Abstract
Background & Objective:
NSCs therapy is considered one of the most potential methods for spinal cord injury (SCI).
Methods:
We build the SCI model rats to investigate the therapeutic effect of fire needle acupuncture in improving the locomotor function of SCI rats and its possible mechanism. BBB scale was used for the motor ability of rats. The expression of Nestin, NSE, Gal-C, and GFAP was detected by immunohistochemistry. Wnt, GSK3β, β-catenin, ERK1/2, CyclinD1, and ngn1 were detected by western blot and PCR. The BBB score of both model group (1.20±0.94, 3.12±0.67, 5.34±1.57, 7.12±1.49) and fire needle group (1.70±0.58, 4.50±1.63, 7.53±2.41, 9.24±0.63) gradually increased after SCI. Furthermore, at d10 and d14, the fire needle group showed a significantly high score compared with that in model group at the same time (P<0.05). Fire needle increased Nestin, NSE, and Gal-C expression inhibited GFAP expression after SCI. Also, fire needle could up-regulate Wnt3a, GSK3β, β-catenin, and ngn1, and down-regulate ERK1/2, cyclinD1 gene and protein expression.
Conclusion:
In conclusion, fire needle could improve lower limb locomotor function of SCI rats. Also, fire needles could promote endogenous NSCs proliferation differentiating into neurons, and the mechanism might be mediated by promoting the activation of Wnt/β-catenin and inhibiting the overexpression of ERK.
Keywords: Fire needle acupuncture, Spinal cord injury, Neural stem cell, Neurons, Wnt, ERK
1. INTRODUCTION
Spinal cord injury (SCI) is a serious central nervous system (CNS) injury, causing loss of muscle function, sensation, or autonomic function in parts of the body served by the spinal cord below the level of the lesion due to the disruption of neuronal axons passing through the damaged segments [1, 2]. Currently, the treatment of SCI mainly focused on two aspects [3, 4]: one is neuroprotection, by reducing or eliminating secondary pathological response to protect residual axons and neuronal cells, such as steroids [5], topiramate [6], nimodipine, Chinese herbs, and acupuncture [7-9]; the other one is nerve regeneration and repairment, by promoting the regeneration of neurons and rebuilding the synaptic connections, such as brain-derived neurotrophic factor (BDNF) [10] treatment, gene therapy, and neural stem cell therapy [11, 12].
Among them, neural stem cells (NSCs) therapy is considered one of the most prospective methods for the treatment of SCI [12]. NSCs are primitive cells in the CNS which have showed potentials for self-renewal and multidirectional differentiation into neurons, astrocytes, and oligodendrocytes. As the main component of the central nervous system, neurons are also the basic material for its function. Numerous studies have shown that the pathological apoptosis of neurons is one of the major pathological basis of secondary injury to SCI [13, 14], while appropriately inducing endogenous NSCs to proliferate and differentiate into neurons to compensate for the loss of neurons is a potential and effective method to reduce pathological injury, and to promote nerve regeneration and repairment against SCI [15, 16].
The differentiation of endogenous NSCs into neurons is mediated by multiple signal transduction pathways. Wnt signaling pathway is a highly complex signaling pathway widely in multicellular eukaryotes and plays an important role in the proliferation and differentiation of neural stem cells [17, 18]. The Wnt/β-catenin signaling pathway is the most classic, and it plays a key role in the proliferation, differentiation and axon guidance of neural stem cells [17, 19]. Wnt3a could not only exhibit a mitogenic effect in developing spinal cord neural progenitor cells [20] but also plays an important role in the specialization of neurons in the dorsal spinal cord [21]. It could increase the number of neurons after spinal cord injury, and promote axon conduction and neurological improvement. Mitogen-activated protein kinase (MAPK) is one of the most important signal transduction pathways in mammals and it is involved in the regulation of physiological and pathological processes [22] in various cellular responses, including cell growth, proliferation and differentiation. An extracellular signal-regulated kinase (ERK) is an important subfamily of MAPK family, including ERK1 and ERK2, collectively referred to as ERK1/2 [23]. Studies have shown that the activation of ERK signaling pathway is associated with spinal cord degeneration and dysfunction induced by spinal cord injury in rats [24], and ERK1/2 inhibitors can significantly reduce secondary spinal cord injury and improve neurological function in rats [25].
Due to the networked state of transmission between signals, there exists a complicated crosstalk effect, that is, activated signal molecules in one signal transduction pathway can regulate signal molecules in another signal transduction pathway, and the activated signal molecule is the key target of crosstalk [26]. Similarly, there is a crosstalk between Wnt/β-catenin and ERK1/2. Both activated Wnt and ERK1/2 protein could phosphate Glycogen Synthase Kinase 3beta (GSK3β) to regulate β-catenin to induce cyclinD1 and neurogenin1(ngn1), to promote NSCS to differentiate into neurons.
Acupuncture, one of the oldest practices of traditional Chinese medicine, has been applied as a symptomatic treatment for SCI for years, and the results are encouraging. The effect of acupuncture on improving sensory and motor skills in SCI patients has been proved [7, 27]. Fire needle acupuncture, also known as fire needle, is an acupuncture technique which involves quickly inserting a red hot needle into areas on the body [28]. It combines conventional acupuncture and cauterization with heated needles therapy. Clinically, fire needle has been found to show significant effects on neurological damage such as SCI and sciatica [29]. Studies also proved that the neuroprotective effects of fire needle on the neuronal injury, especially SCI [30, 31]. Our previous studies showed that fire needle could not only significantly increase the expression of BDNF in spinal cord anterior horn of SCI rats, but also promote the recovery of motor neuron function, and they were positively correlated [32]. Serum of SCI rats after fire needle intervention also showed the tendency of inducing the proliferation of NSCs and differentiating into neurons [33].
Based on that, we proposed the following hypothesis: fire needle therapy might function in regulating Wnt/β-Catenin and ERK1/2 multi-signaling pathway to promote the differentiation of endogenous NSCs into neurons, to play a nerve repair effect against SCI. In order to verify the above hypothesis, we intend to build SCI model rats, to observe the neurological improvement after intervened by fire needle therapy by behavior, to detect the expression of Nestin, NSE, Gal-C, and GFAP by double-label immunohistochemistry, and the key targets of Wnt/β-Catenin and ERK1/2 multi-signal transduction pathway Wnt, GSK3β, β-catenin, ERK1/2, cyclinD1, and ngn1 by western blot and PCR.
2. MATERIALS AND METHODS
2.1. Animals and Groups
120 healthy SD female rats, weighing about 260-300 grams, were provided by the China Institute for Food and Drug Control (License No.SCXK 2009-0017), and all the rats were maintained at 23±2°C and 60±10% humidity under a 12-h light/dark cycle with ad libitum access to water and food, acclimatized for 7 days before the experiments. All animal experiments met the guidelines of the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Documentation 55, 2001), and were approved by the local ethical committee at Tianjin University of Traditional Chinese Medicine.
The rats were randomly divided into blank group (10), sham operation group (10), model group (50), and fire needle group (50). Rats in fire needle group and model group were then divided into five-time points: 1d, 3d, 7d, 10d and 14d, 10 in each time point. Rats in blank group didn’t receive any operation, and rats in sham operation group received only a laminectomy at the T9-T11 levels. While rats in model group and fire needle group were established as SCI models, but only rats in fire needle groups were treated by fire needle.
2.2. Establishment of SCI Model and Assessment of Motor Function
The modified Allen's method was applied to rats in the model group and fire needle group to build SCI models [34]. All rats were anesthetized with 10% chloral hydrate (300 mg/kg), and a laminectomy was performed at the T9 level to expose the cord without disrupting the dura mater. The exposed dorsal surface of the cord was subjected to contusion injury (10 g×60 mm) using a modified Allen’s Impactor. Only T9 and T10 vertebral plates were moved without smashing the spinal cord of rats in sham operation group. After modeling, bladders were manually emptied twice a day until spontaneous voiding occurred. The Basso Beattie Bresnahan scale (BBB scale), on a 21 point rating scale, with a score of 21 representing normal movement and 0 representing complete paralysis of the hindlimb, was used for evaluating the motor ability of rats before modeling and at each time point [35].
2.3. Fire Needle Treatment
He’s fire needle (1 inch in length, 0.5mm in diameter) was applied on Jiaji points of T7, T8, T11, and T12 (according to Atlas of acupuncture and moxibustion points on animals by the Chinese Acupuncture and Moxibustion Society and experimental acupuncture and moxibustion) to treat SCI rats. Rats were kept without anesthesia in an immobilization apparatus after sterilizing the skin of the acupoints. Taking the alcohol lamp by the left hand as close as possible to the acupoints, the performer held the handle of the fire needle with right hand, heated the pinpoint and body of the needle to red, and then pricked swiftly into the point and withdrew the needle out quickly. The fire needle was performed only once for each point in 1/3 second, with a depth of 3-5mm, once a day for each rat. Rats in blank, sham operation and model group were not treated by fire needle.
2.4. Double-label Immunohistochemistry
The rats (n=4) in each group were intraperitoneally injected with 1% 5-Bromo-2-deoxyuridine (BrdU) saline solution at 50 mg/ (kg) 48h, 36h, and 24h before sacrificing to mark cells in differentiation. When sacrificing, rats were deeply anesthetized by an intraperitoneal injection of 10% chloral hydrate (0.3g/kg) and were perfused intracardially with 4% paraformaldehyde in a 0.1 M sodium phosphate buffer (PB, pH57.4) at d1, d3, d7, d10, d14, respectively. A 20 mm section of the spinal cord centering at the injured area was dissected out. Then the spinal cord tissues were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin wax, and serially sectioned using an RM2065-type paraffin section machine (Leica Inc). After deparaffinization and rehydration, the tissues were soaked in 0.01M PBS for 5 minutes, heated repair, and incubated in 0.1% TritonX-100 for 15 minutes at room temperature. After incubated in 3% H2O2 at room temperature for 10 minutes and blocked for 15 minutes, the following primary anti-antibody were used: mouse anti-BrdU monoclonal antibody (1:1000) and rabbit anti-Nestin polyclonal antibody (1:500) for BrdU/nestin double-label staining, mouse anti-BrdU monoclonal antibody (1:1000) and rabbit anti-NSE polyclonal antibody (1:500) for BrdU/NSE double-label staining, mouse anti-BrdU monoclonal antibody (1:1000) and rabbit anti-GFAP polyclonal antibody (1: 200) for BrdU/GFAP double-label staining, mouse anti-BrdU monoclonal antibody (1:1000) and rabbit anti-Gal-C polyclonal antibody (1:500) for BrdU/Gal-C double-label staining). The tissues were then incubated with a biotinylated secondary antibody solution for 15 minutes at 37°C; and then they were washed, dripped with horseradish enzyme-labeled streptavidin working solution, incubated for 15 minutes at 37°C, colored with DAB; washed again with distilled water, stained by hematoxylin; washed with tap water, 1% hydrochloric acid-ethanol for 5 seconds; washed with tap water, dehydrated, and blocked. Fluorescent images were observed and recorded under fluorescence microscopy. Images were processed using Image-pro Plus Software to measure the densities of the double-labeled positive cells.
2.5. Western Blot Analysis
Rats (n=3 for each group) were anesthetized as described above at d1, d3, d7, d10, d14, respectively. Each segment of spinal cord was removed from an injured area quickly for extracting protein samples. Protein samples (40 μg each) was loaded into each well of a sodium dodecyl sulfate-polyacrylamide gel (10% polyacrylamide gels) and separated by electrophoresis. The proteins were then transferred to nitrocellulose membranes (Amersham, USA), blocked with 5% bovine serum albumin and prepared in a Tris-buffered saline (TBS) overnight at 4°C, followed by incubation with antibodies against Wnt3a (1:1000), GSK3β (1:1000), β-catenin (1:1000), ERK1 (1:1000), ERK2 (1:1000), cyclinD1 (1:1000), ngn1 (1:1000), and β-Actin (1:1000). After washing, the membranes were incubated with secondary antibodies for 1h. Immunoreactive bands were visualized using the BeyoECL Plus (Beyotime Company, China), and the resulting membranes were imaged using the VersaDoc MP5000 imaging system (BIO-RAD,USA). β-Actin was used as an internal control. Relative intensity of each band was measured and analyzed by Quantity One.
2.6. Quantitative Real-time Polymerase Chain Reaction (PCR) Analysis
The anesthesia of rats (n=3 in each group) and dissection of spinal cord segments were as described above. RNA isolation and cDNA synthesis were performed following Trizol Kit (Gibco). The reverse transcription reaction system one (RNA 2μL, dNTP 2μL, Oligo(dT) 2μL, DEPC H2O 8.5μL) was prepared and pre-incubated at 70°C for 5 min, then was chilled on ice for 2 min, followed by adding the reaction system two (M-MLV 1μL, RRI 0.5μL, Buffer 4μL) to each sample. After fully mixed and incubated at 42°C for 50 min, 95°C for 5 min, the real-time PCR (SYBR Mix 12.5μL [CWBIO, China; CW0957], forward prime 0.5μL, reverse prime 0.5μL, double distilled water 9.5μL, cDNA 2μL) was used to carry out quantitative PCR (qPCR) analyses using Corbett Rotor-Gene 6000 (Corbett, Australia). The primer sequences are shown in Table 1. The mRNA expression was detected using the 2-ΔΔCt method.
Table 1. Primer sequences for quantitative real time PCR.
| Gene | Primer Sequence |
|---|---|
| Wnt3a | F: 5'-CCCCAACCTCATTTCCACAT-3' R:5'-TCATACGCATCCCATCTCTCC-3' |
| β-catenin | F: 5'-AAAGCGGCTGTTAGTCACTGG-3' R: 5'-GACTTGGGAGGTATCCACATCC-3' |
| GSK3β | F: 5'-CCTTAACCTGGTGCTGGACT -3' R: 5'-AGCTCTGGTGCCCTGTAGAT-3' |
| ERK1 | F: 5'-GCACGCTGAGAGAAATCCAG-3' R: 5'-CAGCTTGTACAGGTCCGTCTC-3' |
| ERK2 | F: 5'-CTAATCTCTCGTACATCGGAG-3' R: 5'-CTGACAGTAGGTCTGGTGCTC-3' |
| cyclinD1 | F: 5'-AGGCAGCGCGCGTCAGCAGCC-3' R: 5'-TCCATGGCGCGGCCGTCTGGG-3' |
| ngn1 | F: 5'-CAGTAGTCCCTCGGCTTCAG-3' R: 5'-GTCGTGTGGAGCAGGTCTTT-3' |
| β-actin | F: 5'-CAGCCTTCCTTCCTGGGTATG-3' R: 5'-TAGAGCCACCAATCCACACAG-3' |
Note: F: forward; R: reverse.
2.7. Statistical Analysis
SPSS 20.0 software was applied for statistical analysis, and the measurement data were represented as Mean±SD. Differences within groups at multiple time points were assessed with one-way analysis of variance (ANOVA), and P<0.05 was considered statistically significant.
3. RESULTS
3.1. Effects of fire needle on Neural Function
All rats showed limb function and obtained a BBB score of 21 before SCI (d0). The rats in blank and sham operation group showed no locomotor dysfunction, each obtaining a score of 21 at all the time points. Complete paralysis (0.00±0.00) of lower limbs in rats of model and fire needle group was identified at d1 compared with that in the sham group (p<0.05). In the following days, the BBB score of both model group (1.20±0.94, 3.12±0.67, 5.34±1.57, 7.12±1.49) and fire needle group (1.70±0.58, 4.50±1.63, 7.53±2.41, 9.24±0.63) gradually increased after SCI. Furthermore, at d10 and d14, the fire needle group showed significantly higher scores compared with those in the model group (P<0.05).
3.2. Fire Needle Therapy Increased Nestin, NSE, and Gal-C Expression Inhibits GFAP Expression after SCI
BrdU double label results showed that only a few nestin-positive cells could be observed in the blank group and the sham operation group. One day after SCI, there was a significant increase of nestin-positive cells in the model group and the fire needle group, and the increasing tendency continued to d14. Moreover, the expression of nestin-positive cells in fire needle group was significantly higher than that in model group at d7, d10, and d14. It showed that fire needling could promote the activation, migration, and proliferation of endogenous NSCs in the injury area Fig. (2A).
Fig. (2).
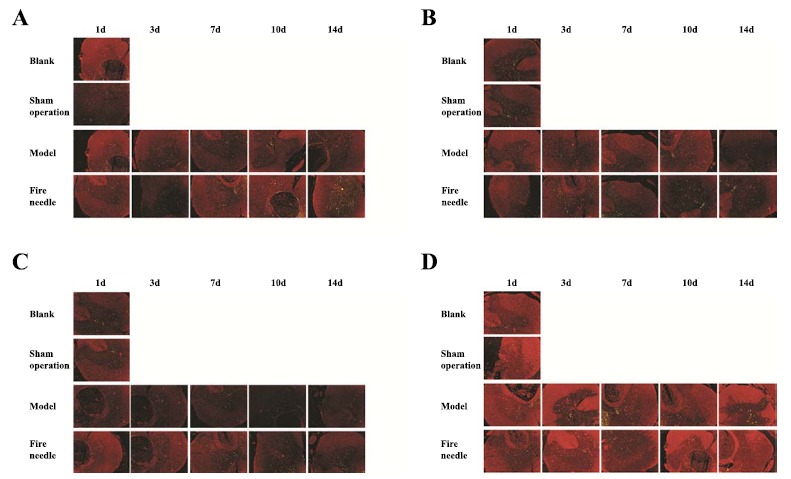
BrdU double label results of Nestin, NSE, Gal-C, and GFAP expression of rats in each group at different time point after SCI. (A) Nestin expression of rats in each group at different time point after SCI; (B) NSE expression of rats in each group at different time point after SCI; (C) Gal-C expression of rats in each group at different time point after SCI; (D) GFAP expression of rats in each group at different time point after SCI. Images were processed using Image-pro Plus Software to measure the densities of the double-labeled positive cells.
Compared with that in the blank group and the sham operation group, NSE positive cells in model group and fire needle group significantly decreased at d1, and at d3 it still decreased in the model group; while in the fire needle group, NSE expression significantly increased at d3 compared with that at d1. The expression of positive cells in fire needle group at d7, d10, d14 was significantly higher than that in the model group, suggesting that fire needle could promote the differentiation of endogenous NSCs into neurons after SCI Fig. (2B). The expression of Gal-C positive cells showed a similar trend with NSE in each group at the different time point Fig. (2C), suggesting that fire needles could also promote the differentiation of endogenous NSCs into microglia after SCI.
The GFAP positive cells in model group and fire needle group significantly increased compared with that in blank group and sham operation group. The GFAP positive cells in the model group were significantly higher at d3 and d7 than that at d1, and there was a tendency of decrease at d10 and d14. In fire needle group, the GFAP positive cells slightly decrease at d3 and d7, but it’s significantly less at d10 and d14 than that in model group, suggesting that the fire needle might have the inhibitory effect on NSCs differentiating into astrocytes after SCI.
3.3. Fire Needle Therapy Regulated Wnt3a, GSK3β, β-Catenin, ngn1, ERK1, ERK2, and cyclinD1 Protein Expression
One way ANOVA results showed that there were significant changes of all the proteins except for cyclinD1 and ngn1 in model group and fire needle group at 1d compared with those in sham operation group (P<0.05). For Wnt3a, GSK3β, β-catenin, the expression showed the same tendency at different time points in model group: increasing at d1 and d3, a sudden decrease at d7, and a significant increase at d10, then decreasing at d14. It was similar to that in the fire needle group, except that for β-catenin there showed a significant increase at d7 compared with that at d3. Comparison at the same time point showed that the protein expression in fire needle group was significantly higher than that in model group at the following time points: Wnt3a at d1, d7 and d10 Fig. (3A), GSK3β at d10 Fig. (3B), β-catenin at d7 Fig. (3C).
Fig. (3).
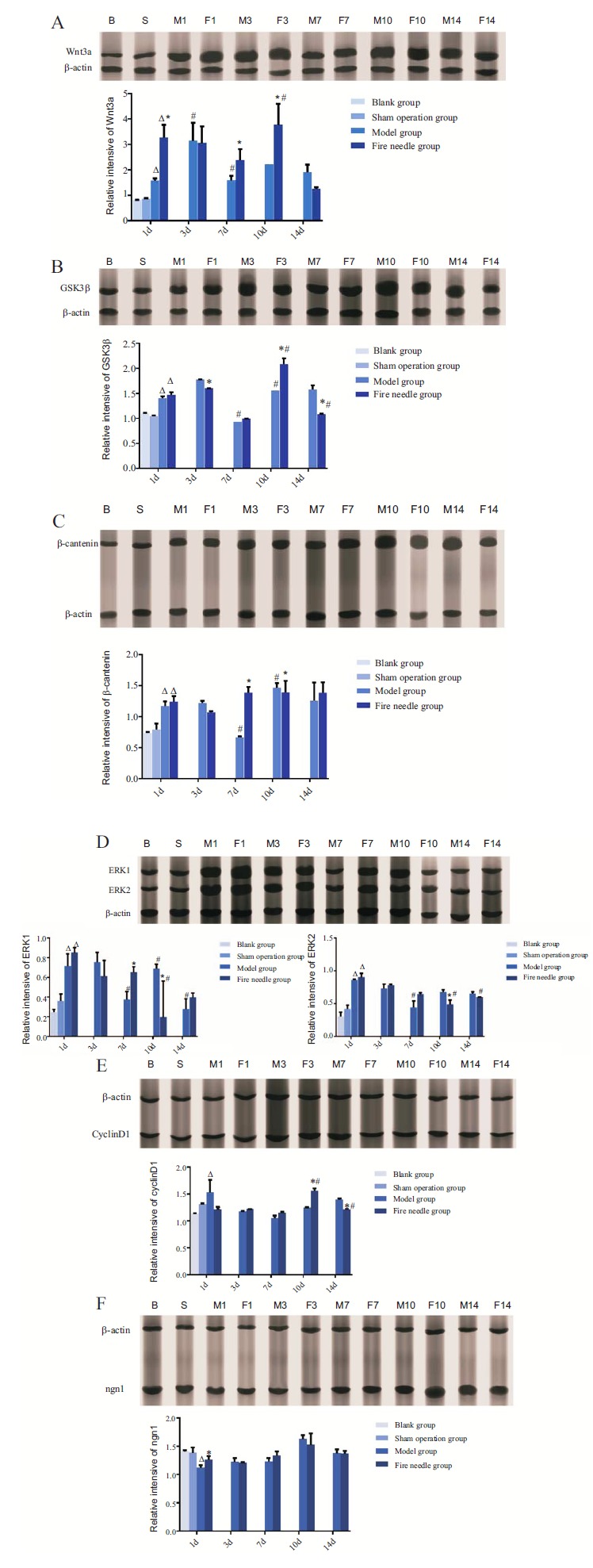
Protein Expression of Wnt3a, GSK3β, β-catenin, ERK1, ERK2, cyclinD1, and ngn1 in each group of different time point (cropped blots from different gels). Notes: B: Blank group; S: Sham operation group; M1: Model group at d1; F1:Fire needle group at d1; M3: Model group at d3; F3:Fire needle group at d3; M7: Model group at d7; F7:Fire needle group at d7; M10: Model group at d10; F10:Fire needle group at d10; M14: Model group at d14; F14:Fire needle group at d14. *indicates that the difference between fire needle group and model group at the same time point is statistically significant (P<0.05). # indicates that the difference between a time point and the point before that in the same group is statistically significant (P<0.05). Δ indicates at d1, there is a significant difference among model group, fire needless group, and sham operation group (P<0.05).
For ERK1 and ERK2 Fig. (3D), they both significantly increased at d1 in model and fire needle group compared with that in the sham operation group. There was a decreasing trend following different time point in the two groups. At d10, the expression of ERK1/2 in fire needle group was significantly lower than that in the model group. The CyclinD1 protein in the model group showed a significant increase at d1, then a slight decrease at d3 and d7, followed by an insignificant increase at d10 and d14. While in fire needle group, the CyclinD1 expression showed a nearly steady stage at d1, d3, and d7 Fig. (3E), with a significant increase at d10. Moreover, the expression of CyclinD1 at d10 in fire needle group was significantly higher than that in the model group. For ngn1 Fig. (3F), the expression in both model and fire needle group was lower than that in the sham operation group. Then there was a steady but insignificant increase at d3, d7, and d10 in both groups. Interestingly, the difference between groups showed statistically significant only at d1.
3.4. Fire Needle Therapy Up-regulated Wnt3a, GSK3β, β-catenin, and ngn1, Down-regulated ERK1, ERK2, cyclinD1 Gene Expression
The levels of Wnt3a, GSK3β, β-catenin, ERK1, ERK2, and cyclinD1 mRNA in model group and fire needle group all significantly increased d1 after SCI compared with that in sham operation group (P<0.05), while the levels of ngn1 decreased (P<0.05). The gene expression of Wnt3a, GSK3β, and β-catenin mRNA in both model group and fire needle group showed the similar tendency at different time point: increasing at d1, d3, and d7, and then decreasing at d10 and d14. But the statistical significance of the increase only showed in fire needle group on Wnt3a and GSK3β3 days after SCI. For Wnt3a, though the levels in fire needle group were higher than that in the model group at d3, d7, and d14 Fig. (4A), there was no significant difference (P>0.05). The expression of GSK3β mRNA at d3 Fig. (4B), and β-catenin mRNA at d1, d3, d7 Fig. (4C) in fire needle group was significantly higher than that in model group (P<0.05). The expression of ERK1 and ERK2 mRNA showed a tendency of decrease since d3, till d14. Moreover, in the fire needle group, ERK1 mRNA levels at d1 and d3 Fig. (4D), ERK2 mRNA levels at d1, d3, d7, and d10 Fig. (4E) were significantly lower than that in model group (P<0.05). The expression of cyclinD1 mRNA increased at d1, d3, and d7, and then decreased at d10 and d14 in both groups, and the changes of different time were not statistically significant (P>0.05). However, in fire needle group, the cyclinD1 mRNA levels at d7, d10, and d14 were significantly lower than that in the model group (P<0.05, Fig. (4F). For ngn1 mRNA, compared to d1, the levels increased at the following time point, but still less than that in sham operation and blank group, even at d14. At d3 and d10, the levels in fire
Fig. (4).
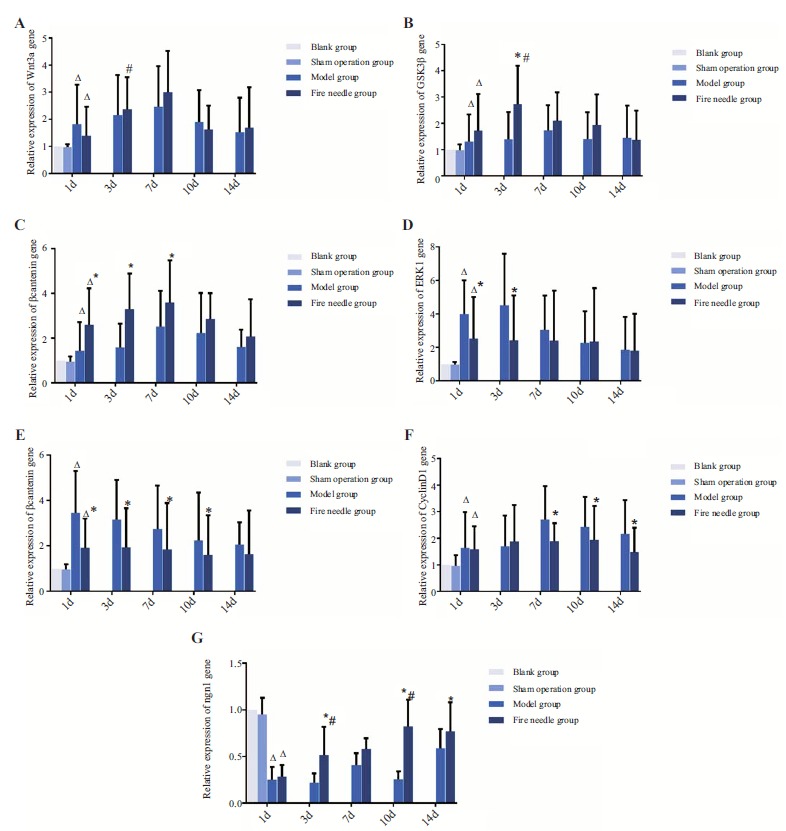
Gene expression of Wnt3a, GSK3β, β-catenin, ERK1, ERK2, cyclinD1, and ngn1 in each group of different time point.
Notes: *indicates that the difference between fire needle group and model group at the same time point is statistically significant (P<0.05).
# indicates that the difference between a time point and the point before that in the same group is statistically significant (P<0.05).
Δ indicates at d1, there is a significant difference among model group, fire needless group and sham operation group (P<0.05).
needle group were significantly higher than those at d1 and d7, respectively, and the levels at d3, d10, and d14 were significantly higher than those in model group (P<0.05, Fig. 4G).
4. DISCUSSION
Acupuncture has been commonly used for relieving pain in Chinese cultures [36], and it has gradually gained increasing popularity in the United States as a treatment for pain [37], because of its lower incidence of adverse effects. A prospective clinical trial found that acupuncture was effective in decreasing chronic musculoskeletal shoulder pain in SCI subjects [38]. Several systematic reviews (including meta-analysis) have proved that acupuncture exerts a beneficial effect in neurological recovery, motor function recovery, and it might have some positive effect in decreasing postvoid residual (PVR) and in improving response rates for chronic urinary retention (CUR) due to spinal cord injury (SCI) [39].
Previous literature has reported various forms of acupuncture used for SCI, such as filiform needle acupuncture [40], electro-acupuncture [41], auricular acupuncture [42], scalp acupuncture [43], and laser acupuncture [44]. The potential mechanisms have been studied in a large number of animal experiments, which might be involved with reducing glial fibrillary acidic protein levels in the injured cord to inhibit reactive astrocyte proliferation. Also, acupuncture could act as an antioxidant, anti-inflammation, and antiapoptosis agent to promote axonal regeneration to inducing neuronal function recovery [45-48]. Fire needle is also a traditional form of acupuncture, which combines conventional acupuncture and cauterization with heated needles therapy. Studies showed that fire needle could effectively inhibit neuron apoptosis to improve neuronal function recovery of SCI rats [30].
In this study, we found that fire needle puncturing the Jiaji points surrounding the injured cord on the back could significantly increase the locomotor function of lower limbs in SCI rats during the 14-day treatment. In traditional Chinese medicine, stimulation at Jiaji points could harmonize the Governing Vessel (GV) and bladder meridian (BL). In anatomy, there is a high concentration of nerve endings and dense microvasculature around the points. To stimulate Jiaji points, it could largely activate local immune-inflammatory systems to induce higher visceral vasodilation than to stimulate the local points [49, 50]. The research found that acupuncture at Jiaji point could improve locomotor function evaluated by the BBB score at different time points after SCI [48], which is consistent with our results.
Inducing endogenous NSCs to proliferate and differentiate into neurons to compensate for the loss of neurons has been considered to be a potential and effective method to promote nerve regeneration and repair locomotor function after SCI [12]. Acupuncture also shows the ability to promote NSCs proliferation [48, 51] and to inhibit neurons apoptosis [52]. In our study, we chose nestin, which was highly expressed in actively proliferating NSCs, as the biomarker for NSCs [39, 53, 54]. NSCs are normally in a quiescent state in non-pathological conditions, which is also confirmed in our study: the expression of nestin was not detected in the spinal cord tissue of the blank group and the sham group. One day after spinal cord injury, the expression of nestin-positive cells in the injured spinal cord tissue of the model group and the fire needle group was significantly increased, indicating the activation of NSCs after SCI. At the following time points, the expression of nestin-positive cells in the fire needle group was significantly higher than that in the model group, suggesting that the fire needle could promote the activation, migration and proliferation of local NSCs after SCI.
NSE, Gal-C, and GFAP are chosen to be specific biomarkers in our study to reflect the expression of neurons, oligodendrocytes, and astrocytes, respectively [55-58]. In the process of self-repairment of spinal cord tissue, only a few NSCs will differentiate into neurons without external intervention. Oligodendrocytes could maintain and protect the normal function of axons, but it would cause a large number of oligodendrocyte apoptosis and axonal demyelination to hinder neurological function recovery after SCI [59, 60]. The reactively over-activated and overgrowing astrocyte could lead to glial scars and generate astrocytic barrier to regrowing axons to inhibit nerve regeneration after SCI [61]. In our study, the positive expressions of NSE and Gal-C were significantly reduced in the model group 1 day after SCI, and GFAP increased significantly at each time point, while fire needle could upregulate the expression of NSE and Gal-C, and inhibit GFAP, suggesting that the fire needle could promote endogenous NSCs differentiating into neurons and oligodendrocytes, and inhibit its differentiation into astrocytes in SCI rats.
Wnt and ERK signal transduction pathways play important roles in mediating endogenous NSCs differentiating into neurons [62-64] and they are closely related to its recovery after SCI [65-67]. There exists a crosstalk effect between Wnt and ERK pathways: both activated Wnt/β-catenin and ERK could phosphate GSK3β to regulate β-catenin to induce cyclinD1 and neurogenin1(ngn1). Ngn1 is a member of the Neurogenins family, the increased expression of ngn1 could induce the differentiation of NSCs into neurons [68, 69]. Cyclin D plays an important role in cyclins, and as the most important subunit of cell cycle regulation, CyclinD1 is highly expressed in many neural differentiation models and is also involved in the proliferation and differentiation of NSCs [70]. The increased expression of CyclinD1 could promote the proliferation of NSCs. In our study, we found that fire needle could increase the expression of Wnt3a, β-catenin protein and mRNA in Wnt/β-catenin pathway, to promote the proliferation of endogenous NSCs and its differentiation into neurons; also, fire needle could inhibit the over-expression of ERK1/2 protein and mRNA in ERK1/2 signaling pathway after SCI to prevent its over-activation. As for the common factor in two signal pathways, fire needle could positively promote protein and mRNA expression of GSK3β. It can be seen that fire needle could regulate Wnt/ERK multi-signal pathways bidirectionally, that is, promote the activation of Wnt/β-catenin signaling pathway and inhibit the overexpression of ERK signaling pathway after SCI. we also found that fire needle could increase the expression of ngn1 and cyclinD1 genes and proteins, thereby to promote the proliferation of NSCs and differentiation into neurons.
CONCLUSION
To our knowledge, this is the first study to investigate the fire needle regulating Wnt/ERK muti-signal pathways to promote the proliferation of NSCs and differentiation into neurons. Conclusively, we first demonstrate the therapeutic efficacy of fire needle in improving lower limb locomotor function of SCI rats. Also, fire needles could promote endogenous NSCs proliferation and differentiate into neurons and oligodendrocytes, and inhibit its differentiation into astrocytes in SCI rats. The mechanism of its promoting NSCs to differentiate into neurons might be mediated by promoting the activation of Wnt/β-catenin signaling pathway and inhibiting the overexpression of ERK signaling pathway after SCI.
Fig. (1).
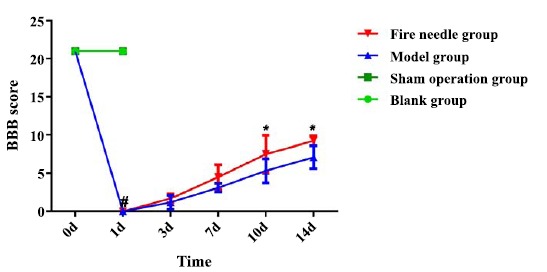
Basso, Beattie, and Bresnahan (BBB) scores of rats in each group at different time points postinjury.
Notes: #indicates compared with that in the sham operation group, p<0.05.
* indicates compared with that in the model group, p<0.05.
ACKNOWLEDGEMENTS
This work is supported by National Natural Science Foundation of China (grant numbers: 81373839).
Jiachun Xu, Suli Cheng, Zhaohua Jiao, Zhiheng Zhao, and Yan Li made substantial contributions to conception, design, and performing of the experiment. Zhimin Cai, Nan Su, Baohong Liu, and Zhen Zhou analyze the data and are involved in drafting the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the local ethical committee at Tianjin University of Traditional Chinese Medicine, China.
HUMAN AND ANIMAL RIGHTS
No human were used in this study. All animal experiments met the guidelines of the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Documentation 55, 2001).
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hawasli A., Rutlin J., Roland J.L., et al. Spinal cord injury disrupts resting-state networks in the human brain. J. Neurotrauma. 2017 doi: 10.1089/neu.2017.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iorio-Morin C., Noonan V.K., White B., et al. Quality of life and health utility scores among Canadian traumatic spinal cord injury patients - A national cross-sectional study. Spine. 2017 doi: 10.1097/BRS.0000000000002492. [DOI] [PubMed] [Google Scholar]
- 3.Cristante A.F., Barros Filho T.E., Marcon R.M., et al. Therapeutic approaches for spinal cord injury. Clinics (São Paulo) 2012;67(10):1219–1224. doi: 10.6061/clinics/2012(10)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raspa A., Pugliese R., Maleki M., et al. Recent therapeutic approaches for spinal cord injury. Biotechnol. Bioeng. 2016;113(2):253–259. doi: 10.1002/bit.25689. [DOI] [PubMed] [Google Scholar]
- 5.Dhaliwal P. Spinal cord injury, steroids and Latin America. World Neurosurg. 2016;90:636–637. doi: 10.1016/j.wneu.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 6.Gensel J.C., Tovar C.A., Bresnahan J.C., et al. Topiramate treatment is neuroprotective and reduces oligodendrocyte loss after cervical spinal cord injury. PLoS One. 2012;7(3):e33519. doi: 10.1371/journal.pone.0033519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heo I., Shin B.C., Kim Y.D., et al. Acupuncture for spinal cord injury and its complications: a systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2013;2013:364216. doi: 10.1155/2013/364216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng Q., Liu X., Shan Q., et al. Acupuncture for treatment of secondary osteoporosis in patients with spinal cord injury: a controlled study. Acupunct. Med. 2014;32(5):381–386. doi: 10.1136/acupmed-2013-010463. [DOI] [PubMed] [Google Scholar]
- 9.Ma R., Liu X., Clark J., et al. The impact of acupuncture on neurological recovery in spinal cord injury: a systematic review and meta-analysis. J. Neurotrauma. 2015;32(24):1943–1957. doi: 10.1089/neu.2014.3866. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Torres V., Gransee H.M., Mantilla C.B., et al. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury. J. Neurophysiol. 2017;117(2):537–544. doi: 10.1152/jn.00654.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H., Ahuja C.S., Salewski R.P., et al. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One. 2017;12(8):e0182339. doi: 10.1371/journal.pone.0182339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y., Uezono N., Yasui T., et al. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev. Dyn. 2017;247(1):75–84. doi: 10.1002/dvdy.24558. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Su B., Zhu H., et al. Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-kappaB and p38 MAPK. Exp. Ther. Med. 2016;12(6):3607–3613. doi: 10.3892/etm.2016.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H., Chen S., Gao K., et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 15.De Filippis L., Sudhof T.C., Pang Z.P. Harness the power of endogenous neural stem cells by biomaterials to treat spinal cord injury. Sci. China Life Sci. 2015;58(11):1167–1168. doi: 10.1007/s11427-015-4943-z. [DOI] [PubMed] [Google Scholar]
- 16.Gregoire C.A., Goldenstein B.L., Floriddia E.M., et al. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63(8):1469–1482. doi: 10.1002/glia.22851. [DOI] [PubMed] [Google Scholar]
- 17.Azim K., Fischer B., Hurtado-Chong A., et al. Persistent Wnt/beta-catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells. 2014;32(5):1301–1312. doi: 10.1002/stem.1639. [DOI] [PubMed] [Google Scholar]
- 18.Shitasako S., Ito Y., Ito R., et al. Wnt and Shh signals regulate neural stem cell proliferation and differentiation in the optic tectum of adult zebrafish. Dev. Neurobiol. 2017;77(10):1206–1220. doi: 10.1002/dneu.22509. [DOI] [PubMed] [Google Scholar]
- 19.Huang G.H., Yang X.T., Chen K., et al. Porf-2 inhibits neural stem cell proliferation through Wnt/beta-catenin pathway by Its GAP domain. Front. Cell. Neurosci. 2016;10:85. doi: 10.3389/fncel.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickinson M.E., McMahon A.P. The role of Wnt genes in vertebrate development. Curr. Opin. Genet. Dev. 1992;2(4):562–566. doi: 10.1016/s0959-437x(05)80172-8. [DOI] [PubMed] [Google Scholar]
- 21.Ring A., Kim Y.M., Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. 2014;10(4):512–525. doi: 10.1007/s12015-014-9515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giafis N., Katsoulidis E., Sassano A., et al. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res. 2006;66(13):6763–6771. doi: 10.1158/0008-5472.CAN-05-3699. [DOI] [PubMed] [Google Scholar]
- 23.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol. Res. 2012;66(2):105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Yu C.G., Yezierski R.P., Joshi A., et al. Involvement of ERK2 in traumatic spinal cord injury. J. Neurochem. 2010;113(1):131–142. doi: 10.1111/j.1471-4159.2010.06579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genovese T., Esposito E., Mazzon E., et al. Evidence for the role of mitogen-activated protein kinase signaling pathways in the development of spinal cord injury. J. Pharmacol. Exp. Ther. 2008;325(1):100–114. doi: 10.1124/jpet.107.131060. [DOI] [PubMed] [Google Scholar]
- 26.Jobe E.M., McQuate A.L., Zhao X. Crosstalk among epigenetic pathways regulates neurogenesis. Front. Neurosci. 2012;6:59. doi: 10.3389/fnins.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong A.M., Leong C.P., Su T.Y., et al. Clinical trial of acupuncture for patients with spinal cord injuries. Am. J. Phys. Med. Rehabil. 2003;82(1):21–27. doi: 10.1097/00002060-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Peng W., Clarke J., et al. Acupuncture for uterine fibroids. Cochrane Database Syst. Rev. 2010;13(1):CD007221. doi: 10.1002/14651858.CD007221.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Zhou Z., He X., et al. Therapeutic effect of fire-needle therapy on arthralgia syndrome. Chinese Moxibustion. 2003;23(07):34–37. [Google Scholar]
- 30.Sun L. Effect of fire needle on apoptotic cells in rats with spinal cord injury. J Clin Moxibustion. 2011;27:58–61. [Google Scholar]
- 31.Li Y., Li P., Zhou Z., et al. Effect of fire on COX-2, IL-1β and BDNF expression in sciatic nerve injury model rats. J Clin Moxibustion. 2007;23(12):36–39. [Google Scholar]
- 32.Li Y., Zhou Z., Cheng S., et al. Effect of fire acupuncture on motor function and BDNF expression after spinal cord injury in rats. Tianjin J Tradition Med. 2012;29(06):545–547. [Google Scholar]
- 33.Li Y., Zhou Z., Cheng S., et al. Effect of fire needle treated serum on the proliferation and differentiation of neural stem cells after spinal cord injury in rats. Tianjin J Tradition Med. 2013;30(1):25–27. [Google Scholar]
- 34.Chen S., Xu J., Wang J., et al. Improvement and behavioral evaluation of modified Allen 's modeling method in rats with acute spinal cord injury. J Tradition Med. 2015;33(10):43–45. [Google Scholar]
- 35.Scheff S.W., Saucier D.A., Cain M.E. A statistical method for analyzing rating scale data: the BBB locomotor score. J. Neurotrauma. 2002;19(10):1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- 36.Robinson N., Lorenc A., Ding W., et al. Exploring practice characteristics and research priorities of practitioners of traditional acupuncture in China and the EU-A survey. J. Ethnopharmacol. 2012;140(3):604–613. doi: 10.1016/j.jep.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 37.Green S., Buchbinder R., Hetrick S. Acupuncture for shoulder pain. Cochrane Database Syst. Rev. 2005;2:CD005319. doi: 10.1002/14651858.CD005319. [DOI] [PubMed] [Google Scholar]
- 38.Dyson-Hudson T.A., Shiflett S.C., Kirshblum S.C., et al. Acupuncture and Trager psychophysical integration in the treatment of wheelchair user’s shoulder pain in individuals with spinal cord injury. Arch. Phys. Med. Rehabil. 2001;82(8):1038–1046. doi: 10.1053/apmr.2001.24888. [DOI] [PubMed] [Google Scholar]
- 39.Wang J., Zhai Y., Wu J., et al. Acupuncture for chronic urinary retention due to spinal cord injury: a systematic review. Evid. Based Complement. Alternat. Med. 2016;2016:9245186. doi: 10.1155/2016/9245186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Q., Zhao Y., Hu D., et al. Clinical observation of acupuncture at trigone of urinary bladder for urination dysfunction induced by spinal cord injury. Zhongguo Zhenjiu. 2015;35(1):21–24. [PubMed] [Google Scholar]
- 41.Baoyan Q., Xiguo C., Yujuan M., et al. An observation on effects of electroacupuncture at Shu and Mu points in patients with neurogenic bladder after spinal cord injury. J. Rehabil. Med. 2016;31(01):50–53. [Google Scholar]
- 42.Estores I., Chen K., Jackson B., et al. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J. Spinal Cord Med. 2017;40(4):432–438. doi: 10.1080/10790268.2016.1141489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widrin C. Scalp acupuncture for the treatment of motor function in acute spinal cord injury: a case report. J. Acupunct. Meridian Stud. 2018;11(2):74–76. doi: 10.1016/j.jams.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Wong Y.M. Effect of laser acupuncture on heart rate variability of nonpatients and patients with spinal cord injury. J. Acupunct. Meridian Stud. 2017;10(1):53–54. doi: 10.1016/j.jams.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Choi D.C., Lee J.Y., Moon Y.J., et al. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol. Dis. 2010;39(3):272–282. doi: 10.1016/j.nbd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Lee J.Y., Choi D.C., Oh T.H., et al. Analgesic effect of acupuncture is mediated via inhibition of JNK activation in astrocytes after spinal cord injury. PLoS One. 2013;8(9):e73948. doi: 10.1371/journal.pone.0073948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juarez Becerril O., Salgado Ceballos H., Anguiano Solis C., et al. Electro-Acupuncture at GV.4 improves functional recovery in paralyzed rats after a traumatic spinal cord injury. Acupunct. Electrother. Res. 2015;40(4):355–369. [PubMed] [Google Scholar]
- 48.Wei Z., Wang Y., Zhao W., et al. Electro-acupuncture modulates L1 adhesion molecule expression after mouse spinal cord injury. Am. J. Chin. Med. 2017;45(1):37–52. doi: 10.1142/S0192415X17500045. [DOI] [PubMed] [Google Scholar]
- 49.Omura Y. Patho-physiology of acupuncture treatment: effect of acupuncture on cardiovascular and nervous systems. Acupunct. Electrother. Res. 1975;41(6):378–384. [Google Scholar]
- 50.Kendall D.E. A scientific model for acupuncture. Am. J. Acupunct. 1989;17(4):343–360. [Google Scholar]
- 51.Wu H., Hu M., Yuan D., et al. Electroacupuncture promotes the proliferation of endogenous neural stem cells and oligodendrocytes in the injured spinal cord of adult rats. Neural Regen. Res. 2012;7(15):1138–1144. doi: 10.3969/j.issn.1673-5374.2012.15.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J., Wu Y. Electro-acupuncture-modulated miR-214 prevents neuronal apoptosis by targeting Bax and inhibits sodium channel Nav1.3 expression in rats after spinal cord injury. Biomed. Pharmacother. 2017;89:1125–1135. doi: 10.1016/j.biopha.2017.02.077. [DOI] [PubMed] [Google Scholar]
- 53.Park D., Xiang A.P., Mao F.F., et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells. 2010;28(12):2162–2171. doi: 10.1002/stem.541. [DOI] [PubMed] [Google Scholar]
- 54.Xu R., Wu C., Tao Y., et al. Nestin-positive cells in the spinal cord: a potential source of neural stem cells. Int. J. Dev. Neurosci. 2008;26(7):813–820. doi: 10.1016/j.ijdevneu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Min S.K., Kim C.R., Jung S.M., et al. Astrocyte behavior and GFAP expression on Spirulina extract-incorporated PCL nanofiber. J. Biomed. Mater. Res. 2013;101(12):3467–3473. doi: 10.1002/jbm.a.34654. [DOI] [PubMed] [Google Scholar]
- 56.Doucette R., Devon R. Elevated intracellular levels of cAMP induce olfactory ensheathing cells to express GAL-C and GFAP but not MBP. Glia. 1995;13(2):130–140. doi: 10.1002/glia.440130206. [DOI] [PubMed] [Google Scholar]
- 57.Mercier E., Tardif P.A., Cameron P.A., et al. Prognostic value of neuron-specific enolase (NSE) for prediction of post-concussion symptoms following a mild traumatic brain injury: a systematic review. Brain Inj. 2018;32(1):29–40. doi: 10.1080/02699052.2017.1385097. [DOI] [PubMed] [Google Scholar]
- 58.Shinohara Y., Ohtani T., Konno A., et al. Viral vector-based evaluation of regulatory regions in the neuron-specific enolase (nse) promoter in mouse cerebellum in vivo. Cerebellum. 2017;16(5-6):913–922. doi: 10.1007/s12311-017-0866-5. [DOI] [PubMed] [Google Scholar]
- 59.Imai T., Katoh H., Suyama K., et al. Amiloride promotes oligodendrocyte survival and remyelination after spinal cord injury in rats. J. Clin. Med. 2018;7(3):E46. doi: 10.3390/jcm7030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J., Xiong L.L., Wang Y.C., et al. Oligodendrocyte precursor cell transplantation promotes functional recovery following contusive spinal cord injury in rats and is associated with altered microRNA expression. Mol. Med. Rep. 2018;17(1):771–782. doi: 10.3892/mmr.2017.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okada S., Hara M., Kobayakawa K., et al. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018;126:39–43. doi: 10.1016/j.neures.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Zhang W.M., Zhang Z.R., Yang X.T., et al. Overexpression of miR21 promotes neural stem cell proliferation and neural differentiation via the Wnt/betacatenin signaling pathway in vitro. Mol. Med. Rep. 2018;17(1):330–335. doi: 10.3892/mmr.2017.7856. [DOI] [PubMed] [Google Scholar]
- 63.Yang X.T., Huang G.H., Li H.J., et al. Rac1 Guides Porf-2 to Wnt Pathway to Mediate Neural Stem Cell Proliferation. Front. Mol. Neurosci. 2017;10:172. doi: 10.3389/fnmol.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan H., Zhang X., Luo S., et al. Effects of homocysteine on ERK signaling and cell proliferation in fetal neural stem cells in vitro. Cell Biochem. Biophys. 2013;66(1):131–137. doi: 10.1007/s12013-012-9461-z. [DOI] [PubMed] [Google Scholar]
- 65.Crown E.D., Ye Z., Johnson K.M., et al. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006;199(2):397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J., Li S., Wu Y. Recovery of spinal cord injury following electroacupuncture in rats through enhancement of Wnt/beta-catenin signaling. Mol. Med. Rep. 2017;16(2):2185–2190. doi: 10.3892/mmr.2017.6801. [DOI] [PubMed] [Google Scholar]
- 67.Yamagami T., Pleasure D.E., Lam K.S., et al. Transient activation of Wnt/beta-catenin signaling reporter in fibrotic scar formation after compression spinal cord injury in adult mice. Biochem. Biophys. Res. Commun. 2018;496(4):1302–1307. doi: 10.1016/j.bbrc.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reier P.J. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1(4):424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satoh J., Obayashi S., Tabunoki H., et al. Stable expression of neurogenin 1 induces LGR5, a novel stem cell marker, in an immortalized human neural stem cell line HB1.F3. Cell. Mol. Neurobiol. 2010;30(3):415–426. doi: 10.1007/s10571-009-9466-3. [DOI] [PubMed] [Google Scholar]
- 70.Kumar D.U., Devaraj H. Expression of Wnt 3a, beta-catenin, cyclin D1 and PCNA in mouse dentate gyrus subgranular zone (SGZ): a possible role of Wnt pathway in SGZ neural stem cell proliferation. Folia Biol. 2012;58(3):115–120. [PubMed] [Google Scholar]


