Key Points
Question
What is the effect of systematic depression screening with and without provision of enhanced depression care on quality-adjusted life-years and depressive symptoms among survivors of acute coronary syndromes?
Findings
In this randomized clinical trial that included 1500 survivors of acute coronary syndromes, there were no differences in quality-adjusted life-years or depression-free days in those who were and were not screened for depression, even when depression screening was followed by enhanced depression care.
Meaning
Contrary to recommendations from professional societies, systematic depression screening of survivors of acute coronary syndromes may not be warranted.
Abstract
Importance
Patients with acute coronary syndrome (ACS) and elevated depressive symptoms are at increased risk for recurrent cardiovascular events and mortality, worse quality of life, and higher health care costs. These observational findings prompted multiple scientific panels to advise universal depression screening in survivors of ACS prior to evidence from randomized screening trials.
Objective
To determine whether systematically screening for depression in survivors of ACS improves quality of life and depression compared with usual care.
Design, Setting, and Participants
A 3-group multisite randomized trial enrolled 1500 patients with ACS from 4 health care systems between November 1, 2013, and March 31, 2017, with follow-up ending July 31, 2018. Patients were eligible if they had been hospitalized for ACS in the previous 2 to 12 months and had no prior history of depression. All analyses were performed on an intention-to-treat basis.
Interventions
Patients with ACS were randomly assigned 1:1:1 to receive (1) systematic depression screening using the 8-item Patient Health Questionnaire, with notification of primary care clinicians and provision of centralized, patient-preference, stepped depression care for those with positive screening results (8-item Patient Health Questionnaire score ≥10; screen, notify, and treat, n = 499); (2) systematic depression screening, with notification of primary care clinicians for those with positive screening results (screen and notify, n = 501); and (3) usual care (no screening, n = 500).
Main Outcomes and Measures
The primary outcome was change in quality-adjusted life-years. The secondary outcome was depression-free days. Adverse effects and mortality were assessed by patient interview and hospital records.
Results
A total of 1500 patients (424 women and 1076 men; mean [SD] age, 65.9 [11.5] years) were randomized in the 18-month trial. Only 71 of 1000 eligible survivors of ACS (7.1%) had elevated 8-item Patient Health Questionnaire scores indicating depressive symptoms at screening. There were no differences in mean (SD) change in quality-adjusted life-years (screen, notify and treat, –0.06 [0.20]; screen and notify, −0.06 [0.20]; no screen, −0.06 [0.18]; P = .98) or cumulative mean (SD) depression-free days (screen, notify and treat, 343.1 [179.0] days; screen and notify, 351.3 [175.0] days; no screen, 339.0 [176.6] days; P = .63). Harms including death, bleeding, or sleep difficulties did not differ among groups.
Conclusions and Relevance
In patients with ACS without a history of depression, systematic depression screening with or without providing depression treatment did not alter quality-adjusted life-years, depression-free days, or harms.
Trial Registration
ClinicalTrials.gov identifier: NCT01993017
This randomized clinical trial examines whether systematically screening for depression in survivors of acute coronary syndromes improves quality of life and depression compared with usual care.
Introduction
Many patients with acute coronary syndromes (ACSs) experience clinically significant depressive symptoms after the acute cardiac event,1,2 and approximately 10% of patients with ACS meet the criteria for major depressive disorder.3 Depression in patients with ACS is associated with twice the risk of mortality or recurrent cardiovascular events,4,5 lower quality of life,6 and greater health care costs.7 Citing these observational data and the availability of effective depression treatments,3 multiple professional societies have recommended depression screening in patients with ACS, followed by comprehensive treatment when depression is detected.1,8,9,10,11,12
Controversy exists about recommendations to screen for depression in patients with ACS.13 Studies demonstrating the effectiveness of depression treatment in patients with ACS have enrolled only patients seeking treatment, limiting generalizability to patients whose depression is detected by screening. To our knowledge, no depression screening trials have been conducted in patients with ACS.14 Some have argued that until a randomized depression screening trial demonstrates improvements in depressive symptoms or other outcomes, depression screening recommendations are premature.13,15 In the meantime, few cardiologists or primary care providers (PCPs; physicians and nurse practitioners) have implemented routine depression screening in patients with ACS.16,17 Primary reasons cited for delayed implementation include lack of evidence from randomized screening trials and harms of screening.18,19 In addition to cost, potential harms of depression screening include adverse psychological effects from depression labeling and adverse biological effects related to increased use of antidepressant medications in those whose screening results are positive.20,21,22
We report here on CODIACS-QoL (Comparison of Depression Interventions After Acute Coronary Syndrome: Quality of Life), a depression screening trial of 1500 patients with ACS from 4 diverse health care systems. Patients were randomized to 1 of 3 groups: (1) no depression screening, (2) screening and notifying the PCP, and (3) screening, notifying the PCP, and providing enhanced depression treatment that had previously been shown to be effective in patients with ACS, albeit not in a population that underwent screening for depression.23,24 We sought to determine if depression screening after ACS improved quality-adjusted life-years (QALYs), and, secondarily, depressive symptoms and depression-free days.
Methods
Study Design and Setting
This multicenter randomized clinical trial had 3 groups: (1) systematic depression screening with notification of PCPs and provision of patient-preference, stepped-care depression treatment for those with positive screening results (screen, notify, and treat group); (2) systematic depression screening with notification of PCPs only (screen and notify group); and (3) usual care (no screen group). Patients were recruited from 4 geographically diverse health systems: HealthPartners (Minneapolis, Minnesota), Duke University Health System (Durham, North Carolina), Kaiser Permanente Northwest (Portland, Oregon), and New York–Presbyterian/Columbia University Irving Medical Center (New York, New York; recruiting and coordinating site). Patients were screened for eligibility starting November 1, 2013, with enrollment ending March 31, 2017, and 18-month follow-up ending July 31, 2018. The institutional review boards of all 4 recruitment centers approved this study. Oral consent was obtained from all patients. Those who were randomized to the screen, notify, and treat group were later asked to provide written consent. The trial protocol and statistical analysis plans25 are available in Supplement 1.
Patients were eligible if they were age 21 years or older, spoke English or Spanish, and had documented ACS within 2 to 12 months of enrollment based on standardized International Classification of Diseases, Ninth Revision, or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, discharge codes for acute myocardial infarction and unstable angina.26 Eligibility was confirmed via medical record review and baseline telephone interview. Patients were excluded if they were currently receiving treatment for depression or had a prior history of depression; had a life expectancy less than 1 year; had a prior or current history of bipolar disorder, suicidal risk, or psychosis; had current substance abuse; had dementia; were currently pregnant; had severe arthritis or rheumatologic illness; had advanced liver disease requiring frequent hospitalizations; had advanced heart failure; had advanced lung disease needing oxygen at home; had advanced HIV infection or AIDS; or had advanced cancer of any kind.
Patients were randomized in a 1:1:1 ratio to 1 of 3 groups, stratified by site. A web-based random number generator integrated into the patient tracking system produced a blocked randomization assignment within strata with randomly selected block sizes of 3, 6, or 9. Randomization assignment became visible to an unblinded coordinator only after all baseline data had been entered. Concealment was ensured as group allocation occurred in real time using the web-based computer algorithm. Unblinded coordinators then completed depression screening, notification, and referral to treatment if indicated by group assignment and result of depression screening. Blinded coordinators conducted all outcome assessments while masked to group allocation.
Intervention and Control Procedures
Patients assigned to the screen, notify, and treat group were screened for depression using the 8-item Patient Health Questionnaire (PHQ-8), a research-grade, validated, sensitive, and specific depression screening tool.27 Patients with clinically significant depressive symptoms (PHQ-8 score ≥10) had their PCP and/or treating cardiologist notified of their elevated depressive symptoms via letter. Unblinded site coordinators provided patients with education regarding depression treatment options and offered a free-of-charge 6-month course of patient preference–driven stepped-care depression treatment (problem-solving therapy, antidepressant, both, or none), which adhered to a previously published format and algorithm.28 Depression treatment was provided without first requiring a clinical diagnosis of major depression, as even subsyndromal symptoms of depression have been associated with risk of adverse prognosis,29 and prior studies have verified the effectiveness of depression treatment in such patients.23,24 Intervention elements were delivered by a centralized, licensed clinical social worker with expertise in telephone-delivered problem-solving therapy and/or a site psychiatrist, internist, or psychiatric nurse practitioner with prescribing privileges.
Patients assigned to the screen and notify group were systematically screened for depression using the PHQ-8, with notification of patients’ cardiologists and/or PCPs if a patient had positive screening results. Subsequent depression treatment decisions were made by patients’ treating clinicians, and patients were responsible for out-of-pocket costs of depression treatment.
Patients assigned to the no screen group were not systematically screened for depression using the PHQ-8. Patients received usual care from their treating clinicians and were eligible to seek mental health screening and/or treatments at their own expense.
Data Collection
After completing an eligibility questionnaire, eligible patients completed consent, baseline assessment, and randomization by telephone. The assessment included age, sex, race, ethnicity, educational level, nativity status, preferred language, partner status, employment status, health insurance coverage, the 10-item Center for Epidemiologic Studies Depression scale (CESD-10), and a health-related quality-of-life measure (the 12-Item Short-Form Health Survey, version 2).30 Follow-up data collection telephone calls were conducted at 6, 12, and 18 months after baseline assessments. At these follow-up assessments, patients completed the CESD-10; the 12-Item Short-Form Health Survey, version 2; a symptom checklist assessing potential adverse consequences from depression screening and treatment; and items assessing receipt of depression treatment since the prior study visit. At 18 months, patients also completed the PHQ-8.
Outcomes
The prespecified primary outcome was change in QALYs from baseline through 18 months after randomization. Utility scores, an overall assessment of well-being on a scale from 0 (death) to 1 (perfect health), were estimated using the Short Form-6 dimension, with scores derived from responses to the 12-Item Short-Form Health Survey, version 2, at baseline and 6, 12, and 18 months.31,32,33,34,35,36 Quality-adjusted life-years for the period from baseline to 18 months were calculated as the area under the curve by linearly interpolating the utility scores at the 4 assessments. Change in QALYs was then obtained by subtracting the baseline QALY from the observed QALY for an 18-month period, where baseline QALY was calculated under the assumption that the baseline utility score remained constant during the 18-month period.
The prespecified secondary outcome was depression-free days based on the CESD-10, a nondiagnostic, epidemiologic, reliable, and valid measurement for depressive symptoms.37 A CESD-10 score of 10 or greater indicates elevated depressive symptoms.38 Depression-free days was calculated using linear interpolation to estimate daily depression severity at each of the 4 assessments.
Additional outcomes included depressive symptoms as measured by the CESD-10 and the PHQ-8, although the PHQ-8 was not assessed at baseline in the no screen group. Harms included potential adverse effects from antidepressant medications (ie, appetite problems, sleep problems, gastrointestinal upset, and bleeding) and mortality, assessed by surveying patient surrogates and through review of the electronic medical record.
Statistical Analysis
Baseline characteristics were examined as means (SD) or percentages by randomization assignment to assess for a balanced allocation. The primary comparison of change in QALYs among the 3 groups was conducted using a 2-step gatekeeping test procedure. An omnibus F test using analysis of variance comparing the 3 groups with one another was first performed, and pairwise comparisons using a 2-sided t test at 5% nominal significance were planned only if the omnibus F test had a P value of less than .05. This procedure would control the familywise type I error rate at 5%.39,40 In the secondary outcome analysis, depression-free days of the 3 groups were compared using the same gatekeeping procedure.
All analyses used the principle of intention to treat. The missing patterns of QALYs, CESD-10 scores, and PHQ-8 scores at each telephone follow-up were tested to see if they met the assumption of missing at random using the Little test. As this assumption was met, missing data were handled using multiple imputation, with the point estimate derived from the mean of 5 data sets and the pooled variance calculated using the Rubin formula.41 Sensitivity analyses were performed using (1) worst outcome imputation and (2) last observation carried forward. Sex-stratified analyses were also conducted for these outcomes.
Exploratory analyses of change in utility scores over time were performed in the framework of linear mixed models with a random intercept to account for intrapatient correlation. These models included time and randomization group as main effects and the time-by-group interaction.
Sample size was calculated to detect a clinically significant difference in change in QALYs. We determined that a sample size per group of 500, assuming 5% loss to follow-up, would yield 80% power for a 2-sided t test at the 5% level. These calculations were based on an assumed SD for QALYs of 0.17,42 expected prevalence of screening-detected depression of 20%, and assumed net improvement in QALYs of 0.155 over 18 months for individuals with depression who received treatment for depression in the screen, notify, and treat group.43
Analyses were performed in R, version 3.4.3 (R Foundation for Statistical Computing). All P values were from 2-sided tests, and results were deemed statistically significant at P < .05. Given the use of the 2-step gatekeeping procedure, no adjustment for multiple comparisons was performed for the primary or secondary outcomes.
Results
Participants
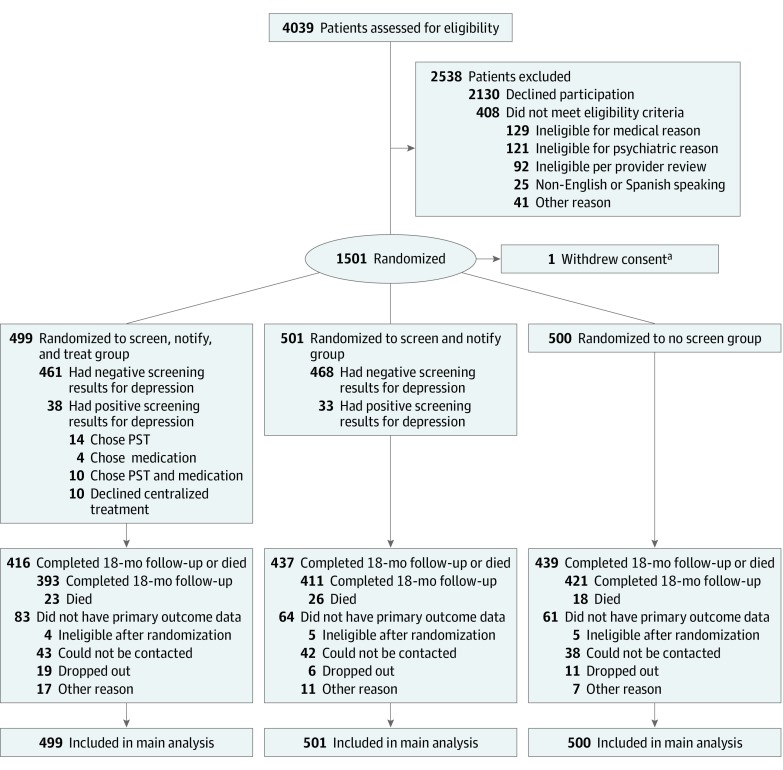
There were 5332 patients identified as potentially eligible for depression screening after ACS based on review of the electronic medical record, and 4039 patients were reached by telephone for assessment (Figure 1). Of these patients, 2130 (52.7%) declined to participate in the study, and 1501 were ultimately found to be eligible. One patient asked to have data withdrawn, such that the final sample size was 1500. The mean (SD) age of patients was 65.9 (11.5) years; 424 were women, 1076 were men, and 244 were Hispanic (Table 1). There were no substantial differences in demographic, depression, or health status characteristics of patients in the 3 groups.
Figure 1. CONSORT Flowchart.
PST indicates problem-solving therapy.
aFrom the Screen, Notify, and Treat group.
Table 1. Baseline Characteristicsa.
| Characteristic | Overall (N = 1500) | Screen, Notify, and Treat Group (n = 499) | Screen and Notify Group (n = 501) | No Screen Group (n = 500) |
|---|---|---|---|---|
| Age, mean (SD), y | 65.9 (11.5) | 66.2 (11.3) | 65.8 (11.7) | 65.8 (11.7) |
| Male sex | 1076 (71.7) | 357 (71.5) | 364 (72.7) | 355 (71.0) |
| Race | ||||
| White | 1080 (72.0) | 353 (70.7) | 368 (73.5) | 359 (71.8) |
| Black | 130 (8.7) | 47 (9.4) | 37 (7.4) | 46 (9.2) |
| Other | 259 (17.3) | 92 (18.4) | 82 (16.4) | 85 (17.0) |
| Refused or unknown | 31 (2.1) | 7 (1.4) | 14 (2.8) | 10 (2.0) |
| Ethnicity | ||||
| Hispanic | 244 (16.3) | 82 (16.4) | 88 (17.6) | 74 (14.8) |
| Non-Hispanic | 1218 (81.2) | 406 (81.4) | 402 (80.2) | 410 (82.0) |
| Refused or unknown | 38 (2.5) | 11 (2.2) | 11 (2.2) | 16 (3.2) |
| Born in the United States | 1169 (77.9) | 392 (78.6) | 392 (78.2) | 385 (77.0) |
| English as first language | 1282/1495 (85.8) | 426/497 (85.7) | 427/501 (85.2) | 429/497 (86.3) |
| Educational level, No./total No. (%) | ||||
| High school or lower | 536/1487 (36.0) | 191/493 (38.7) | 178/498 (35.7) | 167/496 (33.7) |
| Some college | 336/1487 (22.6) | 105/493 (21.3) | 119/498 (23.9) | 112/496 (22.6) |
| College and higher | 615/1487 (41.4) | 197/493 (40.0) | 201/498 (40.4) | 217/496 (43.8) |
| Married | 966/1489 (64.9) | 318/495 (64.2) | 336/498 (67.5) | 312/496 (62.9) |
| Employed | 606/1488 (40.7) | 205/492 (41.7) | 198/500 (39.6) | 203/496 (40.9) |
| Covered by health insurance | 1432/1491 (96.0) | 475/496 (95.8) | 482/499 (96.6) | 475/496 (95.8) |
| PHQ-8 score ≥10 | NA | 38/494 (7.7) | 33/501 (6.6) | NA |
| CESD-10 score, mean (SD) | 4.8 (4.8) | 4.9 (5.1) | 4.8 (4.8) | 4.7 (4.6) |
| CESD-10 score ≥10 | 212/1489 (14.2) | 73/493 (14.8) | 66/500 (13.2) | 73/496 (14.7) |
| SF-12 Mental score, mean (SD) | 54.0 (9.0) | 54.0 (9.6) | 53.8 (9.0) | 54.2 (8.4) |
| SF-12 Physical score, mean (SD) | 42.9 (11.8) | 42.5 (11.8) | 44.2 (11.5) | 42.2 (11.9) |
Abbreviations: CESD-10, 10-item Center for Epidemiologic Studies Depression Scale; NA, not applicable; PHQ-8, 8-item Patient Health Questionnaire; SF-12, 12-Item Short-Form Health Survey.
Data are presented as number (percentage) of patients unless otherwise specified. Less than 1% of data are missing for some categorical variables such that totals may add up to less than 100%.
Among 499 patients randomized to the screen, notify, and treat group, 38 of 494 (7.7%) had positive screening results for depression (ie, PHQ-8 score ≥10), and 28 of these patients with positive screening results (73.7%) agreed to the patient-preference stepped-care intervention; 4 chose antidepressants, 14 chose problem-solving therapy, and 10 chose both. Of 501 patients randomized to the screen and notify group, 33 (6.6%) had positive screening results for depression.
Change in QALYs
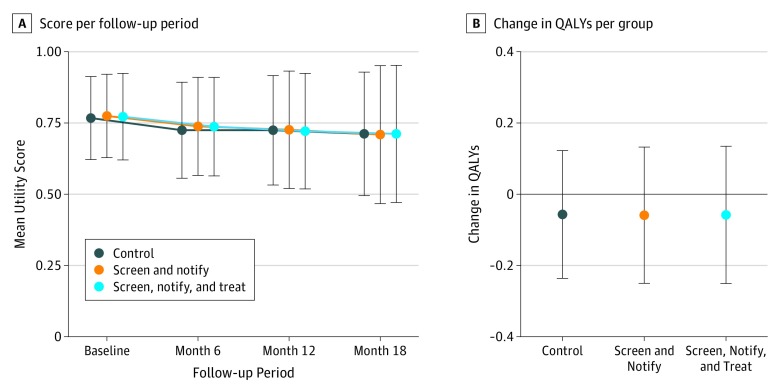
There were no significant differences in the change in mean (SD) QALYs between the 3 groups (screen, notify, and treat, −0.06 [0.20]; screen and notify, −0.06 [0.20]; no screen, −0.06 [0.18]; P = .98) (Figure 2; eTable 1 in Supplement 2). Quality-adjusted life-years declined modestly between baseline and 18 months in all 3 groups. Results remained unchanged when conducting sensitivity analyses in which worst outcome imputation and last observation carried forward were used to impute missing data. There were no differences in outcomes when analyses were stratified by sex (eTable 2 in Supplement 2).
Figure 2. Quality-of-Life Utility Score and Change in Quality-Adjusted Life-Years (QALYs) From Baseline to 18 Months.
Error bars indicate SD.
When examining utility scores derived from quality-of-life scores at all 4 time points, utilities gradually declined from baseline to 18 months (Figure 2; eTable 1 in Supplement 2). A similar pattern was observed in all 3 groups. Using a mixed model (secondary analysis), we found no differences in change in utility scores from baseline to 18 months among the 3 groups.
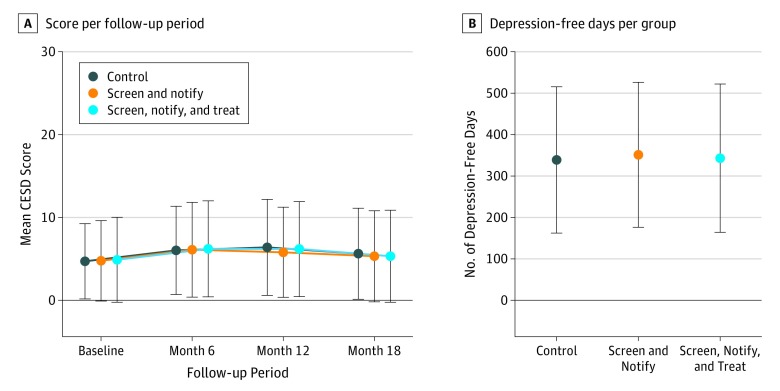
Change in Depression-Free Days and Depressive Symptoms
There were no differences between the groups in cumulative mean (SD) depression-free days (screen, notify, and treat, 343.1 [179.0] days; screen and notify, 351.3 [175.0] days; no screen, 339.0 [176.6] days; P = .63) or change in CESD-10 depressive symptoms from baseline to 18 months (Figure 3). There were also no differences in depressive symptoms as measured by the mean (SD) PHQ-8 score at 18 months (screen, notify, and treat, 3.63 [4.40]; screen and notify, 3.60 [4.14]; no screen, 3.69 [4.21]; P = .99). There were also no differences in depression-free days or depressive symptoms when analyses were stratified by sex (eTable 2 in Supplement 2). At follow-up visits, there were no differences in the percentage of patients who reported taking antidepressant medication or seeing a mental health clinician (eTable 3 in Supplement 2).
Figure 3. Depression-Free Days and Depressive Symptoms From Baseline to 18 Months.
Error bars indicate SD. CESD indicates 10-item Center for Epidemiologic Studies Depression scale.
Harms of Depression Screening
There were no differences in mortality between the 3 groups, with 67 patients (4.5%) confirmed deceased (Figure 1). There were also no differences in patient-reported harms potentially attributable to the use of antidepressant medications (Table 2).
Table 2. Harms Associated With Depression Screening From 6 to 18 Months.
| Outcome | Patients, No./Total No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At 6 mo | At 12 mo | At 18 mo | ||||||||||
| Screen, Notify, and Treat | Screen and Notify | Control | P Value | Screen, Notify, and Treat | Screen and Notify | Control | P Value | Screen, Notify, and Treat | Screen and Notify | Control | P Value | |
| Any bleeding | 61/450 (13.6) | 51/455 (11.2) | 72/457 (15.8) | .13 | 51/423 (12.1) | 50/429 (11.7) | 50/427 (11.7) | .98 | 40/394 (10.2) | 45/410 (11.0) | 40/419 (9.5) | .79 |
| Increased appetite | 83/450 (18.4) | 84/455 (18.5) | 91/457 (19.9) | .81 | 71/423 (16.8) | 77/431 (17.9) | 76/427 (17.8) | .90 | 76/394 (19.3) | 62/410 (15.1) | 60/418 (14.4) | .12 |
| Decreased appetite | 72/450 (16.0) | 74/455 (16.3) | 76/457 (16.6) | .97 | 63/423 (14.9) | 65/431 (15.1) | 76/427 (17.8) | .43 | 53/394 (13.5) | 45/410 (11.0) | 62/419 (14.8) | .26 |
| Drowsiness | 218/450 (48.4) | 212/455 (46.6) | 217/458 (47.4) | .86 | 179/423 (42.3) | 187/431 (43.4) | 198/427 (46.4) | .47 | 152/394 (38.6) | 177/410 (43.2) | 171/419 (40.8) | .42 |
| Gastrointestinal upset | 128/450 (28.4) | 116/454 (25.6) | 112/457 (24.5) | .38 | 95/423 (22.5) | 95/431 (22.0) | 107/427 (25.1) | .53 | 98/394 (24.9) | 99/410 (24.1) | 100/418 (23.9) | .95 |
Discussion
In this 3-group, depression screening randomized clinical trial, we found no difference in change in QALYs among patients with ACS who were randomized to depression screening and notification with or without provision of enhanced depression care compared with a control group that did not undergo depression screening. There was also no evidence for differences in depressive symptoms, depression-free days, or patient-reported harms of screening among the 3 groups.
There is compelling evidence that depression is a cardiotoxic risk marker in patients with ACS, strongly and consistently associated with health-related quality of life, independent of traditional factors associated with quality of life.44 For example, patients with a history of depression have twice the rate of angina, triple the reported physical limitations, and almost triple the risk of diminished health-related quality of life after ACS.45 Of multiple factors associated with 1-year quality of life in a study of patients with myocardial infarction that included sociodemographic variables, severity of disease, and other factors, depression was the most substantial factor.46 Despite the risk associated with depression, our data suggest that a strategy of systematically screening patients with ACS for depression is unlikely to lead to substantial population-level benefits in terms of improved quality of life or depression-free days.
There are several possible explanations as to why depression screening did not improve quality of life or depressive symptoms. To begin with, a smaller than expected proportion of study patients had positive screening results for depression. Our study intentionally excluded individuals with a history of depression, even if they were not recently or currently being treated for depression. As depression is a relapsing and remitting disorder that is often undertreated even when it is recognized, it is possible that expanding depression assessments to encompass patients with ACS with a history of depression could case-find additional patients with ACS who could benefit from enhanced depression treatment. Nearly 25% of those who had positive screening results in the screen, notify, and treat group declined the enhanced depression care treatment, which may have limited the potential for this screening approach to have an effect on quality of life and depression. It is possible that, even among those who initiated depression treatment, those who were identified as depressed by the screening may have been less motivated or interested in treatment and, in turn, less engaged, limiting treatment effectiveness. Overall, by 6 months, there were no differences in the percentage of patients in each group who had received depression treatment. Future studies could investigate if motivation, interest, adherence, and/or engagement differ between patients with depression detected by screening and patients with depression who are seeking treatment.
Currently, multiple professional societies recommend depression screening in patients with ACS. These advisories are based on observational evidence that depression is prevalent for patient with ACS3 and is associated with worse morbidity and mortality outcomes,4,46,47 worse quality-of-life outcomes,6 and greater health care costs.7 Yet, evidence-based guidelines also rate the strength of evidence for clinical recommendations, with the strongest recommendations reserved for meta-analyses of randomized clinical trials. Our study provides a single, well-conducted randomized clinical trial that provides evidence against depression screening in patients with ACS. Our findings suggest that existing guidelines advising routine depression screening in patients with ACS may need to be reconsidered.
Our findings were consistent with recent research on the effect of depression screening in other contexts. A cluster randomized clinical trial in England evaluated the effect of a point-of-care electronic prompt for general practitioners to screen for depression, anxiety, and pain vs for pain alone among patients with osteoarthritis.48 The electronic prompt did not result in lower depressive symptoms. From 2006 to 2013, the United Kingdom Quality and Outcomes Framework financially incentivized systematic depression screening of primary care patients with coronary heart disease or diabetes.49,50 It was estimated that 976 patients had to be screened for each new diagnosis of depression and 687 patients screened for each new antidepressant prescription. Based on this low yield, depression screening was discontinued as a quality indicator in the United Kingdom.
Strengths and Limitations
There were many strengths of our study, including successful accrual of patients across multiple health care settings and representation of socioeconomically diverse patients with ACS in the sample. There were also limitations. It is possible that patients assigned to the no screen group became more aware of depressive symptoms through participation in the study, which, in turn, led to increased depression recognition and treatment in this group. There have been public health efforts to increase screening for depression in primary care settings, which may have led to smaller differences in depression screening between the screened and no screen groups. Several patients with depression in the screen, notify, and treat group declined the remotely delivered depression treatment provided by the study and reported pursuing treatment from their usual care providers. It is possible that a higher proportion would have agreed to the study depression treatment if it were better integrated into their usual clinical care.51 Nevertheless, given the low prevalence of positive results of the depression screening and modest effectiveness of depression treatments, better integration would be unlikely to substantially affect change in QALYs associated with screen, notify, and treat. Approximately half of the patients approached declined to enroll in the study. The applicability of this study to depression screening conducted outside the context of a trial may be different.
Conclusions
Providing universal depression screening and notifying treating clinicians of positive results of screening either with or without provision of enhanced depression care did not substantially alter quality of life, depression-free days, depressive symptoms, mortality, or patient-reported harms in patients with ACS.
Trial Protocol
eTable 1. Utility Scores and Depressive Symptoms from Baseline to 18 Months
eTable 2. Sex-Specific Analyses of Key Outcomes of CODIACS-QOL Trial
eTable 3. Patient-Reported Receipt of Mental Health Treatment Since the Prior Study Visit From 6 Months to 18 Months
Data Sharing Statement
References
- 1.Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. ; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association . Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768-1775. doi: 10.1161/CIRCULATIONAHA.108.190769 [DOI] [PubMed] [Google Scholar]
- 2.Thombs BD, Bass EB, Ford DE, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21(1):30-38. doi: 10.1111/j.1525-1497.2005.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieuwsma JA, Williams JW Jr, Namdari N, et al. Diagnostic accuracy of screening tests and treatment for post–acute coronary syndrome depression: a systematic review. Ann Intern Med. 2017;167(10):725-735. doi: 10.7326/M17-1811 [DOI] [PubMed] [Google Scholar]
- 4.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763-2774. doi: 10.1093/eurheartj/ehl338 [DOI] [PubMed] [Google Scholar]
- 5.Lichtman JH, Froelicher ES, Blumenthal JA, et al. ; American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing . Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350-1369. doi: 10.1161/CIR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 6.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study [see comment]. JAMA. 2003;290(2):215-221. doi: 10.1001/jama.290.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutledge T, Vaccarino V, Johnson BD, et al. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2009;53(2):176-183. doi: 10.1016/j.jacc.2008.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosca L, Banka CL, Benjamin EJ, et al. ; Expert Panel/Writing Group; American Academy of Physician Assistants; American Association for Clinical Chemistry; American Association of Cardiovascular and Pulmonary Rehabilitation; American College of Chest Physicians; American College of Emergency Physicians; American Diabetes Association; American Geriatrics Society; American Society for Preventive Cardiology; American Society of Echocardiography; American Society of Nuclear Cardiology; Association of Women’s Health, Obstetric and Neonatal Nurses; Global Alliance for Women’s Health; Mended Hearts, Inc; National Black Nurses Association; National Black Women’s Health Imperative; National Women’s Health Resource Center; North American Menopause Society; Partnership for Gender-Specific Medicine at Columbia University; Preventive Cardiovascular Nurses Association; Society for Vascular Medicine and Biology; Society for Women’s Health Research; Society of Geriatric Cardiology; Women in Thoracic Surgery; WomenHeart: the National Coalition for Women with Heart Disease . Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49(11):1230-1250. doi: 10.1016/j.jacc.2007.02.020 [DOI] [PubMed] [Google Scholar]
- 9.Post-Myocardial Infarction Depression Clinical Practice Guideline Panel AAFP guideline for the detection and management of post–myocardial infarction depression. Ann Fam Med. 2009;7(1):71-79. doi: 10.1370/afm.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham I, Atar D, Borch-Johnsen K, et al. ; European Society of Cardiology (ESC) Committee for Practice Guidelines (CPG) . European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2007;28(19):2375-2414. doi: 10.1093/eurheartj/ehm316 [DOI] [PubMed] [Google Scholar]
- 11.National Collaborating Centre for Mental Health (UK) Depression in Adults With Chronic Physical Health Problems. London, UK: National Institute for Health and Clinical Excellence; 2009. [Google Scholar]
- 12.Smith SC Jr, Benjamin EJ, Bonow RO, et al. ; World Heart Federation and the Preventive Cardiovascular Nurses Association . AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-2473. doi: 10.1161/CIR.0b013e318235eb4d [DOI] [PubMed] [Google Scholar]
- 13.Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease: never mind. J Am Coll Cardiol. 2009;54(10):886-890. doi: 10.1016/j.jacc.2009.01.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300(18):2161-2171. doi: 10.1001/jama.2008.667 [DOI] [PubMed] [Google Scholar]
- 15.Thombs BD, Roseman M, Coyne JC, et al. Does evidence support the American Heart Association’s recommendation to screen patients for depression in cardiovascular care? an updated systematic review. PLoS One. 2013;8(1):e52654. doi: 10.1371/journal.pone.0052654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smolderen KG, Spertus JA, Reid KJ, et al. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2(4):328-337. doi: 10.1161/CIRCOUTCOMES.109.868588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolderen KG, Buchanan DM, Amin AA, et al. Real-world lessons from the implementation of a depression screening protocol in acute myocardial infarction patients: implications for the American Heart Association depression screening advisory. Circ Cardiovasc Qual Outcomes. 2011;4(3):283-292. doi: 10.1161/CIRCOUTCOMES.110.960013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thombs BD, de Jonge P, Ziegelstein RC. Depression screening in patients with heart disease—reply. JAMA. 2009;301(13):1338. doi: 10.1001/jama.2009.409 [DOI] [PubMed] [Google Scholar]
- 19.Hasnain M, Vieweg WVR, Lesnefsky EJ, Pandurangi AK. Depression screening in patients with coronary heart disease: a critical evaluation of the AHA guidelines. J Psychosom Res. 2011;71(1):6-12. doi: 10.1016/j.jpsychores.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 20.Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JP. There are no randomized controlled trials that support the United States Preventive Services Task Force Guideline on screening for depression in primary care: a systematic review. BMC Med. 2014;12(1):13. doi: 10.1186/1741-7015-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laporte S, Chapelle C, Caillet P, et al. Bleeding risk under selective serotonin reuptake inhibitor (SSRI) antidepressants: a meta-analysis of observational studies. Pharmacol Res. 2017;118:19-32. doi: 10.1016/j.phrs.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 22.Rieckmann N, Kronish IM, Shapiro PA, Whang W, Davidson KW. Serotonin reuptake inhibitor use, depression, and long-term outcomes after an acute coronary syndrome: a prospective cohort study. JAMA Intern Med. 2013;173(12):1150-1151. doi: 10.1001/jamainternmed.2013.910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2010;170(7):600-608. doi: 10.1001/archinternmed.2010.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson KW, Bigger JT, Burg MM, et al. Centralized, stepped, patient preference–based treatment for patients with post-acute coronary syndrome depression: CODIACS vanguard randomized controlled trial. JAMA Intern Med. 2013;173(11):997-1004. doi: 10.1001/jamainternmed.2013.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moise N, Davidson KW, Cheung YKK, et al. Rationale, design, and baseline data for a multicenter randomized clinical trial comparing depression screening strategies after acute coronary syndrome: the Comparison of Depression Identification after Acute Coronary Syndromes-Quality of Life and Cost Outcomes (CODIACS-QOL) trial. Contemp Clin Trials. 2019;84:105826. doi: 10.1016/j.cct.2019.105826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luepker RV, Apple FS, Christenson RH, et al. ; AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543-2549. doi: 10.1161/01.CIR.0000100560.46946.EA [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 28.Whang W, Burg MM, Carney RM, et al. Design and baseline data from the vanguard of the Comparison of Depression Interventions after Acute Coronary Syndrome (CODIACS) randomized controlled trial. Contemp Clin Trials. 2012;33(5):1003-1010. doi: 10.1016/j.cct.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813. doi: 10.1097/01.psy.0000146332.53619.b2 [DOI] [PubMed] [Google Scholar]
- 30.Ware J, Kolinski M, Keller S. How to Score the SF-12 Physical and Mental Health Summaries: A User’s Manual. Boston, MA: The Health Institute, New England Medical Centre; 1995. [Google Scholar]
- 31.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851-859. doi: 10.1097/01.mlr.0000135827.18610.0d [DOI] [PubMed] [Google Scholar]
- 32.De Smedt D, Clays E, Annemans L, De Bacquer D. EQ-5D versus SF-12 in coronary patients: are they interchangeable? Value Health. 2014;17(1):84-89. doi: 10.1016/j.jval.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19(2):179-186. doi: 10.1093/oxfordjournals.pubmed.a024606 [DOI] [PubMed] [Google Scholar]
- 34.Ware J Jr, Kosinski M, Keller SDA. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Han Y, Zhao FL, Zhou J, Chen Z, Sun H. Validation and comparison of EuroQoL-5 dimension (EQ-5D) and Short Form-6 Dimension (SF-6D) among stable angina patients. Health Qual Life Outcomes. 2014;12:156. doi: 10.1186/s12955-014-0156-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grochtdreis T, Brettschneider C, Wegener A, et al. Cost-effectiveness of collaborative care for the treatment of depressive disorders in primary care: a systematic review. PLoS One. 2015;10(5):e0123078. doi: 10.1371/journal.pone.0123078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 38.Vannoy SD, Arean P, Unützer J. Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatr Serv. 2010;61(2):160-163. doi: 10.1176/ps.2010.61.2.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung YK. Sequential implementation of stepwise procedures for identifying the maximum tolerated dose. J Am Stat Assoc. 2007;102(480):1448-1461. doi: 10.1198/016214507000000699 [DOI] [Google Scholar]
- 40.Hochberg Y, Tamhane AC, eds. Multiple Comparison Procedures. New York, NY: John Wiley & Sons, Inc; 1987. doi: 10.1002/9780470316672 [DOI] [Google Scholar]
- 41.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Vol 81 New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 42.Davis JC, Marra CA, Najafzadeh M, Liu-Ambrose T. The independent contribution of executive functions to health related quality of life in older women. BMC Geriatr. 2010;10(1):16. doi: 10.1186/1471-2318-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40-48. doi: 10.1016/S0140-6736(08)60991-5 [DOI] [PubMed] [Google Scholar]
- 44.Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. J Psychosom Res. 2007;62(4):401-410. doi: 10.1016/j.jpsychores.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 45.Rumsfeld JS, Magid DJ, Plomondon ME, et al. History of depression, angina, and quality of life after acute coronary syndromes. Am Heart J. 2003;145(3):493-499. doi: 10.1067/mhj.2003.177 [DOI] [PubMed] [Google Scholar]
- 46.Lane D, Carroll D, Ring C, Beevers DG, Lip GY. Mortality and quality of life 12 months after myocardial infarction: effects of depression and anxiety. Psychosom Med. 2001;63(2):221-230. doi: 10.1097/00006842-200103000-00005 [DOI] [PubMed] [Google Scholar]
- 47.Frasure-Smith N, Lespérance F. Reflections on depression as a cardiac risk factor. Psychosom Med. 2005;67(suppl 1):S19-S25. doi: 10.1097/01.psy.0000162253.07959.db [DOI] [PubMed] [Google Scholar]
- 48.Mallen CD, Nicholl BI, Lewis M, et al. The effects of implementing a point-of-care electronic template to prompt routine anxiety and depression screening in patients consulting for osteoarthritis (the Primary Care Osteoarthritis Trial): a cluster randomised trial in primary care. PLoS Med. 2017;14(4):e1002273. doi: 10.1371/journal.pmed.1002273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton C, Simpson C, Anderson N. Diagnosis and treatment of depression following routine screening in patients with coronary heart disease or diabetes: a database cohort study. Psychol Med. 2013;43(3):529-537. doi: 10.1017/S0033291712001481 [DOI] [PubMed] [Google Scholar]
- 50.Thombs BD, Ziegelstein RC. Primary care doctors should not screen their patients for depression. Expert Rev Neurother. 2017;17(7):645-647. doi: 10.1080/14737175.2017.1327356 [DOI] [PubMed] [Google Scholar]
- 51.Moise N, Falzon L, Obi M, et al. Interventions to increase depression treatment initiation in primary care patients: a systematic review. J Gen Intern Med. 2018;33(11):1978-1989. doi: 10.1007/s11606-018-4554-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Utility Scores and Depressive Symptoms from Baseline to 18 Months
eTable 2. Sex-Specific Analyses of Key Outcomes of CODIACS-QOL Trial
eTable 3. Patient-Reported Receipt of Mental Health Treatment Since the Prior Study Visit From 6 Months to 18 Months
Data Sharing Statement