA coated on microneedles.
Inkjet Printing.
In this method the material to be coated is ejected directly onto the surface of the microneedle in the form of tiny liquid droplets from piezo-driven nozzles (Uddin et al., 2015; Boehm et al., 2016; Pere et al., 2018). Fig. 3F-i shows a piezoelectric nozzle coating a microneedle on an array, and Fig. 3F-ii shows a microneedle array coated with this approach. In contrast to the dip-coating method, for inkjet printing the coating solution viscosity must be relatively low to prevent nozzle clogging and to successfully generate small-sized drops (Uddin et al., 2015). The inkjet printing method can precisely deposit the droplets onto the microneedle surface; however, since the droplets are small, multiple drops maybe required to cover the desired length of the microneedle. Unlike dip coating, inkjet printing is unidirectional, thus at a given time only one side of the microneedle can be coated with a nozzle. A second coating cycle is required if both sides of a microneedle are to be coated. In this coating method, if multiple microneedles are to be coated simultaneously, then multiple nozzles are required. While this is achievable, however, if the microneedle spacing is small, then placement of multiple nozzles with appropriate spacing could become challenging.
Coating Methods Shown to Coat Both the Microneedle Shafts and the Microneedle Array Base Substrate
Immersion Coating.
In this method the entire microneedle array is immersed into a coating solution. Upon withdrawal of the array, the microneedles and the base substrate both get coated (Fig. 4, A-i and A-ii). It should be noted that in this approach, both the back and the front sides of the base of the array also get coated. This was the first technique reported in literature to coat microneedles (Matriano et al., 2002) and has also been used by others (Raja et al., 2013; van der Maaden et al., 2014; Zeng et al., 2017; Chandler et al., 2018). This is the simplest of all coating techniques, but it leads to significant wastage of the active molecule since coatings are produced on the entire microneedle patch.
Fig. 4.
Microneedle coating methods that have been shown to coat both the microneedle shafts and the base substrate of the array. (A-i and ii) Fluorescent micrographs of titanium microneedles after immersion in a solution containing fluorescently-labeled ovalbumin (Matriano et al., 2002), (B-i) illustration of a drop placed on a microneedle array to coat the microneedles, (B-ii) top view of a silicon microneedle array after coating with a drop containing fluorescein isothiocyanate, white arrows point to tip of a microneedle (Vrdoljak et al., 2012), (C-i) concept of using a gas jet-assisted coating in which gas is blown at an angle theta (θ) to dry the drop of coating formulation placed on a microneedle array (Chen et al., 2011), (C-ii) fluorescent micrograph of a silicon microneedle array coated with polio virus labeled with red fluorescent DyLight 550, white arrows point to coated microneedles (Muller et al., 2017), (D-i) concept of spray-coating microneedles (McGrath et al., 2011), (D-ii) scanning electron micrograph of a silicon microneedle array spray-coated with a coating formulation containing fluorescein isothiocyanate (Vrdoljak et al., 2012), (D-iii) fluorescent micrograph of a single microneedle spray-coated with a coating formulation containing fluorescein isothiocyanate (Vrdoljak et al., 2012), (E) schematic illustrating the concept of electrohydrodynamic spray coating, (F) confocal micrograph of a microneedle array coated using the layer-by-layer method with alternate layers of diphtheria toxoid (green fluorescently labeled with AlexaFluor 488) and N-trimethyl chitosan, a cationic adjuvant that was red fluorescently labeled with rhodamine B isothiocyanate (Schipper et al., 2017), (G) scanning electron micrographs of poly(lactic-co-glycolic acid) microneedles coated using the layer-by-layer method with alternate layers of a poly(β-amino ester) and cross-linked vesicles (DeMuth et al., 2012). Figures used with permission from publishers.
Drop Coating.
In this method, instead of dipping the microneedles in a coating solution, a drop of the coating liquid is placed on the microneedle array thereby submerging all the microneedles (Fig. 4B-i). This approach prevents coating of the backside of the base substrate but still leads to coating deposition on the front surface of the microneedle array base substrate. As the solvent evaporates, the dissolved solid is expected to conformally coat the microneedles and the base substrate. However, under ambient conditions, the rate of evaporation of the solvent (water in most cases) is not fast enough, and consequently the liquid segregates away from the microneedle tip and accumulates on the substrate between the microneedles. As a result, conformal uniform coatings that cover the microneedle shafts and the base substrate are not achieved (Chen et al., 2009; Vrdoljak et al., 2012) (Fig. 4B-ii). Instead, the coatings are produced primarily on the base substrate in between the microneedle shafts. To address this limitation, the rate of solvent evaporation can be increased by heating the patch (Gittard et al., 2013) or by drying under vacuum (DeMuth et al., 2013). Another approach called gas jet–assisted drying coating has been developed to accelerate the drying process with the expectation that the dissolved solids can produce a conformal coating preferentially on the microneedles (Fig. 4C-i). Chen et al. (2009) first reported this technique and used a gas jet at 6–8 m/s and an incident angle of 20° to the horizontal to direct the coating liquid onto the microneedles and away from the base of the microneedles. The 6–8 m/s jet flow was followed by a faster gas-jet of about 10 m/s to blow away the excess coating solution from the base of the microneedles (Chen et al., 2009). While this coating method can preferentially coat the microneedles, it does not completely prevent the base substrate from being coated (Fig. 4C-ii).
Spray Coating.
Conventional spray coating, which uses fluid pressure as the driving force to create droplets (Fig. 4D-i), has also been used to coat microneedles (McGrath et al., 2011; Vrdoljak et al., 2012). An important consideration in achieving conformal coatings via spray coating is to promote coalescence of the drops deposited on the microneedles and the substrate. It was found that a balance between the viscosity and surface tension of the coating liquid and the droplet size (smaller is better) is essential to achieve conformal coatings (McGrath et al., 2011; Vrdoljak et al., 2012). McGrath et al. found that using this method, conformal coatings were produced on both the microneedle surface and the base substrate (McGrath et al., 2011). In another study, Vrdoljak attempted to spray-coat onto a silicon microneedle patch, and found that instead of a conformal coating, the coatings localized at the base of the microneedles (Fig. 4, D-ii and D-iii) (Vrdoljak et al., 2012). As a variation to the conventional spray-coating method, which uses fluid pressure to form droplets, an electrospraying technique called electrohydrodynamic spray method has also been used to coat microneedles. In this approach electrical potential is used to create droplets. To achieve droplet formation, the microneedle array is grounded while the liquid nozzle is provided a high voltage (Fig. 4E). Khan et al. (2014) coupled this method with a sacrificial insulating mask that contained holes and slid it over the microneedles to protect the base substrate from being coated. Although the insulating mask prevented coating of the base substrate, it did not prevent wastage of drug that is deposited on the mask instead of the base substrate. Readers are referred to a recent review by Haj-Ahmad (Haj-Ahmad et al., 2015), which contains a more detailed explanation of spray coatings.
Layer-by-Layer Coating
Layer-by-layer coating is not necessarily a different method of coating microneedles, it differs rather in the principle by which coatings are produced on microneedles. In layer-by-layer method, instead of relying on solution viscosity, electrostatic interactions are used to create a layered coating on microneedle surfaces. First the microneedle surface is prepared so that it can acquire the desired charge polarity. This is often done by either chemically modifying the microneedle surface (van der Maaden et al., 2015; Duong et al., 2018) or by precoating multiple alternate layers of negatively and positively charged polymers through the standard layer-by-layer coating protocols (Saurer et al., 2010). The charge of the top polymer layer dictates the final charge of the microneedles. Subsequently, alternate layers of drug and a suitably charged material is coated using the layer-by-layer method (Saurer et al., 2010; DeMuth et al., 2012; van der Maaden et al., 2015; Schipper et al., 2017; Zeng et al., 2017). The layer-by-layer protocol typically involves either submerging the entire microneedle array into the solution (DeMuth et al., 2012; Zeng et al., 2017) or placing a coating liquid drop on the array to submerge the microneedles (van der Maaden et al., 2015). The contact time of the liquid drop and microneedle array varies anywhere between a few minutes (Saurer et al., 2010) to hours (van der Maaden et al., 2015). To coat nanoparticles, a spray method has also been used (DeMuth et al., 2012). Some exemplary microneedles coated with layer-by-layer method are shown in Fig. 4, F and G. The layer-by-layer approach cannot be used to coat the microneedles selectively, as the base substrate is also contaminated.
Coating Solution Excipients
To successfully create coatings on microneedles, different coating methods require different values of coating solution viscosities and surface tension. To impart these desirable properties to the coating liquid, different excipients have been added to the coating liquid. The materials that have been used as viscosity enhancers can be classified as synthetic polymers such as polyvinylpyrrolidone (Seok et al., 2017) and polyphosphazene (Andrianov et al., 2009) or natural polymers such as carboxymethylcellulose (Gill and Prausnitz, 2007a), although small molecules such as sucrose (Widera et al., 2006) have also been used. The different materials used as viscosity enhancers are tabulated in Table 1. The surfactants that have been used include polymers such as poloxamer Lutrol F68 (Gill and Prausnitz, 2007a,b) and the more conventional Tween class of molecules (Widera et al., 2006), although some unconventional molecules such as Quil-A have also been used as a dual-use material for its adjuvant effect and surfactant properties (Fernando et al., 2012). The different surfactant materials used in microneedle coatings are summarized in Table 2. Besides adding surfactants to the coating liquid, the microneedle surfaces have also been subjected to different treatments with the goal of changing the microneedle surface energy such that proper microneedle wetting can be achieved with coating liquids without the use of a surfactant. For example, plasma treatment was helpful in reducing contact angle of the coating solution on stainless steel microneedle surfaces (Uddin et al., 2015), and a coating of silicon dioxide on stainless steel microneedles also allowed for an excipient-free coating (Gill and Prausnitz, 2007b).
TABLE 1.
Different excipients used to enhance viscosity of a coating solution
| Viscosity Enhancer | Range Used |
|---|---|
| Carboxymethylcellulose sodium salt | 0.25% (w/v) (Kim et al., 2010b) to 10% (w/v) (Baek et al., 2017) |
| 3.2% (w/w) (Song et al., 2015) to 14% (w/w) (Lee et al., 2017a) | |
| Carrageenan | 2% (Kim et al., 2016a)a |
| Fish sperm DNA | 2% (w/v) (Seok et al., 2017) |
| Glycol chitosan | 0.5% (Kim et al., 2016a)a |
| 1% (w/v) (Seok et al., 2017) | |
| Gum ghatti | 1% (w/v) (Kim et al., 2010b) |
| Hyaluronic acid | 0.5% (w/v) (Gill and Prausnitz, 2007b) |
| 5% (Katsumi et al., 2017)a | |
| Hydroxyethylcellulose | 0.5% (w/v) (Kim et al., 2016a) |
| Hydroxypropyl methyl cellulose | 1% (w/v) (Chen et al., 2015) to 12% (w/v) (McGrath et al., 2011) |
| Karaya gum | 1% (w/v) (Kim et al., 2010b) |
| Methylcellulose | −0.5% (Kim et al., 2016a)a |
| −4% (w/v) (Chen et al., 2009) | |
| −2% (w/w) (Caudill et al., 2018) to 3.7% (w/w) (Song et al., 2015) | |
| Pectin | −0.5% (Kim et al., 2016a)a |
| Poly[di(carboxylatophenoxy) phosphazene] | 0.5% (w/v) (Andrianov et al., 2009) |
| Polyethylene glycol | −20% (w/w) (Ma and Gill, 2014) to 80% (w/w) (Ma and Gill, 2014) |
| Polyvinyl alcohol | 11% (w/w) (Chen et al., 2017) to 26% (w/w) (Chen et al., 2017) |
| Polyvinylpyrrolidone | 1% (w/v) (Kim et al., 2010b) to 30% (w/v) (Chen et al., 2015) |
| 10% (w/w) (Li et al., 2018) | |
| Sodium alginate/Alginic acid | 0.3% (w/v) (Choi et al., 2015) to 5% (w/v) (Baek et al., 2017) |
| Sucrose | 25% (w/v) (Gill and Prausnitz, 2007b) to 50% (w/v) (Chen et al., 2015) |
| 52% (w/w) (Gill and Prausnitz, 2007b) | |
| Xanthan gum | 0.075% (w/v) (Choi et al., 2015) to 1% (w/v) (Kim et al., 2010b) |
Unit not mentioned in the reference.
TABLE 2.
Different surfactants used in coating formulations
| Surfactant | Range Used |
|---|---|
| Lutrol F68 NF/Poloxamer 188/Pluronic F68 | 0.5% (w/v) (Serpe et al., 2016) to 2% (w/v) (Hiraishi et al., 2011) |
| Polysorbate 20/Tween 20 | 0.01% (v/v) (Pearson et al., 2013) |
| 0.2% (w/w) (Peters et al., 2012) | |
| Quil-A | 0.2% (w/v) (Chen et al., 2009) |
| Tween 80 | −0.02% (v/v) (Vrdoljak et al., 2012) to 0.5% (v/v) (Vrdoljak et al., 2012) |
| 1% (w/v) (McGrath et al., 2011) |
In addition to viscosity enhancers and surfactants, stabilizers molecule have also been added to help stabilize and protect active molecules such as proteins, inactivated viruses, and virus-like particles from desiccation forces and denaturation. The stabilizer molecules have typically been selected from materials already known for their ability to serve as stabilizers in freeze-drying processes. A study investigated the effect of different excipients and stabilizers and found that instead of carboxymethylcellulose, hydroxyethylcellulose and methylcellulose without surfactant but with trehalose were better able to stabilize hemagglutinin titers in viruses (Kim et al., 2016a). Use of disaccharides such as trehalose, sucrose, and maltose as excipients has helped to stabilize a broad range of materials, such as DNA (Pearton et al., 2012), live measles virus (Edens et al., 2013), erythropoietin (Peters et al., 2012), live adenovirus and modified vaccinia virus Ankara (Vrdoljak et al., 2012), inactivated influenza virus (Quan et al., 2009; Kim et al., 2010a; Kommareddy et al., 2013), inactivated rotavirus (Moon et al., 2013), human growth hormone (Ameri et al., 2014), live bacillus Calmette-Guérin vaccine (Hiraishi et al., 2011), influenza virus-like particles (Kim et al., 2010b), ebola virus glycoprotein (Liu et al., 2018), and inactivated canine influenza virus (Choi et al., 2018). A list of stabilizers with their respective ranges that have been added to coating liquids are presented in Table 3.
TABLE 3.
Different stabilizers added to coating solution
| Stabilizer | Range Used |
|---|---|
| Arginine | 5% (Kim et al., 2016a)a |
| Dextran | 15% (w/v) (Kim et al., 2010b) |
| Fish gelatin | 1.25% (Edens et al., 2013)a |
| Glucose | 15% (w/v) (Kim et al., 2010b) |
| I-Phenylalanine | 2% (w/v) (Witting et al., 2015) |
| Inulin from dahlia tubers and chicory | 15% (w/v) (Kim et al., 2010b) |
| Lactose | 15% (Kim et al., 2016a)a |
| Maltodextrin | 15% (Kim et al., 2016a)a |
| Maltose | 1.5% (w/v) (Pearton et al., 2012) to 3% (w/v) (Pearton et al., 2012) |
| 15% (Kim et al., 2016a)a | |
| Mannitol | 0.35% (Muller et al., 2017)a |
| Mannose | 10% (Kim et al., 2016a)a |
| Myo-inositol | 7.5% (Edens et al., 2013)a |
| Raffinose | 15% (Kim et al., 2016a)a |
| Sucrose | 15% (w/w) (Peters et al., 2012) to 48% (w/w) (Widera et al., 2006) |
| 0.75% (Muller et al., 2017)a | |
| 5% (w/v) (Witting et al., 2015) to 15% (w/v) (Kim et al., 2010b) | |
| Trehalose | 0.75% (Muller et al., 2017)a |
| 30% (w/v) (Kim et al., 2010b) |
Unit not mentioned in the reference.
Properties to Consider When Selecting Coating Excipients
When selecting a coating excipient for coating microneedles, a few important criteria should be considered. First, the material should be biocompatible, preferably it should already be approved for use as an excipient in injectable formulations by local regulatory agencies, such as the Food and Drug Administration in the United States. Prior approval for use of the coating excipients for injectables can reduce the regulatory hurdle in bringing the coated microneedle product to market. Often the “generally recognized as safe” label is used to motivate the notion that the excipient added to coating formulations is safe. It should be noted though that it applies to food additives and not to injectable excipients. Second, the excipients should be in solid state at room temperature. This is important so that they can get deposited as solids on the microneedle surface when the solvent evaporates. If excipients are in liquid state at room temperature, then the coatings will stay wet even after evaporation of the solvent. Tween 20, which is a viscous liquid at room temperature, has been used as a surfactant by many investigators (Table 2). However, it has been added in low amounts (typically 0.2% w/w), and thus its presence has not adversely impacted the integrity of the coated solid film. Third, the coating excipients should be used in as small an amount as possible. If larger amounts are used, this automatically reduces the fraction of the active component in the coatings, and thus lowers the drug-carrying capacity of the microneedle array.
In Vitro Characterization of Coated Microneedles
Once coatings have been deposited on microneedles, it is important to determine the uniformity of coating, the amount of drug that has been coated, and the delivery efficiency.
Coating Uniformity
A visual analysis of the coated microneedles under a microscope (often a stereomicroscope) can provide details on coating uniformity, and whether coatings are contaminating the base substrate of the microneedle array. This is typically done by adding a colored compound or a fluorescent dye into the coating solution, or by conjugating a fluorescent dye onto the active molecule being coated. This aids in visualization of coatings under a stereomicroscope (Fig. 3, A-iii, D-ii, and D-iii; Fig. 4, A-i and A-ii), or a confocal microscope (Fig. 3B-ii-right; Fig. 4, C-ii and F-i). Alternatively, coated microneedles can also be imaged using a scanning electron microscope (Fig. 3, B-ii-left, Fig. 3, C-ii, E-ii, and F-ii; Fig. 4, D-ii and G).
What Is the “Effective Amount” of Drug That Is Coated on a Microneedle Array?
Since only microneedle shafts penetrate the skin, the drug coated just on the microneedle shafts should be considered as the “effective amount” of drug coated on a microneedle array. For microneedle arrays in which the drug coating is situated and localized on just the microneedles shafts, the drug on the entire microneedle array becomes the “effective drug.” To make this quantification, coated microneedle arrays are often immersed in water to allow for the coating to completely dissolve, and the amount of drug in the solution is then determined. However, when the drug is coated on microneedle shafts and on the base substrate of the microneedle array, it is not feasible to determine the effective drug loading of the microneedle array. This is because once drug from the patch has been dissolved into the extraction liquid, the fraction of drug that emanates from the microneedle shafts cannot be discriminated. In such cases, the amount of drug that is actually delivered into the skin provides a measure of the “effective amount” of drug coated on a microneedle array.
As shown in Table 4, the “effective amounts” of active materials that have been coated on microneedles has ranged from nanograms to hundreds of micrograms to a couple of milligrams. The approximate weight ranges of the different materials that have been coated are: viruses and bacteria, 0.43 ng (Corbett et al., 2010) to 30–45 μg (Kommareddy et al., 2013); virus-like particles, 0.4 μg (Song et al., 2010) to 10 μg (Pearton et al., 2013); oligonucleotides, 4 ng (Kask et al., 2010) to 40 μg (Chong et al., 2013); peptides and proteins, 0.05 μg (Schipper et al., 2017) to 500 μg (Ameri et al., 2014); and small molecules, 132 ng (Chen et al., 2017) to 1.9 and 3.8 mg (Kellerman et al., 2017). Because the dip-coating method provides control and precision in coating, it has been successfully used to coat materials ranging from a few nanograms to milligrams. On the other hand, drop coating and layer-by-layer methods have produced coatings that contain relatively lower amounts of active materials.
TABLE 4.
Representative different active materials coated on microneedle patches This is not an exhaustive list. The objective of this table is to provide an assortment of active materials that have been coated on microneedles, along with their respective ranges.
| Active Material Coated | Coating Method | Total Amount Coated (Total Microneedle Shafts in the Patch That Are Coated)a/b |
|---|---|---|
| Viruses and bacteria | ||
| Bacillus Calmette-Guérin vaccine | Dip | 0.83 μg (5 microneedles)a (Hiraishi et al., 2011) |
| Francisella novicida | Immersion | 2.5 × 106 CFU/ml (77 microneedles)b (Chandler et al., 2018) |
| Human papillomavirus: Gardasil | Drop–gas jet | 300ng–0.43 ng HPV vaccine (3364 microneedles)b (Corbett et al., 2010) |
| Inactivated chikungunya virus | Drop–gas jet | 0.38 μg (3364 microneedles)b (Prow et al., 2010) |
| Inactivated polio virus | Drop–gas jet | 1 D-antigen units (10,000 microneedles)b (Muller et al., 2016) |
| Inactivated split influenza virus | Dip | 3 μg (5 microneedles)a (Koutsonanos et al., 2012) |
| Inactivated split influenza virus | Dip | 30–45 μg (320 microneedles)a (Kommareddy et al., 2013) |
| Inactivated split influenza virus | Drop–gas jet | 37 ng (3364 microneedles)b (McNeilly et al., 2014) |
| Inactivated split influenza virus | Drop–gas jet | 15 μg hemagglutinin (10,000 microneedles)b (Fernando et al., 2018) |
| Inactivated whole influenza virus | Dip | 0.4 μg (5 microneedles)a (Quan et al., 2009) |
| Inactivated whole influenza virus | Dip | 3 or 10 μg (5 microneedles)a (Koutsonanos et al., 2009) |
| Inactivated rotavirus vaccine | Dip | 5 or 0.5 μg (5 microneedles)a (Moon et al., 2013) |
| Live measles vaccine virus | Dip | 200 or 1000 TCID50 (5 microneedles)a (Edens et al., 2013) |
| Pneumococcal-conjugate vaccine | Drop–gas jet | 1 μg (21,400 microneedles)b (Pearson et al., 2015) |
| Trivalent inactivated whole influenza viruses | Dip | 6 μg ((5 microneedles)a (Kim et al., 2016b) |
| Virus-like particles (VLPs) | ||
| Influenza VLPs | Dip | 0.4 μg (5 microneedles)a (Song et al., 2010) |
| Influenza VLPs | Dip | 10 μg (5 microneedles)a (Pearton et al., 2013) |
| Oligonucleotides | ||
| DNA vaccine (hepatitis C virus, nonstructural 3/4 A protein) | Dip | 1.6 μg (5 microneedles)a (Gill et al., 2010) |
| DNA vaccine (herpes simplex virus type 2 US6) | Drop–gas jet | 400–4 ng (3364 microneedles)b (Kask et al., 2010) |
| DNA vaccine (influenza virus hemagglutinin) | Dip | 3.6 μg DNA (5 microneedles)a (Kim et al., 2012) |
| DNA vaccine (influenza virus nucleoprotein) | Drop–gas jet | 10 μg (3364 microneedles)b (Fernando et al., 2016) |
| DNA vaccine (Leishmania) | Dip | 20 μg DNA (10 microneedles)a (Moreno et al., 2017) |
| Oligonucleotide | Dip | 0.136 nmol (5 microneedles)a (Luo et al., 2013) |
| siRNA | Dip | 40 μg (10 microneedles)a (Chong et al., 2013) |
| Peptides and proteins | ||
| Bevacizumab (antibody) | Dip | 1.1 μg (5 microneedles)a (Kim et al., 2014) |
| Bovine serum albumin (protein) | Dip | 30 μg (64 microneedles)a (Caudill et al., 2018) |
| Bovine serum albumin (protein) | Dip | 20–100 μg (50 microneedles)a (Andrianov et al., 2009) |
| Der p1 (house dust mite allergen protein) | Dip | 25 ± 25 μg CpG (57 microneedles)a (Shakya et al., 2018) |
| Desmopressin (peptide) | Dip | 82 μg (642 microneedles)a (Cormier et al., 2004) |
| Diphtheria toxoid (protein) | Layer-by-layer | 0.05–0.6 μg (576 microneedles)b (Schipper et al., 2017) |
| Ebola virus glycoprotein | Dip | 0.63 μg (5 microneedles)a (Liu et al., 2018) |
| Erythropoietin (protein) | Dip | 200 μg (1950 microneedles)a (Peters et al., 2012) |
| Exendin-4 (peptide) | Dip | 0.5–4 μg (140 microneedles)a (Liu et al., 2016) |
| M2e-flagellin (fusion protein) | Dip | 1.4 μg (5 microneedles)a (Wang et al., 2014) |
| Ovalbumin (protein) | Dip | 25 ± 25 μg CpG (57 microneedles)a (Shakya et al., 2017) |
| Ovalbumin (protein) | Drop–gas jet | 5 μg OVA (3364 microneedles)b (Ng et al., 2012) |
| Parathyroid hormone peptide | Dip | 20, 30, 40 μg (1300 microneedles)a (Daddona et al., 2011) |
| Peptides and CpG (oligonucleotide) | Layer-by-layer | 5 to 6 μg (77 microneedles)b (Zeng et al., 2017) |
| Recombinant human growth hormone (protein) | Dip | 500 μg (1740 microneedles)a (Ameri et al., 2014) |
| Salmon calcitonin (cyclic polypeptide) | Dip | 1.5 μg (5 microneedles)a (Tas et al., 2012) |
| Small molecules | ||
| 5-Aminolevulinic acid | Dip | 206–680 μg (57 microneedles)a (Jain et al., 2016) |
| Alendronate | Dip | 15 μg (190 microneedles)a (Katsumi et al., 2017) |
| Bleomycin | Dip | 62–518 μg (100 microneedles)a (Lee et al., 2017a) |
| Calcein | Dip | 0.13–21 μg (144 microneedles)a (Chen et al., 2015) |
| Curcumin, 5-fluorouracil, sodium fluorescein | Inkjet | 24–118 μg (50 microneedles)a (Uddin et al., 2015) |
| Doxorubicin encapsulated in particles | Dip | 0.6 μg (5 microneedles)a (Ma et al., 2015) |
| Lidocaine | Dip | 45–94 μg (316 microneedles)a (Zhang et al., 2012b) |
| Lidocaine | Dip | 290 μg (256 microneedles)a (Baek et al., 2017) |
| Lidocaine | Dip (molten drug) | 15 μg (5 microneedles)a (Ma and Gill, 2014) |
| Paclitaxel | Dip | 1 μg (9 microneedles)a (Lee et al., 2014) |
| Sulforhodamine | Dip | 132–835 ng (25 microneedles)a (Chen et al., 2017) |
| Sulforhodamine | Dip | 15 μg (57 microneedles)a (Serpe et al., 2016) |
| Zolmitriptan | Dip | 1.9 mg (1987 microneedles)a (Spierings et al., 2018) |
| 3.8 mg (microneedle number unclear)a (Kellerman et al., 2017) |
Amount coated on microneedle shafts.
Amount delivered into skin (for coating methods that generate coatings on microneedle shaft and base of patch).
Delivery Efficiency
Delivery efficiency is defined as the fraction of the drug coated on a microneedle array that gets delivered into the skin. Although this is an important parameter, during our review we found that not all published studies have determined it. Delivery efficiency is often computed by finding the mass of the drug coated on microneedles, and then subtracting the mass of the drug left on the microneedle array after its insertion into the skin and the mass of the residual drug that maybe left behind on the skin. For microneedles coated with the dip-coating process, the delivery efficiency has been reported in the range of 60%–90% for skin (Hiraishi et al., 2011; Zhang et al., 2012b; Zhao et al., 2016; Baek et al., 2017; Shakya et al., 2017; Nguyen et al., 2018), about 65%–90% for the oral cavity (Ma et al., 2014; Serpe et al., 2016), and about 50%–74% for the eye (Jiang et al., 2007; Kim et al., 2014). Delivery efficiencies from microneedle arrays coated via drop coating (including gas jet–assisted drying coating and layer-by-layer) have been reported to be in the range of 7%–67% for skin (Prow et al., 2010; Fernando et al., 2012; Pearson et al., 2015; Schipper et al., 2017) and about 31% for oral mucosa (McNeilly et al., 2014). For spray coated microneedle arrays, a delivery efficiency of about 40% was achieved (Vrdoljak et al., 2012).
Factors Affecting Delivery Efficiency.
Deposition of coatings only on microneedle shafts.
Since only microneedle shafts penetrate the skin, any drug coated on the microneedle array but away from the microneedle shafts is undeliverable and contributes toward an inefficient delivery process. The contribution of the microneedle array base area toward the inefficiency can be calculated. For example, the typical microneedle patch design used in gas jet–assisted drying coating comprises a 4 × 4 mm patch with 3364 microneedles (Chen et al., 2011). Each microneedle has a base diameter of 25 μm and a length of 110 μm. Although these microneedles are not conical, for an approximate calculation, the microneedles are assumed to be conical in shape, and their total surface area is then about 14.6 mm2 and the surface area of the space between microneedles is about 14.3 mm2. Thus, if drug is uniformly coated on the patch, about 50% of the drug is wasted since it coats the base substrate of the microneedle array. Thus, to achieve higher delivery efficiencies, the base substrate of the microneedle patch should remain uncoated.
Mass of material coated on microneedles.
It has been observed that as the amount of material coated is increased, then after a certain threshold amount, the delivery efficiency decreases (Widera et al., 2006; Kim et al., 2014; Jain et al., 2016; Abdalla et al., 2019). In a study done by Widera et al. (2006) it was seen that the delivery efficiency in hairless guinea pig skin using microneedles coated with radiolabeled ovalbumin was a function of the amount coated. A delivery efficiency of 27% was observed when a high dose (25 μg) was coated; however, the delivery efficiency increased to 50% when a lower dose (5 μg) was coated on the same patch. Likewise, Jain et al. (2016) found that although the amount of 5-aminolevulinic acid on a microneedle patch increased from 206–350 to 458–680 μg/patch, the delivery efficiency was found to have fallen from about 90% to 60% across the dose range. Abdalla et al. (2019) also found a similar threshold effect. When they coated tramadol onto microneedles, they saw that at 20% or 30% (w/v) of tramadol in the coating solution, the resulting delivery efficiencies were similar and measured at 73% and 72%, translating into delivery of 139 and 185 μg tramadol, respectively. However, when the mass of tramadol in the coating solution was increased to 50% (w/v), then, despite the presence of a higher mass of tramadol in the coatings, the delivery efficiency fell to 26% and a much lower mass of tramadol got delivered (78 μg). A similar phenomenon was observed upon insertion of microneedles into the cornea of anesthetized rabbits (Kim et al., 2014). It was seen that although the amount of bevacizumab coated on microneedles increased from 1.1 to 7.6 μg, the delivery efficiency was reduced from around 52% to 44%.
Residence time of coated microneedles in tissues.
It has also been observed that if the coated amount is fixed but the wear time of the patch is increased, the delivery efficiency increases (Cormier et al., 2004; Baek et al., 2017; Lee et al., 2017a). In a study done by Peters et al. (2012) a delivery efficiency of 75% was achieved when a patch coated with 100 μg of erythropoietin was placed in skin for 15 minutes and was further enhanced to 90% when the patch was placed for 2 hours. In another study, Zhang et al. (2012a) coated lidocaine on microneedles and observed a similar effect. The delivery efficiency for a lower dose (approx. 90 μg/array) increased from 53% to 71% as the wear time was increased from 1 to 4 minutes. At a higher dose (approx. 225 μg/array), a much lower delivery efficiency was seen after a 1-minute wear time (approx. 16%), which increased to 28% with a wear time of 4 minutes; however, it was still significantly lower compared with the low dose group. One possible explanation for this phenomenon is that if a larger amount of material is delivered into the skin using coatings, then the material does not immediately dissolve in the limited local interstitial fluid available in the tissue. As a result, if the microneedles are removed while there is still undissolved material at the deposition site, then the microneedle can pull back a fraction of this material, leading to lower delivery efficiencies. However, if the microneedle is allowed to stay inserted in the skin, then over time, the material dissolves in the local tissue fluid and dissipates away from the delivery site.
Other factors.
Delivery efficiency can also be affected by hydrophobicity of the active molecule. In one study, keeping all parameters same, use of low, medium, and high water-soluble peptides led to significantly different delivery efficiencies of about 46%, 59%, and 90%, respectively (Zhao et al., 2017). Delivery efficiency can also vary on the basis of the site of insertion. For example, it was observed by Ma et al. (2014) that using microneedles coated with ovalbumin, a delivery efficiency of 64% was achieved in rabbit lip, whereas a significantly higher delivery efficiency of 91% was observed when the patch was inserted in the tongue. The flat area available to insert a microneedle patch is comparatively smaller in the lip compared with the tongue, which could have reduced the delivery efficiency.
Material of Microneedle Fabrication
A variety of materials have been used to make the coated microneedles. The most dominant material reported in literature is stainless steel, followed by silicon, titanium, and other materials, including polymers. Since microneedles are often dipped in aqueous solutions for coating, they should not be constructed from water-soluble materials. A few studies have, however, reported microneedles made from hyaluronic acid (Liu et al., 2016; Choi et al., 2018). In these studies, to coat microneedles made from hyaluronic acid, the microneedles were dipped rapidly into the aqueous coating solutions to minimize microneedle dissolution (Liu et al., 2016; Choi et al., 2018). In one study however, the microneedles were first frozen to reduce their solubility in water (Choi et al., 2018).
Biomedical Applications, Animal Models, and Human Studies Pursued with Coated Microneedles
Not only is the skin an attractive anatomical site for vaccine delivery, but it also provides an attractive pathway for the delivery of other active compounds. Drugs delivered via coated microneedles bypass the hepatic first-pass effect and can also eliminate adverse effects such as gastric irritation, which can arise if drugs are delivered orally. A broad range of active materials have been coated on microneedles. These include small molecules, peptides, proteins, short nucleic acids, DNA, viruses, virus-like particles, polymeric particles, and insoluble inorganic particles (Cormier et al., 2004; Gill and Prausnitz, 2007a; Kim et al., 2010b; Zhang et al., 2012b). More specifically, coated microneedles have been widely investigated to study influenza vaccination (Kim et al., 2011; McNeilly et al., 2014; Wang et al., 2014; Fernando et al., 2016; Choi et al., 2018). Additionally, coated microneedles have also been used to deliver antigens for other infectious diseases such as diphtheria (Schipper et al., 2017; Du et al., 2018), ebola (Liu et al., 2018), hepatitis (Andrianov et al., 2009; Gill et al., 2010), herpes simplex virus (Chen et al., 2010; Kask et al., 2010), human immunodeficiency virus (Ma et al., 2014; Caucheteux et al., 2016), human papillomavirus (Kines et al., 2015), Leishmania (Moreno et al., 2017), measles (Edens et al., 2013), pneumonia (Pearson et al., 2015), polio (Muller et al., 2016), severe fever with thrombocytopenia syndrome (Jung et al., 2017), smallpox (Hooper et al., 2007), tuberculosis (Hiraishi et al., 2011), West Nile virus (Prow et al., 2010), and chikungunya (Prow et al., 2010). Microneedles have also been used for treatment of allergy (Shakya and Gill, 2015), local delivery of anesthetics (Zhang et al., 2012a), for treatment of anemia (Peters et al., 2012), treatment of type 2 diabetes (Liu et al., 2016), treatment of intimal hyperplasia (Lee et al., 2017b), corneal neovascularization (Kim et al., 2014), treatment of enuresis (Cormier et al., 2004), and treatment of cancer (Ma et al., 2015; Jain et al., 2016). Coated microneedles have also been used in immunotherapy of type 1 diabetes (Zhao et al., 2017) and for treatment of osteoporosis (Katsumi et al., 2017) and restenosis (Lee et al., 2014).
In addition to mice and rats, larger animals whose skin better resembles that of humans have also been used for in vivo investigation of coated microneedles. These larger animals include guinea pigs/hairless guinea pigs (Kommareddy et al., 2013), pigs (Andrianov et al., 2009; Zhang et al., 2012b), and macaques (DeMuth et al., 2013). Rabbits have also been used to deliver drugs and vaccines into the eye (Jiang et al., 2007) and the mouth (Ma and Gill, 2014).
Coated microneedles have been tested in humans. For example, coated microneedles have been used to study treatment of warts (Ryu et al., 2018), osteoporosis (Daddona et al., 2011), migraine (Spierings et al., 2018), and influenza vaccination (Fernando et al., 2018). The tolerability and acceptability of excipient-coated microneedles in humans has also been evaluated. Griffin et al. (2017) noted redness of the skin (erythema) in humans, which lasted day 3 to day 7 post–microneedle application. Pin-prick bleeding was observed in some subjects but it lasted only until 10 minutes post-application. It was found that most of the subjects preferred microneedle patch application over needle and syringe (Griffin et al., 2017). A safety study (Ono et al., 2017) and pain evaluation from uncoated microneedles (Gill et al., 2008) has also been done in humans.
Factors to Consider When Deciding Suitability of Coated Microneedles for New-Drug Delivery Applications
When deciding whether coated microneedles are a suitable drug delivery system for a new application, the drug physical properties at room temperature, drug potency, and drug water solubility are some of the important factors to consider. For obvious reasons, drug that is in a liquid state cannot be delivered using solid microneedles since the liquid will wipe off on the skin surface during insertion. The physical state of the drug at room temperature must be a solid so that when the solvent in the coating solution evaporates, the drug that remains behind can produce a solid film on the microneedle shaft. One of the most important questions to ask in deciding suitability of coated microneedles for drug delivery is the amount of drug that is to be delivered. This is because the delivery capacity of coated microneedles is limited. In general, a microneedle patch of about 1 cm2 can carry as much as a few hundred micrograms of drug. Thus, potent drugs are a good candidate for delivery via coated microneedles. However, if multiple microneedle patches are used or the microneedle patch size is increased, then much higher amounts of drug can be delivered. For example, in a human study, 3.8 mg of drug (zolmitriptan) was delivered using two 5-cm2 patches each coated with 1.9 mg of drug, and also with a single 10-cm2 patch that was coated with 3.8 mg, and the drug pharmacokinetics were found to be similar (Kellerman et al., 2017). Another factor to consider is the water solubility of the drug. Drug water solubility is important because the coating solution often has water as the solvent, and thus it is important that the drug should be water soluble to allow its dissolution in the coating liquid. However, it should be noted that materials such as viruses, bacteria, nanoparticles, and microparticles, although not water-soluble, can nevertheless form a stable dispersion in water and thus be coated on microneedles. These dispersions do not have to be indefinitely stable but should stay stable at least during the coating process. Although it is preferable that drugs should be water soluble, even hydrophobic materials can be coated on microneedles by creating special coating formulations, such as by using cosolvents for dissolving the material in the coating solution (Zhao et al., 2017). The drug stability is an important factor, which is especially relevant to proteins, and must be considered when making a decision to pursue drug delivery using coated microneedles.
Conclusion and Perspective on Microneedle Coatings
Of the different coating methods, some are better than others at achieving the two important properties desirable in a coating process, i.e., achievement of uniformity in coating and the ability to selectively coat just the microneedle shafts without coating the microneedle array base substrate. The dip-coating methods and the inkjet-coating method are precision coating methods that coat just the microneedle shafts without causing wasteful depositions on the substrate. The gas jet–drying coating version of drop coating has improved over the years and offers better selectivity in coating the microneedle shafts; however, its delivery efficiencies are still lower than the microneedles coated with the dip-coating approaches. The spray-coating method indiscriminately sprays the drug on the microneedle patch, and although a sacrificial mask can be used to prevent the coating of the substrate of the microneedle, the drug that gets coated on the sacrificial mask is still wasted. In the layer-by-layer method, selective coating of just the microneedles is not achieved; instead, the coatings are also deposited on the base substrate. Thus, the layer-by-layer coating approach also inherently suffers from poor drug delivery efficiencies. However, if the dip-coating approach is used for layer-by-layer coatings, higher drug delivery efficiencies could be achieved. In terms of time taken to coat a microneedle array, the layer-by-layer method on average is expected to take longer than the other coating methods.
Delivery efficiency of a coated microneedle is an important and biologically relevant design parameter because it directly affects the dose of the active material that is administered in vivo. The delivery efficiencies of microneedle arrays coated via the dip-coating method average around 70%–90%, but this number is reduced to around 30%–50% for immersion coating, drop-coating, spray-coating, and layer-by-layer coating methods. Delivery efficiency is a critical parameter, however, during our review of the literature, we found that many studies failed to report delivery efficiency of their coated-microneedle array. To facilitate cross-study comparisons and to promote rigor, the quantification and reporting of delivery efficiencies of coated-microneedle patches should be considered essential. It should be noted that quantification of proteins and oligonucleotides on microneedles after their insertion into tissues, via nonspecific assays that rely on quantifying total proteins and oligonucleotides can lead to erroneous results and should be avoided. This is because when the coated microneedles are removed from tissues, they can transport out native proteins and DNA from the tissues, and these molecules can erroneously increase the test readings. Fluorescent or radiolabeled tags or tracer molecules are better approaches to facilitate evaluation of delivery efficiencies (Widera et al., 2006; Crichton et al., 2010; Hiraishi et al., 2011; Chen et al., 2012; Luo et al., 2013). Inherent spectroscopic properties of the active material have also been used to specifically quantify the active materials (Peters et al., 2012; Ameri et al., 2014; Jain et al., 2016).
Coated microneedles offer a versatile approach for the delivery of therapeutics. Importantly, different researchers have shown that by using the same base microneedle structure and with almost no change in coating excipients, a few nanograms to hundreds of micrograms of a broad assortment of active materials can be coated including small molecules, proteins, DNA, viruses, and micro- and nanoparticles. For example, in our own coating studies at Texas Tech University (Lubbock, TX), we have continued to use carboxymethylcellulose (1% w/v) as the viscosity enhancer and Lutrol F68 (0.5% w/v) as the surfactant in all our experiments. Likewise, the Prausnitz laboratory at Georgia Institute of Technology (Atlanta, GA) has also been using these same excipients for most of their studies. They have also added trehalose at varying amounts to confer stability on the more labile active materials that are at risk of denaturation upon drying. The work coming out of 3M and Zosana Pharma, likewise, have used sucrose in varying amounts as the viscosity enhancer, with Zosana Pharma also using polysorbate 20 at 0.2% (w/w) as the surfactant. The Kendall group at the University of Queensland (St. Lucia, Australia) has chiefly used methylcellulose as the viscosity enhancer for coating a broad range of materials. The significance of this observation is that when faced with a situation of coating a new active material, the same coating formulation without modification can often be used as a good starting point, and the targeted mass in the coating can be achieved by simply changing the weight fraction of the active material in the coating solution. This suggests that not only the same base microneedle array but also the same coating formulation could be used to develop new products based on coated microneedles, which can help to accelerate product development.
Recently, the concept of individually coated microneedles (Caudill et al., 2018; Li et al., 2018) has emerged as a means of coating different drug molecules on the same microneedle patch. Using this concept, it should be possible to provide combination drug therapies through a single microneedle patch. In addition to their use in the delivery of active molecules, coated microneedles are now being developed for use in sampling the skin and the interstitial fluid for detection and sensing purposes (Mandal et al., 2018; Samant and Prausnitz, 2018).
In addition to water-based coating solutions, a molten coating solution without a solvent has also been reported. To coat water-insoluble drugs, Ma and Gill (2014) used a mixture of polyethylene glycol (PEG)+lidocaine (water-insoluble base form of lidocaine), heated it to 120°C to melt the PEG and lidocaine, and dip-coated the microneedles in it. PEG was added as an excipient to serve as a water-soluble matrix so that upon insertion into skin PEG would dissolve and facilitate detachment of the PEG+lidocaine coating from the microneedle surface. Such approaches could be used to develop formulations for coating water-insoluble small-molecule drugs onto microneedles, which could increase the scope of materials that can be delivered using coated microneedles.
From a commercialization viewpoint, four companies, namely Zosana Pharma (https://www.zosanopharma.com/, accessed: April 1, 2019), 3M (https://www.3m.com/3M/en_US/drug-delivery-systems-us/technologies/microneedle/solid/, accessed: April 1, 2019), Vaxxas (http://www.vaxxas.com/, accessed: April 1, 2019), and Moonlight Therapeutics (https://www.moonlighttx.com/, accessed: April 1, 2019) are operating in the coated-microneedle space. Zosana Pharma and Moonlight Therapeutics use metal microneedles as their base platform, 3M uses polymer microneedles, and Vaxxas use silicon microneedles. These four companies are seeking to develop products with coated microneedle technology as a basis. Zosana Pharma, 3M, and Moonlight Therapeutics use the dip-coating approach to coat microneedles, whereas Vaxxas uses the drop gas jet–assisted drying coating method. Zosana Pharma has a coated microneedle patch for treatment of migraine, and they have completed a phase 3 trial in humans. Vaxxas is developing coated microneedles for vaccine delivery and for use in oncology. 3M on the other hand has developed capability to coat microneedles and works directly with partners for custom applications. Moonlight Therapeutics is developing coated microneedles for treatment of allergies and is focusing on peanut allergy as their first product.
In conclusion, a wide range of microneedle coating methods have been developed. For most applications, controlled coating of microneedle shafts without contaminating the base substrate is desirable. It can, however, be argued that in some instances, e.g., when the drug is inexpensive, contamination of the base substrate may be tolerated. So far, the dip-coating and inkjet-coating methods have exhibited most promise in facilitating the selective coating of microneedle shafts without contaminating the base substrate. The most widely studied use of coated microneedles has been for the delivery of infectious disease vaccines; however, other applications are being actively investigated. Although additional fundamental research on the coating processes can benefit the field of coated microneedles, there is now a strong effort, after 17 years from when the first article on coated microneedles was published (Matriano et al., 2002), to bring the first coated microneedle–based product to market.
Abbreviations
- PEG
polyethylene glycol
Authorship Contributions
Participated in research design: Ingrole, Gill.
Performed data analysis: Ingrole, Gill.
Wrote or contributed to the writing of the manuscript: Ingrole, Gill.
Footnotes
This publication was supported by the National Institute of Allergy and Infectious Diseases National Institutes of Health [Award Numbers R01AI135197 and R01AI121322 to H.S.G.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Financial Disclosures: HSG is a coinventor on a patent related to coated microneedles and has stock ownership in a startup company called Moonlight Therapeutics that is developing microneedles for food allergy immunotherapy. This conflict of interest has been disclosed and is being managed by Texas Tech University. No financial support was provided by Moonlight Therapeutics for this work.
References
- Abdalla HB, Jain AK, Napimoga MH, Clemente-Napimoga JT, Gill HS. (2019) Microneedles coated with tramadol exhibit anti-nociceptive effect in a rat model of temporomandibular hypernociception. J Pharmacol Exp Ther 10.1124/jpet.119.256750 [DOI] [PubMed]
- Ameri M, Fan SC, Maa YF. (2010) Parathyroid hormone PTH(1-34) formulation that enables uniform coating on a novel transdermal microprojection delivery system. Pharm Res 27:303–313. [DOI] [PubMed] [Google Scholar]
- Ameri M, Kadkhodayan M, Nguyen J, Bravo JA, Su R, Chan K, Samiee A, Daddona PE. (2014) Human growth hormone delivery with a microneedle transdermal system: preclinical formulation, stability, delivery and PK of therapeutically relevant doses. Pharmaceutics 6:220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, Babiuk LA, Townsend H, Mutwiri G. (2009) Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci USA 106:18936–18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SH, Shin JH, Kim YC. (2017) Drug-coated microneedles for rapid and painless local anesthesia. Biomed Microdevices 19:2. [DOI] [PubMed] [Google Scholar]
- Bierwagen GP. (1991) Surface defects and surface flows in coatings. Prog Org Coating 19:59–68. [Google Scholar]
- Birchall JC, Clemo R, Anstey A, John DN. (2011) Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res 28:95–106. [DOI] [PubMed] [Google Scholar]
- Boehm RD, Jaipan P, Skoog SA, Stafslien S, VanderWal L, Narayan RJ. (2016) Inkjet deposition of itraconazole onto poly(glycolic acid) microneedle arrays. Biointerphases 11:011008. [DOI] [PubMed] [Google Scholar]
- Bouwstra JA, Ponec M. (2006) The skin barrier in healthy and diseased state. Biochimica et Biophysica Acta 1758:2080–2095. [DOI] [PubMed] [Google Scholar]
- Caucheteux SM, Mitchell JP, Ivory MO, Hirosue S, Hakobyan S, Dolton G, Ladell K, Miners K, Price DA, Kan-Mitchell J, et al. (2016) Polypropylene sulfide nanoparticle p24 vaccine promotes dendritic cell-mediated specific immune responses against HIV-1. J Invest Dermatol 136:1172–1181. [DOI] [PubMed] [Google Scholar]
- Caudill CL, Perry JL, Tian S, Luft JC, DeSimone JM. (2018) Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J Control Release 284:122–132. [DOI] [PubMed] [Google Scholar]
- Chandler CE, Harberts EM, Laemmermann T, Zeng Q, Opene BN, Germain RN, Jewell CM, Scott AJ, Ernst RK. (2018) In vivo intradermal delivery of bacteria by using microneedle arrays. Infect Immun 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Qiu Y, Zhang S, Yang G, Gao Y. (2015) Controllable coating of microneedles for transdermal drug delivery. Drug Dev Ind Pharm 41:415–422. [DOI] [PubMed] [Google Scholar]
- Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, Corbett HJ, Primiero CA, Ansaldo AB, Frazer IH, et al. (2011) Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release 152:349–355. [DOI] [PubMed] [Google Scholar]
- Chen X, Fernando GJ, Raphael AP, Yukiko SR, Fairmaid EJ, Primiero CA, Frazer IH, Brown LE, Kendall MA. (2012) Rapid kinetics to peak serum antibodies is achieved following influenza vaccination by dry-coated densely packed microprojections to skin. J Control Release 158:78–84. [DOI] [PubMed] [Google Scholar]
- Chen X, Kask AS, Crichton ML, McNeilly C, Yukiko S, Dong L, Marshak JO, Jarrahian C, Fernando GJ, Chen D, et al. (2010) Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J Control Release 148:327–333. [DOI] [PubMed] [Google Scholar]
- Chen X, Prow TW, Crichton ML, Jenkins DW, Roberts MS, Frazer IH, Fernando GJ, Kendall MA. (2009) Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release 139:212–220. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen BZ, Wang QL, Jin X, Guo XD. (2017) Fabrication of coated polymer microneedles for transdermal drug delivery. J Control Release 265:14–21. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Song JM, Bondy BJ, Compans RW, Kang SM, Prausnitz MR. (2015) Effect of osmotic pressure on the stability of whole inactivated influenza vaccine for coating on microneedles. PLoS One 10:e0134431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IJ, Kang A, Ahn MH, Jun H, Baek SK, Park JH, Na W, Choi SO. (2018) Insertion-responsive microneedles for rapid intradermal delivery of canine influenza vaccine. J Control Release 286:460–466. [DOI] [PubMed] [Google Scholar]
- Chong RH, Gonzalez-Gonzalez E, Lara MF, Speaker TJ, Contag CH, Kaspar RL, Coulman SA, Hargest R, Birchall JC. (2013) Gene silencing following siRNA delivery to skin via coated steel microneedles: in vitro and in vivo proof-of-concept. J Control Release 166:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. (2010) Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One 5:e13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, Daddona P. (2004) Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release 97:503–511. [DOI] [PubMed] [Google Scholar]
- Crichton ML, Ansaldo A, Chen X, Prow TW, Fernando GJ, Kendall MA. (2010) The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials 31:4562–4572. [DOI] [PubMed] [Google Scholar]
- Daddona PE, Matriano JA, Mandema J, Maa YF. (2011) Parathyroid hormone (1-34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res 28:159–165. [DOI] [PubMed] [Google Scholar]
- DeMuth PC, Li AV, Abbink P, Liu J, Li H, Stanley KA, Smith KM, Lavine CL, Seaman MS, Kramer JA, et al. (2013) Vaccine delivery with microneedle skin patches in nonhuman primates. Nat Biotechnol 31:1082–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. (2012) Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano 6:8041–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Woythe L, van der Maaden K, Leone M, Romeijn S, Kros A, Kersten G, Jiskoot W, Bouwstra JA. (2018) Coated and hollow microneedle-mediated intradermal immunization in mice with diphtheria toxoid loaded mesoporous silica nanoparticles. Pharm Res 35:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong HTT, Kim NW, Thambi T, Giang Phan VH, Lee MS, Yin Y, Jeong JH, Lee DS. (2018) Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J Control Release 269:225–234. [DOI] [PubMed] [Google Scholar]
- Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. (2013) Measles vaccination using a microneedle patch. Vaccine 31:3403–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando GJ, Chen X, Primiero CA, Yukiko SR, Fairmaid EJ, Corbett HJ, Frazer IH, Brown LE, Kendall MA. (2012) Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J Control Release 159:215–221. [DOI] [PubMed] [Google Scholar]
- Fernando GJ, Zhang J, Ng HI, Haigh OL, Yukiko SR, Kendall MA. (2016) Influenza nucleoprotein DNA vaccination by a skin targeted, dry coated, densely packed microprojection array (Nanopatch) induces potent antibody and CD8(+) T cell responses. J Control Release 237:35–41. [DOI] [PubMed] [Google Scholar]
- Fernando GJP, Hickling J, Jayashi Flores CM, Griffin P, Anderson CD, Skinner SR, Davies C, Witham K, Pryor M, Bodle J, et al. (2018) Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). Vaccine 36:3779–3788. [DOI] [PubMed] [Google Scholar]
- Gill HS, Denson DD, Burris BA, Prausnitz MR. (2008) Effect of microneedle design on pain in human volunteers. Clin J Pain 24:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Prausnitz MR. (2007a) Coated microneedles for transdermal delivery. J Control Release 117:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Prausnitz MR. (2007b) Coating formulations for microneedles. Pharm Res 24:1369–1380. [DOI] [PubMed] [Google Scholar]
- Gill HS, Söderholm J, Prausnitz MR, Sällberg M. (2010) Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther 17:811–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittard SD, Chen B, Xu H, Ovsianikov A, Chichkov BN, Monteiro-Riviere NA, Narayan RJ. (2013) The effects of geometry on skin penetration and failure of polymer microneedles. J Adhes Sci Technol 27:227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P, Elliott S, Krauer K, Davies C, Rachel Skinner S, Anderson CD, Forster A. (2017) Safety, acceptability and tolerability of uncoated and excipient-coated high density silicon micro-projection array patches in human subjects. Vaccine 35 (48 Pt B):6676–6684. [DOI] [PubMed] [Google Scholar]
- Gutfinger C, Tallmadge JA. (1965) Films of non-Newtonian fluids adhering to flat plates. AIChE J 11:403–413. [Google Scholar]
- Haj-Ahmad R, Khan H, Arshad MS, Rasekh M, Hussain A, Walsh S, Li X, Chang M-W, Ahmad Z. (2015) Microneedle coating techniques for transdermal drug delivery. Pharmaceutics 7:486–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, Morrissey A, Birchall JC. (2009) Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices 11:35–47. [DOI] [PubMed] [Google Scholar]
- Henry S, McAllister DV, Allen MG, Prausnitz MR. (1998) Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci 87:922–925. [DOI] [PubMed] [Google Scholar]
- Hiraishi Y, Nandakumar S, Choi SO, Lee JW, Kim YC, Posey JE, Sable SB, Prausnitz MR. (2011) Bacillus Calmette-Guérin vaccination using a microneedle patch. Vaccine 29:2626–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper JW, Golden JW, Ferro AM, King AD. (2007) Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine 25:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Lee CH, Gill HS. (2016) 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J Control Release 239:72–81. [DOI] [PubMed] [Google Scholar]
- Jiang J, Gill HS, Ghate D, McCarey BE, Patel SR, Edelhauser HF, Prausnitz MR. (2007) Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci 48:4038–4043. [DOI] [PubMed] [Google Scholar]
- Jung D, Rejinold NS, Kwak JE, Park SH, Kim YC. (2017) Nano-patterning of a stainless steel microneedle surface to improve the dip-coating efficiency of a DNA vaccine and its immune response. Colloids Surf B Biointerfaces 159:54–61. [DOI] [PubMed] [Google Scholar]
- Kask AS, Chen X, Marshak JO, Dong L, Saracino M, Chen D, Jarrahian C, Kendall MA, Koelle DM. (2010) DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine 28:7483–7491. [DOI] [PubMed] [Google Scholar]
- Katsumi H, Tanaka Y, Hitomi K, Liu S, Quan YS, Kamiyama F, Sakane T, Yamamoto A. (2017) Efficient transdermal delivery of alendronate, a nitrogen-containing bisphosphonate, using tip-loaded self-dissolving microneedle arrays for the treatment of osteoporosis. Pharmaceutics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman DJ, Ameri M, Tepper SJ. (2017) Rapid systemic delivery of zolmitriptan using an adhesive dermally applied microarray. Pain Manag 7:559–567. [DOI] [PubMed] [Google Scholar]
- Khan H, Mehta P, Msallam H, Armitage D, Ahmad Z. (2014) Smart microneedle coatings for controlled delivery and biomedical analysis. J Drug Target 22:790–795. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Shin JH, Noh JY, Song CS, Kim YC. (2016a) Development of the novel coating formulations for skin vaccination using stainless steel microneedle. Drug Deliv Transl Res 6:486–497. [DOI] [PubMed] [Google Scholar]
- Kim YC, Grossniklaus HE, Edelhauser HF, Prausnitz MR. (2014) Intrastromal delivery of bevacizumab using microneedles to treat corneal neovascularization. Invest Ophthalmol Vis Sci 55:7376–7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee SH, Choi WH, Choi HJ, Goo TW, Lee JH, Quan FS. (2016b) Microneedle delivery of trivalent influenza vaccine to the skin induces long-term cross-protection. J Drug Target 24:943–951. [DOI] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. (2010a) Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release 142:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. (2010b) Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech 11:1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. (2011) Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm Res 28:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Song JM, Vunnava A, Yoo DG, Park KM, Compans RW, Kang SM, Prausnitz MR. (2010c) Influenza immunization with trehalose-stabilized virus-like particle vaccine using microneedles. Procedia in vaccinology 2:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. (2009) Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine 27:6932–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. (2010d) Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis 201:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Yoo DG, Compans RW, Kang SM, Prausnitz MR. (2013) Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles. J Control Release 172:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Song JM, Lipatov AS, Choi SO, Lee JW, Donis RO, Compans RW, Kang SM, Prausnitz MR. (2012) Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur J Pharm Biopharm 81:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines RC, Zarnitsyn V, Johnson TR, Pang YY, Corbett KS, Nicewonger JD, Gangopadhyay A, Chen M, Liu J, Prausnitz MR, et al. (2015) Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PLoS One 10:e0120797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommareddy S, Baudner BC, Bonificio A, Gallorini S, Palladino G, Determan AS, Dohmeier DM, Kroells KD, Sternjohn JR, Singh M, et al. (2013) Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine 31:3435–3441. [DOI] [PubMed] [Google Scholar]
- Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. (2009) Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One 4:e4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, Taherbhai MT, Prausnitz MR, Compans RW, Skountzou I. (2012) Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep 2:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Ryu HR, Roh JY, Park JH. (2017a) Bleomycin-coated microneedles for treatment of warts. Pharm Res 34:101–112. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim DH, Lee KJ, Seo IH, Park SH, Jang EH, Park Y, Youn YN, Ryu W. (2017b) Transfer-molded wrappable microneedle meshes for perivascular drug delivery. J Control Release 268:237–246. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Park SH, Lee JY, Joo HC, Jang EH, Youn YN, Ryu W. (2014) Perivascular biodegradable microneedle cuff for reduction of neointima formation after vascular injury. J Control Release 192:174–181. [DOI] [PubMed] [Google Scholar]
- Li S, Li W, Prausnitz M. (2018) Individually coated microneedles for co-delivery of multiple compounds with different properties. Drug Deliv Transl Res 8:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wu D, Quan YS, Kamiyama F, Kusamori K, Katsumi H, Sakane T, Yamamoto A. (2016) Improvement of transdermal delivery of exendin-4 using novel tip-loaded microneedle arrays fabricated from hyaluronic acid. Mol Pharm 13:272–279. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ye L, Lin F, Gomaa Y, Flyer D, Carrion R, Jr, Patterson JL, Prausnitz MR, Smith G, Glenn G, et al. (2018) Intradermal vaccination with adjuvanted ebola virus soluble glycoprotein subunit vaccine by microneedle patches protects mice against lethal ebola virus challenge. J Infect Dis 218 (suppl_5):S545–S552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Ye T, Ma Y, Gill HS, Nitin N. (2013) Microprecision delivery of oligonucleotides in a 3D tissue model and its characterization using optical imaging. Mol Pharm 10:2868–2879. [DOI] [PubMed] [Google Scholar]
- Ma Y, Boese SE, Luo Z, Nitin N, Gill HS. (2015) Drug coated microneedles for minimally-invasive treatment of oral carcinomas: development and in vitro evaluation. Biomed Microdevices 17:44. [DOI] [PubMed] [Google Scholar]
- Ma Y, Gill HS. (2014) Coating solid dispersions on microneedles via a molten dip-coating method: development and in vitro evaluation for transdermal delivery of a water-insoluble drug. J Pharm Sci 103:3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. (2014) Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm Res 31:2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Boopathy AV, Lam LKW, Moynihan KD, Welch ME, Bennett NR, Turvey ME, Thai N, Van JH, Love JC, et al. (2018) Cell and fluid sampling microneedle patches for monitoring skin-resident immunity. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. (2002) Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res 19:63–70. [DOI] [PubMed] [Google Scholar]
- McGrath MG, Vrdoljak A, O’Mahony C, Oliveira JC, Moore AC, Crean AM. (2011) Determination of parameters for successful spray coating of silicon microneedle arrays. Int J Pharm 415:140–149. [DOI] [PubMed] [Google Scholar]
- McNeilly CL, Crichton ML, Primiero CA, Frazer IH, Roberts MS, Kendall MA. (2014) Microprojection arrays to immunise at mucosal surfaces. J Control Release 196:252–260. [DOI] [PubMed] [Google Scholar]
- Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. (2013) Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine 31:3396–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, Schwartz J, Calvo A, Blanco L, Larrea E, Irache JM, Sanmartín C, Coulman SA, Soto M, Birchall JC, et al. (2017) Skin vaccination using microneedles coated with a plasmid DNA cocktail encoding nucleosomal histones of Leishmania spp. Int J Pharm 533:236–244. [DOI] [PubMed] [Google Scholar]
- Muller DA, Fernando GJP, Owens NS, Agyei-Yeboah C, Wei JCJ, Depelsenaire ACI, Forster A, Fahey P, Weldon WC, Oberste MS, et al. (2017) High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Sci Rep 7:12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DA, Pearson FE, Fernando GJ, Agyei-Yeboah C, Owens NS, Corrie SR, Crichton ML, Wei JC, Weldon WC, Oberste MS, et al. (2016) Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci Rep 6:22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair K, Whiteside B, Grant C, Patel R, Tuinea-Bobe C, Norris K, Paradkar A. (2015) Investigation of plasma treatment on micro-injection moulded microneedle for drug delivery. Pharmaceutics 7:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HI, Fernando GJ, Kendall MA. (2012) Induction of potent CD8+ T cell responses through the delivery of subunit protein vaccines to skin antigen-presenting cells using densely packed microprojection arrays. J Control Release 162:477–484. [DOI] [PubMed] [Google Scholar]
- Nguyen J, Lewis H, Queja A, Diep AN, Hochart G, Ameri M. (2018) Pharmacokinetics and skin tolerability of intracutaneous zolmitriptan delivery in swine using adhesive dermally applied microarray. J Pharm Sci 107:2192–2197. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Park JH. (2018) Human studies with microneedles for evaluation of their efficacy and safety. Expert Opin Drug Deliv 15:235–245. [DOI] [PubMed] [Google Scholar]
- Ono A, Azukizawa H, Ito S, Nakamura Y, Asada H, Quan YS, Kamiyama F, Katayama I, Hirobe S, Okada N. (2017) Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. Int J Pharm 532:374–383. [DOI] [PubMed] [Google Scholar]
- Pearson FE, McNeilly CL, Crichton ML, Primiero CA, Yukiko SR, Fernando GJ, Chen X, Gilbert SC, Hill AV, Kendall MA. (2013) Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One 8:e67888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson FE, Muller DA, Roalfe L, Zancolli M, Goldblatt D, Kendall MA. (2015) Functional anti-polysaccharide IgG titres induced by unadjuvanted pneumococcal-conjugate vaccine when delivered by microprojection-based skin patch. Vaccine 33:6675–6683. [DOI] [PubMed] [Google Scholar]
- Pearton M, Pirri D, Kang SM, Compans RW, Birchall JC. (2013) Host responses in human skin after conventional intradermal injection or microneedle administration of virus-like-particle influenza vaccine. Adv Healthc Mater 2:1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearton M, Saller V, Coulman SA, Gateley C, Anstey AV, Zarnitsyn V, Birchall JC. (2012) Microneedle delivery of plasmid DNA to living human skin: formulation coating, skin insertion and gene expression. J Control Release 160:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pere CPP, Economidou SN, Lall G, Ziraud C, Boateng JS, Alexander BD, Lamprou DA, Douroumis D. (2018) 3D printed microneedles for insulin skin delivery. Int J Pharm 544:425–432. [DOI] [PubMed] [Google Scholar]
- Peters EE, Ameri M, Wang X, Maa YF, Daddona PE. (2012) Erythropoietin-coated ZP-microneedle transdermal system: preclinical formulation, stability, and delivery. Pharm Res 29:1618–1626. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR. (2004) Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 56:581–587. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR. (2017) Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng 8:177–200. [DOI] [PubMed] [Google Scholar]
- Prow TW, Chen X, Prow NA, Fernando GJ, Tan CS, Raphael AP, Chang D, Ruutu MP, Jenkins DW, Pyke A, et al. (2010) Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small 6:1776–1784. [DOI] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. (2009) Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One 4:e7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn HL, Kearney M-C, Courtenay AJ, McCrudden MTC, Donnelly RF. (2014) The role of microneedles for drug and vaccine delivery. Expert Opin Drug Deliv 11:1769–1780. [DOI] [PubMed] [Google Scholar]
- Raja WK, Maccorkle S, Diwan IM, Abdurrob A, Lu J, Omenetto FG, Kaplan DL. (2013) Transdermal delivery devices: fabrication, mechanics and drug release from silk. Small 9:3704–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AM, Cordeiro AS, Kissenpfennig A, Donnelly RF. (2018) Microneedle arrays for vaccine delivery: the possibilities, challenges and use of nanoparticles as a combinatorial approach for enhanced vaccine immunogenicity. Expert Opin Drug Deliv 15:851–867. [DOI] [PubMed] [Google Scholar]
- Ryu HR, Jeong HR, Seon-Woo HS, Kim JS, Lee SK, Kim HJ, Baek JO, Park JH, Roh JY. (2018) Efficacy of a bleomycin microneedle patch for the treatment of warts. Drug Deliv Transl Res 8:273–280. [DOI] [PubMed] [Google Scholar]
- Rzhevskiy AS, Singh TRR, Donnelly RF, Anissimov YG. (2018) Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J Control Release 270:184–202. [DOI] [PubMed] [Google Scholar]
- Samant PP, Prausnitz MR. (2018) Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc Natl Acad Sci USA 115:4583–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer EM, Flessner RM, Sullivan SP, Prausnitz MR, Lynn DM. (2010) Layer-by-layer assembly of DNA- and protein-containing films on microneedles for drug delivery to the skin. Biomacromolecules 11:3136–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper P, van der Maaden K, Groeneveld V, Ruigrok M, Romeijn S, Uleman S, Oomens C, Kersten G, Jiskoot W, Bouwstra J. (2017) Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J Control Release 262:28–36. [DOI] [PubMed] [Google Scholar]
- Scriven LE. (1988) Physics and applications of dip coating and spin coating. MRS Online Proc 121:717–729 DOI: 10.1557/PROC-121-717. [DOI] [Google Scholar]
- Seok H, Noh JY, Lee DY, Kim SJ, Song CS, Kim YC. (2017) Effective humoral immune response from a H1N1 DNA vaccine delivered to the skin by microneedles coated with PLGA-based cationic nanoparticles. J Control Release 265:66–74. [DOI] [PubMed] [Google Scholar]
- Serpe L, Jain A, de Macedo CG, Volpato MC, Groppo FC, Gill HS, Franz-Montan M. (2016) Influence of salivary washout on drug delivery to the oral cavity using coated microneedles: An in vitro evaluation. Eur J Pharm Sci 93:215–223. [DOI] [PubMed] [Google Scholar]
- Shakya AK, Gill HS. (2015) A comparative study of microneedle-based cutaneous immunization with other conventional routes to assess feasibility of microneedles for allergy immunotherapy. Vaccine 33:4060–4064. [DOI] [PubMed] [Google Scholar]
- Shakya AK, Lee CH, Gill HS. (2017) Cutaneous vaccination with coated microneedles prevents development of airway allergy. J Control Release 265:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya AK, Lee CH, Gill HS. (2018) Coated microneedle-based cutaneous immunotherapy prevents Der p 1-induced airway allergy in mice. J Allergy Clin Immunol 142:2007–2011.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CI, Jeong SD, Rejinold NS, Kim YC. (2017) Microneedles for vaccine delivery: challenges and future perspectives. Ther Deliv 8:447–460. [DOI] [PubMed] [Google Scholar]
- Song HB, Lee KJ, Seo IH, Lee JY, Lee SM, Kim JH, Kim JH, Ryu W. (2015) Impact insertion of transfer-molded microneedle for localized and minimally invasive ocular drug delivery. J Control Release 209:272–279. [DOI] [PubMed] [Google Scholar]
- Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG, Park KM, Chen LM, Quan FS, Birchall JC, et al. (2010) Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol 17:1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierings EL, Brandes JL, Kudrow DB, Weintraub J, Schmidt PC, Kellerman DJ, Tepper SJ. (2018) Randomized, double-blind, placebo-controlled, parallel-group, multi-center study of the safety and efficacy of ADAM zolmitriptan for the acute treatment of migraine. Cephalalgia 38:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas C, Mansoor S, Kalluri H, Zarnitsyn VG, Choi SO, Banga AK, Prausnitz MR. (2012) Delivery of salmon calcitonin using a microneedle patch. Int J Pharm 423:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MJ, Scoutaris N, Klepetsanis P, Chowdhry B, Prausnitz MR, Douroumis D. (2015) Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int J Pharm 494:593–602. [DOI] [PubMed] [Google Scholar]
- van der Maaden K, Sekerdag E, Schipper P, Kersten G, Jiskoot W, Bouwstra J. (2015) Layer-by-Layer assembly of inactivated poliovirus and N-trimethyl chitosan on pH-sensitive microneedles for dermal vaccination. Langmuir 31:8654–8660. [DOI] [PubMed] [Google Scholar]
- van der Maaden K, Varypataki EM, Romeijn S, Ossendorp F, Jiskoot W, Bouwstra J. (2014) Ovalbumin-coated pH-sensitive microneedle arrays effectively induce ovalbumin-specific antibody and T-cell responses in mice. Eur J Pharm Biopharm 88:310–315. [DOI] [PubMed] [Google Scholar]
- Vrdoljak A, McGrath MG, Carey JB, Draper SJ, Hill AV, O’Mahony C, Crean AM, Moore AC. (2012) Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J Control Release 159:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BZ, Gill HS, He C, Ou C, Wang L, Wang YC, Feng H, Zhang H, Prausnitz MR, Compans RW. (2014) Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J Control Release 178:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M. (2006) Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine 24:1653–1664. [DOI] [PubMed] [Google Scholar]
- Witting M, Obst K, Pietzsch M, Friess W, Hedtrich S. (2015) Feasibility study for intraepidermal delivery of proteins using a solid microneedle array. Int J Pharm 486:52–58. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Gammon JM, Tostanoski LH, Chiu YC, Jewell CM. (2017) In vivo expansion of melanoma-specific T cells using microneedle arrays coated with immune-polyelectrolyte multilayers. ACS Biomater Sci Eng 3:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K. (2012a) Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res 29:170–177. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Siebenaler K, Brown K, Dohmeier D, Hansen K. (2012b) Adjuvants to prolong the local anesthetic effects of coated microneedle products. Int J Pharm 439:187–192. [DOI] [PubMed] [Google Scholar]
- Zhao X, Birchall JC, Coulman SA, Tatovic D, Singh RK, Wen L, Wong FS, Dayan CM, Hanna SJ. (2016) Microneedle delivery of autoantigen for immunotherapy in type 1 diabetes. J Control Release 223:178–187. [DOI] [PubMed] [Google Scholar]
- Zhao X, Coulman SA, Hanna SJ, Wong FS, Dayan CM, Birchall JC. (2017) Formulation of hydrophobic peptides for skin delivery via coated microneedles. J Control Release 265:2–13. [DOI] [PubMed] [Google Scholar]