Key Points
Question
Does fluoxetine compared with placebo reduce obsessive-compulsive behaviors among children and adolescents with autism spectrum disorders?
Findings
In this randomized clinical trial that involved 146 participants, mean scores for obsessive-compulsive behaviors at 16 weeks (as measured by the Children’s Yale-Brown Obsessive Compulsive Scale–Modified for Pervasive Developmental Disorders; range, 0-20 points; minimal clinically important difference, 2 points) were 9.02 points in the fluoxetine group and 10.89 points in the placebo group, a difference that was statistically significant. However, prespecified analyses that accounted for potentially confounding factors and baseline imbalances were null, with CIs that included the minimal clinically important difference.
Meaning
In this preliminary study of children and adolescents with autism spectrum disorders, treatment with fluoxetine resulted in significantly lower scores for obsessive-compulsive behaviors at 16 weeks, although interpretation is limited by null findings and imprecise estimates from prespecified analyses that accounted for confounding factors and baseline imbalances.
Abstract
Importance
Selective serotonin receptor inhibitors are prescribed to reduce the severity of core behaviors of autism spectrum disorders, but their efficacy remains uncertain.
Objective
To determine the efficacy of fluoxetine for reducing the frequency and severity of obsessive-compulsive behaviors in autism spectrum disorders.
Design, Setting, and Participants
Multicenter, randomized, placebo-controlled clinical trial. Participants aged 7.5-18 years with autism spectrum disorders and a total score of 6 or higher on the Children’s Yale-Brown Obsessive Compulsive Scale, modified for pervasive developmental disorder (CYBOCS-PDD) were recruited from 3 tertiary health centers across Australia. Enrollment began November 2010 and ended April 2017. Follow-up ended August 2017.
Interventions
Participants were randomized to receive fluoxetine (n = 75) or placebo (n = 71). Study medication was commenced at 4 or 8 mg/d for the first week, depending on weight, and then titrated to a maximum dose of 20 or 30 mg/d over 4 weeks. Treatment duration was 16 weeks.
Main Outcomes and Measures
The primary outcome was the total score on the CYBOCS-PDD (scores range from 0-20; higher scores indicate higher levels of maladaptive behaviors; minimal clinically important difference, 2 points) at 16 weeks postrandomization, analyzed with a linear regression model adjusted for stratification factors (site, age at baseline, and intellectual disability), with an additional prespecified model that included additional adjustment for baseline score, sex, communication level, and imbalanced baseline and demographic variables.
Results
Among the 146 participants who were randomized (85% males; mean age, 11.2 years), 109 completed the trial; 31 in the fluoxetine group and 21 in the placebo group dropped out or did not complete treatment. The mean CYBOCS-PDD score from baseline to 16 weeks decreased in the fluoxetine group from 12.80 to 9.02 points (3.72-point decrease; 95% CI, −4.85 to −2.60) and in the placebo group from 13.13 to 10.89 points (2.53-point decrease; 95% CI, −3.86 to −1.19). The between-group mean difference at 16 weeks was −2.01 (95% CI, −3.77 to −0.25; P = .03) (adjusted for stratification factors), and in the prespecified model with further adjustment, it was −1.17 (95% CI, −3.01 to 0.67; P = .21).
Conclusions and Relevance
In this preliminary study of children and adolescents with autism spectrum disorders, treatment with fluoxetine compared with placebo resulted in significantly lower scores for obsessive-compulsive behaviors at 16 weeks. Interpretation is limited by the high dropout rate, null findings of prespecified analyses that accounted for potentially confounding factors and baseline imbalances, and CIs for the treatment effect that included the minimal clinically important difference.
Trial Registration
anzctr.org.au Identifier: ACTRN12608000173392
This randomized clinical trial compares the effects of fluoxetine vs placebo for obsessive-compulsive behaviors in children and adolescents with autism spectrum disorders.
Introduction
Autism spectrum disorders (ASDs) are characterized by impairments in communication and social relatedness and restricted and repetitive interests and behaviors. In 2012, ASDs had a reported prevalence of 1.7% in the United States,1 and the lifelong effects of the condition constitute a significant health disability burden for individuals and their families.2
Restricted and repetitive behaviors frequently interfere with everyday functioning and include circumscribed preoccupations, ritualistic behaviors, stereotypic motor movements, repetitive nonfunctional use of objects, unusual sensory interests, and difficulty coping with change, which often manifests as anxiety, irritability, aggression, and self-injury.3
Published reports indicate that more than half of children and adolescents with ASDs are prescribed medication, with 21% to 32% receiving selective serotonin receptor inhibitors (SSRIs) despite inconclusive evidence for their efficacy.4,5,6
The efficacy of 4 SSRIs (fluoxetine, fluvoxamine, fenfluramine, and citalopram) for ASDs was examined in a Cochrane review.7 Nine randomized clinical trials involving 320 participants were evaluated, with 17 different outcome measures reported. Most data were unsuitable for meta-analysis, except for proportion improved on the Clinical Global Impression–Improvement scale, for 2 trials of fluoxetine and fluvoxamine in adults, which favored SSRIs over placebo (relative risk, 12.58; 95% CI, 1.77-89.33).8,9 One of these studies also showed improvements in measures of aggressive behavior, and another small study of adults showed improvements in anxiety.9,10 The largest high-quality trial in children showed no evidence of a positive effect of citalopram.11 The authors concluded there was no evidence of effectiveness of SSRIs for ASDs in children and limited evidence in adults.
The objective of the current study was to determine the efficacy for reducing the frequency and severity of restricted, repetitive, and stereotypic behaviors and tolerability of low-dose fluoxetine, a commonly prescribed SSRI agent, through a multicenter randomized clinical trial, the Fluoxetine for Autistic Behaviors study (FAB study).
Methods
The trial was approved by the human research ethics committees at each of the 3 sites. Written informed consent was provided by the parent or primary caregiver of each participant.
The trial design has previously been reported12 and the protocol is available in Supplement 1; the main details are described below. Participants were recruited through pediatricians, child and adolescent psychiatrists, psychologists, and general practitioners at 3 Australian sites: The Royal Children’s Hospital (Melbourne), The Sydney Children’s Hospitals Network, and the State Child Development Centre, Perth.
Eligibility Criteria for Participants
Children and adolescents eligible for the trial were aged 7.5 to 18 years, with a diagnosis of an ASD according to the Autism Diagnostic Interview Revised13 and the Diagnostic and Statistical Manual of Mental Disorders, which aggregates the former Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision conditions of autistic disorder, Asperger disorder, and pervasive developmental disorder, not otherwise specified.14 The eligibility age was originally 8 years but was reduced to 7.5 years with a protocol amendment during the trial to aid recruitment. Children and adolescents were required to have a total score of 6 or greater on the Children’s Yale-Brown Obsessive Compulsive Scale–Modified for pervasive developmental disorders (CYBOCS-PDD).15 Medication for attention-deficit/hyperactivity disorder was permitted.
Children and adolescents were excluded from the trial if they had a diagnosis of Rett syndrome, childhood disintegrative disorder, schizophrenia, or major depression; had previously received fluoxetine; or were currently prescribed or had received in the 6-week period before study entry other SSRIs, psychotropic medications (including typical and atypical antipsychotics, mood stabilizers, and anxiolytics), monoamine oxidase inhibitors or pimozide, antidepressants, or St John’s wort. Children with significant comorbid medical conditions were also excluded.
Randomization and Intervention
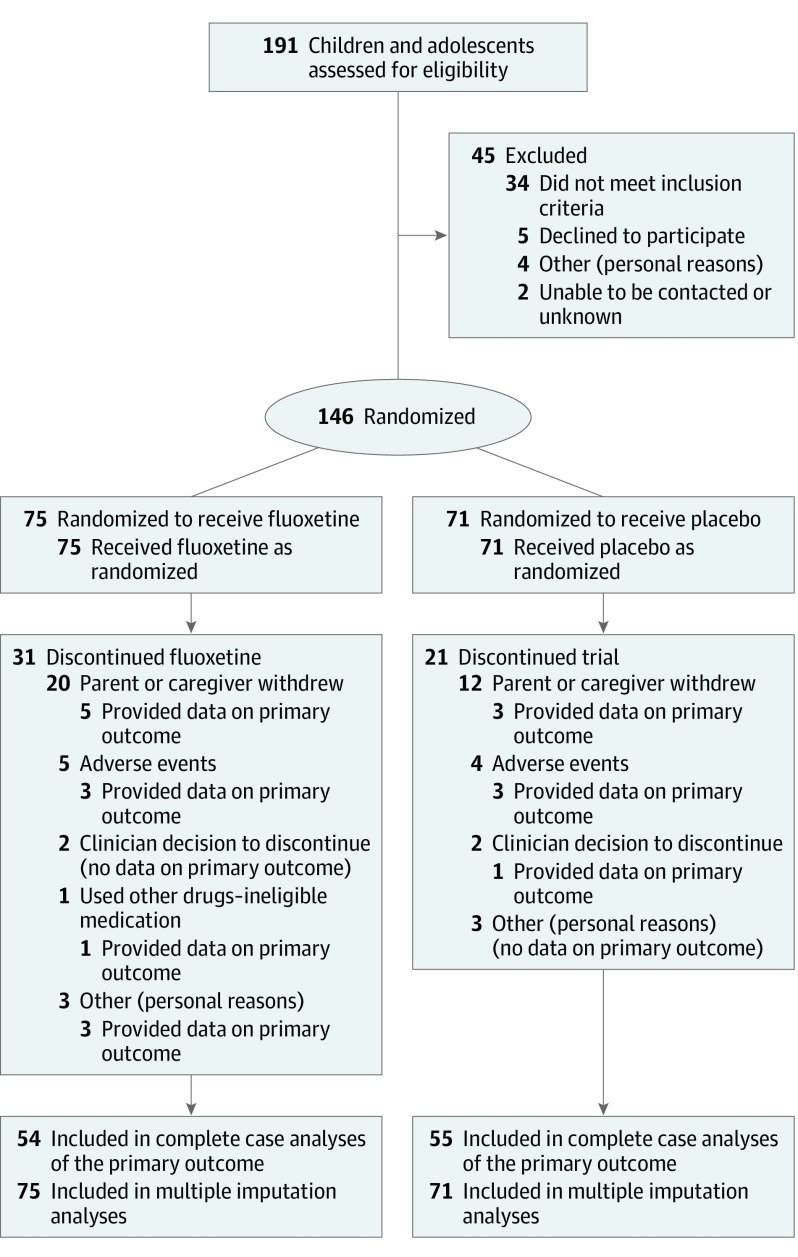
Potential participants were assessed for eligibility by their treating clinician. After consent, participants were randomized to receive fluoxetine (fluoxetine hydrochloride dissolved in Methocel [DuPont] base) or placebo (Methocel base only, which was indistinguishable from the active treatment) in a 1:1 ratio (Figure).
Figure. Trial Profile, Including Assessment, Randomization, and Follow-up Through the Study of Fluoxetine for Obsessive-Compulsive Behaviors in Children and Adolescents With Autism Spectrum Disorders.
An independent statistician created a computer-generated randomization schedule, using block randomization with randomly selected block sizes of 2 and 4, stratified by site, age (7.5 to < 12 years vs 12-18 years), and IQ (≥ 70 vs < 70), giving a total of 12 strata (4 per site). The randomization schedule was provided to the clinical trials pharmacist at each site, who arranged a sequential stock of trial medication for each stratum, labeled with only the study number, strata, and instructions for use. This schedule remained confidential throughout the study. The independent statistician retained a copy of the master randomization schedule to check for any discrepancies. Participants and their families, clinicians, and the research team assessing outcomes remained blind to the randomization schedule throughout the study.
Study medication was administered orally once daily in the morning and dosed according to the participant’s weight, commencing at 4 or 8 mg/d for the first week (4 mg if <40 kg; 8 mg if ≥40 kg) and then titrated weekly, over the subsequent 3 weeks, using a flexible titration schedule directed by a study physician. The maximum dose was 20 mg/d (participants <40 kg) or 30 mg/d (participants ≥40 kg). No further dose increases were made after week 4 of the trial. The dose was decreased (or the medication ceased) at any time during the trial if significant adverse effects occurred. Medication was given for a total of 16 weeks. At 16 weeks, participants had a follow-up assessment. The medication was then tapered under physician supervision during 4 weeks. Participants were involved in the trial for a total of 22 weeks.
Clinical follow-up with completion of scales for the primary and secondary outcomes was performed at baseline and 16 weeks. Telephone monitoring for adverse events occurred weekly during upward titration and weaning of the study medication and every 2 weeks during weeks 4 to 16. Each adverse event, time of onset, duration, severity, and relationship to treatment was established and recorded.
Primary Outcome Measure
The primary outcome was the difference between groups in the total score on the CYBOCS-PDD at 16 weeks. This scale comprises a detailed symptom checklist of possible obsessions and compulsions, rated from zero to 4 across 5 items, with a focus on stereotypic and complex behaviors typically associated with ASDs (time spent on obsessions, interference, distress, resistance, and degree of control).15,16 The total score can range from 0 to 20, with higher scores indicating higher levels of maladaptive behaviors. The CYBOCS-PDD has been widely used in clinical drug trials for ASDs11,17 and was selected in the current trial as the primary outcome in accordance with previous evidence in this population of sensitivity to change.8,9,10,11
Secondary Outcomes
Secondary outcomes were the difference between groups at 16 weeks in the following scales.
Repetitive Behavior Scale–Revised
This scale captures the breadth of repetitive behaviors in ASDs and consists of 6 subscales: stereotypic, self-injurious, compulsive, ritualistic, sameness, and restricted behaviors, which are summed to give a total score. Higher scores indicate higher severity. The scale has 2 scores: total number of items endorsed (range, 0-43) and total score (sum of 5 subscale scores: range, 0-129). The scale has been validated in both children and adults with ASDs.18
Spence Children’s Anxiety Scale
This 38-item parent-report checklist assesses symptoms and experiences of anxiety.19 It contains 6 subscales (panic/agoraphobia, social anxiety, separation anxiety, generalized anxiety, obsessions/compulsions, and fear of physical injury) and provides a total score (range, 0-114), with higher scores indicating greater severity. The validity and reliability of the Spence Children’s Anxiety Scale have been established for both anxiety-disordered and typically developing populations.20
Aberrant Behavior Checklist–Community Version
This 58-item rating scale measures inappropriate behaviors in individuals with developmental or intellectual impairments. Items are grouped into 5 subscales: irritability/agitation (range, 0-45), lethargy/social withdrawal (range, 0-48), stereotypic behavior (range, 0-21), hyperactivity/noncompliance (range, 0-48), and inappropriate speech (range, 0-12). Higher scores indicate higher severity of maladaptive behaviors.21 The Aberrant Behavior Checklist has been widely used in clinical drug trials in ASDs.11,17
Clinical Global Impression Scale–Global Improvement and Efficacy Index
This rating scale is widely used to assess treatment response in psychiatric conditions and in evaluating randomized clinical trials.22 It is a 3-item scale (severity of illness [7-point scale] and global improvement [7-point scale], with the final scale [efficacy index] rated on the basis of the drug effect only) and is scored along a 4 × 4 matrix, with one axis representing therapeutic effect (unchanged to marked effect) and the other representing adverse effects (none to outweighs therapeutic effect). Lower scores are associated with improvement and higher ones are associated with worsening of the condition. It has been widely used in clinical drug trials in autism and has been deemed to be sensitive to change, capturing improvements in a range of behavioral disorders.8,9,10,11,17
Disruptiveness Assessment
This assessment collects information about anger/aggression, anxiety, sadness/depression, excitability/overexcitement, and attention-deficit/hyperactivity disorder, each of which is coded as 0 = not a problem, 1 = some problem, or 2 = significant problem. The outcome of interest is the total score of disruptiveness, which is a sum of the 5 scores (range, 0-10). Because there was no suitable measure for disruptiveness in the literature, this assessment was formulated specifically for this study, after the study protocol was finalized.
Adverse Events
The frequency and type of adverse events occurring during the 16 weeks, the 4 weeks of weaning, and 2 weeks postcompletion were assessed.
Sample Size
The validation of the CYBOCS-PDD in 172 medication-free children with an ASD found a mean total score of 14.4, with a standard deviation of 3.86.17 A difference of 2 on the CYBOCS-PDD, based on investigator experience, was considered to represent a clinically important improvement in repetitive behaviors. Consequently, this study was powered to find an effect size of 0.5 (corresponding to a difference of 2 on the CYBOCS, based on a standard deviation of 3.9). With 80% power and a 2-sided α of .05, this required a sample size of 64 per treatment group. Allowing for 15% dropout, we therefore needed to recruit 73 participants per treatment group, 146 participants in total.
Statistical Methods
Participants were retained in their originally assigned treatment group for analysis, which was conducted only for those with available outcome data. Baseline characteristics are presented separately for children in the fluoxetine and placebo groups, with means and standard deviations for continuous data (or medians and interquartile ranges for nonnormal data) and proportions for categorical data. The primary outcome (total score on the CYBOCS-PDD at 16 weeks) was compared for the fluoxetine and placebo groups with linear regression adjusted for the stratification factors (site, age, and intellectual disability), with results presented as a mean difference, its 95% CI, and a (2-sided) P value, fitted to all participants with outcome data available (primary analysis). Results were deemed statistically significant if P < .05. An additional prespecified analysis of the primary outcome involved rerunning the same linear regression model with additional adjustment for the following potentially confounding factors: CYBOCS-PDD total score at baseline, sex, communication level (verbal vs nonverbal), and baseline and demographic variables for which an imbalance between groups was found (secondary analysis). Two additional factors were prespecified but not adjusted for because of low numbers (frequency of seizures and stimulant medication at baseline). Only 2 participants in the fluoxetine group and 3 in the placebo group had epilepsy, with frequency less than 1 time per year in 4 participants and unknown in the fifth participant, and only 2 participants in the fluoxetine group and none in the placebo group were receiving stimulant medication at baseline, with some missing data.
Secondary outcomes were compared with linear regression adjusted for stratification factors, except for adverse events, which are reported descriptively. It was anticipated that the Spence Children’s Anxiety Scale might be affected by the participant communication level, so this outcome was also analyzed for verbal and nonverbal children separately. As a sensitivity analysis on secondary outcomes, regression models were repeated adjusted for sex, whether verbal or nonverbal, the baseline score on the questionnaire of interest, and baseline variables in which an imbalance was found (secondary analysis). Further details are available in the statistical analysis plan in Supplement 2. In accordance with the preplanned statistical analysis plan, all analyses were conducted with complete case analysis, but a sensitivity analysis was also conducted with multiple imputation to handle the missing data. Multiple imputation was conducted separately in the 2 treatment groups, using chained equations applied to all outcomes (only total scores for the outcomes with subscales and total scores available) simultaneously, including baseline measures as auxiliary variables. Fifty imputed data sets were generated. Because of the number of outcomes considered, analyses of secondary end points should be regarded as exploratory. All analyses were conducted with Stata version 15.23
Results
There were 146 children and adolescents with ASDs who were enrolled in the trial during 7 years (75 fluoxetine, 71 placebo). The mean age of participating children was 11.2 years (SD, 2.9 years), 85% were male children and adolescents, and 30% had an intellectual disability. Some differences in the baseline characteristics between the fluoxetine and placebo groups were noted: in the fluoxetine group, there was a slightly higher proportion of male children and adolescents and participants with a family history of ASDs (Table 1). The placebo group had higher scores on the Repetitive Behavior Scale–Revised and the Aberrant Behavior Checklist lethargy scale than the fluoxetine groups, by 9.11 units and 4.23 units, respectively (Table 2). Forty-one percent of participants in the fluoxetine group and 30% in the placebo group did not complete the treatment in accordance with protocol; reasons for treatment discontinuation included parent/caregiver decision to drop out of the study (20 fluoxetine, 12 placebo), adverse events (5 fluoxetine, 4 placebo), clinician decision to discontinue treatment (2 fluoxetine, 2 placebo), commencement of study-excluded medication (1 fluoxetine), and a small number with other personal reasons (3 fluoxetine, 3 placebo).
Table 1. Summary of Baseline Characteristics by Treatment Group.
| No. (%) | ||
|---|---|---|
| Fluoxetine (n = 75) | Placebo (n = 71) | |
| Site | ||
| Royal Children’s Hospital | 19 (25) | 17 (24) |
| State Child Development Centre | 8 (11) | 9 (13) |
| Westmead | 48 (64) | 45 (63) |
| Age stratification, y | ||
| 13-18 | 27 (36) | 25 (35) |
| 7.5-12 | 48 (64) | 46 (65) |
| Demographics | ||
| Age at consent, mean (SD), y | 11.3 (2.9) | 11.0 (2.8) |
| Height with no shoes, mean (SD) [No.], cm | 148.0 (17.1) [60] | 146.6 (15.3) [67] |
| Weight with no shoes, mean (SD) [No.], kg | 42.4 (16.4) [75] | 42.9 (16.6) [70] |
| Sex | ||
| Boys | 69 (92) | 55 (78) |
| Girls | 6 (8) | 16 (23) |
| Autism History | ||
| Primary diagnosis | ||
| Autism | 32 (43) | 33 (47) |
| Asperger syndrome | 12 (16) | 9 (13) |
| ASD | 31 (41) | 29 (41) |
| Intellectual disability | 23 (31) | 21 (30) |
| Communication level, No. | 75 | 70 |
| Normal language for age | 49 (65) | 48 (69) |
| Impaired speech and language | 22 (29) | 17 (24) |
| Has no speech | 4 (5) | 5 (7) |
| Current medications (including alternative therapies)a | 33 (44) | 29 (41) |
| Receiving stimulant medications at baseline | 2 (3) | 0 |
| Family history of ASD, No. | 75 | 70 |
| No | 35 (47) | 42 (60) |
| Yes | 28 (37) | 22 (31) |
| Uncertain | 12 (16) | 6 (9) |
| History of seizures, No. | 75 | 70 |
| No | 73 (97) | 67 (96) |
| Yes | 2 (3) | 3 (4) |
Abbreviation: ASD, autism spectrum disorder.
Current medications included antiasthma medications, laxatives, and vitamins.
Table 2. Comparison of Outcomes at 16 Weeks With a Complete Case Analysis.
| Mean (SD) [No.] | Primary Analysisa | Secondary Analysisb | ||||||
|---|---|---|---|---|---|---|---|---|
| Fluoxetine | Placebo | |||||||
| Baseline | 16 Weeks | Baseline | 16 Weeks | Mean Difference (95% CI) | P Value | Mean Difference (95% CI) | P Value | |
| CYBOCS-PDDc | 12.80 (3.41) [75] | 9.02 (4.84) [54] | 13.13 (3.36) [71] | 10.89 (4.92) [55] | −2.01 (−3.77 to −0.25) | .03 | −1.17 (−3.01 to 0.67) | .21 |
| RBSd | ||||||||
| Total items | 20.75 (9.67) [75] | 16.89 (9.35) [53] | 24.08 (10.01) [71] | 21.64 (11.69) [53] | −5.06 (−9.15 to −0.97) | .02 | −1.01 (−4.83 to 2.81) | .60 |
| Total score | 36.79 (22.86) [75] | 27.09 (21.60) [54] | 45.90 (24.26) [71] | 36.40 (24.61) [53] | −9.93 (−18.74 to −1.12) | .03 | −1.64 (−9.69 to 6.42) | .69 |
| Spence Children’s Anxiety totale | 31.24 (21.72) [74] | 20.58 (18.17) [53] | 32.17 (20.15) [71] | 24.70 (17.11) [53] | −4.04 (−10.75 to 2.66) | .23 | −2.86 (−7.94 to 2.21) | .27 |
| Verbal subgroupf | 32.59 (21.55) [70] | 21.76 (18.40) [49] | 33.63 (20.18) [65] | 24.08 (17.04) [50] | −2.35 (−9.39 to 4.69) | .51 | −2.37 (−7.63 to 2.88) | .37 |
| ABCg | ||||||||
| I, irritability | 18.57 (10.00) [75] | 12.28 (9.19) [53] | 17.87 (11.80) [71] | 13.34 (11.29) [53] | −1.54 (−5.47 to 2.39) | .44 | −0.39 (−3.87 to 3.09) | .82 |
| II, lethargy | 12.57 (8.03) [75] | 9.49 (6.77) [53] | 16.80 (10.60) [71] | 13.85 (9.32) [53] | −4.41 (−7.61 to −1.21) | .007 | −0.67 (−3.69 to 2.35) | .66 |
| III, stereotypy | 6.05 (4.91) [75] | 5.08 (4.50) [53] | 7.20 (5.63) [71] | 5.08 (4.57) [53] | −0.21 (−1.85 to 1.42) | .80 | 0.62 (−0.99 to 2.22) | .45 |
| IV, hyperactivity | 21.71 (11.11) [75] | 16.21 (10.48) [53] | 21.28 (11.51) [71] | 17.60 (12.52) [53] | −2.09 (−6.38 to 2.21) | .34 | −0.92 (−4.70 to 2.86) | .63 |
| V, inappropriate speech | 4.48 (3.65) [75] | 3.60 (3.18) [53] | 4.80 (3.37) [71] | 4.04 (3.44) [53] | −0.52 (−1.78 to 0.73) | .41 | 0.61 (−0.50 to 1.73) | .28 |
| CGIh | ||||||||
| Severity of illness | 3.25 (1.13) [55] | 3.26 (1.15) [53] | −0.09 (−0.45 to 0.26) | .60 | −0.03 (−0.41 to 0.35) | .88 | ||
| Global improvement | 3.22 (1.12) [55] | 3.38 (1.10) [53] | −0.18 (−0.61 to 0.24) | .40 | −0.18 (−0.66 to 0.30) | .46 | ||
| Efficacy index | 9.76 (3.97) [55] | 10.69 (3.17) [54] | −0.97 (−2.33 to 0.38) | .16 | −0.80 (−2.30 to 0.71) | .30 | ||
| Disruptiveness assessment, totali | 3.95 (2.24) [74] | 2.98 (2.14) [43] | 4.40 (2.16) [70] | 3.69 (2.09) [49] | −0.76 (−1.59 to 0.08) | .08 | −0.06 (−0.82 to 0.71) | .89 |
Abbreviations: ABC, Aberrant Behavior Checklist; CGI, Clinical Global Impression Scale; CYBOCS-PDD, Children’s Yale-Brown Obsessive Compulsive Scale–modified for pervasive developmental disorders; RBS, Repetitive Behavior Scale.
Results for the primary analysis are adjusted for site, age (8 to < 12 years and 12 to 18 years), and intellectual ability (IQ ≥ 70 and IQ < 70), as used in randomization.
Secondary analysis was in accordance with the primary analysis but also adjusted for sex, verbal vs nonverbal, baseline measure of the outcome of interest, baseline measures of RBS total items, RBS total score, and ABC II, lethargy.
CYBOCS-PDD range was 0 to 20, with higher scores indicating higher severity.
RBS total items range was 0 to 43; and total score range, 0 to 129. Higher scores indicate higher severity.
Range was 0 to 114, with higher scores indicating higher severity.
Because of the small sample in the nonverbal subgroup (9 participants at baseline, of whom 7 provided data at 16 weeks), the analysis of the Spence Children’s Anxiety Scale was conducted only on the verbal subgroup.
ABC irritability range was 0 to 45; lethargy/social withdrawal range, 0 to 48; stereotypic behavior range, 0 to 21; hyperactivity/noncompliance range, 0 to 48; and inappropriate speech range, 0 to 12. Higher scores indicate higher severity.
CGI severity of illness range was 0 to 7; global improvement range, 0 to 7; and efficacy index range, 1 to 16. Higher scores are associated with worsening of the condition.
Disruptiveness assessment: range was 0 to 10, with higher scores indicating greater severity.
Twenty-five percent of the participants did not provide data on the primary outcome (n = 21 and n = 16 in the fluoxetine and placebo groups, respectively), leaving 109 participants in the complete case analysis (Figure). In comparing the characteristics between individuals who completed the CYBOCS-PDD and those who did not, scores on the Repetitive Behavior Scale–Revised were slightly higher for those with outcome data (eTable 1 in Supplement 3), which suggested that the data were not missing completely at random.
Primary Outcome
Scores on the CYBOCS-PDD were lower at 16 weeks after fluoxetine than they were after placebo in the primary analysis (fluoxetine: baseline = 12.80, 16 weeks = 9.02; placebo: baseline = 13.13, 16 weeks = 10.89; mean difference at 16 weeks, −2.01; 95% CI, −3.77 to −0.25; P = .03) (Table 2). The strength of evidence for this difference weakened in the sensitivity analyses and the difference became nonsignificant when sex, verbal ability, baseline measure of the CYBOCS-PDD, and imbalances found at baseline in the Repetitive Behavior Scale total items and total score measures and the Aberrant Behavior Checklist lethargy subscale were taken into account (secondary analysis: mean difference, −1.17; 95% CI, −3.01 to 0.67; P = .21) (Table 2); and the same applies to the multiple imputation analysis (mean difference, −1.82; 95% CI, −3.71 to 0.06; P = .06) (eTable 2 in Supplement 3).
Secondary Outcomes
Although there was some evidence of a difference in the Repetitive Behavior Scale total items and total score measures and the Aberrant Behavior Checklist lethargy subscale in the primary analysis, there were no significant differences in the sensitivity analyses after adjustment for the baseline imbalances (Table 2, secondary analysis) and in the multiple imputation analyses (eTable 2, secondary analysis). There were no significant differences between fluoxetine and placebo in any other secondary outcome or analyses.
Adverse Events
Forty-five percent of the fluoxetine group and 42% of the placebo group experienced adverse events (Table 3; eTable3 in Supplement 3), most commonly mood disturbance, particularly irritability (9 fluoxetine, 12 placebo), gastrointestinal problems such as nausea and diarrhea (10 fluoxetine, 7 placebo), and sleep disorders (13 fluoxetine, 16 placebo). Two participants in the placebo group and none in the fluoxetine group experienced serious adverse events (suicidality).
Table 3. Summary of Adverse Events by Treatment Group.
| No. (%) | ||
|---|---|---|
| Fluoxetine (n = 75) | Placebo (n = 71) | |
| Participants with at least 1 AEa | 34 (45) | 30 (42.3) |
| Total No. of AEs | ||
| Mean (SD) | 2.5 (1.6) | 2.6 (2.1) |
| 1 | 14 (41) | 13 (43) |
| 2 | 3 (9) | 6 (20) |
| 3 | 7 (21) | 3 (10) |
| 4 | 7 (21) | 3 (10) |
| 5 | 1 (3) | 1 (3) |
| 6 | 2 (6) | 2 (7) |
| 7 | 0 | 1 (3) |
| 9 | 0 | 1 (3) |
Abbreviation: AE, adverse event.
Adverse events, assessed at 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 17, 18, 19, 20, and 22 weeks, were most commonly irritability, mood disturbance, nausea, vomiting, and sleep disorders.
Discussion
Among children and adolescents with ASDs, treatment with fluoxetine compared with placebo resulted in significant improvement in obsessive-compulsive behaviors at 16 weeks in the primary analysis. However, an additional prespecified analysis showed no significant difference between groups, which should potentially be given more weight because it controlled for baseline scores, correcting for the imbalance in mean baseline scores, and offered greater statistical power than the primary analysis model. Moreover, repeating the analyses with multiple imputation to handle the missing data, arguably a preferable analysis, also failed to show evidence of benefit of fluoxetine compared with placebo irrespective of adjustment for the baseline imbalance. Although cautious interpretation of the results from the primary analysis is warranted, all analyses of the primary outcome yielded 95% CIs that extended well above the minimum clinically important difference of 2 points, indicating that fluoxetine may reduce the frequency and severity of obsessive-compulsive behaviors in children and adolescents with ASDs. Given the large amount of missing data, the study may have been underpowered to detect the minimum clinically important difference of 2 points. There was little evidence of differences between the fluoxetine and placebo groups in any of the secondary outcomes.
The CYBOCS-PDD may not be the most valid measure of repetitive behaviors because they commonly occur in ASDs. This measure was selected as the primary outcome for the current trial in accordance with the findings of a previous trial of fluvoxamine in children with ASDs, which showed that the CYBOCS-PDD was sensitive to change across the treatment period.17 However, although the measure was not developed specifically for the ASD population, Scahill and colleagues24 have referenced the reliability and validity of this measure in ASD.
The rationale for the use of SSRIs is based on evidence that serotonin plays a contributory role in the pathophysiology of ASDs. There is converging support from genetic, biological, and neuroimaging studies indicating that individuals with ASDs have higher serotonin levels than those without such disorders.25 Depletion of the serotonin precursor tryptophan has also been shown to induce an increase in the severity of symptoms in adults with ASDs.26 Such a relationship was supported in a study of 18 children with ASDs that found a correlation between the SSRI fluvoxamine and the L variant genotype of the serotonin transporter gene (SLC6A4).27 However, a more recent study of escitalopram administered to 44 participants with ASDs did not observe this relationship.28 It is feasible that serotonin has an important role in ASDs, but the mechanism of reuptake inhibition is ineffective, supported by findings that drugs with serotonin antagonistic properties (eg, risperidone, aripiprazole) are effective in reducing the severity of some of the core behaviors associated with ASDs.29,30
The strengths of the current study include the randomized clinical trial design, the relatively large sample size, and the predefined statistical analyses. Low-dose fluoxetine was selected as recommended previously,7 given that it has a favorable safety profile. The maximum dose of 20 mg for children and adolescents weighing less than 30 kg and 30 mg for those weighing 40 kg or greater was chosen because both clinical experience and reports from the literature suggest that higher doses may be associated with an increased risk of behavioral activation, particularly agitation and aggression.31 Although the use of citalopram as in a previous randomized clinical trial was considered, it was reported to be associated with significant adverse events.10 Two-dose schedules were used in the current trial to account for differing participant weights, with dose escalation undertaken by a study physician experienced in ASDs. In addition, the current study focused on a clinically important outcome that affects quality of life: the frequency and severity of restricted, repetitive, and stereotypic behaviors. However, smaller studies of SSRIs have reported reductions in anxiety and aggressive behaviors.8,10 Also, participation was enabled by provision of free car parking, provision of most communication by telephone or e-mail, and coordination of clinical appointments with study visits. Future studies should use these strategies, but should also include families in study design and implementation, who will be helpful in providing advice about recruitment and retention. Investigation of higher doses of fluoxetine, subgroups of children, and other outcomes such as anxiety and aggression may be warranted.
Limitations
This study has several limitations. First, there was a chance baseline imbalance in some of the key behavioral measures of ASDs, indicating that the placebo group had a comparatively more severe behavioral phenotype than the fluoxetine group. This difference was accounted for in the sensitivity analyses, which provided important caveats to the positive treatment effect identified in the primary analysis. Second, recruitment and retention were difficult because many families did not want to hazard being randomized to the placebo group because fluoxetine was available off label from community pediatricians, making it difficult for parents to understand why they should participate in a trial. Once agreeing to participate, some families failed to complete the treatment and follow-up visits, resulting in moderately high dropout rates. Perceived failure of treatment; adverse events being attributed to the medication, prompting family withdrawal from the study; and difficulties in encouraging children to accept the medication contributed to the high dropout rate. Although more participants dropped out in the fluoxetine group, the adverse events were similar in the 2 groups. It is well known that maternal depression and family stress levels are high in this population and that families often have other priorities, adding to the difficulties of both recruitment and retention.
Conclusions
In this preliminary study of children and adolescents with autism spectrum disorders, treatment with fluoxetine compared with placebo resulted in significantly lower scores for obsessive-compulsive behaviors at 16 weeks. Interpretation is limited by the high dropout rate, null findings of prespecified analyses that accounted for potentially confounding factors and baseline imbalances, and CIs for the treatment effect that included the minimal clinically important difference.
Trial Protocol
Statistical Analysis Plan
eTable 1. Summary of baseline characteristics in those who did and did not complete the CYBOCS-PDD at 16 weeks by treatment group
eTable 2. Comparison of outcomes at 16 weeks between the treatment groups using multiple imputation to handle the missing data
eTable 3. Comparison of Repetitive Behavior Scale – Revised subscales and outcomes Spence Children’s Anxiety subscales at 16 weeks using a complete case analysis
eTable 4. Summary of adverse events by treatment group and category
Data Sharing Statement
References
- 1.Christensen DL, Braun KVN, Baio J, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2018;65(13):1-23. doi: 10.15585/mmwr.ss6513a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721-728. doi: 10.1001/jamapediatrics.2014.210 [DOI] [PubMed] [Google Scholar]
- 3.Soorya L, Kiarashi J, Hollander E. Psychopharmacologic interventions for repetitive behaviors in autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2008;17(4):753-771, viii. doi: 10.1016/j.chc.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 4.Aman MG, Lam KSL, Collier-Crespin A. Prevalence and patterns of use of psychoactive medicines among individuals with autism in the Autism Society of Ohio. J Autism Dev Disord. 2003;33(5):527-534. doi: 10.1023/A:1025883612879 [DOI] [PubMed] [Google Scholar]
- 5.Langworthy-Lam KS, Aman MG, Van Bourgondien ME. Prevalence and patterns of use of psychoactive medicines in individuals with autism in the Autism Society of North Carolina. J Child Adolesc Psychopharmacol. 2002;12(4):311-321. doi: 10.1089/104454602762599853 [DOI] [PubMed] [Google Scholar]
- 6.Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2007;17(3):348-355. doi: 10.1089/cap.2006.17303 [DOI] [PubMed] [Google Scholar]
- 7.Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;8(8):CD004677. doi: 10.1002/14651858.CD004677.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollander E, Soorya L, Chaplin W, et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am J Psychiatry. 2012;169(3):292-299. doi: 10.1176/appi.ajp.2011.10050764 [DOI] [PubMed] [Google Scholar]
- 9.McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996;53(11):1001-1008. doi: 10.1001/archpsyc.1996.01830110037005 [DOI] [PubMed] [Google Scholar]
- 10.Buchsbaum MS, Hollander E, Haznedar MM, et al. Effect of fluoxetine on regional cerebral metabolism in autistic spectrum disorders: a pilot study. Int J Neuropsychopharmacol. 2001;4(2):119-125. doi: 10.1017/S1461145701002280 [DOI] [PubMed] [Google Scholar]
- 11.King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590. doi: 10.1001/archgenpsychiatry.2009.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouti A, Reddihough D, Marraffa C, et al. Fluoxetine for Autistic Behaviors (FAB trial): study protocol for a randomized controlled trial in children and adolescents with autism. Trials. 2014;15:230. doi: 10.1186/1745-6215-15-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutter M, Le Couteur A, Lord C. ADI-R: Autism Diagnostic Interview Revised. WPS Edition Manual. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 14.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 15.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):844-852. doi: 10.1097/00004583-199706000-00023 [DOI] [PubMed] [Google Scholar]
- 16.Scahill L, McDougle CJ, Williams SK, et al. ; Research Units on Pediatric Psychopharmacology Autism Network . Children’s Yale-Brown Obsessive Compulsive Scale modified for pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1114-1123. doi: 10.1097/01.chi.0000220854.79144.e7 [DOI] [PubMed] [Google Scholar]
- 17.Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582-589. doi: 10.1038/sj.npp.1300627 [DOI] [PubMed] [Google Scholar]
- 18.Lam KSL, Aman MG. The Repetitive Behavior Scale–Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37(5):855-866. doi: 10.1007/s10803-006-0213-z [DOI] [PubMed] [Google Scholar]
- 19.Spence SH. A measure of anxiety symptoms among children. Behav Res Ther. 1998;36(5):545-566. doi: 10.1016/S0005-7967(98)00034-5 [DOI] [PubMed] [Google Scholar]
- 20.Nauta MH, Scholing A, Rapee RM, Abbott M, Spence SH, Waters A. A parent-report measure of children’s anxiety: psychometric properties and comparison with child-report in a clinic and normal sample. Behav Res Ther. 2004;42(7):813-839. doi: 10.1016/S0005-7967(03)00200-6 [DOI] [PubMed] [Google Scholar]
- 21.Brinkley J, Nations L, Abramson RK, et al. Factor analysis of the aberrant behavior checklist in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37(10):1949-1959. doi: 10.1007/s10803-006-0327-3 [DOI] [PubMed] [Google Scholar]
- 22.Guy W. ECDEU Assessment Manual for Psychopharmacology. Bethesda, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 23.StataCorp Stata Statistical Software: Release 15. College Station, TX: StataCorp LP; 2017. [Google Scholar]
- 24.Scahill L, Dimitropoulos A, McDougle CJ, et al. Children’s Yale-Brown obsessive compulsive scale in autism spectrum disorder: component structure and correlates of symptom checklist. J Am Acad Child Adolesc Psychiatry. 2014;53(1):97-107. doi: 10.1016/j.jaac.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook EH Jr, Rowlett R, Jaselskis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry. 1992;31(4):739-745. doi: 10.1097/00004583-199207000-00024 [DOI] [PubMed] [Google Scholar]
- 26.McDougle CJ, Naylor ST, Cohen DJ, Aghajanian GK, Heninger GR, Price LH. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiatry. 1996;53(11):993-1000. doi: 10.1001/archpsyc.1996.01830110029004 [DOI] [PubMed] [Google Scholar]
- 27.Sugie Y, Sugie H, Fukuda T, et al. Clinical efficacy of fluvoxamine and functional polymorphism in a serotonin transporter gene on childhood autism. J Autism Dev Disord. 2005;35(3):377-385. doi: 10.1007/s10803-005-3305-2 [DOI] [PubMed] [Google Scholar]
- 28.Najjar F, Owley T, Mosconi MW, et al. Pharmacogenetic study of serotonin transporter and 5HT2A genotypes in autism. J Child Adolesc Psychopharmacol. 2015;25(6):467-474. doi: 10.1089/cap.2014.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the Autism Network of the Research Units on Pediatric Psychopharmacology. Am J Psychiatry. 2005;162(6):1142-1148. doi: 10.1176/appi.ajp.162.6.1142 [DOI] [PubMed] [Google Scholar]
- 30.Ghanizadeh A, Tordjman S, Jaafari N. Aripiprazole for treating irritability in children and adolescents with autism: a systematic review. Indian J Med Res. 2015;142(3):269-275. doi: 10.4103/0971-5916.166584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord. 2000;30(5):427-435. doi: 10.1023/A:1005551523657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Summary of baseline characteristics in those who did and did not complete the CYBOCS-PDD at 16 weeks by treatment group
eTable 2. Comparison of outcomes at 16 weeks between the treatment groups using multiple imputation to handle the missing data
eTable 3. Comparison of Repetitive Behavior Scale – Revised subscales and outcomes Spence Children’s Anxiety subscales at 16 weeks using a complete case analysis
eTable 4. Summary of adverse events by treatment group and category
Data Sharing Statement