Abstract
The use of drug delivery systems (DDS) is an attractive approach to facilitate uptake of therapeutic agents at the desired site of action, particularly when free drug has poor pharmacokinetics/biodistribution (PK/BD) or significant off-site toxicities. Successful translation of DDS into the clinic is dependent on a thorough understanding of the in vivo behavior of the carrier, which has, for the most part, been an elusive goal. This is, at least in part, due to significant differences in the mechanisms controlling pharmacokinetics for classic drugs and DDSs. In this review, we summarize the key physiologic mechanisms controlling the in vivo behavior of DDS, compare and contrast this with classic drugs, and describe engineering strategies designed to improve DDS PK/BD. In addition, we describe quantitative approaches that could be useful for describing PK/BD of DDS, as well as critical steps between tissue uptake and pharmacologic effect.
Introduction
Modern pharmacotherapy uses an expanded roster of distinct classes of therapeutic, prophylactic, imaging, and other agents ranging in size and complexity from diatomic gases, oxygen, and nitric oxide to cellular fragments and cells themselves—natural or modified chemically or genetically. In between these extremes, therapeutics can be divided into classic small drugs and biologicals or biotherapeutics, such as proteins, nucleic acids, and other biomolecules.
Both small molecules and biologicals have issues with delivery in the organism of a patient, from administration site to the desirable site of action. Accordingly, diverse drug delivery systems (DDS; liposomes, nanocarriers, affinity drug conjugates, and so on) are devised to enable or improve delivery of some of these agents. In addition, in some cases, DDS themselves have additional functions and even therapeutic action. In this review, we highlight critical factors that affect the behavior of DDS following injection into an organism. The majority of the work discussed focuses on liposomes, as these have been the most extensively studied DDS to date; however, the critical parameters affecting in vivo behavior are likely relevant to many types of DDS.
Each type of these agents—small drugs, biologicals, and DDS—has advantages and challenges, some of which are outlined in Table 1. Here, we attempt a comparative review of the main parameters of their behavior in the body, which we colloquially call pharmacokinetics (PK). PK is often defined simply as “what the body does to the drug,” and is typically described using four critical processes: absorption, distribution, metabolism, and elimination, or ADME. The interactions between the drug molecule (or drug delivery system) and the body control the relative rates and efficiencies of each of these processes and body compartments involved.
TABLE 1.
Comparison of features of small-molecule drugs, biotherapeutic proteins, and multimolecular DDS
| Small Molecules | Proteins | DDSs | |
|---|---|---|---|
| Size | <5 kDa | 10–300 kDa | Above 1000 kDa |
| <1 nm | 1–10 nm | 10–1000 nm | |
| Advantages | Stability | Multifunctional | |
| Utility | Precision | High drug load | |
| Low cost | Catalytic power | Controlled release | |
| Quality control | Natural activity | Regulation of PK/BD | |
| High purity | Targeting | Delivery of nucleic acids | |
| Routes of administration | Targeting | ||
| Challenges | High cost | As for proteins | |
| Off-target effects | Parenteral routes | RES overload | |
| Limited efficacy | Immunologic issues | Host defense reactions | |
| Limited mechanisms | Precise delivery needed | Biologic barriers |
Although these processes are well understood and described for small-molecule drugs and for many protein therapeutics, a thorough understanding of PK (and underlying mechanisms) is often lacking for DDS. This is likely due to several reasons, including, but not limited to, assay limitations, interspecies differences in processes controlling PK, and a smaller overall body of work on PK of DDS, particularly in the clinic. In this review, we discuss differences in ADME processes for small-molecule drugs, protein biotherapeutics, and DDS. In addition, the key features of DDS that can be tuned to modulate PK and analysis of DDS PK are discussed in detail.
ADME Processes
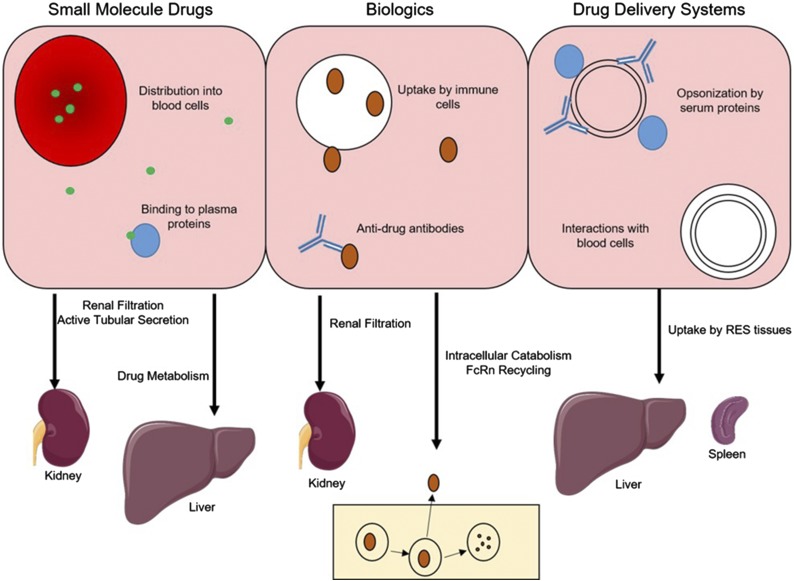
One challenge in the characterization of the in vivo behavior of DDS is the differences in mechanisms controlling PK and biodistribution compared with small-molecule drugs and biologics. As the purpose of this review is not to provide a detailed description of the ADME of small molecules and biologics but rather to highlight their differences from DDS, only a brief overview of mechanisms controlling their in vivo behavior is provided (Fig. 1; Table 2).
Fig. 1.
Mechanisms controlling the behavior of small-molecule drugs (left), protein therapeutics (center), and drug delivery systems (right) in blood (top) and in eliminating organs (bottom). FcRn, neonatal Fc receptor.
TABLE 2.
Comparison of mechanisms controlling pharmacokinetic processes
| Drug Class | Absorption | Distribution | Metabolism/Elimination |
|---|---|---|---|
| Small molecules | Gut wall permeability | Plasma protein binding | Renal filtration |
| Active transport | Diffusion | Active transport | |
| Drug metabolism | Active transport | Drug metabolism | |
| Biologics | Renal filtration | ||
| Lymph flow | Diffusion | Intracellular catabolism | |
| FcRn binding | Bulk fluid flow | Target-mediated clearance | |
| Nanoparticles | N/A | Bulk fluid flow | Reticuloendothelial system |
| Target-mediated clearance |
FcRn, neonatal Fc receptor; N/A, not applicable.
Absorption.
For drugs administered via an extravascular route, the first barrier to reaching the site of action is absorption into the bloodstream, which can be controlled by both properties of the drug and the site of administration. For small-molecule drugs, absorption most frequently occurs in the gastrointestinal (GI) tract following oral administration. In brief, following dosing, the dosage form must disintegrate and the drug has to dissolve and permeate across the GI wall. The rate and extent of this process can vary widely between drugs, although predictions can often be made based on physicochemical properties of the drug molecule (Palm et al., 1997; Lipinski et al., 2001). It should be noted, however, that interactions with transporters (Estudante et al., 2013) and drug-metabolizing enzymes (Peters et al., 2016) in the GI tract can significantly modulate the passive absorption profile that would be predicted using molecular descriptors.
On the other hand, in general, biologics are poorly absorbed following oral absorption, and as such, are often administered intravenously; however, subcutaneous dosing of protein therapeutics has become more popular in recent years. Absorption from this space is generally a slow process (hours to days) due to the pathway through the lymphatic system that most proteins follow after subcutaneous dosing (Supersaxo et al., 1990; Bittner et al., 2018). Although determinants of the efficiency of subcutaneous administration for protein therapeutics are not as well understood as oral absorption of small molecules, it is appreciated that molecular properties of the protein (e.g., size, charge), affinity for the neonatal Fc receptor (Deng et al., 2012; Zheng et al., 2012; Richter et al., 2018), and addition of absorption enhancers to the formulation (e.g., buffer components, hyaluronidase) can impact bioavailability (Fathallah et al., 2015; Bittner et al., 2018).
Finally, for DDS, absorption is not typically a process that is considered, as the efficiency of uptake into the systemic circulation after extravascular delivery is very low. There have been many preclinical investigations of oral delivery of nanoparticles; however, absorption is often low due to poor permeation across the GI wall. Following extravascular injection (e.g., subcutaneous or intramuscular) of DDS, bioavailability would likely be very low due to efficient uptake by resident immune cells in the lymph nodes collecting fluid draining from the injection site; however, this may be an efficient route of administration for local delivery (Kaledin et al., 1982).
Distribution.
Following entry into the systemic circulation, the movement of drugs between blood and tissues is a critical factor controlling the efficacy and toxicities associated with therapy. As with absorption, distribution varies widely between drug classes both in kinetics and in mechanism. The distribution of small-molecule drugs, in particular, may range from being confined to the plasma space to being distributed throughout the entire body. This variability can, in part, be described using molecular descriptors and binding to plasma proteins (Poulin and Theil, 2002a,b). Distribution of small-molecule drugs can be modulated by interactions with uptake and/or efflux transporters expressed in certain tissues (Giacomini et al., 2010).
The efficiency of distribution of protein therapeutics into tissues is highly dependent on the molecular weight of the protein, with smaller proteins entering tissues more efficiently than larger proteins, due to enhanced diffusion and improved permeation through paracellular pores (convective uptake) (Sarin, 2010). Additionally, tissue uptake can be increased via receptor-mediated transcytosis for proteins with high affinity for receptors such as the transferrin receptor (Friden et al., 1991; Pardridge et al., 1991).
As most DDS are much larger than typical pores between endothelial cells, distribution is often limited to the vascular space (Allen et al., 1989) in the absence of specific pathologies or affinity for receptors. However, in tissues with larger endothelial pores (e.g., fenestrations in liver and spleen), tissue uptake via bulk fluid flow (convection) may be favorable. In a similar manner to biologics, DDS with affinity for receptors that undergo transcytosis may have enhanced tissue uptake at sites of target expression (Cerletti et al., 2000; Hatakeyama et al., 2004).
Metabolism/Elimination.
As with the previous processes, elimination of drugs from the system occurs via different mechanisms and at different rates for various types of molecules. For small molecules, there are two primary routes of elimination. Renal clearance is controlled by the relative efficiencies of glomerular filtration, active secretion into the urine, and reabsorption (active and passive) from the tubules (Dave and Morris, 2015). Metabolic clearance, occurring primarily in the liver for most drugs, is dependent on recognition of the drug molecule by a drug-metabolizing enzyme (e.g., cytochrome P450). Following metabolism, the metabolite can be further metabolized, cleared via the bile ducts into the feces, or eliminated in the urine.
For peptides and small-protein therapeutics, renal clearance may be significant when molecular mass is smaller than the glomerular filtration threshold (∼60 kDa). However, for proteins that are not eliminated in the urine, catabolic breakdown can occur throughout the body, typically following uptake into the endo-lysosomal pathway. The efficiency of this breakdown can be enhanced if a protein with high affinity for an internalizing receptor is taken up via receptor-mediated endocytosis in a process often referred to as target-mediated drug disposition (TMDD) (Levy, 1994; Mager and Jusko, 2001a). For proteins containing an Fc region [e.g., monoclonal antibodies (mAbs) and Fc fusion proteins], elimination may be blunted via interactions with the neonatal Fc receptor, which protects IgG and albumin from degradation, allowing them to have long circulating half-lives (∼3 weeks in humans) (Ghetie et al., 1996; Israel et al., 1996; Junghans and Anderson, 1996).
For drug delivery systems, the primary route of elimination is via tissues of the reticuloendothelial system (RES), such as the liver, spleen, bone marrow, and lung. These tissues contain large amounts of phagocytic cells (e.g., macrophages) that recognize nanoparticles as foreign bodies and efficiently remove them from the circulation. The efficiency of this pathway can be enhanced by opsonization of the nanoparticle by serum proteins (e.g., immunoglobulins and complement proteins), which cause more efficient recognition by phagocytes (Devine and Marjan, 1997). On the contrary, this clearance mechanism can be slowed by enhancing the “stealthiness” of nanoparticles via approaches such as conjugation of polyethylene glycol (PEG) (Klibanov et al., 1990) (see DDS Design Parameters). Similar to targeted protein therapeutics, specific interactions with the receptors (TMDD) can be a significant route of elimination for targeted DDS.
Physiologic Factors Affecting DDS Pharmacokinetics
To mechanistically describe the in vivo behavior of any drug (or drug carrier), understanding how physiology may control disposition is critical. In this section, we provide a high-level overview of physiologic processes that contribute to the ADME of DDS.
Cardiovascular System.
Following systemic injection, drugs are immediately present in the bloodstream. While often described as a simple, well mixed space in quantitative representations of pharmacokinetics, the cardiovascular system is, in reality, a dynamic space that significantly impacts PK. Almost immediately following injection, nanomaterials are typically coated with a layer of plasma proteins in a process referred to as opsonization, or protein corona formation. While the exact determinant of the protein corona is highly complex, and likely specific to a given nanoparticle, species, and individual, it typically will include complement proteins and immunoglobulins, which lead to more efficient elimination of the particle by immune cells (Devine and Marjan, 1997; Yan et al., 2005).
In addition to the coating of nanoparticles by proteins, there is the potential for dynamic interactions between particles and blood cells (e.g., erythrocytes, platelets, leukocytes). Although this is not an area that has been studied extensively, flow cytometry has been used to demonstrate rapid association of liposomes with erythrocytes and platelets in mice following intravenous injection (Constantinescu et al., 2003).
DDSs aggregation (or initially large size, usually >200–300 nm) leads to rapid mechanical and charge-mediated entrapment in the microvasculature and clearing compartments. This may either impede delivery (Shuvaev et al., 2011b) or enable rather fortuitous accumulation in the vasculature of organs of interest (Myerson et al., 2016).
Reticuloendothelial System.
Since the earliest studies of the in vivo disposition of liposomes, it has been appreciated that injected particles are rapidly taken up by the liver (Gregoriadis and Ryman, 1971, 1972). The mechanism for this efficient clearance pathway in liver and other tissues of the RES (e.g., spleen, bone marrow, lung) is via phagocytic uptake of particles by cells accessible from the vascular space (e.g., hepatic Kupffer cells). This clearance pathway is saturable at doses of 0.1–10 mg of lipid, and saturation of the primary RES organs by increasing doses of liposomes has been shown to lead to decreased uptake in liver and shifting uptake to spleen (lower doses) and lung (higher doses) (Abra and Hunt, 1981; Souhami et al., 1981). In fact, preblocking of the RES with empty liposomes has been investigated as a strategy to improve circulation time (Ellens et al., 1982; Dave and Patel, 1986) and enhance uptake in target tissues (Sun et al., 2017; Liu et al., 2018). Additionally, Chow et al. (1989) have demonstrated that the saturability of the RES not only leads to redistribution to other tissues, but also allows for altered distribution within the liver, shifting uptake from Kupffer cells to hepatocytes.
Target Epitope Properties.
Uptake of DDS at the desired site is often obtained via either active targeting or taking advantage of pathologic alterations in the target tissue that lead to advantageous distribution in the site of injury. For example, in conditions such as inflammation and solid tumors, vascular leakiness is increased, which may lead to improved uptake into target tissues via bulk fluid flow. In the case of solid tumors, many studies have used this enhanced permeability and retention effect in mouse models to obtain delivery of drug into the tumor (Maeda et al., 2013); however, it should be noted that the magnitude of the enhanced permeability and retention effect is likely highly variable and may not exist in all tumors (Wilhelm et al., 2016).
In the case of active targeting, selection of the target epitope can be critical in obtaining optimal delivery to the desired site. While many targets are selectively upregulated in pathologies, expression is still likely to occur in healthy tissues. The relative target expression in diseased and healthy tissues is a critical parameter that defines drug targeting (Scherpereel et al., 2002; Shuvaev et al., 2011b).
Additionally, a critical parameter in active targeting is the accessibility of the target, as this will lead to drastically different concentrations of targeting ligand available to interact with the target. For example, for a target expressed constitutively on the surface of the vascular endothelium, the entire concentration of the affinity ligand in the bloodstream will be able to bind; however, if the target is located at an extravascular site, then the relevant concentration will be that which has extravasated into the tissue. This concentration will likely be folds lower than the concentration within the bloodstream due to generally poor uptake of particles into tissues, and the limiting step in targeting may be tissue uptake rather than target binding (Chacko et al., 2011; Howard et al., 2014).
Finally, following binding of DDS to target molecules, it is possible that the DDS-target complex will be internalized. In some cases, the features of DDS induce internalization even though the DDS is anchored on a cellular receptor normally not involved in internalization (Muzykantov, 2013; Han et al., 2015). In general, internalization of DDS is desirable, as most DDS release drugs within the endo-lysosomal space. However, for chronic administration of DDS, internalization of the complex may lead to reduced target available on subsequent doses, leading to diminished targeting and efficacy on later doses. Although not demonstrated to date for nanomedicines, this principle has previously been shown for mAbs (Meijer et al., 2002).
DDS Design Parameters
To reach the desired site of action, DDS must evade major clearance mechanisms (e.g., RES uptake) and bypass distributional barriers to reach the desired site of action. The use of DDS dates back nearly 50 years to early publications using liposomes as delivery vehicles (Gregoriadis et al., 1971). Over this nearly half-century, a myriad of approaches has been proposed to modulate the in vivo behavior of DDS, with varying degrees of success. In this section, we highlight some of the most commonly studied strategies for the design of DDS, mainly focusing on liposomes as a model DDS.
“Classic” Design Parameters.
From the early days of liposome research, it has been appreciated that modulating the liposome properties can lead to alterations in blood clearance (Juliano and Stamp, 1975). One parameter that has been studied in detail for liposomes is the effect of size. Liu et al. (1992) performed a detailed characterization of the PK and biodistribution of liposomes and found that maximal blood concentrations and minimal liver concentrations were observed for liposomes in the size range of 100–200 nm. This “sweet spot” of liposome size has been hypothesized to be due to efficient extravasation of small (diameter <100 nm) liposomes in the liver, allowing for hepatocyte uptake, and rapid clearance of large (diameter ∼500 nm) liposomes by Kupffer cells and splenic macrophages (Rahman et al., 1982). In addition to size, the impact of liposome charge has also received a great deal of investigation for its impacts on PK and distribution. In their early work, Juliano and Stamp (1975) observed that cationic liposomes were cleared more rapidly than anionic or neutral liposomes. Litzinger et al. (1996) demonstrated that cationic liposomes were rapidly taken up in the liver [60% injected dose (ID) at 5 minutes], mainly in Kupffer cells. However, much like with size, it has been hypothesized that there is a “sweet spot” for cationic charge. In rats, it was shown that liposomes with a zeta potential of ∼15 mV had enhanced PK relative to those with zeta potentials of −5 to +10 mV and >+25 mV. These results were hypothesized to be due to balanced electrostatic interactions with erythrocytes (favoring circulation) and Kupffer cells (favoring clearance) (Aoki et al., 1997).
Due to the observation that liposomes were primarily cleared by cells of the innate immune system, several approaches were put forward to create “stealth” liposomes with natural abilities to evade uptake by phagocytic cells. An early method proposed to extend liposome circulation was to mimic the outer surface of a naturally long-circulating particle, erythrocytes, by including sphingomyelin and ganglioside (GM1) in the liposome. This approach led to large increases in blood and tumor uptake, with significant decreases in RES clearance (Gabizon and Papahadjopoulos, 1988; Allen et al., 1989).
In the early 1990s, multiple groups observed that modifying lipids with PEG provided similar evasion of RES clearance and extended circulation time (Klibanov et al., 1990; Allen et al., 1991). This approach, termed PEGylation, was used in the development of the first approved liposomal product, liposomal doxorubicin (Doxil). However, it has been observed that following repeated injections of PEGylated liposomes, clearance and RES uptake were significantly increased (Dams et al., 2000), which was shown to be due to formation of an antibody response against PEG (Ishida et al., 2006; Wang et al., 2007).
“Modern” Design Parameters.
In recent years, as the field has gained tighter control over the ability to reproducibly manipulate nanomaterials, more intricate design features have been used to alter the pharmacokinetics of DDS. Within the last 15 years, there have been several investigations of the impact of nanoparticle shape on biodistribution and pharmacokinetics, dating to the observation that long, worm-like filomicelles have extended circulation time relative to spherical carriers (Geng et al., 2007; Shuvaev et al., 2011a). Similarly, it has been shown for mesoporous silica nanoparticles (Huang et al., 2011) and for gold nanoparticles (Arnida et al., 2011) that an extended (rod-like) configuration leads to extended blood circulation and reduced RES uptake. For filomicelles, it was suggested that their hydrodynamic properties allowed them to better align with blood flow and remain in circulation (Geng et al., 2007). While not exhaustive, these examples highlight the potential for engineering of nanoparticle shape to modulate interactions with clearance organs and prolong circulation.
With increasing interest in polymeric nanoparticles, there has been an increased ability to tune not only size and shape but also mechanical properties, creating “soft” and “hard” nanoparticles. Anselmo et al. (2015) used polymeric hydrogels to demonstrate that increased nanoparticle flexibility led to extended circulation time in mice and decreased uptake in several types of cells (macrophage, endothelial, tumor). Similarly, Guo et al. (2018) showed that by tuning the elasticity of nanolipogels, uptake into tumor and RES organs could be controlled. Our group has also demonstrated that lysozyme-dextran nanogels were highly deformable and allowed for targeting of caveolar targets that were otherwise inaccessible to rigid particles of a similar size (Myerson et al., 2018), demonstrating the impact that particle flexibility could have not only on pharmacokinetics but also on active targeting.
Targeted DDS Design Parameters.
Instead of merely relying on passive uptake to guide delivery of DDS to their intended sites, active targeting using mAbs, antibody fragments, peptides, and small molecules has been extensively studied. By coating the surface of a particle with a targeting ligand, very high affinity and avidity for target epitopes can be achieved. It is possible that by modulating targeting ligand properties, the degree of uptake in the desired site of action can be controlled.
The most straightforward approach to modulating targeting properties would be to modify the density of targeting ligand coating on the nanoparticle. In the simplest scenario, it would be expected that by maximizing coating density, targeting to the desired site would be enhanced, which does appear to hold true in certain cases (Calderon et al., 2011). However, increased targeting ligand density could also lead to delivery to less desirable (e.g., off-target) sites or reduced sensitivity to changes in target expression (Zern et al., 2013).
Additionally, in the specific scenario where receptor-mediated transcytosis is the desired outcome, high-avidity nanoparticles have been shown to have reduced transcytosis due to poor release from the endothelial surface following exocytosis (Wiley et al., 2013). In general, caution should be applied when tuning nanoparticle avidity, and in vivo experiments to assess the impact of changes in avidity on targeting should be performed.
When selecting targeting ligands, the potential impact of the properties of the ligand on pharmacokinetics and biodistribution should also be considered. Classically, mAbs have been used to target nanoparticles, but with recent advances in molecular biology, the ability to make antibody fragments (e.g., frament antigen binding (Fab), single-chain variable fragment etc.) that can be conjugated to the surface of particles is enhanced. By coupling full-length mAbs to the surface of nanoparticles, the potential for significant exposure of Fc fragments is present, potentially leading to increased immune-mediated clearance (Koning et al., 2001). The clearance of liposomes displaying a high density of Fc fragments was inhibited in mice by injection of an anti-Fc receptor mAb, demonstrating the potential role of Fc receptor in the PK of immunoliposomes (Aragnol and Leserman, 1986). By using antibody fragments that do not contain an Fc fragment, enhanced delivery of nanoparticle cargo to tumor was obtained in lymphoma (Cheng and Allen, 2008) and breast cancer (Duan et al., 2018) models, which was hypothesized to be due to decreased Fc-dependent clearance.
Design of In Vivo Studies
Quantitatively accurate, objective, and methodologically reliable characterization of carrier behavior in vivo [both PK and biodistribution (BD)] is necessary. Nonspecific PK/BD influences DDS in many ways and may ultimately override a proposed targeting mechanism. Without knowledge of PK/pharmacodynamics (PD), data obtained from animal models are of limited translation value, as lack of knowledge of these parameters may lead to erroneous interpretation of the mechanism(s) of delivery and effect. Therefore, it is critical to define the relative contributions of the designed targeting mechanism and other factors in delivery and effects of DDS.
Interaction with components of the blood may lead to uptake by blood cells, aggregation, opsonization, degradation, or other alterations to DDS, which may alter PK/PD differentially in normal versus diseased organisms. Additionally, drugs and biologically active components of the DDS may affect PK/PD. To account for all of these scenarios, the following formulations should be tested in vivo:
targeted versus untargeted (coated by inactive ligand) carriers; pristine characters are not a proper comparison group, as they may have different size, charge, and surface properties;
naïve animals versus animal model(s) of disease; and
empty versus drug-loaded DDS.
Available methodologies to study PK vary, and no single method is sufficient to address all potential questions related to in vivo behavior. By tracing DDS labeled with optical probes, localization within the tissue at the microscopic level at postmortem and macroscopically in real time in sufficiently transparent sites is feasible (Pollinger et al., 2013). However, optical methods are subjective, relatively low throughput, and difficult to analyze quantitatively.
The use of molecular imaging approaches, such as positron emission tomography, single-photon emission computed tomography, and magnetic resonance imaging, is insufficient to analyze subtissue localization, but these clinically useful technologies allow for real-time imaging of isotope-labeled components of DDS (Danilov et al., 1989; Rossin et al., 2008; Brinkhuis et al., 2012; Zern et al., 2013) at a macroscopic level, with the ability for quantitative approximation of the intensity of a signal from a region of interest (partially subjective).
Labeling of a DDS may alter PK/PD features and lead to artifacts due to dissociation of the labeled component from the DDS. To mitigate this, ideally, both the drug cargo and carrier (but not targeting moiety) should be stably traced by conjugated labels (Simone et al., 2012). Direct measurement of the isotope level in drawn blood samples and tissue specimens postmortem is arguably the most reliable approach for PK studies (Danilov et al., 1991; Muzykantov et al., 1991, 1996, 1999; Shuvaev et al., 2011a; Pan et al., 2013). It allows for accurate, quantitative analysis of key parameters of PK, targeting, and biodistribution, including percentage of ID (%ID) in tissues, localization ratio (or ratio of %ID per gram of tissue to that in blood), and immunospecificity index (or ratio of localization ratio for targeted versus untargeted formulations) (Muzykantov et al., 1995, 1996).
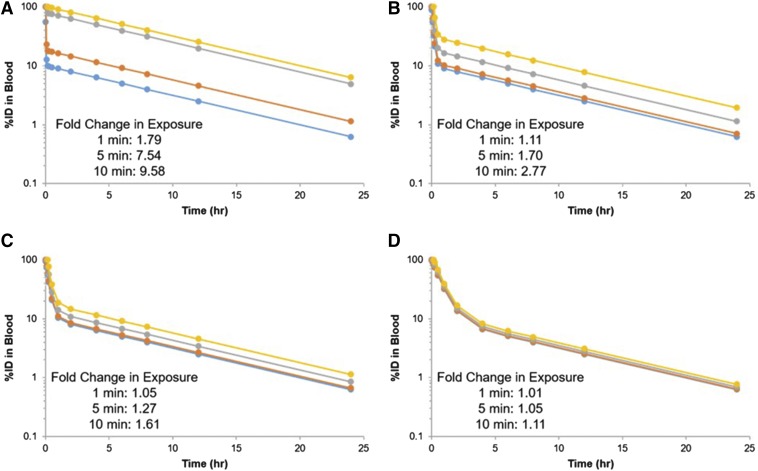
It is critical that PK/BD data be normalized to the injected dose of DDS. Using the concentration in the first blood draw because 100%ID is not acceptable, a significant fraction of DDS may be eliminated within seconds. This can lead to artifacts in blood and tissue concentrations when analyzing PK/BD data (Fig. 2).
Fig. 2.
Apparent pharmacokinetics for a theoretical DDS with a terminal half-life of 6 hours when normalizing to injected dose (blue) and blood concentration 1 (orange), 5 (gray), or 10 (yellow) minutes postinjection for a DDS with 50% of injected dose eliminated in 1 (A), 5 (B), 10 (C), or 30 (D) minutes.
A useful approach to increase the throughput of PK/BD studies would be to inject in the same animal a mixture of both targeted and untargeted formulations labeled by different isotopes. This can help to minimize individual variability and significantly reduce efforts. However, caution should be taken to not administer a cumulative dose of DDS that would lead to saturation of nonspecific clearance processes (e.g., RES uptake).
Quantitative Descriptions of DDS Pharmacokinetic
“Nonmechanistic” Approaches.
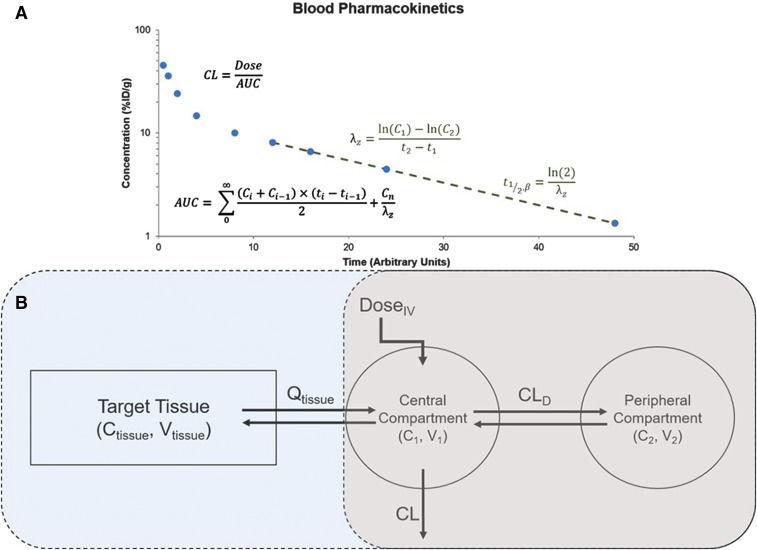
For simple comparison of the blood kinetics of DDS formulations, simple, nonmechanism-based approaches are often sufficient. The simplest of these, termed noncompartmental analysis, simply utilizes values that can be extracted from the concentration versus time curve to characterize the PK of drugs (Fig. 3A). Common parameters that are obtained from noncompartmental analysis include the terminal half-life (t1/2), volume of distribution (Vss, Vd), clearance, area under the curve, and mean residence time. This approach is useful for obtaining estimates of parameters related to drug exposure and distribution. To obtain a further description of the concentration versus time curve, simple mammillary models can be used (Fig. 3B). In brief, these models link compartments representing volumes in rapid and slow equilibrium with the blood stream via distributional clearance terms (CLD) and assume all elimination occurs from the central compartment (in rapid equilibrium with blood). These models can be used with either linear or nonlinear (saturable) clearance kinetics. While there have been many models proposed for liposomes, many of them are used to describe the kinetics of the loaded and free cargo as opposed to the particle (Harashima et al., 1999; Hempel et al., 2003; Fetterly et al., 2008). However, there are several examples of models proposed to describe the PK of the particle in rodents (Kume et al., 1991; Palatini et al., 1991; Decker et al., 2013), suggesting the potential utility of simple, mammillary models in describing the PK of DDS.
Fig. 3.
Pharmacokinetic data analysis. (A) Sample concentration versus time curve showing calculations for select parameters derived using noncompartmental analysis. (B) Pharmacokinetic model structure for a simple two-compartment mammillary model (gray) and a semiphysiologic (blue + gray) model. AUC, area under the curve; CL, clearance.
Mechanism-Based Modeling.
To make meaningful extrapolations from modeling analyses, some degree of mechanism should be included in the model. Simple TMDD models developed via inclusion of parameters related to target binding, expression, and turnover in a mammillary model structure are a common approach used to describe nonlinear PK of targeted therapeutics (e.g., mAbs) (Mager and Jusko, 2001a). To date, there have been no descriptions of the use of TMDD models for DDS in a mammillary model; however, with the large number of studies of targeted liposomes, this model structure could potentially be useful for those seeking to characterize target-specific parameters without needing to build a physiologically based model.
A more elegant, and possibly predictive, approach to describe the in vivo behavior of DDS would be to build pharmacokinetic models including some degree of physiologic relevance. One such example, semiphysiologically pharmacokinetic modeling, adds a tissue of interest onto a mammillary model (Fig. 3B). This tissue is described using physiologically relevant volumes and flow rates and is used to describe the tissue concentration versus time profile of a drug. This approach was used previously to describe the blood, liver, and tumor PK of radiolabeled liposomes detected by positron emission tomography imaging (Qin et al., 2010) and more recently to describe the processes controlling tumor exposure to nanoparticle-encapsulated drugs (Benchimol et al., 2019). In addition, we recently used a semiphysiologic model to describe the pharmacokinetics of vascular-targeted nanocarriers in a mouse model of acute respiratory distress syndrome. Using this model, we were able to predict the heterogeneous distribution of nanocarriers across the lung and support experimental hypotheses regarding the mechanisms controlling lung distribution (Brenner et al., 2017).
The “gold standard” for prediction of drug behavior in an in vivo setting is the full physiologically based pharmacokinetic (PBPK) model, which has been widely applied both for small molecules (Jones et al., 2015; Sager et al., 2015) and for biologics (Wong and Chow, 2017; Glassman and Balthasar, 2019). In brief, these models include all tissues of the body and are parameterized with physiologically relevant values (e.g., blood flow, tissue volume, receptor expression, etc.). To date, there have been several reviews describing the potential utility of PBPK in nanomedicine; however, there are relatively few examples of applications of this approach (Li et al., 2010, 2017; Yang et al., 2010; Moss and Siccardi, 2014; Yuan et al., 2019). Kagan et al. (2014) were among the first to demonstrate the use of PBPK for DDS. In their paper, they considered the blood and tissue PK of AmBisome (liposomal amphotericin) in mice, rats, and humans and ultimately used their model to predict the clinical PK of AmBisome over a multiple-dosing regimen. Key features of their model included 1) dual-level modeling of encapsulated and released drug, 2) consideration of saturable uptake by phagocytic cells of the RES, and 3) interspecies scaling to predict the clinical behavior of liposomal drug (Kagan et al., 2014). More recently, Carlander et al. (2016) proposed an extension to the model developed by Li et al. (2014) for PEGylated polyacrylamide nanoparticles to consider several types of nanomaterials (polyacrylamide, gold, TiO2). In this model, the authors considered saturable uptake by phagocytic cells in all tissues of the body, potentially providing a platform that could be used to describe the redistribution of nanoparticles from the liver and spleen at doses that would saturate RES clearance (Carlander et al., 2016). Further development of PBPK models incorporating critical determinants of DDS disposition would be desirable for prediction of the behavior of DDS in pathologies or for optimization of dosing regimens.
Pharmacodynamics of DDS
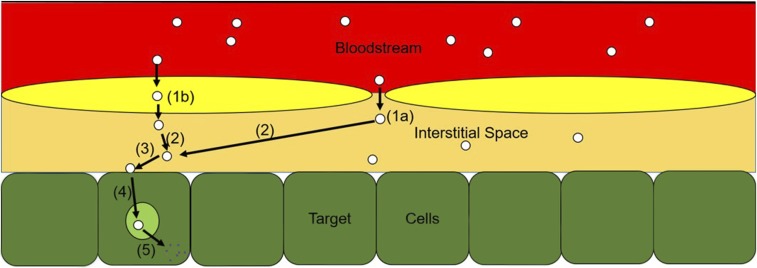
Beyond merely understanding what the body does to the DDS (e.g., pharmacokinetics), it is just as important to characterize what the DDS does to the body (e.g., pharmacodynamics). This is generally a less well understood process; however, by delineating the key steps required to move from uptake into tissues to therapeutic effect, one can gain an appreciation for the complexity of the underlying mechanisms and potentially gain insights into the kinetics of each individual step (Fig. 4).
Fig. 4.
Transduction steps between DDS arrival in system and pharmacologic effect. Extravasation via endothelial pores into tissue interstitium (1a), transendothelial uptake into the interstitium (1b), diffusion within the interstitial space (2), binding to target epitope (3), internalization into endosomes and subcellular sorting (4), and drug release into cell allowing for pharmacologic activity (5).
Following uptake into the tissue of interest, the journey of a DDS (and its cargo) is not complete. Although merely understanding total tissue concentrations, or concentrations in a pathologically altered region of tissue, may be sufficient to generate a dose-response relationship, the pharmacologically relevant concentration is likely to be within a subset of that space. For most DDS, the site of action is within the intracellular space of a target cell (e.g., tumor cell). Therefore, following extravasation into the target tissue, the first critical processes are binding to (generally rapid for highly avid particles) and internalization by target cells (dependent on target epitope). For the therapeutic payload (cargo) to reach its intracellular destination, release of drug should occur from the DDS within the endo-lysosomal route, often via breakdown of the particle, allowing the payload to diffuse to its target organelle and elicit a pharmacologic effect.
From this simplified schematic of DDS processing and drug release, it becomes apparent that a critical step in the pharmacodynamics of drugs loaded into DDS is the release from the particle. For most delivery systems, drug release is optimally slow in the circulation and rapid inside of target cells. In general, burst release from the particle within the endo-lysosomal space is ideal for molecules that are stable within this harsh environment, whereas for macromolecules (e.g., proteins and nucleic acids), release into the cytoplasm would be desirable.
To tune release within intracellular compartments, several strategies have been proposed, including 1) incorporation of pH-sensitive lipids into the bilayer, which destabilize the liposome at acidic pH and/or induce fusion with the endosomal membrane (Connor and Huang, 1985, 1986; Straubinger et al., 1985); 2) incorporation of endosomal escape peptides (Parente et al., 1988; Mandal and Lee, 2002; Kakimoto et al., 2009) or lipids (Du et al., 2014; Sabnis et al., 2018) into the nanoparticle to facilitate cytoplasmic release; or 3) reliance on natural breakdown of the liposome in the harsh lysosomal environment. Each of these methods may provide different kinetics and efficiencies of release of therapeutic payload into the cell, potentially leading to differential kinetics of pharmacologic effect.
In general, these transduction steps between delivery to target cells and pharmacologic effect are hidden away in a “black box” due to poor understanding of the kinetics of each individual step. With this level of knowledge, the best-case scenario for describing pharmacodynamics would be to link an estimated total or receptor-bound target tissue concentration to a therapeutic outcome using a signal transduction model (Sun and Jusko, 1998; Mager and Jusko, 2001b; Lobo and Balthasar, 2002). However, to open this “black box” of transduction compartments, recent developments in cellular pharmacokinetic/pharmacodynamic models could be repurposed in nanomedicine, leveraging in vitro cellular processing data to predict in vivo effects following receptor binding. In particular, models developed for antibody-drug conjugates could be of particular utility, as they consider similar processes as would be required for nanoparticle-based DDS (Cilliers et al., 2016; Singh et al., 2016; Singh and Shah, 2017).
Conclusions
Successful use of drug delivery systems in clinical medicine has been hampered by poor understanding of the mechanisms controlling pharmacokinetics and biodistribution, as well as the kinetics of each of these processes. In this review, we provided an overview of critical differences in ADME processes for small-molecule drugs, protein therapeutics, and DDS, focusing on the physiologic mechanisms relevant for DDS. By understanding the interplay between the organism and the DDS, engineering strategies can be applied to the drug carrier to modulate the efficiency of various ADME processes. Well designed PK/BD studies for DDS coupled with quantitative approaches for describing PK can be useful in predicting the pharmacologic effect (pharmacodynamics) and ultimately allow for the design of better drug delivery systems.
Abbreviations
- ADME
absorption, distribution, metabolism, and elimination
- BD
biodistribution
- DDS
drug delivery system
- GI
gastrointestinal
- ID
injected dose
- mAb
monoclonal antibody
- PBPK
physiologically based pharmacokinetic
- PD
pharmacodynamics
- PEG
polyethylene glycol
- PK
pharmacokinetics
- RES
reticuloendothelial system
- TMDD
target-mediated drug disposition
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Glassman, Muzykantov.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01HL126874, R01HL125462, and T32HL007971].
References
- Abra RM, Hunt CA. (1981) Liposome disposition in vivo. III. Dose and vesicle-size effects. Biochim Biophys Acta 666:493–503. [DOI] [PubMed] [Google Scholar]
- Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. (1991) Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta 1066:29–36. [DOI] [PubMed] [Google Scholar]
- Allen TM, Hansen C, Rutledge J. (1989) Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta 981:27–35. [DOI] [PubMed] [Google Scholar]
- Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S. (2015) Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 9:3169–3177. [DOI] [PubMed] [Google Scholar]
- Aoki H, Tottori T, Sakurai F, Fuji K, Miyajima K. (1997) Effects of positive charge density on the liposomal surface on disposition kinetics of liposomes in rats. Int J Pharm 156:163–174. [Google Scholar]
- Aragnol D, Leserman LD. (1986) Immune clearance of liposomes inhibited by an anti-Fc receptor antibody in vivo. Proc Natl Acad Sci USA 83:2699–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnida, Janát-Amsbury MM, Ray A, Peterson CM, Ghandehari H. (2011) Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm 77:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchimol MJ, Bourne D, Moghimi SM, Simberg D. (2019) Pharmacokinetic analysis reveals limitations and opportunities for nanomedicine targeting of endothelial and extravascular compartments of tumours. J Drug Target:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner B, Richter W, Schmidt J. (2018) Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs 32:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner JS, Bhamidipati K, Glassman PM, Ramakrishnan N, Jiang D, Paris AJ, Myerson JW, Pan DC, Shuvaev VV, Villa CH, et al. (2017) Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions. Nanomedicine (Lond) 13:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhuis RP, Stojanov K, Laverman P, Eilander J, Zuhorn IS, Rutjes FP, van Hest JC. (2012) Size dependent biodistribution and SPECT imaging of (111)In-labeled polymersomes. Bioconjug Chem 23:958–965. [DOI] [PubMed] [Google Scholar]
- Calderon AJ, Bhowmick T, Leferovich J, Burman B, Pichette B, Muzykantov V, Eckmann DM, Muro S. (2011) Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J Control Release 150:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlander U, Li D, Jolliet O, Emond C, Johanson G. (2016) Toward a general physiologically-based pharmacokinetic model for intravenously injected nanoparticles. Int J Nanomedicine 11:625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti A, Drewe J, Fricker G, Eberle AN, Huwyler J. (2000) Endocytosis and transcytosis of an immunoliposome-based brain drug delivery system. J Drug Target 8:435–446. [DOI] [PubMed] [Google Scholar]
- Chacko AM, Hood ED, Zern BJ, Muzykantov VR. (2011) Targeted nanocarriers for imaging and therapy of vascular inflammation. Curr Opin Colloid Interface Sci 16:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WW, Allen TM. (2008) Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab’ fragments and single chain Fv. J Control Release 126:50–58. [DOI] [PubMed] [Google Scholar]
- Chow DD, Essien HE, Padki MM, Hwang KJ. (1989) Targeting small unilamellar liposomes to hepatic parenchymal cells by dose effect. J Pharmacol Exp Ther 248:506–513. [PubMed] [Google Scholar]
- Cilliers C, Guo H, Liao J, Christodolu N, Thurber GM. (2016) Multiscale modeling of antibody-drug conjugates: connecting tissue and cellular distribution to whole animal pharmacokinetics and potential implications for efficacy. AAPS J 18:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J, Huang L. (1985) Efficient cytoplasmic delivery of a fluorescent dye by pH-sensitive immunoliposomes. J Cell Biol 101:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J, Huang L. (1986) pH-sensitive immunoliposomes as an efficient and target-specific carrier for antitumor drugs. Cancer Res 46:3431–3435. [PubMed] [Google Scholar]
- Constantinescu I, Levin E, Gyongyossy-Issa M. (2003) Liposomes and blood cells: a flow cytometric study. Artif Cells Blood Substit Immobil Biotechnol 31:395–424. [DOI] [PubMed] [Google Scholar]
- Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC. (2000) Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther 292:1071–1079. [PubMed] [Google Scholar]
- Danilov SM, Martynov AV, Klibanov AL, Slinkin MA, Sakharov IYu, Malov AG, Sergienko VB, Vedernikov AYu, Muzykantov VR, Torchilin VP. (1989) Radioimmunoimaging of lung vessels: an approach using indium-111-labeled monoclonal antibody to angiotensin-converting enzyme. J Nucl Med 30:1686–1692. [PubMed] [Google Scholar]
- Danilov SM, Muzykantov VR, Martynov AV, Atochina EN, Sakharov IYu, Trakht IN, Smirnov VN. (1991) Lung is the target organ for a monoclonal antibody to angiotensin-converting enzyme. Lab Invest 64:118–124. [PubMed] [Google Scholar]
- Dave J, Patel HM. (1986) Differentiation in hepatic and splenic phagocytic activity during reticuloendothelial blockade with cholesterol-free and cholesterol-rich liposomes. Biochim Biophys Acta 888:184–190. [DOI] [PubMed] [Google Scholar]
- Dave RA, Morris ME. (2015) Quantitative structure-pharmacokinetic relationships for the prediction of renal clearance in humans. Drug Metab Dispos 43:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C, Schubert H, May S, Fahr A. (2013) Pharmacokinetics of temoporfin-loaded liposome formulations: correlation of liposome and temoporfin blood concentration. J Control Release 166:277–285. [DOI] [PubMed] [Google Scholar]
- Deng R, Meng YG, Hoyte K, Lutman J, Lu Y, Iyer S, DeForge LE, Theil FP, Fielder PJ, Prabhu S. (2012) Subcutaneous bioavailability of therapeutic antibodies as a function of FcRn binding affinity in mice. MAbs 4:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DV, Marjan JM. (1997) The role of immunoproteins in the survival of liposomes in the circulation. Crit Rev Ther Drug Carrier Syst 14:105–131. [PubMed] [Google Scholar]
- Du Z, Munye MM, Tagalakis AD, Manunta MD, Hart SL. (2014) The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci Rep 4:7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Wang A, Ni L, Zhang L, Yan X, Jiang Y, Mu H, Wu Z, Sun K, Li Y. (2018) Trastuzumab- and Fab’ fragment-modified curcumin PEG-PLGA nanoparticles: preparation and evaluation in vitro and in vivo. Int J Nanomedicine 13:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellens H, Mayhew E, Rustum YM. (1982) Reversible depression of the reticuloendothelial system by liposomes. Biochim Biophys Acta 714:479–485. [DOI] [PubMed] [Google Scholar]
- Estudante M, Morais JG, Soveral G, Benet LZ. (2013) Intestinal drug transporters: an overview. Adv Drug Deliv Rev 65:1340–1356. [DOI] [PubMed] [Google Scholar]
- Fathallah AM, Turner MR, Mager DE, Balu-Iyer SV. (2015) Effects of hypertonic buffer composition on lymph node uptake and bioavailability of rituximab, after subcutaneous administration. Biopharm Drug Dispos 36:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterly GJ, Grasela TH, Sherman JW, Dul JL, Grahn A, Lecomte D, Fiedler-Kelly J, Damjanov N, Fishman M, Kane MP, et al. (2008) Pharmacokinetic/pharmacodynamic modeling and simulation of neutropenia during phase I development of liposome-entrapped paclitaxel. Clin Cancer Res 14:5856–5863. [DOI] [PubMed] [Google Scholar]
- Friden PM, Walus LR, Musso GF, Taylor MA, Malfroy B, Starzyk RM. (1991) Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc Natl Acad Sci USA 88:4771–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon A, Papahadjopoulos D. (1988) Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci USA 85:6949–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. (2007) Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. (1996) Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol 26:690–696. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman PM, Balthasar JP. (2019) Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development. Drug Metab Pharmacokinet 34:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G, Leathwood PD, Ryman BE. (1971) Enzyme entrapment in liposomes. FEBS Lett 14:95–99. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G, Ryman BE. (1971) Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J 124:58P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G, Ryman BE. (1972) Fate of protein-containing liposomes injected into rats. An approach to the treatment of storage diseases. Eur J Biochem 24:485–491. [DOI] [PubMed] [Google Scholar]
- Guo P, Liu D, Subramanyam K, Wang B, Yang J, Huang J, Auguste DT, Moses MA. (2018) Nanoparticle elasticity directs tumor uptake. Nat Commun 9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Shuvaev VV, Davies PF, Eckmann DM, Muro S, Muzykantov VR. (2015) Flow shear stress differentially regulates endothelial uptake of nanocarriers targeted to distinct epitopes of PECAM-1. J Control Release 210:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima H, Tsuchihashi M, Iida S, Doi H, Kiwada H. (1999) Pharmacokinetic/pharmacodynamic modeling of antitumor agents encapsulated into liposomes. Adv Drug Deliv Rev 40:39–61. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Akita H, Maruyama K, Suhara T, Harashima H. (2004) Factors governing the in vivo tissue uptake of transferrin-coupled polyethylene glycol liposomes in vivo. Int J Pharm 281:25–33. [DOI] [PubMed] [Google Scholar]
- Hempel G, Reinhardt D, Creutzig U, Boos J. (2003) Population pharmacokinetics of liposomal daunorubicin in children. Br J Clin Pharmacol 56:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MD, Hood ED, Zern B, Shuvaev VV, Grosser T, Muzykantov VR. (2014) Nanocarriers for vascular delivery of anti-inflammatory agents. Annu Rev Pharmacol Toxicol 54:205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Li L, Liu T, Hao N, Liu H, Chen D, Tang F. (2011) The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 5:5390–5399. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H. (2006) Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release 112:15–25. [DOI] [PubMed] [Google Scholar]
- Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. (1996) Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology 89:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, Snoeys J, Upreti VV, Zheng M, Hall SD. (2015) Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther 97:247–262. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Stamp D. (1975) The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem Biophys Res Commun 63:651–658. [DOI] [PubMed] [Google Scholar]
- Junghans RP, Anderson CL. (1996) The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA 93:5512–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan L, Gershkovich P, Wasan KM, Mager DE. (2014) Dual physiologically based pharmacokinetic model of liposomal and nonliposomal amphotericin B disposition. Pharm Res 31:35–45. [DOI] [PubMed] [Google Scholar]
- Kakimoto S, Hamada T, Komatsu Y, Takagi M, Tanabe T, Azuma H, Shinkai S, Nagasaki T. (2009) The conjugation of diphtheria toxin T domain to poly(ethylenimine) based vectors for enhanced endosomal escape during gene transfection. Biomaterials 30:402–408. [DOI] [PubMed] [Google Scholar]
- Kaledin VI, Matienko NA, Nikolin VP, Gruntenko YV, Budker VG, Vakhrusheva TE. (1982) Subcutaneously injected radiolabeled liposomes: transport to the lymph nodes in mice. J Natl Cancer Inst 69:67–71. [PubMed] [Google Scholar]
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. (1990) Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268:235–237. [DOI] [PubMed] [Google Scholar]
- Koning GA, Morselt HW, Gorter A, Allen TM, Zalipsky S, Kamps JA, Scherphof GL. (2001) Pharmacokinetics of differently designed immunoliposome formulations in rats with or without hepatic colon cancer metastases. Pharm Res 18:1291–1298. [DOI] [PubMed] [Google Scholar]
- Kume Y, Maeda F, Harashima H, Kiwada H. (1991) Saturable, non-Michaelis-Menten uptake of liposomes by the reticuloendothelial system. J Pharm Pharmacol 43:162–166. [DOI] [PubMed] [Google Scholar]
- Levy G. (1994) Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther 56:248–252. [DOI] [PubMed] [Google Scholar]
- Li D, Johanson G, Emond C, Carlander U, Philbert M, Jolliet O. (2014) Physiologically based pharmacokinetic modeling of polyethylene glycol-coated polyacrylamide nanoparticles in rats. Nanotoxicology 8 (Suppl 1):128–137. [DOI] [PubMed] [Google Scholar]
- Li M, Al-Jamal KT, Kostarelos K, Reineke J. (2010) Physiologically based pharmacokinetic modeling of nanoparticles. ACS Nano 4:6303–6317. [DOI] [PubMed] [Google Scholar]
- Li M, Zou P, Tyner K, Lee S. (2017) Physiologically based pharmacokinetic (PBPK) modeling of pharmaceutical nanoparticles. AAPS J 19:26–42. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26. [DOI] [PubMed] [Google Scholar]
- Litzinger DC, Brown JM, Wala I, Kaufman SA, Van GY, Farrell CL, Collins D. (1996) Fate of cationic liposomes and their complex with oligonucleotide in vivo. Biochim Biophys Acta 1281:139–149. [DOI] [PubMed] [Google Scholar]
- Liu D, Mori A, Huang L. (1992) Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta 1104:95–101. [DOI] [PubMed] [Google Scholar]
- Liu F, Han L, Huang X, Sang M, Liu B, Li C, Ma C, Liu W, Feng F, Qu W. (2018) Reticuloendothelial system pre-block strategy to improve tumor targeting efficacy for hyaluronic acid related drug delivery system. J Biomed Nanotechnol 14:1731–1743. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Balthasar JP. (2002) Pharmacodynamic modeling of chemotherapeutic effects: application of a transit compartment model to characterize methotrexate effects in vitro. AAPS PharmSci 4:E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Nakamura H, Fang J. (2013) The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 65:71–79. [DOI] [PubMed] [Google Scholar]
- Mager DE, Jusko WJ. (2001a) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532. [DOI] [PubMed] [Google Scholar]
- Mager DE, Jusko WJ. (2001b) Pharmacodynamic modeling of time-dependent transduction systems. Clin Pharmacol Ther 70:210–216. [DOI] [PubMed] [Google Scholar]
- Mandal M, Lee KD. (2002) Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim Biophys Acta 1563:7–17. [DOI] [PubMed] [Google Scholar]
- Meijer RT, Koopmans RP, ten Berge IJ, Schellekens PT. (2002) Pharmacokinetics of murine anti-human CD3 antibodies in man are determined by the disappearance of target antigen. J Pharmacol Exp Ther 300:346–353. [DOI] [PubMed] [Google Scholar]
- Moss DM, Siccardi M. (2014) Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modelling. Br J Pharmacol 171:3963–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov VR. (2013) Targeted drug delivery to endothelial adhesion molecules. ISRN Vascular Medicine 2013:1–27. [Google Scholar]
- Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. (1996) Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci USA 93:5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. (1999) Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA 96:2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov VR, Gavriluk VD, Reinecke A, Atochina EN, Kuo A, Barnathan ES, Fisher AB. (1995) The functional effects of biotinylation of anti-angiotensin-converting enzyme monoclonal antibody in terms of targeting in vivo. Anal Biochem 226:279–287. [DOI] [PubMed] [Google Scholar]
- Muzykantov VR, Puchnina EA, Atochina EN, Hiemish H, Slinkin MA, Meertsuk FE, Danilov SM. (1991) Endotoxin reduces specific pulmonary uptake of radiolabeled monoclonal antibody to angiotensin-converting enzyme. J Nucl Med 32:453–460. [PubMed] [Google Scholar]
- Myerson JW, Anselmo AC, Liu Y, Mitragotri S, Eckmann DM, Muzykantov VR. (2016) Non-affinity factors modulating vascular targeting of nano- and microcarriers. Adv Drug Deliv Rev 99 (Pt A):97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson JW, Braender B, Mcpherson O, Glassman PM, Kiseleva RY, Shuvaev VV, Marcos-Contreras O, Grady ME, Lee HS, Greineder CF, et al. (2018) Flexible nanoparticles reach sterically obscured endothelial targets inaccessible to rigid nanoparticles. Adv Mater 30:e1802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P, Viola G, Bigon E, Menegus AM, Bruni A. (1991) Pharmacokinetic characterization of phosphatidylserine liposomes in the rat. Br J Pharmacol 102:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm K, Stenberg P, Luthman K, Artursson P. (1997) Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm Res 14:568–571. [DOI] [PubMed] [Google Scholar]
- Pan H, Myerson JW, Hu L, Marsh JN, Hou K, Scott MJ, Allen JS, Hu G, San Roman S, Lanza GM, et al. (2013) Programmable nanoparticle functionalization for in vivo targeting. FASEB J 27:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Buciak JL, Friden PM. (1991) Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther 259:66–70. [PubMed] [Google Scholar]
- Parente RA, Nir S, Szoka FC., Jr (1988) pH-dependent fusion of phosphatidylcholine small vesicles. Induction by a synthetic amphipathic peptide. J Biol Chem 263:4724–4730. [PubMed] [Google Scholar]
- Peters SA, Jones CR, Ungell AL, Hatley OJ. (2016) Predicting drug extraction in the human gut wall: assessing contributions from drug metabolizing enzymes and transporter proteins using preclinical models. Clin Pharmacokinet 55:673–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollinger K, Hennig R, Ohlmann A, Fuchshofer R, Wenzel R, Breunig M, Tessmar J, Tamm ER, Goepferich A. (2013) Ligand-functionalized nanoparticles target endothelial cells in retinal capillaries after systemic application. Proc Natl Acad Sci USA 110:6115–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin P, Theil FP. (2002a) Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci 91:129–156. [DOI] [PubMed] [Google Scholar]
- Poulin P, Theil FP. (2002b) Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J Pharm Sci 91:1358–1370. [DOI] [PubMed] [Google Scholar]
- Qin S, Seo JW, Zhang H, Qi J, Curry FR, Ferrara KW. (2010) An imaging-driven model for liposomal stability and circulation. Mol Pharm 7:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman YE, Cerny EA, Patel KR, Lau EH, Wright BJ. (1982) Differential uptake of liposomes varying in size and lipid composition by parenchymal and kupffer cells of mouse liver. Life Sci 31:2061–2071. [DOI] [PubMed] [Google Scholar]
- Richter WF, Christianson GJ, Frances N, Grimm HP, Proetzel G, Roopenian DC. (2018) Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice. MAbs 10:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossin R, Muro S, Welch MJ, Muzykantov VR, Schuster DP. (2008) In vivo imaging of 64Cu-labeled polymer nanoparticles targeted to the lung endothelium. J Nucl Med 49:103–111. [DOI] [PubMed] [Google Scholar]
- Sabnis S, Kumarasinghe ES, Salerno T, Mihai C, Ketova T, Senn JJ, Lynn A, Bulychev A, McFadyen I, Chan J, et al. (2018) A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther 26:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. (2015) Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos 43:1823–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin H. (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, et al. (2002) Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther 300:777–786. [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Ilies MA, Simone E, Zaitsev S, Kim Y, Cai S, Mahmud A, Dziubla T, Muro S, Discher DE, et al. (2011a) Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano 5:6991–6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou-Solomidou M, Albelda SM, Muzykantov VR. (2011b) Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J Control Release 149:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone EA, Zern BJ, Chacko AM, Mikitsh JL, Blankemeyer ER, Muro S, Stan RV, Muzykantov VR. (2012) Endothelial targeting of polymeric nanoparticles stably labeled with the PET imaging radioisotope iodine-124. Biomaterials 33:5406–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Maass KF, Betts AM, Wittrup KD, Kulkarni C, King LE, Khot A, Shah DK. (2016) Evolution of antibody-drug conjugate tumor disposition model to predict preclinical tumor pharmacokinetics of trastuzumab-emtansine (T-DM1). AAPS J 18:861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Shah DK. (2017) Measurement and mathematical characterization of cell-level pharmacokinetics of antibody-drug conjugates: a case study with trastuzumab-vc-MMAE. Drug Metab Dispos 45:1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souhami RL, Patel HM, Ryman BE. (1981) The effect of reticuloendothelial blockade on the blood clearance and tissue distribution of liposomes. Biochim Biophys Acta 674:354–371. [DOI] [PubMed] [Google Scholar]
- Straubinger RM, Düzgünes N, Papahadjopoulos D. (1985) pH-sensitive liposomes mediate cytoplasmic delivery of encapsulated macromolecules. FEBS Lett 179:148–154. [DOI] [PubMed] [Google Scholar]
- Sun X, Yan X, Jacobson O, Sun W, Wang Z, Tong X, Xia Y, Ling D, Chen X. (2017) Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics 7:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YN, Jusko WJ. (1998) Transit compartments versus gamma distribution function to model signal transduction processes in pharmacodynamics. J Pharm Sci 87:732–737. [DOI] [PubMed] [Google Scholar]
- Supersaxo A, Hein WR, Steffen H. (1990) Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res 7:167–169. [DOI] [PubMed] [Google Scholar]
- Wang X, Ishida T, Kiwada H. (2007) Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release 119:236–244. [DOI] [PubMed] [Google Scholar]
- Wiley DT, Webster P, Gale A, Davis ME. (2013) Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci USA 110:8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. (2016) Analysis of nanoparticle delivery to tumours. Nat Rev Mater 1:16014. [Google Scholar]
- Wong H, Chow TW. (2017) Physiologically based pharmacokinetic modeling of therapeutic proteins. J Pharm Sci 106:2270–2275. [DOI] [PubMed] [Google Scholar]
- Yan X, Scherphof GL, Kamps JA. (2005) Liposome opsonization. J Liposome Res 15:109–139. [DOI] [PubMed] [Google Scholar]
- Yang RS, Chang LW, Yang CS, Lin P. (2010) Pharmacokinetics and physiologically-based pharmacokinetic modeling of nanoparticles. J Nanosci Nanotechnol 10:8482–8490. [DOI] [PubMed] [Google Scholar]
- Yuan D, He H, Wu Y, Fan J, Cao Y. (2019) Physiologically based pharmacokinetic modeling of nanoparticles. J Pharm Sci 108:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zern BJ, Chacko AM, Liu J, Greineder CF, Blankemeyer ER, Radhakrishnan R, Muzykantov V. (2013) Reduction of nanoparticle avidity enhances the selectivity of vascular targeting and PET detection of pulmonary inflammation. ACS Nano 7:2461–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Tesar DB, Benincosa L, Birnböck H, Boswell CA, Bumbaca D, Cowan KJ, Danilenko DM, Daugherty AL, Fielder PJ, et al. (2012) Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. MAbs 4:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]