Abstract
Ovarian cancer (OC) is the seventh most common cancer in women worldwide. Standard therapeutic treatments involve debulking surgery combined with platinum-based chemotherapies. Of the patients with advanced-stage cancer who initially respond to current treatments, 50%–75% relapse. Immunotherapy-based approaches aimed at boosting antitumor immunity have recently emerged as promising tools to challenge tumor progression. Treatments with inhibitors of immune checkpoint molecules have shown impressive results in other types of tumors. However, only 15% of checkpoint inhibitors evaluated have proven successful in OC due to the immunosuppressive environment of the tumor and the transport barriers. This limits the efficacy of the existing immunotherapies. Nanotechnology-based delivery systems hold the potential to overcome such limitations. Various nanoformulations including polymeric, liposomes, and lipid–polymer hybrid nanoparticles have already been proposed to improve the biodistribution and targeting capabilities of drugs against tumor-associated immune cells, including dendritic cells and macrophages. In this review, we examine the impact of immunotherapeutic approaches that are currently under consideration for the treatment of OC. In this review, we also provide a comprehensive analysis of the existing nanoparticle-based synthetic strategies and their limitations and advantages over standard treatments. Furthermore, we discuss how the strength of the combination of nanotechnology with immunotherapy may help to overcome the current therapeutic limitations associated with their individual application and unravel a new paradigm in the treatment of this malignancy.
Introduction
Ovarian cancer (OC) ranks as the seventh leading cause of death in women worldwide. According to the American Cancer Society, 14,070 deaths and approximately 22,240 new cases were predicted for 2018 in the United States (Siegel et al., 2018). Of the patients with advanced-stage cancer who initially respond to current treatments, 50%–75% relapse. The asymptomatic nature of early-stage ovarian cancer is the main reason for its late diagnosis, which normally occurs at a metastatic stage, drastically reducing the chances of a successful outcome of the treatment (Das and Bast, 2008; Rauh-Hain et al., 2011). Despite the continuous improvement in screening methods, OC-associated mortality rates remain high due to the absence of routine early detection approaches. The lack of specificity of the available tests and the limitations associated with the application of imaging techniques further complicate the diagnostic process (Sarojini et al., 2012; Terry et al., 2016; Russell et al., 2017). OC comprises five histologic subtypes: low-/high-grade serous, mucinous, clear cells, and endometrioid cancer. Serous OC represents the most common carcinoma and accounts for more than 50% of all cases. It is associated with specific genetic mutations (i.e., BRCA1, BRCA2, MMR, TP53, BARD1, CHEK2, RAD51, and PALB2) spanning from single nucleotide polymorphisms to high frequency of somatic gene copies or epigenetic features, indicative of defects in homologous recombination repair and gene methylations (Kaldawy et al., 2016; Ducie et al., 2017). These subtypes metastasize to the same area within the peritoneal cavity.
Currently, the treatment of OC includes debulking surgeries, which are meant to excise tumor masses, coupled with extensive chemotherapy, radiotherapy, or a combination of the three depending on the stage and type of cancer. Recommended first-line treatments for OC are platinum-based and taxol drugs (www.nccn.org/guidelines). In some cases, after genetic screening, patients may be eligible for monoclonal antibody therapies such as bevacizumab, which blocks tumoral angiogenesis by inhibiting the vascular endothelial growth factor signaling. Other approaches include using olaparib, rucaparib, and niraparib, known as inhibitors of the poly(adenosine diphosphate [ADP]-ribose) polymerases and involved in DNA repair. The use of the latter treatments has been specifically recommended for patients with BRCA gene mutations (Coward et al., 2015). Table 1 explains the current therapies available for OC, including standard and targeted chemotherapies. The state-of-the-art nanotherapies currently being used or tested in clinical trials are also mentioned.
TABLE 1.
List of current therapies for ovarian cancer
Chemotherapies and targeted therapies are FDA approved. Some of the nanotherapies mentioned are already used in clinics, but the majority of them are still undergoing clinical trials.
| Drug Name |
Drug Class |
Formulation |
FDA Approved/Clinical Trial Phase |
Reference |
|---|---|---|---|---|
| Gold standard chemotherapeutic | ||||
| Doxorubicin | Antibiotics/antineoplastics | 1995 | Bolis et al., 1978 | |
| Carboplatin | Alkylating agents | 1989 | Adams et al., 1989 | |
| Paclitaxel | Mitotic inhibitors | 1998 | Khanna et al., 2015 | |
| Cyclophosphamide | Alkylating agents | 1959 | Handolias et al., 2016 | |
| Gemcitabine | Antimetabolites | 2006 | Lorusso et al., 2006 | |
| Melphalan | Alkylating agents | 2001 | Hasan and Jayson, 2003 | |
| Cisplatin | Alkylating agents | 1978 | Monneret, 2011 | |
| Topotecan | Miscellaneous antineoplastics | 1996 | Seiden et al., 2004 | |
| Etoposide | Mitotic inhibitors | 1998 | Long et al., 2005 | |
| Thiotepa | Alkylating agents | 2001 | Gordinier et al., 2002 | |
| Targeted therapies | ||||
| Bevacizumab | VEGF/VEGFR inhibitors | 2004 | Rossi et al., 2017 | |
| Olaparib | PARP inhibitors | 2017 | Moore et al., 2018 | |
| Niraparib | PARP inhibitors | 2017 | Essel and Moore, 2018 | |
| Rucaparib | PARP inhibitors | 2018 | Dal Molin et al., 2018 | |
| Current nanotechnology treatments and ongoing trials | ||||
| Doxil | Antibiotics/antineoplastics | Pegylated liposomal doxorubicin | 1999 | Pisano et al., 2013 |
| Lipodox | Antibiotics/antineoplastics | Pegylated liposomal doxorubicin | 2012 | Chou et al., 2006 |
| Genexol-PM | Mitotic inhibitors | PEG-PLA polymeric micellar paclitaxel | Phase II | Lee et al., 2017 |
| LEP-ETU | Mitotic inhibitors | Liposomal paclitaxel | Phase I | Damjanov et al., 2005 |
| Paclical | Mitotic inhibitors | Paclitaxel micelles | Phase III | NCT00989131 |
| OSI-211 | Antineoplastics | Liposomal lurtotecan | Phase II | Seiden et al., 2004 |
PARP, poly(adenosine diphosphate [ADP]-ribose) polymerase; PLA, polylactic acid; VEGF, vascular endothelial growth factor.
The 5-year survival rate for women with advanced-stage OC is approximately 40% (Timmermans et al., 2018; Torre et al., 2018) but increases if the ovarian tumor has more infiltrating T cells (Zhang et al., 2003). The lack of a curative therapeutic regimen, the frequency of relapse, and the mortality levels underlie the effort needed to refine the current treatment options and improve patient outcomes. The diversity of physiopathology (Nezhat et al., 2015) between OC types and the heterogeneity of cells infiltrating the peritoneum calls for the identification of effective approaches to maintain the bioactivity of the payload, precisely aim at the target, and preferentially accumulate the drug at the site of interest while reducing cytotoxicity.
Nanomedicines are frequently used as engineered drug delivery systems that support the prolonged circulation of drugs, maintain their bioactivity, reduce their side effects, and selectively target diseased cells (Blanco et al., 2015). Targeted nanomedicines include liposomal nanocarriers [small interfering RNA (siRNA)–EphA2, OSI-211, Myocet (liposome-encapsulated doxorubicin citrate; Ben Venue Laboratories)] (Seiden et al., 2004; Shen et al., 2013; Eitan et al., 2014), polymeric nanoparticles (Abraxane [Protein-bound Paclitaxel; Celgene], CRLX101) (Srinivasan et al., 2014; Pham et al., 2015), and antibody-drug conjugates (Howard et al., 2016). Nanotechnology-based strategies for diagnostic tools have been also developed to detect biomarkers and genetic mutations (Engelberth et al., 2014), as well as to combine nano-enabled therapeutic and diagnostic capabilities, giving rise to “nano-theranostics” (Yaari et al., 2016).
In this review, we discuss the potential of cancer immunotherapy, a recently developed field that aims at treating cancer patients by restimulating their immune system. Particular emphasis is given to its applications and pitfalls in OC. We also review how a nanomedicine approach to immunotherapy may overcome the current therapeutic limitations of the treatment of OC and unravel a new paradigm in the cure of this malignancy.
Immunotherapy and Cancer
Cancer immunotherapy aims at stimulating the immune system to provide cancer prevention and treatment. The first discoveries of the crucial role played by the immune regulation in cancer progression have recently led to the 2018 Nobel Prize for Medicine and Physiology to Dr. James P. Allison and Dr. Tasuku Honjo (www.nobelprize.org). Their studies unraveled fundamental mechanisms that govern immune cell (specifically T cells) responses to cancer and provided insights to overcome immune system evasion by cancer. Since then, the use of immune checkpoint blockade has been widely recognized as an effective cancer treatment. In particular, Dr. Allison and his research group have been the first to identify the cytotoxic T-lymphocyte antigen 4 (CTLA-4) protein, an immune checkpoint receptor expressed on the surface of activated T cells that is believed to regulate their proliferation. When the CTLA-4 pathway is activated by costimulatory molecules (CD80, CD86), the result is hindrance of T-cell function, which inhibits the T-cell strong anticancer potential (Leach et al., 1996). Based on these observations, a specific antibody was developed to retain CTLA-4 activation and maintain T cells in an activated status (Chambers et al., 1996). Almost simultaneously, in 1992 Dr. Honjo’s group discovered programmed death-ligand 1, which also acts as a T-cell retainer, finding an alternative way to defeat the tumor-mediated immune evasion. The insights provided by such inspiring scientists have led to many Food and Drug Administration (FDA)–approved drugs for the treatment of various cancers. These drugs span from sipuleucel-T, approved in 2010 to target the immune system for the treatment of prostate cancer (Cheever and Higano, 2011), to ipilimumab, the first monoclonal antibody against CTLA-4 for metastatic melanoma (Lipson and Drake, 2011). By 2018, eight immunotherapies had been FDA approved for the treatment of several cancers (Table 2), including durvalumab (stage 3 lung cancer), blinatumomab (acute lymphoblastic leukemia), and nivolumab (used in combination with ipilimumab for previously untreated kidney cancers) (https://www.cancer.gov/news-events/cancer-currents-blog/fda-approvals).
TABLE 2.
FDA-approved immunotherapeutics since the beginning of 2018
| Drugs | Mechanism of Action | Targeted Disease | Release Date |
|---|---|---|---|
| Durvalumab (IMFINZI, AstraZeneca) | Checkpoint immunotherapy targeting PD-1/PD-L1 pathway | Stage III non–small-cell lung cancer | February 16, 2018 |
| Brentuximab vedotin (Adcetris, Seattle Genetics, Inc.) | Antibody-drug conjugate targeting the CD30 receptor | Untreated classic Hodgkin lymphoma | March 20, 2018 |
| Blinatumomab (Blincyto, Amgen Inc.) | Bispecific T cell–engaging antibody targeting CD19 receptor | B-cell precursor acute lymphoblastic leukemia | March 29, 2018 |
| Nivolumab (Opdivo, Bristol-Myers Squibb) + ipilimumab (Yervoy, Bristol-Myers Squibb) | Checkpoint immunotherapy targeting PD-1 and CTLA-4 | Advanced renal cell carcinoma | April 16, 2018 |
| Tisagenlecleucel (Kymriah, Novartis) | CAR-T cell immunotherapy targeting CD19 receptor | Relapsed/refractory large B-cell lymphoma | May 01, 2018 |
| Pembrolizumab (Keytruda, Merck) | Checkpoint immunotherapy targeting PD-1 | Cervical cancer | June 12, 2018 |
| Pembrolizumab (Keytruda, Merck) | Checkpoint immunotherapy targeting anti–PD-1 | Adult and pediatric primary mediastinal large B-cell lymphoma | June 13, 2018 |
| Nivolumab (Opdivo,l Bristol-Myers Squibb) + ipilimumab (Yervoy, Bristol-Myers Squibb) | Checkpoint immunotherapy targeting PD-1 and CTLA-4 | Relapsed colorectal cancer with high microsatellite instability or deficient DNA mismatch repair | July 10, 2018 |
| Nivolumab (Opdivo, Bristol-Myers Squibb) | Checkpoint immunotherapy targeting PD-1 | Metastatic small-cell lung cancer | August 17, 2018 |
PD-1, programmed death-ligand 1.
Immunotherapeutic approaches include the use of targeted antibodies and vaccines against immune checkpoint inhibitors directed toward a specific immune cell population (Ventola, 2017). For instance, due to their antigen-presenting capabilities, dendritic cells (DCs) have been used to develop immune vaccines (Sabado et al., 2017). Depending on the molecules used to activate them, DCs are able to reprogram or launch a cell-specific cytotoxic response. Conversely, tumor-associated macrophages (TAMs) have been shown to exert different roles in tumor microenvironment development and flourishing (Mills et al., 2016). Approaches that target this macrophage population are currently being evaluated, especially since the discovery that the blockade of TAMs potentiates immune checkpoint inhibitors’ effect (Ries et al., 2014; Zhu et al., 2014). Adoptive T-cell therapy to re-engineer the T-cell populations against tumor initiation is another strategy that has been widely validated (Dzhandzhugazyan et al., 2018). The chimeric antigen receptors re-engineered T cells (CAR-T) system has been recently approved by the FDA for the treatment of patients with leukemia, large B-cell, and non-Hodgkin lymphomas (Zheng et al., 2018). Other focuses involve the use of a different immune cell population, the natural killer T cells. Natural killer T cells naturally stimulate the innate and adaptive immune system in several ways, such as the release of interferon-γ to activate the CD8+ T-cell population (Mah and Cooper, 2016). They are being investigated as potential immunotherapies both as ex vivo expanded cell vaccines and as combinatorial therapies (Nair and Dhodapkar, 2017).
Ovarian Cancer: A “Cold” Enemy
The characterization of the topographic distribution of immune cells within the tumor in a panel of 177 human samples with different cancer types has recently led to their categorization as inflamed (“hot”), noninflamed (“cold”), and “immune excluded” patterns according to where the cells are positioned (Kather et al., 2018). Cold tumors are malignancies that display a very limited response to immunotherapies compared with other cancer types. OC is considered a “cold” tumor (Preston et al., 2011) despite the significant association between tumor immunity and ovarian patient outcomes and the strong correlation between the presence of infiltrating lymphocytes in the primary tumor and patient survival (Zhang et al., 2003). The reasons behind this lack of effectiveness have yet to be clarified. A possible explanation, proposed for pancreatic cancer, suggests that the difference between hot and cold tumors reflects the way tumor-infiltrating immune cells are recognized by cancer cells or engage in the tumor. If so, the properties of the microenvironment make a tumor hot or cold. Hot tumors are more sensitive to treatments that activate the T-cell population, as they are considered to be the main drivers of the adaptive immune response against tumor initiation (Haanen, 2017).
The tumor microenvironment is a complex hub where different cell types interact with each other and with the extracellular matrix, and it is plausible that other cells, including antigen-presenting cells (APCs), play an active role in downregulating the immune system. APCs, including the aforementioned DCs, are highly responsive to external stimuli, and the tumor surroundings can negatively affect their physiologic behavior. Indeed, it has been shown that endoplasmic reticulum stress is also crucial for triggering cancer resistance mechanisms by activating the unfolded protein response, which in turn disrupts the physiologic immune response (Yadav et al., 2014). Specifically, through the constitutive activation of the endoplasmic reticulum stress response factor XBP1, DCs undergo an abnormal lipid accumulation that leads to their ineffective functioning (Cubillos-Ruiz et al., 2015). While low infiltration of immune cells both inside and outside the tumor is found in OC samples, the coexistence of different immune microenvironments within the same patient partly explains the heterogeneity in the response to treatment often observed in patients with recurrent disease (Jiménez-Sánchez et al., 2017).
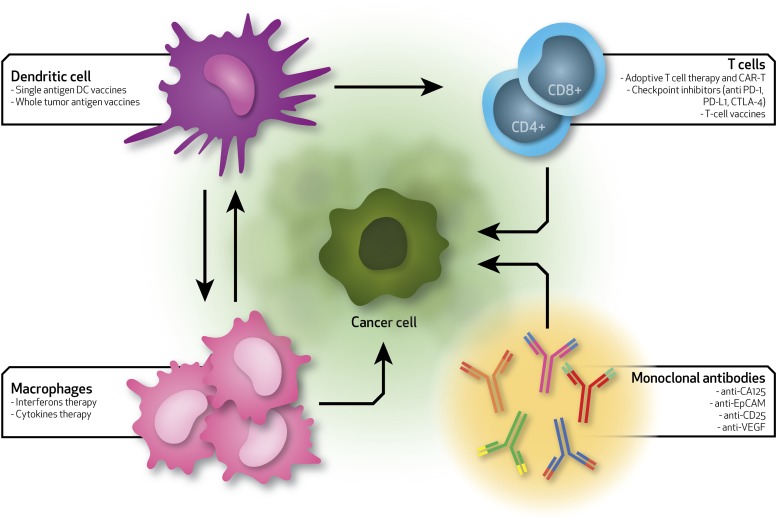
Currently, there are no FDA-approved immunotherapies for OC, although there are several ongoing clinical trials. Of the 98 total clinical trials, 26 have been completed, 40 are actively recruiting patients, and nine have been terminated before their planned end due to the inefficacy determined by the limitations described in the previous paragraphs (https://clinicaltrials.gov). In 2016, Gaillard et al. reported a comprehensive analysis of all clinical trials on checkpoint inhibitors, and discovered that, on average, the efficacy of these treatments is surprisingly poor in OC patients (Gaillard et al., 2016). The positive outcome was found to be around 10%–15%. A schematic representation of the immunotherapy-based approaches used in OC and the interactions between different immune players and tumor cells is provided in Fig. 1.
Fig. 1.
Schematic of the current immunotherapies for ovarian cancer. Arrows show the interactions between immune system players (dendritic cells, T cells, macrophages, and monoclonal antibodies) and ovarian cancer cells. Each specific immune cell type can be used to deliver specific therapies that can differently alter the immune system toward a more efficient activity rate. EpCAM, epithelial cell adhesion molecule; VEGF, vascular endothelial growth factor.
Monoclonal Antibodies in OC.
In the attempt to enhance treatments for OC, a number of monoclonal antibodies capable of inhibiting the function of molecules involved in tumor progression and immune suppression. Catumaxomab is a monoclonal antibody directed against the epithelial cell adhesion molecule, a glycoprotein highly expressed in OC (Tayama et al., 2017). This antibody is currently being evaluated in a phase II clinical study on patients resistant to chemotherapy (Berek et al., 2014). Following the identification of cancer antigen 125, which is the most renowned OC marker (Bast et al., 1981), its role as a suppressant of both natural killer cell activity (Patankar et al., 2005; Tyler et al., 2012) and antibody-dependent cellular toxicity (Kline et al., 2017) has been widely investigated. Several anti–cancer antigen 125 monoclonal antibodies have been developed and tested, including oregovomab (Berek et al., 2009) and abagovomab (Sabbatini et al., 2013), although they did not prove to be effective in improving the outcomes in advanced OC when used as a single-agent maintenance immunotherapy. Anti-CD25 (daclizumab) has been clinically tested for its capacity to suppress the T regulatory cell populations, which are responsible for shorter patient survival rates when infiltrated within the tumor (Barnett et al., 2010). Although the trial has been completed, the results have not been released yet. The translational potential of anti-CD25–based platforms is limited by their nonspecific binding, as CD25 is widely expressed on T-cell populations.
Dendritic Cell Vaccines in OC.
Dendritic cells have a pivotal role in launching the immune response due to their capacity for activating CD4+ or CD8+ T cells (Sallusto and Lanzavecchia, 2002). Their role in the tumor microenvironment is the subject of active contemporary research (Pfirschke et al., 2017). As plastic APCs, DCs are currently harnessed for their potential to boost the immune system against tumor initiation and progression. Scarlett et al. (2012) applied an inducible p53-dependent model of aggressive ovarian carcinoma to demonstrate that DCs display differential immunostimulatory capacity during tumor initiation and escape. These changes correspond to significantly lower levels of major histocompatibility complex II (MHC-II) and CD40 on their surface. DCs are tunable cells, capable of either inducing an immune surveillance effect or releasing malignant growth by activating or suppressing antitumor T-cell activity, respectively. DC-based vaccines have also been conceived in the context of OC, by ex vivo pulsing DCs with tumor-derived components, as single tumor-associated peptides or peptide combinations (Liao and Disis, 2013). Cancer testis antigens (CTAs) that are typically expressed in multiple types of tumors have also gained interest for their potential applicability in immunotherapy (Gjerstorff et al., 2015; Seifi-Alan et al., 2018). NY-ESO-1, a member of the CTA family, has been used to produce either DC-based vaccines (National Clinical Trial [NCT] number NCT02387125) or adoptive T-cell therapies (NCT number NCT01567891). Similar immunotherapeutic approaches are being developed using melanoma antigens (i.e., MAGE-A1, MAGE-A4, MAGE-A3, and MAGE-A10) that represent another subgroup of the CTA category (Daudi et al., 2014). Zitvogel et al. (1996) are among the first researchers to use tumor antigen-pulsed DCs to treat mice with fibrosarcoma. They also demonstrated that patient-derived DCs pulsed with a cocktail of tumor antigens [whole tumor antigen (WTA)] can trigger a tumor growth suppression through the activation of CD4+ and CD8+ T cells when reintroduced into the patient. In their study, the activation of T cells correlated with a better prognosis in patients with recurrent ovarian, fallopian tube, or primary peritoneal cancer (Kandalaft et al., 2013; Tanyi et al., 2018). Recently, a pilot study demonstrated an autologous WTA-pulsed DC-based vaccine to be safe and effective in combination with cyclophosphamide and bevacizumab (Tanyi et al., 2018). By priming DCs with patient-derived WTA, Tanyi et al. (2018) were able to overcome two of the limitations associated with the use of immunotherapy for the treatment of OC: the lack of an efficient antigen-specific active treatment and the inability of tumor-specific T cells to home to tumors.
Adoptive Cell Therapy in OC.
Adoptive cell therapies show potential for the treatment of OC. For example, it has been shown that tumor-infiltrating lymphocytes derived from OC biopsy-derived cells can be expanded ex vivo and be reactivated to produce antitumor cytokines (Owens et al., 2018). Similarly, the abundance of tumor-infiltrating lymphocytes in patients’ ascitic fluid has prompted their evaluation as reinjectable immunotherapies after their demonstrated cytotoxic effect on tumor cells (Abe et al., 2018). CAR-T–based therapy produced by combining programmable antigen receptor specificity with T-cell activation also holds an attractive opportunity for the treatment of OC (Dzhandzhugazyan et al., 2018). The lack of a demonstrable efficacy of this approach is mainly due to the poor T-cell trafficking and the immunosuppressive microenvironment (Zhang et al., 2016; Mirzaei et al., 2017; Jindal et al., 2018). Despite the potential pitfalls of this approach, clinical trials evaluating its efficacy in OC are currently active and specifically target mesothelin (NCT02580747, NCT01583686), MUC16 (NCT02498912), HER2 (NCT01935843), and NY-ESO-1 (NCT02366546), among others (Zhu et al., 2017).
Nanomedicines and Immunotherapy in Ovarian Cancer
Synthetic and natural nanotechnologies are currently being investigated to deliver immunotherapies, as they have the potential to improve patient treatment outcomes and reduce mortality rates (Shen et al., 2017). This includes the use of nanoparticles for the delivery of immunostimulatory and immunosuppressive molecules in combination with chemo- or radiotherapy or as adjuvants to other immunotherapies (Sapiezynski et al., 2016). Nanoparticles have also been designed to produce vaccines to stimulate T-cell response against tumor growth (Fan and Moon, 2015), allowing for the codelivery of antigen and adjuvants (Dunkle et al., 2013), contributing to the inclusion of multiple antigens to activate DC targets (Xia et al., 2015), and guaranteeing the sustained release of antigens for a prolonged immune stimulation (Engelberth et al., 2014).
The literature reports only a few examples of preclinical studies investigating the potential of nanotechnology-based platforms to improve the outcome of immunotherapeutic regimens in OC. These include polymeric nanoparticles (Cubillos-Ruiz et al., 2009; Hanlon et al., 2011; Ortega et al., 2015; Teo et al., 2015), liposomes (Turk et al., 2004; Rajan et al., 2018), and lipid–polymer hybrids (Anwer et al., 2013).
Nanoplatforms for OC have been synthesized primarily to guide the delivery of RNA oligonucleotides to target cells, thus overcoming the current limitations related to the use of RNA therapeutics. Limitations include low bioavailability, poor cellular uptake, cytotoxicity, and the need to evade the phagocytic cellular components of the immune system (Kole et al., 2012). Polymeric nanostructures have been developed to provide additional control over drug release at tumor sites, as they offer the advantage of being able to respond to specific stimuli provided by the tumor environment, such as pH and enzymatic activity (Uthaman et al., 2018). Among the many polymers available, polyethylenimine (PEI) is one of the most used materials in OC treatment, as it is considered a versatile gene carrier (Teo et al., 2013). PEI displays high efficacy for siRNA encapsulation and delivery for both in vitro and in vivo purposes. Its cationic charge enables the loading of siRNA into nanocomplexes and protects it from enzymatic degradation (Zheng et al., 2011; Höbel and Aigner, 2013). The abundant presence of amine groups allows for the functionalization of the platform and favors further modifications of this polymer to improve the bioactive features, such as its targeting ability and cell specificity. Cubillos-Ruiz et al. (2009) investigated PEI-siRNA nanoparticle uptake by tumor-associated DCs and its effect in reprogramming their phenotype from immunosuppressive cells to efficient APCs. The authors found that the changes induced in DCs through the use of PEI-siRNA against immunosuppressive determinants consequently activated tumor-reactive human and murine lymphocytes and exerted a direct tumoricidal activity in aggressive ovarian carcinoma–bearing murine models (Cubillos-Ruiz et al., 2009). The induced T cell–mediated tumor regression and prolonged survival were dependent upon the activation of the myeloid differentiation primary response gene 88 (MyD88). PEI alone was sufficient to mediate the upregulation of MHC-II, MHC-I, and costimulatory molecules in tumor DCs in vivo. This suggests that the intrinsic stimulation of Toll-like receptors 5 and 7 by PEI nanoparticles synergizes with the gene-specific silencing activity of the siRNA to transform tumor-infiltrating regulatory DCs into cells capable of promoting therapeutic antitumor immunity. Cubillos-Ruiz et al. (2012) further optimized the platform to achieve the synthetic enhancement of the specific molecular pathway miR-155 signaling in DCs. This pathway is responsible for boosting a potent antitumor immune response that abrogates the progression of established ovarian cancers (Cubillos-Ruiz et al., 2012). Other researchers have taken advantage of polymeric nanoparticles’ capability to be functionalized, thus improving targeting and, consequently, the therapeutic outcome. By applying a different immunotherapy-based approach, Teo et al. (2015) proposed various folic acid (FA)–functionalized PEI polymers to block programmed death-ligand 1/PD-L1 interactions by delivering PD-L1 siRNA to human epithelial ovarian cancer (EOC) cells (SKOV-3 line) and to sensitize them against T cells. With their hypothesis to target PD-L1, the authors responded to the need for a specific targeted delivery of PD-L1 siRNA to epithelial cancer tissues, as PD-L1 is also expressed on healthy tissues (Liang et al., 2003), including placenta and eyes. The polymer/siRNA nanocomplexes knocked down PD-L1 on a luciferase-expressing SKOV-3, enhancing the efficacy of T-cell immunotherapy for the treatment of EOC compared with the respective PEI–FA and PEI–polyethylene glycol (PEG)–FA/scrambled siRNA treated controls. These data highlight the potential use of PEI–FA as specific gene delivery carriers. The modification of PEI with FA or PEG–FA proved to be a valuable tool to reduce cytotoxicity while improving tumor cell targeting toward EOC cells and uptake, with a striking ≈40%–50% knockdown of PD-L1 expression. Ortega et al. (2015) used click chemistry to produce nanoparticles based on 2-(dimethylamino)ethyl methacrylate polymer further functionalized with the mannose ligand (MnNP). This platform was meant to condense siRNA against the polyoma middle T oncogene and specifically target the mannose receptor (CD206) present on the surface of TAMs (Ortega et al., 2015). MnNP has been demonstrated to be biocompatible in both in vitro and in vivo settings. MnNP is also able to efficiently incorporate and deliver functional siRNA into the cytoplasm of TAMs. This study provides evidence that mannosylation is responsible for TAM selectivity in vivo following intraperitoneal injection with a twofold increase in TAM uptake compared with nontargeted particles and about a 10-fold increase compared with nonmyeloid cells. In this study, the spatial confinement of the MnNP within the peritoneal cavity enhanced the opportunity for the interaction with immune cells associated with OC, and the biodegradability of the system ensured the persistence of the treatment for over 24 hours.
Poly(lactic-co-glycolic acid) nanoparticles (PLGA-NPs) are biodegradable, and their composition can be tuned to temporally control the release of the payload (Corradetti et al., 2012; Minardi et al., 2016). PLGA-NPs have been used as an alternative route to deliver whole WTA to DCs since the injection of soluble antigens presents inherent limitations due to instability and poor internalization rates. These factors result in the transient and inefficient activation of T cells (Hanlon et al., 2011). At the same time, PLGA-NPs protect antigens from enzymatic degradation and maintain their bioactivity, leading to a more efficient presentation of MHC-peptide complexes by recipient cells following uptake and processing. In vitro studies have confirmed the effectiveness of PLGA-NPs in the activation of a CD8+ cell response characterized by a significant increase in the production of inflammatory cytokines and a greater expression of costimulatory molecules, providing encouraging evidence for their potential clinical translation. Interestingly, the delivery of WTAs through PLGA-NPs appeared to facilitate the antigens’ access to the MHC class I compartment in the cytoplasm, providing a reservoir for a prolonged and enhanced Ag presentation.
Liposomes are small, artificial, spherical vesicles synthesized primarily from natural nontoxic phospholipids (Akbarzadeh et al., 2013). Their wide application as drug delivery systems in biomedical settings is due to their biocompatibility, biodegradability, low toxicity, and capability to load both hydrophobic and hydrophilic drugs (Johnston et al., 2007). Moreover, liposomal encapsulation offers the advantage of effectively enhancing the solubility of lipophilic and amphiphilic drugs and improving site-specific drug delivery to tumor tissues through surface functionalization (Hofheinz et al., 2005; Corradetti et al., 2012). The latter aspect is crucial to increase the retention time, which can be modulated by drug-lipid interactions, and permit the accumulation of liposome-encapsulated chemotherapeutic agents at the tumor site (Deshpande et al., 2013).
Doxil (Ben Venue Laboratories) was the first pegylated liposome–based drug to enter the market in 1995. The nanoformulation includes doxorubicin, a DNA intercalating agent used against a variety of cancers, including gynecologic cancers (Howard et al., 2016). While no significant differences were observed in terms of efficacy compared with the free drug, the liposomal formulation allowed the reduction of cardiotoxicities related to the use of doxorubicin and the preferential accumulation of the drug at the tumor site (Green and Rose, 2006). More recently, the FDA approved the use of an RNA interference therapeutic delivered by lipid nanoparticles: patisiran (Adams et al., 2018). Although developed for the treatment of degenerative diseases, patisiran shows promise as a new breakthrough in patient care, as it heralds the arrival of an entirely new class of medicines to treat human diseases. However, despite the wide interest in the use of liposomal formulations for OC treatment, only one group has tested liposomes as nanocarriers for immunotherapy. Turk et al. (2004) developed folate-conjugated liposomes to target intraperitoneal ovarian carcinoma cells, as they overexpress the folate receptor. Data revealed that this formulation was also absorbed by TAM through folate receptor–mediated internalization, with a 10-fold increase in the engulfment of macrophages compared with ascitic tumor cells in vivo, corroborating the need to develop combinatorial strategies aimed at modulating TAM and inhibiting cancer cell growth.
Lipid–polymer hybrid nanoparticles (LPNs) are core–shell nanoparticle structures constituted by a polymeric core and a lipid shell. LPNs have been considered by other researchers to confer a high degree of physical stability to the platform, resulting in a superior in vivo cellular delivery efficacy (Hadinoto et al., 2013) compared with polymeric and liposomal nanoparticles. The combination of the two LPN platforms formulated with a lipopolymer PEG–PEI–cholesterol was used as an effective tool to deliver an interleukin 12 plasmid at the tumor site. Interleukin 12 was chosen for the therapeutic action it plays in OC, which relies on its potential to activate the antitumor immunity (Whitworth and Alvarez, 2011). This approach proved to be safe and effective in platinum-sensitive OC patients treated with intravenous carboplatin and docetaxel (Anwer et al., 2013).
Physical and Biologic Barriers Challenging the Treatment of OC
Innovative immunotherapeutic-targeted strategies mediated by nanotechnology offer the promise of enhancing host antitumor responses which may improve clinical outcomes in women with OC. Although preclinical studies have demonstrated the induction of an antitumor response, there is no clinically effective nanomedicine-based immunotherapy available for OC patients. The biologic barriers that physically and mechanically influence the processes involved in tumor spread and immune cell infiltration must be considered when developing new strategies for the treatment of OC. As mentioned earlier, one of the main mechanisms by which OC cells spread is through transcoelomic metastasis, which involves dissemination throughout the peritoneal cavity (Tan et al., 2006). Ascite formation is determined by the accumulation of cancer cells, growth factors, and immunosuppressive ligands (vascular endothelial growth factor and fibroblastic growth factor β), which increase peritoneal capillary permeability (Ahmed and Stenvers, 2013) and thus the leakage of plasma proteins (i.e., albumin, fibrin, and fibrinogen) from newly developed vessels (Stanojevic et al., 2004). The obstruction of lymphatic vessels by cancer cells also occurs, which leads to an impaired reabsorption of the physiologic peritoneal fluid (Kipps et al., 2013). As a consequence of the compromised lymphatic drainage of the peritoneal cavity, fluid confinement in the peritoneum occurs, contributing to the pathogenesis of malignant ascites. The environment that these biologic and physical processes create impedes immune cell migration and infiltration within the metastatic tumors (Cai and Jin, 2017) and induces a peripheral tolerance that attenuates their function (Kulshrestha et al., 2017). For instance, ascites proved to recruit and immunologically suppress a population of neutrophils through cell contact in a cohort of newly diagnosed OC patients (Singel et al., 2017). The release of macrophage migration inhibitory factor from ascite-derived cancer cells has also been proposed to halt the tumor-killing ability of NK cells by transcriptionally downregulating the expression of the surface receptor NKG2D (Krockenberger et al., 2008). These findings confirm the proactive role of malignant ascitic fluid in physically supplying cells and chemical stimuli to favor an immune-suppressed environment. Additionally, another physical barrier to immune cell penetration is represented by the tumor vascular endothelium (Motz and Coukos, 2013). In a physiologic environment, the presence of adhesion molecules, such as intercellular cell adhesion molecule or vascular cell adhesion molecule, allows T cells to adhere to and travel through the endothelium. In the tumor milieu, the release of angiogenic growth factors prevents T cells from passing through by inhibiting the adhesion molecules’ expression (Bouzin et al., 2007).
The use of nano-sized molecules/structures that are able not only to precisely target and accumulate at the site of interest and maintain the bioactivity of the drug while ensuring its release but also to overcome biologic and physical barriers is pivotal in unveiling the mechanisms behind tumoral immune suppression. The development of approaches capable of capitalizing on the transport oncophysics of the peritoneal cavity will improve the delivery strategies for the treatment of metastatic OC (Nizzero et al., 2018).
Exosomes: An Alternative Tunable and Nanoscopic Strategy
Recently, biologic nanoparticles (called exosomes) have also emerged as a powerful translational platform to be harnessed in the development of naturally inspired delivery systems. Exosomes are nanoscopic lipidic vesicles with a size range spanning from 30 to 150 nm that are released by cells and thus retain their bioactive moieties. Due to their small size and architecture, exosomes can penetrate across the lymphatic vessels and tumor interstitium and reach target organs (Srinivasan et al., 2016). Their composition and cargo can be further modified by conditioning parental cells or by improving their natural potential with the addition of functional drugs, thus giving them additional functions (Conlan et al., 2017). Exosomes play a crucial role in cell-to-cell communication and are characterized by a precise targeting potential that allows for the activation or repression of specific molecular cascades in targeted cells (Syn et al., 2017). Currently, their role in the exchange of information between the tumor and the surrounding microenvironment is being explored (Maia et al., 2018), as is their potential as delivery vessels for both therapeutic and imaging purposes (Luan et al., 2017; Shen et al., 2018).
Recent advances in the field of immunotherapy unveiled the role of appropriately stimulated exosomes released from cancer cells as potent endogenous nanocarriers responsible for the suppression of T cells and the facilitation of tumor growth (Chen et al., 2018). Once injected for therapeutic purposes, exosomes are not susceptible to further modifications determined by the microenvironment, offering a great advantage over the use of CAR-T cells or DCs, which are amenable to acquiring a different phenotype (Yamashita et al., 2018).
Interestingly, they have also been proposed as useful tools to predict the patient response to immunotherapy. On the other hand, exosomes derived from immune cells, APCs, or TAMs are now at the forefront for the development of innovative vaccine strategies for cancer immunotherapy against tumor initiation and are the subject of current clinical trials for the treatment of other tumor types (Hong et al., 2017; Liu et al., 2017).
Conclusions and Perspectives
In this review we discussed the widely recognized impact of immunotherapy in the treatment of cancers, highlighting the challenges researchers face in the effort to overcome the limitations provided by OC. These include its cold nature, determined by the immunosuppressive environment and the transport oncophysics, which urgently calls for the conception of alternative approaches to deliver immunotherapies. Ideally, these approaches are meant to preferentially accumulate the drug at the tumor site; sustain the temporal and spatial release of the payload, thereby reducing cytotoxicity; and selectively target specific cell types to stimulate antitumor immunity (Fig. 2). Nanotechnology offers advantageous drug delivery systems with demonstrated therapeutic efficacy, with a direct or indirect effect on cancer cells. However, the potential of nanomedicines for the treatment of OC has been harnessed to a limited extent. Although capable of identifying and targeting the cell population of interest, none of the nano-enabled strategies proposed have yet shown significant clinical benefits. Furthermore, the literature lacks a comprehensive discussion about the in vivo biodistribution of the proposed nanoplatforms, reinforcing the concept that the drastic changes within the peritoneal cavity in terms of transport oncophysics and metastases heterogeneity largely limit their capability to reach tumor masses. A deep understanding of the role exosomes play in traveling and mediating cell interaction within the OC environment will successfully lead to the development of cutting-edge approaches to prime the body’s immune system against tumor initiation. The continuous advancements in the field of nanotechnology will provide the tools needed to synthesize exosome-resembling particles to be used as alternative immunotherapy treatment of OC. Another approach may include the coupling of naturally derived exosomes with established multistage vectors, demonstrated to achieve efficient delivery of chemotherapeutics to metastatic breast cancer (Xu et al., 2016) and ovarian tumor tissues (Shen et al., 2013). The possibility to exploit the physical properties of the ascitic fluid and the geometry of the peritoneal cavity during metastatic OC to tailor the architecture of multistage vectors paves the way for the fabrication of nanotechnology-based immunotherapies to accomplish the challenge of boosting the anticancer immune system and minimizing tumor relapse.
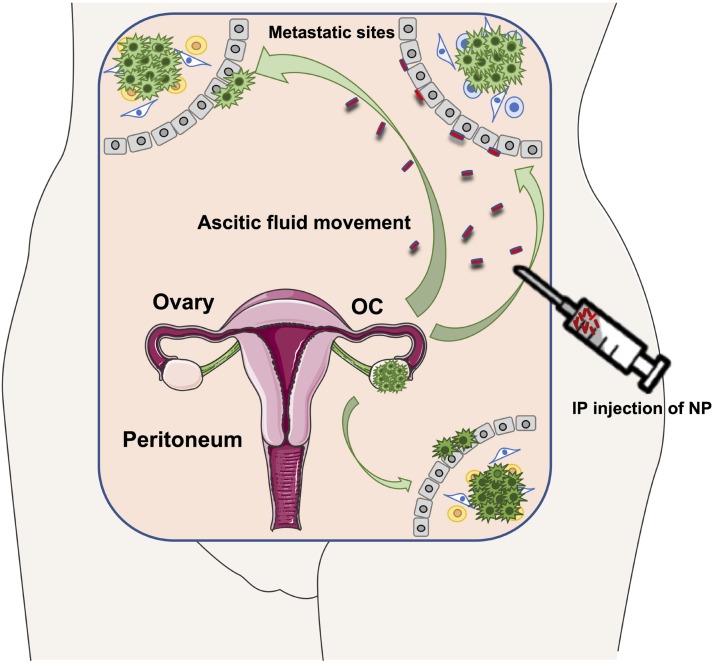
Fig. 2.
Schematic representation of intraperitoneal injection (IP) of nanoparticles able to follow the ascitic fluid movement (green arrows) and reach metastatic sites. Tumor spreading from the ovaries is also shown.
Acknowledgments
We gratefully acknowledge Federica Ferrari and Oscar Velascquez for the editorial assistance and Matthew Landry for the graphical support.
Abbreviations
- APC
antigen-presenting cell
- CAR-T
chimeric antigen receptor engineered T cells
- CTA
cancer testis antigen
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- DC
dendritic cell
- EOC
epithelial ovarian cancer
- FA
folic acid
- FDA
Food and Drug Administration
- LPN
lipid–polymer nanoparticle
- MAGE
melanoma antigen-encoding gene
- MHC
major histocompatibility complex
- Mn
mannose ligand
- NCT
National Clinical Trial
- NP
nanoparticle
- OC
ovarian cancer
- PD-L1
Programmed death-ligand 1
- PEG
polyethylene glycol
- PEI
polyethylenimine
- PLGA
poly(lactic-co-glycolic acid)
- siRNA
small interfering RNA
- TAM
tumor-associated macrophage
- WTA
whole tumor antigen
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Corradetti, Pisano, Conlan, Ferrari.
Footnotes
M.F. is supported through the National Institutes of Health National Cancer Institute [Grants U54CA210181 and R01CA222959], Department of Defense Breast Cancer Research Breakthrough Level IV Award [W81XWH-17-1-0389], and his Ernest Cockrell Jr. Presidential Distinguished Chair at Houston Methodist Research Institute. M.F. serves on the Board of Directors of Arrowhead Pharmaceuticals. B.C. is supported through the Sêr Cymru II programme, funded by the European Commission through the Horizon 2020 Marie Skłodowska-Curie Actions COFUND scheme and the Welsh European Funding Office under the European Regional Development Fund. S.P. is sponsored by the Swansea University (UK)/Houston Methodist Research Institute (US) Joint Initiative.
References
- Abe Y, Kobayashi H, Akizawa Y, Ishitani K, Hashimoto K, Matsui H. (2018) Possible application of ascites-infiltrating gamma-delta T cells for adoptive immunotherapy. Anticancer Res 38:4327–4331. [DOI] [PubMed] [Google Scholar]
- Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, et al. (2018) Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 379:11–21. [DOI] [PubMed] [Google Scholar]
- Adams M, Kerby IJ, Rocker I, Evans A, Johansen K, Franks CR, The Swons Gynaecological Cancer Group (1989) A comparison of the toxicity and efficacy of cisplatin and carboplatin in advanced ovarian cancer. Acta Oncol 28:57–60. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Stenvers KL. (2013) Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol 3:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwer K, Kelly FJ, Chu C, Fewell JG, Lewis D, Alvarez RD. (2013) Phase I trial of a formulated IL-12 plasmid in combination with carboplatin and docetaxel chemotherapy in the treatment of platinum-sensitive recurrent ovarian cancer [published correction appears in Gynecol Oncol (2014) 134:216]. Gynecol Oncol 131:169–173. [DOI] [PubMed] [Google Scholar]
- Barnett JC, Bean SM, Whitaker RS, Kondoh E, Baba T, Fujii S, Marks JR, Dressman HK, Murphy SK, Berchuck A. (2010) Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol 116:556–562. [DOI] [PubMed] [Google Scholar]
- Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. (1981) Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest 68:1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. (2009) Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol 27:418–425. [DOI] [PubMed] [Google Scholar]
- Berek JS, Edwards RP, Parker LP, DeMars LR, Herzog TJ, Lentz SS, Morris RT, Akerley WL, Holloway RW, Method MW, et al. (2014) Catumaxomab for the treatment of malignant ascites in patients with chemotherapy-refractory ovarian cancer: a phase II study. Int J Gynecol Cancer 24:1583–1589. [DOI] [PubMed] [Google Scholar]
- Blanco E, Shen H, Ferrari M. (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolis G, D’Incalci M, Gramellini F, Mangioni C. (1978) Adriamycin in ovarian cancer patients resistant to cyclophosphamide. Eur J Cancer 14:1401–1402. [DOI] [PubMed] [Google Scholar]
- Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O. (2007) Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol 178:1505–1511. [DOI] [PubMed] [Google Scholar]
- Cai DL, Jin L-P. (2017) Immune cell population in ovarian tumor microenvironment. J Cancer 8:2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CA, Krummel MF, Boitel B, Hurwitz A, Sullivan TJ, Fournier S, Cassell D, Brunner M, Allison JP. (1996) The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol Rev 153:27–46. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Higano CS. (2011) PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 17:3520–3526. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H-H, Wang K-L, Chen C-A, Wei L-H, Lai C-H, Hsieh C-Y, Yang YC, Twu NF, Chang TC, Yen MS, Taiwanese Gynecologic Oncology Group (2006) Pegylated liposomal doxorubicin (Lipo-Dox) for platinum-resistant or refractory epithelial ovarian carcinoma: a Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol Oncol 101:423–428. [DOI] [PubMed] [Google Scholar]
- Conlan RS, Pisano S, Oliveira MI, Ferrari M, Mendes Pinto I. (2017) Exosomes as reconfigurable therapeutic systems. Trends Mol Med 23:636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti B, Freile P, Pells S, Bagnaninchi P, Park J, Fahmy TM, de Sousa PA. (2012) Paracrine signalling events in embryonic stem cell renewal mediated by affinity targeted nanoparticles. Biomaterials 33:6634–6643. [DOI] [PubMed] [Google Scholar]
- Coward JI, Middleton K, Murphy F. (2015) New perspectives on targeted therapy in ovarian cancer. Int J Womens Health 7:189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, et al. (2012) Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res 72:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, et al. (2009) Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest 119:2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. (2015) ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161:1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Molin GZ, Omatsu K, Sood AK, Coleman RL. (2018) Rucaparib in ovarian cancer: an update on safety, efficacy and place in therapy. Ther Adv Med Oncol 10:1758835918778483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanov N, Fishman MN, Steinberg JL, Fetterly GJ, Haas A, Grahn A, Lauay C, Dul JL, Sherman JW, Rubin EH. (2005) Final results of a phase I study of liposome entrapped paclitaxel (LEP-ETU) in patients with advanced cancer. J Clin Oncol 23(16_Suppl):2048. [Google Scholar]
- Das PM, Bast RC., Jr (2008) Early detection of ovarian cancer. Biomarkers Med 2:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi S, Eng KH, Mhawech-Fauceglia P, Morrison C, Miliotto A, Beck A, Matsuzaki J, Tsuji T, Groman A, Gnjatic S, et al. (2014) Expression and immune responses to MAGE antigens predict survival in epithelial ovarian cancer. PLoS One 9:e104099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande PP, Biswas S, Torchilin VP. (2013) Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond) 8:1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducie J, Dao F, Considine M, Olvera N, Shaw PA, Kurman RJ, Shih IM, Soslow RA, Cope L, Levine DA. (2017) Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat Commun 8:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle A, Blanchette C, Boone T, Corzett M, Fischer N, Hoeprich P, Driks A, Rasley A. (2013) Co-delivery of adjuvant and subunit antigens via a nanoparticle platform induces tissue-associated and systemic adaptive immune responses (P4409). J Immunol 190:205–214.23183895 [Google Scholar]
- Dzhandzhugazyan KN, Guldberg P, Kirkin AF. (2018) Adoptive T cell cancer therapy. Nat Mater 17:475–477. [DOI] [PubMed] [Google Scholar]
- Eitan R, Fishman A, Meirovitz M, Goldenberg H, Amit A, Koren C, Schneider Y, Rosengarten O, Neuman A, Keren-Rosenberg S, et al. (2014) Liposome-encapsulated doxorubicin citrate (Myocet) for treatment of recurrent epithelial ovarian cancer: a retrospective analysis. Anticancer Drugs 25:101–105. [DOI] [PubMed] [Google Scholar]
- Engelberth SA, Hempel N, Bergkvist M. (2014) Development of nanoscale approaches for ovarian cancer therapeutics and diagnostics. Crit Rev Oncog 19:281–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essel KG, Moore KN. (2018) Niraparib for the treatment of ovarian cancer. Expert Rev Anticancer Ther 18:727–733. [DOI] [PubMed] [Google Scholar]
- Fan Y, Moon JJ. (2015) Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines (Basel) 3:662–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard SL, Secord AA, Monk B. (2016) The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstorff MF, Andersen MH, Ditzel HJ. (2015) Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget 6:15772–15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordinier ME, Kudelka AP, Kavanagh JJ, Wharton JT, Freedman RS. (2002) Thiotepa in combination with cisplatin for primary epithelial ovarian cancer: a phase II study. Int J Gynecol Cancer 12:710–714. [DOI] [PubMed] [Google Scholar]
- Green AE, Rose PG. (2006) Pegylated liposomal doxorubicin in ovarian cancer. Int J Nanomedicine 1:229–239. [PMC free article] [PubMed] [Google Scholar]
- Haanen JBAG. (2017) Converting cold into hot tumors by combining immunotherapies. Cell 170:1055–1056. [DOI] [PubMed] [Google Scholar]
- Hadinoto K, Sundaresan A, Cheow WS. (2013) Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 85 (3 Pt A):427–443. [DOI] [PubMed] [Google Scholar]
- Handolias D, Quinn M, Foo S, Mileshkin L, Grant P, Dutu G, Rischin D. (2016) Oral cyclophosphamide in recurrent ovarian cancer. Asia Pac J Clin Oncol 12:e154–e160. [DOI] [PubMed] [Google Scholar]
- Hanlon DJ, Aldo PB, Devine L, Alvero AB, Engberg AK, Edelson R, Mor G. (2011) Enhanced stimulation of anti-ovarian cancer CD8(+) T cells by dendritic cells loaded with nanoparticle encapsulated tumor antigen. Am J Reprod Immunol 65:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan J, Jayson GC. (2003) Oral melphalan as a treatment for platinum-resistant ovarian cancer. Br J Cancer 88:1828–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höbel S, Aigner A. (2013) Polyethylenimines for siRNA and miRNA delivery in vivo. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:484–501. [DOI] [PubMed] [Google Scholar]
- Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. (2005) Liposomal encapsulated anti-cancer drugs. Anticancer Drugs 16:691–707. [DOI] [PubMed] [Google Scholar]
- Hong C-S, Sharma P, Yerneni SS, Simms P, Jackson EK, Whiteside TL, Boyiadzis M. (2017) Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci Rep 7:14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Garcia-Parra J, Healey GD, Amakiri C, Margarit L, Francis LW, Gonzalez D, Conlan RS. (2016) Antibody-drug conjugates and other nanomedicines: the frontier of gynaecological cancer treatment. Interface Focus 6:20160054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Sánchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, et al. (2017) Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 170:927–938.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal V, Arora E, Gupta S, Lal A, Masab M, Potdar R. (2018) Prospects of chimeric antigen receptor T cell therapy in ovarian cancer. Med Oncol 35:70. [DOI] [PubMed] [Google Scholar]
- Johnston MJW, Semple SC, Klimuk SK, Ansell S, Maurer N, Cullis PR. (2007) Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta 1768:1121–1127. [DOI] [PubMed] [Google Scholar]
- Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA. (2016) Low-grade serous ovarian cancer: a review. Gynecol Oncol 143:433–438. [DOI] [PubMed] [Google Scholar]
- Kandalaft LE, Chiang CL, Tanyi J, Motz G, Balint K, Mick R, Coukos G. (2013) A phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer. J Transl Med 11:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather JN, Suarez-Carmona M, Charoentong P, Weis CA, Hirsch D, Bankhead P, Horning M, Ferber D, Kel I, Herpel E, et al. (2018) Topography of cancer-associated immune cells in human solid tumors. eLife 7: e36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna C, Rosenberg M, Vail DM. (2015) A review of paclitaxel and novel formulations including those suitable for use in dogs. J Vet Intern Med 29:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps E, Tan DSP, Kaye SB. (2013) Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer 13:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline JB, Kennedy RP, Albone E, Chao Q, Fernando S, McDonough JM, Rybinski K, Wang W, Somers EB, Schweizer C, et al. (2017) Tumor antigen CA125 suppresses antibody-dependent cellular cytotoxicity (ADCC) via direct antibody binding and suppressed Fc-γ receptor engagement. Oncotarget 8:52045–52060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole R, Krainer AR, Altman S. (2012) RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov 11:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Hönig A, Häusler S, Voigt H, Becker JC, Leng L, Steinle A, et al. (2008) Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol 180:7338–7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshrestha D, Yeh L-T, Chien M-W, Chou F-C, Sytwu H-K. (2017) Peripheral autoimmune regulator induces exhaustion of CD4+ and CD8+ effector T cells to attenuate autoimmune diabetes in non-obese diabetic mice. Front Immunol 8:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736. [DOI] [PubMed] [Google Scholar]
- Lee S-W, Kim Y-M, Kim YT, Kang SB. (2017) An open-label, multicenter, phase I trial of a cremophor-free, polymeric micelle formulation of paclitaxel combined with carboplatin as a first-line treatment for advanced ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG-3016). J Gynecol Oncol 28:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. (2003) Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol 33:2706–2716. [DOI] [PubMed] [Google Scholar]
- Liao JB, Disis ML. (2013) Therapeutic vaccines for ovarian cancer. Gynecol Oncol 130:667–673. [DOI] [PubMed] [Google Scholar]
- Lipson EJ, Drake CG. (2011) Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 17:6958–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Chen L, Peng Y, Yu S, Liu J, Wu L, Zhang L, Wu Q, Chang X, Yu X, et al. (2017) Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget 9:2887–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HJ, III, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, Miller DS, Eaton LA, Fiorica JV, Gynecologic Oncology Group Study (2005) Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol 23:4626–4633. [DOI] [PubMed] [Google Scholar]
- Lorusso D, Di Stefano A, Fanfani F, Scambia G. (2006) Role of gemcitabine in ovarian cancer treatment. Ann Oncol 17(Suppl 5): v188–v194. [DOI] [PubMed] [Google Scholar]
- Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah AY, Cooper MA. (2016) Metabolic regulation of natural killer cell IFN-γ production. Crit Rev Immunol 36:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. (2018) Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Lenz LL, Harris RA. (2016) A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res 76:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minardi S, Corradetti B, Taraballi F, Sandri M, Martinez JO, Powell ST, Tampieri A, Weiner BK, Tasciotti E. (2016) Biomimetic concealing of PLGA microspheres in a 3D scaffold to prevent macrophage uptake. Small 12:1479–1488. [DOI] [PubMed] [Google Scholar]
- Mirzaei HR, Rodriguez A, Shepphird J, Brown CE, Badie B. (2017) Chimeric antigen receptors T cell therapy in solid tumor: challenges and clinical applications. Front Immunol 8:1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneret C. (2011) Platinum anticancer drugs. From serendipity to rational design. Ann Pharm Fr 69:286–295. [DOI] [PubMed] [Google Scholar]
- Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, et al. (2018) Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495–2505. [DOI] [PubMed] [Google Scholar]
- Motz GT, Coukos G. (2013) Deciphering and reversing tumor immune suppression. Immunity 39:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Dhodapkar MV. (2017) Natural killer T cells in cancer immunotherapy. Front Immunol 8:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhat FR, Apostol R, Nezhat C, Pejovic T. (2015) New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol 213:262–267. [DOI] [PubMed] [Google Scholar]
- Nizzero S, Ziemys A, Ferrari M. (2018) Transport barriers and oncophysics in cancer treatment. Trends Cancer 4:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega RA, Barham WJ, Kumar B, Tikhomirov O, McFadden ID, Yull FE, Giorgio TD. (2015) Biocompatible mannosylated endosomal-escape nanoparticles enhance selective delivery of short nucleotide sequences to tumor associated macrophages. Nanoscale 7:500–510. [DOI] [PubMed] [Google Scholar]
- Owens GL, Price MJ, Cheadle EJ, Hawkins RE, Gilham DE, Edmondson RJ. (2018) Ex vivo expanded tumour-infiltrating lymphocytes from ovarian cancer patients release anti-tumour cytokines in response to autologous primary ovarian cancer cells. Cancer Immunol Immunother 67:1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. (2005) Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol 99:704–713. [DOI] [PubMed] [Google Scholar]
- Pfirschke C, Siwicki M, Liao H-W, Pittet MJ. (2017) Tumor microenvironment: no effector T cells without dendritic cells. Cancer Cell 31:614–615. [DOI] [PubMed] [Google Scholar]
- Pham E, Birrer MJ, Eliasof S, Garmey EG, Lazarus D, Lee CR, Man S, Matulonis UA, Peters CG, Xu P, et al. (2015) Translational impact of nanoparticle-drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin Cancer Res 21:808–818. [DOI] [PubMed] [Google Scholar]
- Pisano C, Cecere SC, Di Napoli M, Cavaliere C, Tambaro R, Facchini G, Scaffa C, Losito S, Pizzolorusso A, Pignata S. (2013) Clinical trials with pegylated liposomal doxorubicin in the treatment of ovarian cancer. J Drug Deliv 2013:898146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CC, Goode EL, Hartmann LC, Kalli KR, Knutson KL. (2011) Immunity and immune suppression in human ovarian cancer. Immunotherapy 3:539–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R, Sabnani MK, Mavinkurve V, Shmeeda H, Mansouri H, Bonkoungou S, Le AD, Wood LM, Gabizon AA, La-Beck NM. (2018) Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. J Control Release 271:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh-Hain JA, Krivak TC, Del Carmen MG, Olawaiye AB. (2011) Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol 4:15–21. [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I. (2014) Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25:846–859. [DOI] [PubMed] [Google Scholar]
- Rossi L, Verrico M, Zaccarelli E, Papa A, Colonna M, Strudel M, Vici P, Bianco V, Tomao F. (2017) Bevacizumab in ovarian cancer: a critical review of phase III studies. Oncotarget 8:12389–12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MR, Graham C, D’Amato A, Gentry-Maharaj A, Ryan A, Kalsi JK, Ainley C, Whetton AD, Menon U, Jacobs I, et al. (2017) A combined biomarker panel shows improved sensitivity for the early detection of ovarian cancer allowing the identification of the most aggressive type II tumours. Br J Cancer 117:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabado RL, Balan S, Bhardwaj N. (2017) Dendritic cell-based immunotherapy. Cell Res 27:74–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini P, Harter P, Scambia G, Sehouli J, Meier W, Wimberger P, Baumann KH, Kurzeder C, Schmalfeldt B, Cibula D, et al. (2013) Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a phase III trial of the AGO OVAR, COGI, GINECO, and GEICO--the MIMOSA study. J Clin Oncol 31:1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. (2002) The instructive role of dendritic cells on T-cell responses. Arthritis Res 27(Suppl 3):S127–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiezynski J, Taratula O, Rodriguez-Rodriguez L, Minko T. (2016) Precision targeted therapy of ovarian cancer. J Control Release 243:250–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini S, Tamir A, Lim H, Li S, Zhang S, Goy A, Pecora A, Suh KS. (2012) Early detection biomarkers for ovarian cancer. J Oncol 2012:709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, et al. (2012) Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med 209:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden MV, Muggia F, Astrow A, Matulonis U, Campos S, Roche M, Sivret J, Rusk J, Barrett E. (2004) A phase II study of liposomal lurtotecan (OSI-211) in patients with topotecan resistant ovarian cancer. Gynecol Oncol 93:229–232. [DOI] [PubMed] [Google Scholar]
- Seifi-Alan M, Shamsi R, Ghafouri-Fard S. (2018) Application of cancer-testis antigens in immunotherapy of hepatocellular carcinoma. Immunotherapy 10:411–421. [DOI] [PubMed] [Google Scholar]
- Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, Zhang G, Guo X, Bai L, Qin G, et al. (2013) Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin Cancer Res 19:1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sun T, Hoang HH, Burchfield JS, Hamilton GF, Mittendorf EA, Ferrari M. (2017) Enhancing cancer immunotherapy through nanotechnology-mediated tumor infiltration and activation of immune cells. Semin Immunol 34:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L-M, Quan L, Liu J. (2018) Tracking exosomes in vitro and in vivo to elucidate their physiological functions: implications for diagnostic and therapeutic nanocarriers. ACS Applied Nano Materials 1:2438–2448. [Google Scholar]
- Siegel RL, Miller KD, Jemal A. (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. [DOI] [PubMed] [Google Scholar]
- Singel KL, Khan ANH, Moysich KB, Odunsi K, Segal BH. (2017) Ovarian cancer ascites induces a T cell suppressive phenotype in mature neutrophils: a potential barrier to anti-tumor immunity. J Immunol 198(1 Suppl). [Google Scholar]
- Srinivasan KN, Rauthan A, Gopal R. (2014) Combination therapy of albumin-bound paclitaxel and carboplatin as first line therapy in a patient with ovarian cancer. Case Rep Oncol Med 2014:940591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Vannberg FO, Dixon JB. (2016) Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep 6:24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojevic Z, Rancic G, Radic S, Potic-Zecevic N, Djordjevic B, Markovic M, Todorovska I. (2004) Pathogenesis of malignant ascites in ovarian cancer patients. Arch Oncol 12:115–118. [Google Scholar]
- Syn NL, Wang L, Chow EK-H, Lim CT, Goh B-C. (2017) Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol 35:665–676. [DOI] [PubMed] [Google Scholar]
- Tan DS, Agarwal R, Kaye SB. (2006) Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol 7:925–934. [DOI] [PubMed] [Google Scholar]
- Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, Baumgartner P, Stevenson BJ, Iseli C, Dangaj D, et al. (2018) Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med 10. [DOI] [PubMed] [Google Scholar]
- Tayama S, Motohara T, Narantuya D, Li C, Fujimoto K, Sakaguchi I, Tashiro H, Saya H, Nagano O, Katabuchi H. (2017) The impact of EpCAM expression on response to chemotherapy and clinical outcomes in patients with epithelial ovarian cancer. Oncotarget 8:44312–44325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo PY, Yang C, Hedrick JL, Engler AC, Coady DJ, Ghaem-Maghami S, George AJ, Yang YY. (2013) Hydrophobic modification of low molecular weight polyethylenimine for improved gene transfection. Biomaterials 34:7971–7979. [DOI] [PubMed] [Google Scholar]
- Teo PY, Yang C, Whilding LM, Parente-Pereira AC, Maher J, George AJ, Hedrick JL, Yang YY, Ghaem-Maghami S. (2015) Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing. Adv Healthc Mater 4:1180–1189. [DOI] [PubMed] [Google Scholar]
- Terry KL, Schock H, Fortner RT, Hüsing A, Fichorova RN, Yamamoto HS, Vitonis AF, Johnson T, Overvad K, Tjønneland A, et al. (2016) A prospective evaluation of early detection biomarkers for ovarian cancer in the european EPIC cohort. Clin Cancer Res 22:4664–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RFPM. (2018) No improvement in long-term survival for epithelial ovarian cancer patients: a population-based study between 1989 and 2014 in The Netherlands. Eur J Cancer 88:31–37. [DOI] [PubMed] [Google Scholar]
- Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk MJ, Waters DJ, Low PS. (2004) Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett 213:165–172. [DOI] [PubMed] [Google Scholar]
- Tyler C, Kapur A, Felder M, Belisle JA, Trautman C, Gubbels JA, Connor JP, Patankar MS. (2012) The mucin MUC16 (CA125) binds to NK cells and monocytes from peripheral blood of women with healthy pregnancy and preeclampsia. Am J Reprod Immunol 68:28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaman S, Huh KM, Park IK. (2018) Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater Res 22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola CL. (2017) Cancer immunotherapy, part 1: current strategies and agents. PT 42:375–383. [PMC free article] [PubMed] [Google Scholar]
- Whitworth JM, Alvarez RD. (2011) Evaluating the role of IL-12 based therapies in ovarian cancer: a review of the literature. Expert Opin Biol Ther 11:751–762. [DOI] [PubMed] [Google Scholar]
- Xia X, Mai J, Xu R, Perez JET, Guevara ML, Shen Q, Mu C, Tung HY, Corry DB, Evans SE, et al. (2015) Porous silicon microparticle potentiates anti-tumor immunity by enhancing cross-presentation and inducing type I interferon response. Cell Rep 11:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, Shen J, Liu H, Hu Z, Chen L, et al. (2016) An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol 34:414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaari Z, da Silva D, Zinger A, Goldman E, Kajal A, Tshuva R, Barak E, Dahan N, Hershkovitz D, Goldfeder M, et al. (2016) Theranostic barcoded nanoparticles for personalized cancer medicine. Nat Commun 7:13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Chae S-W, Kim H-R, Chae HJ. (2014) Endoplasmic reticulum stress and cancer. J Cancer Prev 19:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Takahashi Y, Takakura Y. (2018) Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull 41:835–842. [DOI] [PubMed] [Google Scholar]
- Zhang B-L, Qin D-Y, Mo Z-M, Li Y, Wei W, Wang Y-S, Wang W, Wei YQ. (2016) Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Sci China Life Sci 59:340–348. [DOI] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213. [DOI] [PubMed] [Google Scholar]
- Zheng M, Zhong Y, Meng F, Peng R, Zhong Z. (2011) Lipoic acid modified low molecular weight polyethylenimine mediates nontoxic and highly potent in vitro gene transfection. Mol Pharm 8:2434–2443. [DOI] [PubMed] [Google Scholar]
- Zheng P-P, Kros JM, Li J. (2018) Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov Today 23:1175–1182. [DOI] [PubMed] [Google Scholar]
- Zhu X, Cai H, Zhao L, Ning L, Lang J. (2017) CAR-T cell therapy in ovarian cancer: from the bench to the bedside. Oncotarget 8:64607–64621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74:5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. (1996) Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med 183:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]