Highlights
-
•
Re-irradiation using proton therapy for recurrent breast cancer has excellent local control.
-
•
Re-irradiation using proton beam radiation therapy increases risk of skin toxicity.
-
•
There is minimal increase in the late toxicity due to re-irradiation using proton beam therapy.
Abstract
Purpose
Repeat radiation therapy (RT) using photons/X-rays for locally recurrent breast cancer results in increased short and long-term toxicity. Proton beam RT (PBRT) can minimize dose to surrounding organs, thereby potentially reducing toxicity. Here, we report the toxicity and clinical outcomes for women who underwent re-irradiation to the chest wall for locally recurrent breast cancer using PBRT.
Materials and methods
This was a retrospective study analyzing 16 consecutive patients between 2013 and 2018 with locally recurrent breast cancer who underwent re-irradiation to the chest wall with PBRT. For the recurrent disease, patients underwent maximal safe resection, including salvage mastectomy, wide local excision, or biopsy only per surgeons recommendations. Systemic therapy was used per the recommendation of the medical oncologist. Patients were treated with median dose of 50.4 Cobalt Gray Equivalent (CGyE) in 28 fractions at the time of re-irradiation. Follow-up was calculated from the start of second RT course. Acute toxicities were defined as those occurring during treatment or up to 8 weeks after treatment. Late toxicities were defined as those occurring more than 8 weeks after the completion of therapy. Toxicities were based on CTCAE 4.0.
Results
The median age at original diagnosis and at recurrence was 49.8 years and 60.2 years, respectively. The median time between the two RT courses was 10.2 (0.7–20.2) years. The median follow-up time was 18.7 (2.5–35.2) months. No local failures were observed after re-irradiation. One patient developed distant metastasis and ultimately died. Grade 3–4 acute skin toxicity was observed in 5 (31.2%) patients. Four (25%) patients developed chest wall infections during or shortly (2 weeks) after re-irradiation. Late grade 3–4 fibrosis was observed in only 3 (18.8%) patients. Grade 5 toxicities were not observed. Hyperpigmentation was seen in 12 (75%) patients. Pneumonitis, telangiectasia, rib fracture, and lymphedema occurred in 2 (12.5%), 4 (25%), 1 (6.3%), and 1 (6.3%) patients, respectively.
Conclusions
Re-irradiation with PBRT for recurrent breast cancer has acceptable toxicities. There was a high incidence of acute grade 3–4 skin toxicity and infections, which resolved, however, with skin care and antibiotics. Longer follow-up is needed to determine long-term clinical outcomes.
1. Introduction
Breast cancer is one of the most frequently diagnosed cancer in the world, with approximately 127 new cases per 100,000 persons each year [1]. Due to the improvement in cancer screening and treatment, breast cancer mortality has steadily declined over the last decade [2]. Combined therapy for breast cancer, including surgery with adjuvant radiation therapy (RT), and appropriate systemic therapy has led to further improvement in post-treatment morbidity and mortality with local disease recurrence rates decreasing by 50% with combined therapy [3], [4], [5]. Despite these advances, locally recurrent disease remains an issue for a significant number of patients. Local recurrence rates still remain approximately 10–20% at 10 years, and can be as high as 40% depending on the stage, histology, estrogen/progesterone (ER/PR) receptor status, and Her-2 gene amplification [3], [4], [5], [6].
Uncontrolled locally recurrent breast cancer, especially those that occur on the chest wall, can cause significant morbidity such as ulcerations, bleeding, pain, and an overall decrease in the quality of life for these patients. Treatment options for local control of recurrent disease primarily involves surgery, with adjuvant RT being used in select cases. While surgery plus RT is effective for local recurrence, recent research has shown increased late toxicities including cardiotoxicity as a result of re-irradiation [7], [8]. Due to the concerns of increased short and long-term toxicity, especially lung and cardiac toxicity, as a result of the cumulative RT dose of two RT courses, a second course of RT using X-rays (photons) for locally recurrent breast cancer has been met with resistance among the radiation oncology community [8]. With the introduction of proton beam RT (PBRT), there is now a possibility to reduce the cumulative RT dose to the surrounding normal organs at risk, including the heart and lung. A study by Tommasino et al. has suggested that lower radiation doses can be delivered to cardiac and pulmonary structures with PBRT compared to photon RT [9]. This reduction in dose to surrounding organs can translate to reduced toxicity in the short- and long-term. However, there is currently limited literature describing PRBT for re-treatment of breast cancer. Here, we report the toxicity and outcomes for women who underwent re-irradiation to the chest wall for locally recurrent breast cancer using PBRT.
2. Materials and methods
An IRB approved institutional database was used as a source for this analysis. All consecutive patients between the year 2013 and 2018 who were diagnosed with recurrent breast cancer, and who were re-irradiated with PBRT at our institution were identified. Patients were required to have received previous radiation for ipsilateral breast cancer that overlapped with the current re-irradiation treatment field. As part of this study, patients were only included if re-irradiation was delivered using proton beam radiation therapy. As such, patients treated with X-rays (photons) for re-irradiation were excluded from this analysis. Patients with metastatic disease or patients treated with the primary goal of palliation were also excluded.
Type of surgery (salvage mastectomy, wide local excision, or biopsy only) was generally a maximum safe resection per the recommendation of the surgeon. Similarly, the use of systemic therapy (chemotherapy and/or endocrine therapy) was at the discretion of the medical oncologist. The median RT dose for the first course of RT was 50 Gy with a median boost dose of 10 Gy, and for the second course of treatment, 50.4 Cobalt Gy Equivalent (CGyE) in 28 fractions. A boost of a median dose of 10 CGyE was also delivered in 3 patients. Re-irradiation was delivered using passive scatter proton therapy using Mevion S250 (Mevion Medical Systems, Littleton, MA, USA). A cumulative dose constraint of maximum dose of 66 Gy and V60 < 0.1 cc was used for the brachial plexus. We did not use a specific dose constraints for the lung, heart, or the esophagus as proton therapy adds minimal to no dose to these structures. Concurrent hyperthermia was also utilized for patients with gross disease and close/positive margins, or skin involvement using Sonotherm 1000 (Labthermics Technologies, Inc, Champaign, IL, USA). For hyperthermia, the goal temperature was 41–43 °C for one hour and was delivered weekly within 30 min of RT administration for a total of 6 sessions. For all patients, temperatures were measured with multisensory thermocouple probes on the skin.
Follow up was calculated from the start of second RT course. Local control, distant control, and overall survival were calculated from the date of the first fraction of re-irradiation. Local control, distant control, and overall survival were analyzed using the Kaplan-Meier method. Toxicities were based on the Common Terminology Criteria for Adverse Events (CTCAE) 4.0. Acute toxicities were defined as those occurring during treatment or up to 8 weeks after treatment. Late toxicities were defined as those occurring more than 8 weeks after the completion of therapy. To avoid bias, aggravation of pre-existing toxicity as well as toxicity of uncertain cause were considered to be related to the present treatment and scored accordingly. Statistical analysis was carried out using SPSS version 23. The level of statistical significance was considered <0.05 for all analyses.
3. Results
A total of 16 patients who met the aforementioned inclusion and exclusion criteria were identified and included for the final analysis. The median age at the initial diagnosis of breast cancer was 49.8 years. At the initial diagnosis, 7 patients (43.8%) underwent mastectomy and 9 patients (56.2%) underwent breast conserving surgery. Patient characteristics at the time of initial diagnosis are presented in Table 1. The median age at the time of the diagnosis of recurrence was 60.2 years. The median time between the first and the second course of RT was 10.2 years (0.7–20.2 years). The median tumor size at the time of the recurrence was 1.5 cm (range: 0.9–10.0 cm). A total of 3 (18.8%) patients also had lymph node involvement at the time of recurrence. The majority of the patients (81.2%) had invasive ductal carcinoma. Nine patients (56.3%) had tumors positive for ER/PR receptor, three patients (18.8%) had tumors with Her-2 gene amplification, and four patients (25%) had triple negative breast cancer. Patient characteristics at the time of recurrence are presented in Table 2.
Table 1.
Clinical characteristics at initial diagnosis.
| Patients (n = 16) (%) | |
|---|---|
| Age at first diagnosis | |
| Median (Years) | 49.8 |
| Range (Years) | 26.7–64.5 |
| Race | |
| White | 14 (87.5) |
| African American | 2 (12.5) |
| Laterality | |
| Right | 8 (50.0) |
| Left | 8 (50.0) |
| Receptor Status | |
| ER/PR+ | 12 (75.0) |
| HER2+ | 3 (18.8) |
| Triple Negative | 1 (6.2) |
| Histology | |
| Ductal | 14 (87.5) |
| DCIS | 1 (6.2) |
| Other | 1 (6.2) |
| Nodal Status at Diagnosis | |
| Positive | 11 (68.8) |
| Negative | 5 (31.2) |
| Surgery at Diagnosis | |
| Mastectomy | 7 (43.8) |
| Breast Conserving Surgery | 9 (56.2) |
| Lymph Node Evaluation | |
| Axillary Lymph Node Dissection | 10 (62.5) |
| Sentinel Lymph Node | 5 (31.3) |
| No Lymph Node Evaluation | 1 (6.2) |
| Chemotherapy | |
| Yes | 12 (75.0) |
| No | 4 (25.0) |
| Hormone Therapy | |
| Yes | 12 (75.0) |
| No | 4 (25.0) |
| RT Dose (Gy) | |
| Median (Gy) | 50.0 |
| Range (Gy) | 45.0–50.4 |
| RT Fractions | |
| Median | 26 |
| Range | 25–28 |
| Boost | |
| Yes | 11 (68.8) |
| No | 5 (31.2) |
| Boost Dose (Gy) | |
| Median (Gy) | 10.0 |
| Range (Gy) | 10.0–16.0 |
| Boost Fractions | |
| Median | 5 |
| Range | 5–8 |
| Nodal RT | |
| Yes | 10 (62.5) |
| No | 6 (37.5) |
Table 2.
Clinical characteristics at recurrence.
| Patients (n = 16) (%) | |
|---|---|
| Age at Recurrence (Years) | |
| Median (Years) | 60.2 |
| Range (Years) | 37.5–86.5 |
| Time Between Diagnosis (Years) | |
| Median (Years) | 10.4 |
| Range (Years) | 2.1–22.0 |
| Site of Recurrence | |
| Chest Wall Only | 9 (56.3) |
| Breast Only | 4 (25.0) |
| Nodal Only | 1 (6.2) |
| Chest Wall and Nodal | 0 (0.0) |
| Breast and Nodal | 2 (12.5) |
| Size of Recurrence (cm) | |
| Median (cm) | 1.5 |
| Range (cm) | 0.9–10.0 |
| Histology | |
| Ductal | 13 (81.2) |
| DCIS | 0 |
| Other | 3 (18.8) |
| Receptor Status | |
| ER/PR+ | 9 (56.3) |
| HER2+ | 3 (18.7) |
| Triple Negative | 4 (25.0) |
| Grade | |
| 1 | 0 (0.0) |
| 2 | 9 (56.3) |
| 3 | 7 (43.7) |
| Surgery at Recurrence | |
| Salvage Mastectomy | 6 (37.5) |
| Chest Wall Excision | 8 (50.0) |
| Biopsy Only | 2 (12.5) |
| Margin Status | |
| Negative | 13 (81.2) |
| Positive | 3 (18.8) |
| Lymph Node Assessment | |
| Axillary Lymph Node Dissection | 2 (12.5) |
| Sentinel Lymph Node | 3 (18.7) |
| No Lymph Node Assessment | 11 (68.8) |
| Nodal Status | |
| Positive | 3 (18.8) |
| Negative | 13 (81.2) |
| Chemotherapy | |
| Yes | 4 (25.0) |
| No | 12 (75.0) |
| Hormone Therapy | |
| Yes | 8 (50.0) |
| No | 8 (50.0) |
| Hyperthermia | |
| Yes | 10 (62.5) |
| No | 6 (37.5) |
| Time Between RT Courses (Years) | |
| Median (Years) | 10.2 |
| Range (Years) | 0.7–20.2 |
| RT Target | |
| Chest Wall Only | 12 (75.0) |
| Chest Wall and Regional Nodes | 4 (25.0) |
| RT Dose (CGyE) | |
| Median (CGyE) | 50.4 |
| Range (CGyE) | 41.4–50.4 |
| RT Fractions | |
| Median | 28 |
| Range | 23–28 |
| Boost | |
| Yes | 3 (18.8) |
| No | 13 (81.2) |
| Boost Dose (CGyE) | |
| Median (CGyE) | 10 |
| Range (CGyE) | 10–16 |
| Boost Fractions | |
| Median | 5 |
| Range | 5–8 |
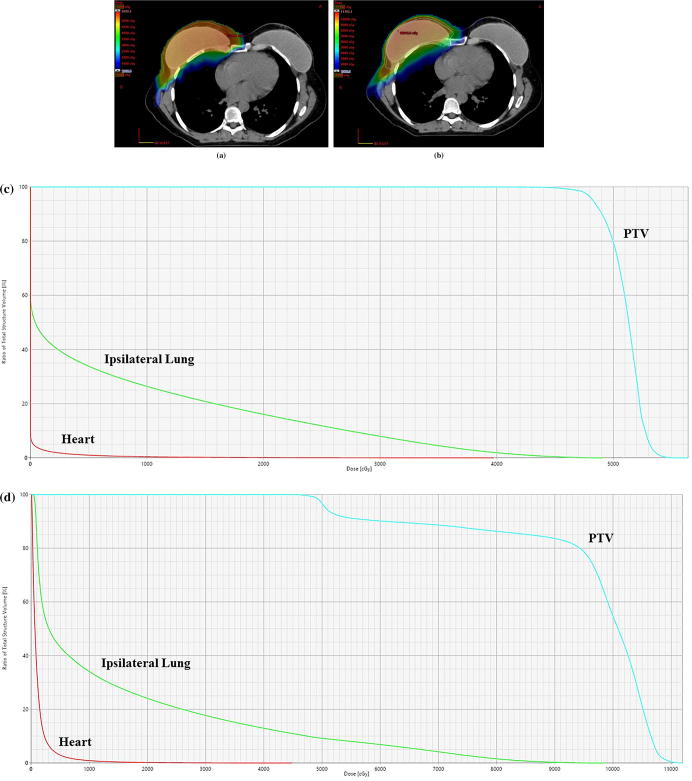
With regards to the type of surgery for the treatment of their recurrent disease, 6 patients underwent salvage mastectomy, 8 patients underwent wide local excision, and 2 patients underwent a biopsy only. The re-irradiation target was the chest wall in 12 (75%) patients, and both chest wall and regional nodes (axillary, supraclavicular, and internal mammary lymph node chain) in 4 (25%) patients. There were no patients who received repeat regional nodal irradiation, however, one patient did receive high tangent treatment for the initial treatment who then went on to receive regional nodal irradiation with protons. The median RT dose was 50.4 CGyE (41.4–50.4 CGyE). A RT boost of 10–16 Gy was delivered to 3 (18.8%) patients. For the proton plan, the median ipsilateral lung V20 was 13% (range 0.2% − 26.8%), ipsilateral mean lung dose was 6.05 Gy (range 0.34–11.7), mean heart dose was 0.24 Gy (range 0.02–2.13), and mean esophagus dose was 0.0 Gy (range 0.0–10.4). Cumulative dose information was available for 9 patients. The median mean heart dose was 4.8 Gy, V5 of 19.8%, V10 of 14.9%, and V25 of 5.8%. The median ipsilateral mean lung dose was 22.8 Gy, V20 of 35.3%, V30 of 27.1%, and V40 of 21.3%. An example patient’s treatment plan showing the proton dose distribution and the cumulative dose distribution is shown in Fig. 1. However, it should be noted that many patients had significant anatomical changes between the two treatment courses and as such, the cumulative dose information may not be accurately represented. Concurrent hyperthermia was utilized in 10 (62.5%) patients. Systemic therapy in the form of chemotherapy was administered to 4 (25%) patients and endocrine therapy was administered to 8 (50%) patients.
Fig. 1.
A. Dose colowash showing the patients current re-treatment plan using proton radiaiton therapy. B. Dose colowash showing the patients composite treatment dose distribution of the current proton plan plus the prior radiation plan. C. Representative dose volume histogram of the proton re-irradiaiton plan. D. Representative dose volume histogram of the composite treatment plan of the prior photon plan and current proton plan.
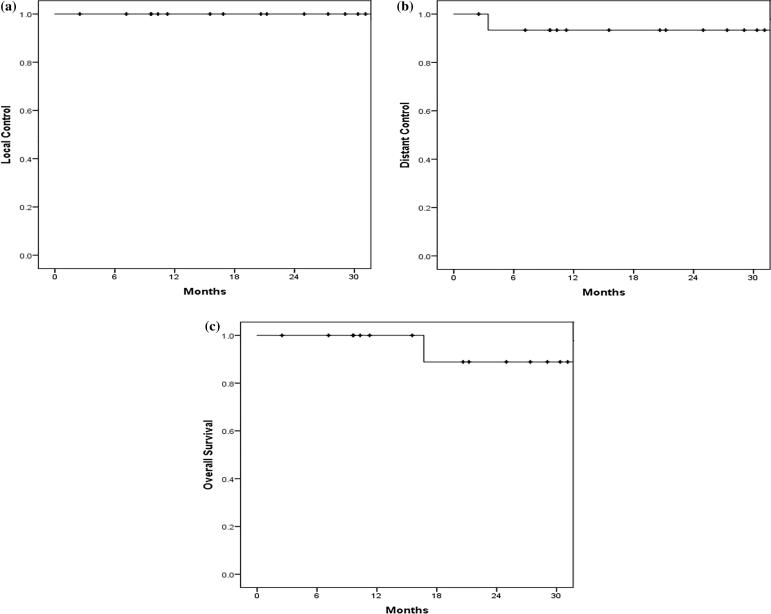
The median follow up time after the start of the 2nd RT course was 18.7 months (range: 2.5–35.2 months). There were no local or regional failures observed after re-irradiation. Only one patient developed distant metastasis and ultimately died as a result of her metastatic disease. However, she lived for 15.6 months after the development of her distant metastases. The 18-month local control, distant control, and overall survival was 100%, 93.3%, and 88.9% respectively (Fig. 2a–c).
Fig. 2.
A. Local Control. B. Distant Control. C. Overall Survival.
Grade 3–4 acute skin toxicity was observed in 5 (31.2%) patients. This was mostly in the form of moist desquamation and ulceration. One patient had a grade 4 ulceration that occurred during treatment and persisted 24 weeks after treatment. There were 4 (25%) patients who developed chest wall infections during or shortly (2 weeks) after re-irradiation. These patients required antibiotics for treatment of their infections. Late grade 3–4 fibrosis was observed in only 3 (18.8%) patients. Hyperpigmentation that persisted through their last follow up was seen in 12 (75%) patients. Other RT related toxicities such as pneumonitis, telangiectasia, rib fracture, and lymphedema occurred in 2 (12.5%), 4 (25%), 1 (6.3%), and 1 (6.3%) patients respectively. The rib fracture observed in one patient was asymptomatic and was incidentally found on a CT scan performed as part of her regular follow up. RT-related pericarditis was not observed in the cohort, nor was any other cardiotoxicity. Furthermore, no Grade 5 toxicity was observed in this cohort. For subjective measures, 14 patients reported a maximum pain score between 0 and 5 (87.5%), while 2 patients reported a score between 6 and 10 (12.5%) during treatment. Toxicity details are presented in Table 3.
Table 3.
Proton beam re-irradiation related toxicities.
| Patients (n = 16) (%) | |
|---|---|
| Acute Skin | |
| Grade 1 | 5 (31.3) |
| Grade 2 | 6 (37.5) |
| Grade 3 | 4 (25.0) |
| Grade 4 | 1 (6.2) |
| Infection | |
| Yes | 4 (25.0) |
| No | 12 (50.0) |
| Max Pain Score | |
| 0–5 | 14 (87.5) |
| 6–10 | 2 (12.5) |
| Fibrosis | |
| Grade 0 | 5 (31.3) |
| Grade 1 | 6 (37.5) |
| Grade 2 | 2 (12.5) |
| Grade 3 | 2 (12.5) |
| Grade 4 | 1 (6.2) |
| Pneumonitis | |
| Yes | 2 (12.5) |
| No | 14 (87.5) |
| Telangiectasia | |
| Yes | 4 (25.0) |
| No | 12 (75.0) |
| Hyperpigmentation | |
| Yes | 12 (75.0) |
| No | 4 (25.0) |
| Rib Fracture | |
| Yes | 1 (6.2) |
| No | 15 (93.8) |
| Brachial Plexopathy | |
| Yes | 1 (6.2) |
| No | 15 (93.8) |
| Lymphedema | |
| Yes | 1 (6.2) |
| No | 15 (93.8) |
4. Discussion
We retrospectively evaluated the clinical outcomes and toxicity after re-irradiation of recurrent breast cancer using PBRT. To our knowledge, this is one of the few reports describing the clinical outcomes and toxicity of re-irradiation of recurrent breast cancer using PBRT. In the 16 patients that were treated with PBRT in this cohort, there were no local recurrences, and only one distant failure and subsequent death from distant metastasis. We observed a high rate of acute grade 3–4 skin toxicity, and chest wall infections. However, they all resolved, with appropriate skin care regimens and antibiotics within 6 weeks after completion of treatment. More importantly, cardiac toxicity was not observed in any of the patients and pneumonitis was rarely observed. Overall, treatment was effective with the 18-month local control and distant control rates of 100% and 93.3%, respectively.
Several studies have discussed toxicities and outcomes in patients who underwent re-irradiation with X-ray/photon radiation after locally recurrent breast cancer [10], [11], [12], [13], [14]. In one of the largest multi-institutional retrospective study by Wahl et al., which recorded outcomes for breast cancer patients with local recurrence after re-irradiation with X-rays, a lower percentage of the cohort experienced acute Grade 3–4 skin toxicities compared to the findings in our study [11]. In a study by Oldenborg et al., the authors reported on 414 pretreated patients with inoperable locally recurrent breast cancer who received re-RT with electrons, photons, or combination of electron and photon therapy along with hyperthermia [14]. Acute grade 3–4 skin toxicity in the form of desquamation and ulceration was noted in 24% of patients, slightly lower than our findings. However, several studies have shown high rates of skin toxicity with proton beam therapy, both in the setting of initial treatment and re-irradiation [15], [16], [17]. For example, a study by Bradley et al. showed 22% rate of grade 3–4 dermatitis, similar to our findings [18]. Similarly, an abstract published by Niska et al. on PBRT for locally recurrent breast cancer in patients without prior RT also showed a 21% rate of acute grade 3+ skin toxicity [19].
Although fewer acute skin toxicities were reported in the study by Wahl et al. and Oldenborg et al. [11], [14] than the findings in the current study, the acute skin toxicities in our study all resolved with appropriate skin care and antibiotic treatment with no long term consequences. A potential reason for high rate of acute grade 3+ skin toxicity in our cohort is that a large proportion of our patients (62.5%) underwent concurrent hyperthermia, which has been a well-established radiosensitizer. For example, a study by Vernon et al. [20] showed improved clinical response and local control with combined use of RT and hyperthermia in breast cancer, however, an increased risk of skin blistering (11% vs. 2%) was found with the addition of hyperthermia to RT. Additionally, they also found a 7% rate of ulceration and 7% rate of necrosis with the use of hyperthermia compared to only 2% and 1% respectively in the RT-only arm [20]. Despite increased risk of acute toxicities, PBRT may still be the more efficacious and less detrimental choice in the long-term as some have claimed that survival can be improved if deaths secondary to cardiac events could be mitigated [21].
In regards to late toxicities, a study by Muller et al. [22] reported moderate incidence of late grade 3 skin toxicity after re-irradiation of the chest wall in breast cancer patients (20%). Pneumonitis was also observed in their cohort after re-irradiation in 10% of patients. Comparatively, we observed a similar rate of late grade 3–4 skin fibrosis in 3 patients (18.8%) and only 1 patient developing pneumonitis. Additionally, the more important late cardiac toxicities were likely not adequately captured in either of the two studies previously discussed due to the short median follow up of 12 months in Wahl et al. and 41 months in Muller et al., as well as 18 months in our study. In regards to the clinical outcomes, the local disease free survival and overall at 1-year in Wahl et al. was only 66% and 64%, respectively compared to 100% and 88.9% at 18-months, respectively in our study [11]. However, longer follow-up will be needed to assess late toxicities such cardiac toxicity of PBRT in this cohort.
One of the clear advantages of PBRT over X-ray/photon therapy is lower RT dose to the heart, thereby translating into a lower risk of cardiac toxicity. While we were unable to find any clinical studies assessing the long term impact of re-irradiation of locally recurrent breast cancer using proton beam therapy, there have been a several dosimetric studies comparing doses delivered to organs at risk, mainly the heart and the lungs secondary to chest wall irradiation using proton versus intensity modulated RT (IMRT) [18], [23], [24], [25]. In one of these studies, the left lung and cardiac doses were reduced by a factor of 2.5 using protons vs. IMRT, and cardiac doses were reduced by a factor of 20 with protons [24]. In another study, the mean heart dose was reduced from 16 Gy with IMRT to 6 Gy with PBRT [23]. Similarly, a study by Bradley et al. showed the median cardiac V5 of only 0.6% with PBRT compared to 16.3% with photon based RT [18]. As such, it can be anticipated that at longer follow up, our study will likely find low rates of cardiac toxicity beyond the risk that these patients already have from their first course of irradiation.
Although the study shows promising results, there are a few limitations to be considered within the study. Firstly, given the retrospective nature of the study, toxicities may not be accurately captured compared to a prospective or a patient reported outcomes study. Median follow-up time after re-irradiation in our study was only 18.7 months, which may have reduced the scope of late toxicities that were observed in our patients. Even though cardiac toxicities were not observed in any patients, longer follow up is needed to conclude about the safety of PBRT as it relates to the heart. Nonetheless, evidence does suggest that PBRT results in minimal heart dose, which theoretically improves the cardiac toxicity profile of re-irradiation [9]. Another limitation is the small sample size, which reduces the power of this study, and the variation of combination treatment within the group. Thus, a larger study with increased cohort population and longer follow-up time, along with analyses excluding potential covariates would yield more definitive results.
There are minimal local treatment options for women with recurrent breast cancer. Often, it is difficult to obtain a negative margin, and rates of local failure are high without RT. The only other currently available treatments are systemic therapy, or palliative doses of RT using photons. With the introduction of PBRT, re-irradiation with a much higher and more definitive dose of RT is now an option without the additional risk of pulmonary or cardiac toxicity. In conclusion, re-irradiation with PBRT for recurrent breast cancer has acceptable toxicities and offers great short-term clinical outcomes. There is a high incidence of grade 3–4 skin toxicity and infections, however, these can be effectively treated with adequate skin care and antibiotics. Further follow up will be needed to determine long-term clinical outcomes and the effects of PBRT on cardiac toxicity.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Noone A.M., Howlader N., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., editors. SEER Cancer Statistics Review, 1975–2015. National Cancer Institute; Bethesda, MD: 2018. https://seercancergov/csr/1975_2015/ [Google Scholar]
- 2.Amin R.W., Fritsch B.A., Retzloff J.E. Spatial clusters of breast cancer mortality and incidence in the contiguous USA: 2000–2014. J General Internal Med. 2019 doi: 10.1007/s11606-018-4824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M., Collins R., Darby S., Davies C., Elphinstone P., Evans V. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 7.Taylor C., Correa C., Duane F.K., Aznar M.C., Anderson S.J., Bergh J. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clinical Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Bronnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 9.Tommasino F., Durante M., D'Avino V., Liuzzi R., Conson M., Farace P. Model-based approach for quantitative estimates of skin, heart, and lung toxicity risk for left-side photon and proton irradiation after breast-conserving surgery. Acta Oncol. 2017;56:730–736. doi: 10.1080/0284186X.2017.1299218. [DOI] [PubMed] [Google Scholar]
- 10.Arthur D.W., Winter K.A., Kuerer H.M., Haffty B.G., Cuttino L.W., Todor D.A. NRG oncology-radiation therapy oncology group study 1014: 1-year toxicity report from a phase 2 study of repeat breast-preserving surgery and 3-dimensional conformal partial-breast reirradiation for in-breast recurrence. Int J Radiat Oncol Biol Phys. 2017;98:1028–1035. doi: 10.1016/j.ijrobp.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahl A.O., Rademaker A., Kiel K.D., Jones E.L., Marks L.B., Croog V. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys. 2008;70:477–484. doi: 10.1016/j.ijrobp.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Hannoun-Levi J.M., Resch A., Gal J., Kauer-Dorner D., Strnad V., Niehoff P. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: multicentric study of the GEC-ESTRO Breast Cancer Working Group. Radiother Oncol. 2013;108:226–231. doi: 10.1016/j.radonc.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Janssen S., Rades D., Meyer A., Fahlbusch F.B., Wildfang I., Meier A. Local recurrence of breast cancer: conventionally fractionated partial external beam re-irradiation with curative intention. Strahlenther Onkol. 2018;194:806–814. doi: 10.1007/s00066-018-1315-1. [DOI] [PubMed] [Google Scholar]
- 14.Oldenborg S., Griesdoorn V., van Os R., Kusumanto Y.H., Oei B.S., Venselaar J.L. Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: size matters. Radiother Oncol. 2015;117:223–228. doi: 10.1016/j.radonc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Kozak K.R., Smith B.L., Adams J., Kornmehl E., Katz A., Gadd M. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys. 2006;66:691–698. doi: 10.1016/j.ijrobp.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Luo L., Cuaron J., Braunstein L., Gillespie E., Kahn A., McCormick B. Early outcomes of breast cancer patients treated with post-mastectomy uniform scanning proton therapy. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 17.McGee L.A., Iftekaruddin Z., Chang J.H.C., Gondi V., Schmidt S., Kaplan D. Postmastectomy chest wall reirradiation with proton therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2017;99:E34–E35. [Google Scholar]
- 18.Bradley J.A., Dagan R., Ho M.W., Rutenberg M., Morris C.G., Li Z. Initial report of a prospective dosimetric and clinical feasibility trial demonstrates the potential of protons to increase the therapeutic ratio in breast cancer compared with photons. Int J Radiat Oncol Biol Phys. 2016;95:411–421. doi: 10.1016/j.ijrobp.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Niska J.R., Thorpe C., Bruso M.E., Kosiorek H.E., McGee L.A., Hartsell W.F. Proton beam therapy for isolated locoregional recurrence of breast cancer after mastectomy without prior radiation therapy: prospective PCG registry analysis. Int J Radiat Oncol Biol Phys. 2018;102 [Google Scholar]
- 20.Vernon C.C., Hand J.W., Field S.B., Machin D., Whaley J.B., van der Zee J. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 21.Plastaras J.P., Berman A.T., Freedman G.M. Special cases for proton beam radiotherapy: re-irradiation, lymphoma, and breast cancer. Semin Oncol. 2014;41:807–819. doi: 10.1053/j.seminoncol.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Muller A.C., Eckert F., Heinrich V., Bamberg M., Brucker S., Hehr T. Re-surgery and chest wall re-irradiation for recurrent breast cancer: a second curative approach. BMC Cancer. 2011;11:197. doi: 10.1186/1471-2407-11-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomax A.J., Cella L., Weber D., Kurtz J.M., Miralbell R. Potential role of intensity-modulated photons and protons in the treatment of the breast and regional nodes. Int J Radiat Oncol Biol Phys. 2003;55:785–792. doi: 10.1016/s0360-3016(02)04210-4. [DOI] [PubMed] [Google Scholar]
- 24.Ares C., Khan S., Macartain A.M., Heuberger J., Goitein G., Gruber G. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys. 2010;76:685–697. doi: 10.1016/j.ijrobp.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 25.Mast M.E., Vredeveld E.J., Credoe H.M., van Egmond J., Heijenbrok M.W., Hug E.B. Whole breast proton irradiation for maximal reduction of heart dose in breast cancer patients. Breast Cancer Res Treat. 2014;148:33–39. doi: 10.1007/s10549-014-3149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]