Abstract
Background:
During the first few trials of gene therapy for Leber’s hereditary optic neuropathy performed by our group, the visual acuity of the patients increased gradually over several months, or even years. However, in the current round of gene therapy for Leber’s hereditary optic neuropathy, we noted that the visual acuity of three patients increased rapidly, within a few days after treatment.
Case presentation:
Three patients who were diagnosed with mitochondrial gene 11778 mutation (associated with a G-to-A transition at Mt-11778 in the ND4 subunit gene of complex I of mitochondrial DNA that changes an arginine to histidine at amino acid 340) by genetic diagnosis were followed up three times before gene therapy, which lasted for 1 year, without spontaneous improvement of vision. Visual acuity in one or both eyes of each of the three patients increased rapidly after the initial gene therapy treatment.
Conclusion:
We suspect that in some patients with Leber’s hereditary optic neuropathy, a portion of the retinal ganglion cells might remain in a “dormant” state for a certain period of time; these may be activated, within an optimal timeframe, during gene therapy for Leber’s hereditary optic neuropathy.
Keywords: Leber’s hereditary optic neuropathy, gene therapy, dormant, time window, visual acuity, retinal ganglion cells
1. INTRODUCTION
Leber’s Hereditary Optic Neuropathy (LHON) is an inherited ocular disorder that is caused by a mutation in mitochondrial DNA (mtDNA). In China, 90% of patients with LHON harbor a point mutation at nucleotide 11778 of the mitochondrial gene (mtDNA G11778A), associated with a G-to-A transition at Mt-11778 in the ND4 subunit gene of complex I of mitochondrial DNA that changes an arginine to histidine at amino acid 340. Clinically, LHON manifests as decreased visual acuity that is not associated with pain. There is currently no effective treatment for LHON [1-3].
Between 2011 and 2012, our group performed gene therapy for nine patients with LHON (mtDNA G11778A). The patients received unilateral intravitreal injection with recombinant adeno-associated virus carrying the nicotinamide adenine dinucleotide (NADH): ubiquinone oxidoreductase subunit 4 gene (rAAV2-ND4). During the 36-month follow-up period, no complications were reported, and the visual acuity in six patients significantly increased (ClinicalTrials.gov number: NCT01267422) [4]. Between 2015 and 2016, Professor Guy treated 14 LHON patients with gene therapy, [5] and the Gensight company in France separately treated 12 patients with gene therapy. In 2017, we conducted another clinical trial of gene therapy for LHON (ClinicalTrials.gov number: NCT03153293), which included 48 patients with LHON who underwent unilateral intravitreal injection of rAAV2-ND4.
Previously, in the six patients who showed significant vision improvement, visual acuity increased gradually and slowly, 3 months after gene therapy [6]. However, three patients in the present gene therapy cohort have shown a rapid increase in visual acuity after treatment. This observation has provided us with a new perspective regarding the mechanism of LHON and may generate a new direction for the treatment of LHON with gene therapy.
2. CASE PRESENTATION
Case 1: A 16-year-old male presented at our hospital on August 30th, 2016, complaining of a sudden onset of blurred vision in both eyes, which had begun two months prior. His family history included a similar occurrence of decreased vision in his uncle. Ophthalmologic examination revealed the following: visual acuity OD 20/50, OS 20/200, optic disc hyperemia in both eyes, sharp border, and a normal foveal reflex. Visual field testing showed dark spots in the center of both eyes and an enlarged blind spot in the right eye. No abnormalities were found on optic disc Optical Coherence Tomography (OCT), macular OCT, or fundus fluorescein angiography. AQP4 antibody testing and cervical spine scan showed no obvious abnormalities. LHON gene test was positive for mtDNA G11778A (+). Final diagnosis: LHON.
During the follow-up period, the patient’s visual acuity decreased in both eyes. The patient met the selection criteria for enrollment in a gene therapy trial [4, 6]. Six months after observation, his visual acuity was stable at OD 20/400 and OS 20/500. On January 12, 2018, the patient underwent unilateral intravitreal injection of rAAV2-ND4 at 0.05 ml (1010 vg) in the right eye. The detailed treatment method was previously described [4].
Within 4 days of undergoing gene therapy treatment, the visual acuity of the injected eye of the patient increased from 20/100 (0.7 LogMAR) to 20/25 (0.1 LogMAR); the visual acuity of the uninjected eye increased from 20/80 (0.6 LogMAR) to 20/50 (0.4 LogMAR). As an increase in visual acuity of ≥ 0.3 log MAR was considered significant [4], we report the visual acuity of the injected eye of this patient as significantly increased, and the visual acuity of the uninjected eye as noticeably increased. The patient was followed up for the first and third month after injection, and his visual acuity improved to 20/33(0.2LogMAR) and 20/40 (0.3LogMAR) at third month after surgery (Fig. 1).
Fig. (1).
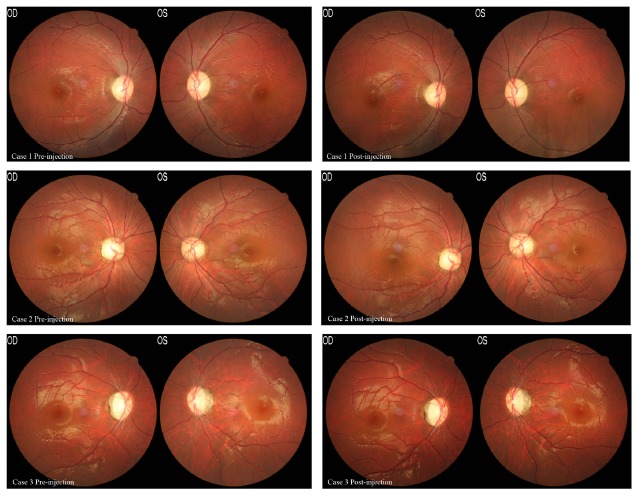
Fundus photographs from Cases 1, 2, and 3, before and after intravitreal injection of rAAV2-ND4. (Left) Fundus photographs of the three patients before surgery. (Right) Fundus photographs of the three patients after surgery. No structural abnormalities of the retina were found in any of the patients.
Case 2: A 25-year-old female who reported a history of a sudden onset of decreased visual acuity in both eyes in January 2004. On August 12, 2017, this patient presented at our hospital for consultation. Ophthalmologic examination revealed the following: visual acuity OD 20/500 OS 20/333, a pale optic disc, sharp border, and a normal foveal reflex. LHON gene testing was positive for mtDNA G11778A (+). Final diagnosis: LHON.
The patient attended regular follow-up and was selected to enroll in a gene therapy trial, as she met the selection criteria [4, 6]. Her visual acuity was stable at OD 20/500 and OS 20/333. On January 26th, 2018, the patient underwent unilateral intravitreal injection of rAAV2-ND4 at 0.05 ml (1010vg) in the right eye. Within 2 days of undergoing gene therapy treatment, the visual acuity of the injected and uninjected eyes of the patient increased to 20/333 (1.2 LogMAR) and 20/200 (1.0 LogMAR), respectively. Within 4 days of undergoing gene therapy treatment, the visual acuity of the injected eye increased from 20/200 (1.0 LogMAR) to 20/166 (0.9 LogMAR), whereas no significant increase was observed in the uninjected eye.The patient was followed up for the first month(Right eye 20/200,Left eye 20/166) after injection. However, she was not followed up on time at the third month (Fig. 2).
Fig. (2).
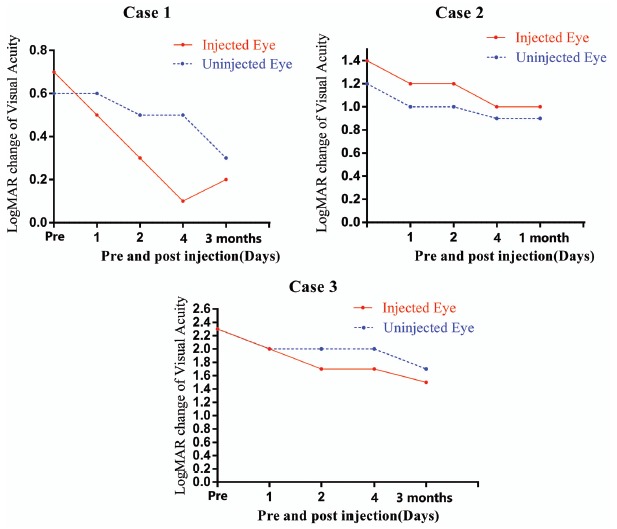
Visual acuity (logMAR) before, 4 days, 1 month(Case 2) and 3 months(Case 1 and Case 3) after injection of rAAV2-ND4 in three patients.
Case 3: An 18-year-old male who reported a history of a simultaneous decrease in visual acuity in both eyes on April 21, 2015. He presented at our hospital on March 16, 2017. Ophthalmologic examination revealed the following: visual acuity OD hand motion (HM)/40 cm, OS HM/40 cm, a pale optic disc, sharp border, and a normal foveal reflex. LHON gene testing was positive for mtDNA G11778A (+). Final diagnosis: LHON.
The patient met the criteria for gene therapy enrollment. His visual acuity was stable at OD HM/40 cm and OS HM/40 cm after 10 months of follow-up observation. On February 2, 2018, the patient underwent unilateral intravitreal injection of rAAV2-ND4 at 0.05 ml (1010vg) in the right eye. Within 1 day of undergoing gene therapy, the visual acuity of the injected eye increased to 20/2000 (2.0 LogMAR). Within 2 days of undergoing gene therapy, the visual acuity of the injected eye had increased from HM/40 cm (2.3 LogMAR) to 20/1000 (1.7 LogMAR), and that of the uninjected eye had increased from HM/40 cm (2.3 LogMAR) to 20/2000 (2.0 LogMAR). Thus, the visual acuity of both injected and uninjected eyes significantly increased (Table 1). The patient was followed up for the first and third month after injection, and his BCVA of her injected and uninjected reached to 20/666(1.5LogMAR) and 20/1000(1.7LogMAR) respectively at the third month after surgery (Fig. 2).
Table 1. Clinical data of three Leber’s hereditary optic neuropathy (LHON) patients.
| Case Number | Sex | Age (Years) |
Disease
Duration (Months) |
Intravitreal Injection Eye | Treatment Date |
Visual Acuity Before Treatment
(Snellen Scale) |
Visual Acuity 4 Days After Treatment
(Snellen Scale) |
Visual Acuity 3 Months After
Treatment (Snellen Scale) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Onset | At Gene Therapy | Injected Eye | Uninjected Eye | Injected Eye | Uninjected Eye | Injected Eye | Uninjected Eye | |||||
| 1 | M | 14 | 16 | 18 | R | 1/12/ 2018 | 20/100 | 20/80 | 20/25 | 20/50 | 20/33 | 20/40 |
| 2 | F | 11 | 25 | 168 | R | 1/ 26/ 2018 | 20/500 | 20/333 | 20/200 | 20/166 | / | / |
| 3 | M | 14 | 18 | 48 | R | 2/2/ 2018 |
Hand motion /40 cm |
Hand motion /40 cm |
20/1000 | 20/2000 | 20/666 | 20/1000 |
3. DISCUSSION
Based on the results from these three patients, and our subsequent observations of rapid increases in visual acuity after gene therapy in multiple other patients, we suggest a new perspective regarding the mechanism of LHON, as follows: the nucleotide at mtDNA11778 is involved in encoding the ND4 protein. The ND4, ND1, and ND6 proteins together form mitochondrial complex I, which transmits electrons and ATP at the mitochondrial surface [7]. A mutation at nucleotide position 11778 causes an amino acid substitution at position 340 of the ND4 protein, from arginine to histidine, resulting in structural instability of mitochondrial complex I [8]. This leads to an insufficient ATP supply to the affected patient’s retinal ganglion cells, thereby inducing oxidative stress and damage in the retinal ganglion cells, which ultimately results in LHON.
Gene therapy for LHON involves delivering the normal ND4 gene to the retinal ganglion cells, using rAAV2 as a carrier. The normal ND4 protein, encoded by the delivered gene, can then enter the mitochondria [2]. Together with other components of the complex that have continued to form properly, a structurally stable mitochondrial complex I is formed, thus providing sufficient ATP for the ganglion cells. In the first gene therapy trial performed by our group, six patients exhibited improved vision after receiving treatment; however, their visual acuity increased slowly. Based on this clinical phenomenon, we speculated at the time that gene therapy had allowed the retinal ganglion cells to receive sufficient ATP, which led to gradual recovery of the optic nerve, followed by gradual improvements in visual acuity. We speculated that this recovery process might require months, or even years.
However, the rapid increase in visual acuity that we report here indicates that the retinal ganglion cells in these patients have recovered rapidly. We speculate that some ganglion cells are not damaged, but may have remained in a “dormant” state. Gene therapy enables these dormant cells to receive sufficient ATP to quickly switch from a “dormant” to a “normal” state, thus enabling them to perform their physiological functions. The clinical result is a rapid increase in the patient’s visual acuity. This new understanding may provide a new course for gene therapy for LHON. The efficacy of the therapy can be increased if it is conducted when the ganglion cells are simply in a dormant state, which might enable rapid recovery of visual functions in the patients. Thus, it is important to determine the optimal “window of time” for treating LHON.
However, the ganglion cells are rapidly damaged in some patients with LHON, suggesting that there is a very small window of time for optimal treatment. There is also a small number of patients whose ganglion cells may remain in a dormant state for an extended period, providing a longer window of time for optimal treatment; this is exemplified in case 2 in the present study. The reason for this difference is not clear; it may be related to various factors, including the copy number of the mutated genes, the tolerance of the retinal ganglion cells, and the disease onset and duration for that particular patient. Our goal is to determine the window of time for optimal treatment for each patient, which may be during the acute phase after disease onset, or at a certain time point after the acute phase. We have designed a new clinical trial (ClinicalTrials.gov number: NCT03428178) to further study this issue.
CONCLUSION
Another interesting observation is that even though patients in the gene therapy trial for LHON underwent unilateral intravitreal injections of rAAV2-ND4, some patients demonstrated increased visual acuity in the uninjected eye [9, 10]. Some researchers have suggested that the recovered optic nerve in the injected eye may grow to meet the contralateral eye, restoring some portion of visual function [11]. However, this theory is likely not applicable for the three patients in this study, as the visual acuity of their uninjected eyes increased rapidly. The reasoning for this observation remains to be explored in future studies.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AAV2-ND4
Adeno-associated virus 2-ND4
- BCVA
Best-corrected visual acuity
- LHON
Leber’s hereditary optic neuropathy
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology and Ezhou Central Hospital (No. TJ-IRB20180316 and No. L2017-K-05), as well as the Human Subjects Review Board at the Department of Ophthalmology, Taihe Hospital, Hubei University of Medicine (No. 201701) China.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. Reported experiments on humans were in accordance with the ethical standards of the committee responsible for human experimentation (institutional national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all patients or their families to participate in the study, as well as for the publication of this report and associated images.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the clinic trial at [https://www.clinicaltrials.gov/ct2/show/ NCT03153293?cond=LHON&cntry=CN&rank=2].
FUNDING
The study was supported by the National Natural Science Foundation of China: 81770969 Huazhong University of Science and Technology Autonomous Innovation Fund: 0118540267. Horizontal Projects Fund: 2017038.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Y-Wai-Man P. Turnbull DM. Chinnery PF. Leber hereditary optic neuropathy. J. Med. Genet. 2002;39:162–169. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peragallo J.H., Newman N.J. Is there treatment for Leber hereditary optic neuropathy? Curr. Opin. Ophthalmol. 2015;26(6):450–457. doi: 10.1097/ICU.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilz Y.L., Bass S.J., Sherman J. A review of mitochondrial optic neuropathies: from inherited to acquired forms. J. Optom. 2017;10(4):205–214. doi: 10.1016/j.optom.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan X., Pei H., Zhao M.J., et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci. Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy J., Feuer W.J., Davis J.L., et al. Gene therapy for Leber hereditary optic neuropathy: Low- and medium-dose visual results. Ophthalmology. 2017;124(11):1621–1634. doi: 10.1016/j.ophtha.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S., Ma S.Q., Wan X., et al. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy. EBioMedicine. 2016;10:258–268. doi: 10.1016/j.ebiom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt U. Energy converting NADH: Quinone oxidoreductase (complex I). Annu. Rev. Biochem. 2006;75:69–92. doi: 10.1146/annurev.biochem.75.103004.142539. [DOI] [PubMed] [Google Scholar]
- 8.Bi R., Zhang A.M., Yu D., Chen D., Yao Y.G. Screening the three LHON primary mutations in the general Chinese population by using an optimized multiplex allele-specific PCR. Clin. Chim. Acta. 2010;411(21-22):1671–1674. doi: 10.1016/j.cca.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Yang S., He H., Zhu Y., et al. Chemical and material communication between the optic nerves in rats. Clin. Exp. Ophthalmol. 2015;43(8):742–748. doi: 10.1111/ceo.12547. [DOI] [PubMed] [Google Scholar]
- 10.Luo X., Salgueiro Y., Beckerman S.R., Lemmon V.P., Tsoulfas P., Park K.K. Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp. Neurol. 2013;247:653–662. doi: 10.1016/j.expneurol.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cen L.P., Ng T.K., Liang J.J., et al. Human periodontal ligament-derived stem cells promote retinal ganglion cell survival and axon regeneration after optic nerve injury. Stem Cells. 2018;36(6):844–855. doi: 10.1002/stem.2812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the article is available in the clinic trial at [https://www.clinicaltrials.gov/ct2/show/ NCT03153293?cond=LHON&cntry=CN&rank=2].


