Abstract
Advanced drug delivery technologies, in general, enable drug reformulation and administration routes, together contributing to life-cycle management and allowing the innovator to maintain the product monopoly. Over the years, there has been a steady shift from mere life-cycle management to drug repurposing—applying delivery technologies to tackle solubility and permeability issues in early stages or safety and efficacy issues in the late stages of drug discovery processes. While the drug and the disease in question primarily drive the choice of route of administration, the oral route, for its compliance and safety attributes, is the most preferred route, particularly when it comes to chronic conditions, including pain, which is not considered a disease but a symptom of a primary cause. Therefore, the attempt of this review is to take a stock of the advances in oral delivery technologies that are applicable for injectable to oral transformation, improve risk-benefit profiles of existing orals, and apply them in the early discovery program to minimize the drug attrition rates.
Introduction
Biomedical research has advanced our understanding of diseases—their causes and remedies. Specifically, the remedies that include approaches to prevent, manage, or treat a particular disease using a drug (e.g., chemical and biologic molecules) or a drug-like (e.g., supplements) compound. The pace at which new diseases, newer pathways of already known diseases, and increasing understanding of the drug-resistant mechanisms are being uncovered is accelerating. This pace is not met by the discovery of new and effective medicines (Silber, 2010). To meet this gap, increasing attention is paid toward drug repositioning, where an existing drug discovered for a specific target is repurposed for other targets (Huang et al., 2018; Pushpakom et al., 2018). The primary advantage of drug repurposing is that scientists already understand the pharmacology and safety profiles that can greatly reduce the risk of attrition in drug development in clinical phases. The drug repurposing aided by artificial intelligence, systems pharmacology, and other computational approaches validate the drug targets (Bai, 2016; Scannell and Bosley, 2016; Peska et al., 2017; Cha et al., 2018). The effective delivery strategies would improve risk-benefit profiles and switch routes of administration (Cipolla and Gonda, 2012; Paulmurugan et al., 2016). Each mode of delivery such as oral, nasal, injection, sublingual, rectal, vaginal, ocular, optic, or nasal has its respective advantages and disadvantages (Mignani et al., 2013). Nevertheless, oral drug delivery (pills, powders, suspensions, and solutions) is the singular, superior method of administration due to its convenience and safety compared with other methods (Mignani et al., 2013). Enterocytes, goblet cells, and Peyer’s patches with M cells make the intestinal epithelium an optimal platform for drug absorption. Especially regarding diseases that require frequent administration for a long duration, patient comfort proves oral drug delivery’s eminence. The advantages of oral drug administration over other methods include ease of use, being painless, lower cost of care, lesser patient supervision, and higher patient compliance.
However, the oral route of drug administration has some disadvantages when it comes to the drug molecules exhibiting low solubility, lesser permeability, and degradation rates. Moreover, the uptake of certain biomolecules/drugs in oral route is largely affected by physiologic barriers such as pH change in gastrointestinal tract (GIT): acidic pH in the stomach followed by basic pH in the intestine and enzymatic degradation (Knipe et al., 2015). Almost 60% of drugs degrade in the harsh gastric environments of the stomach. Many have theorized making salts out of the drugs, thereby increasing its solubility and bioavailability (Serajuddin, 2007; Elder et al., 2013). Unfortunately, in practice, pairing the parent drug with an appropriate counter ion, for sufficient ionic interaction, has proven difficult. Only under ideal thermodynamic conditions will the salt drug precipitate. Furthermore, counter ions increase the weight of the drug but are therapeutically inactive, thus obligating increased and frequent dosage, which has unavoidable side effects on the body. Moreover, these counter ions increase the hygroscopic nature of the drug by forming hydrates, and in consequence, reduce the drug’s shelf life. Some counter ions also exhibit a corrosive nature, like hydrochlorides, and hence reduce the solubility of the drug in the stomach as explained by the counter ion effect (Makary, 2014). These issues motivated researchers to attempt innovative forms of conventional carriers like capsules, tablets, microcapsules, or non-conventional approaches such as intestinal patches and nanoparticles. The successes with non-conventional delivery systems corroborate our belief that oral delivery deserves more attention for its possible advantages.
The recent developments in the area of oral controlled release delivery systems such as dome tablets, dual drug tablets, intestinal patches, polymer nanosystems, or bioinspired delivery systems such as exosomes, have revolutionized the field. This review is an attempt to summarize the oral drug delivery techniques available and under development, with current status for use and future prospects.
Tablets
Oral tablets are the most common, convenient, and easy method for drug administration. Tablets are conventionally made by compressing drug powder with appropriate excipients that result in rapid release of the drug in the body when taken. The drawback of conventional tablets is that rapid drug release makes it difficult to maintain multi-component drug release. Many controlled drug release-based technologies such as matrix tablets, multilayer tablets, Dome-matrix based tablets, core-in-cup devices, three-dimensional (3D) tablets, etc., have been developed or are under development (Moodley et al., 2012; Preis, 2015; Hong et al., 2016) to deal with the drawbacks faced in conventional tablets.
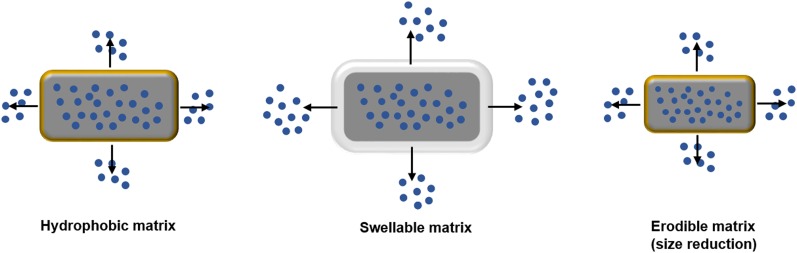
Matrix-tablets have been developed to tackle the controlled drug delivery issues faced in conventional tablets (Fig. 1) (Nokhodchi et al., 2012; Zimmer et al., 2014; Guarascio and Slain, 2015). The advantages of matrix tablets include less frequent dosing, cost effectiveness, side-effect reduction from dose dumping, etc. Matrix-based systems are divided into three types: 1) osmotic pump systems; 2) reservoir matrix systems; and 3) monolith matrix systems.
Fig. 1.
Image showing drug release from the matrix tablets. Adapted and modified from Nokhodchi et al. (2012).
The osmotic pressure plays an important role in osmotic pump systems in which a semipermeable membrane with an orifice controls the drug release. In the case of reservoir matrix systems, a membrane controls the diffusion of the drug from the system, whereas, in monolith matrix systems, the drug has been dispersed or encapsulated in a hydrophobic or hydrophilic system, which controls the drug release.
Multilayered tablets, including bilayered, triple layered, and quadruple layered, etc., can be designed to release multiple drugs at different rates and are superior to conventional tablets. In general, the multilayered tablets consist of a drug core that is surrounded by a hydrophobic or hydrophilic polymer layer that controls the drug release in the GIT. Many multilayered tablets have been developed, such as Geomatrix multilayer tablet technology, Smartrix technology, Sodas multilayer tablet technology, VersaTab bilayered tablet technology, Geolock technology, Procise technology, Chronotropic, CODES, etc. (Table 1) (Moodley et al., 2012; Choonara et al., 2014).
TABLE 1.
Summary of various advanced tablet technologies
Adapted and Modified from Moodley et al. (2012), Choonara et al. (2014).
| Drug | Technology | Design | Factors Affecting Drug Release | Advantages | Reference |
|---|---|---|---|---|---|
| Venlafaxine hydrochloride | Procise | Drug core with a hole | Core geometry | Zero-order kinetics or drug release according to core geometry | Malewar et al. (2015) |
| Diltiazem | Geomatrix | Multilayer tablet | Polymer type, thickness of layer | Zero-order kinetics and controlled drug release | Wilding et al. (1995) |
| Indomethacin | Smartrix | Multilayer tablet with specific shape of core layer | Polymer type, shape of core layer | Zero-order kinetics or drug release according to shape of core layer | Omer et al. (2017) |
| Methylphenidate | Sodas (spheroidal oral drug absorption system) | Multilayer tablet | Layer thickness, shape of core layer | Pulsatile drug release | Biederman et al. (2003) |
| Norfloxacin | Dome matrix | Dome-shaped swellable matrix module | Polymer used, module arrangement | Drug release based on module arrangement | Oliveira et al. (2011) |
| Fenofibrate captopril, glipizide, and nifedipine | 3D printed tablets | Fabrication through 3D inkjet printing or an extrusion-based or fused deposition modelling | Polymer used, drug used | Immediate or controlled release | Khaled et al. (2015); Kyobula et al. (2017) |
| Insulin, camostat mesilate | Chronotropic | Multilayer tablet | Polymer layers | “Two pulse” release and controlled release | Del Curto et al. (2011) |
| Heparin | GIPET | Permeation enhancement technology | Polymer layer, medium fatty acid chains | Immediate or modified release | Leonard et al. (2006) |
| Calcitonin | Peptelligence | Permeation enhancement technology | Polymer layers, a permeation enhancer and the main excipient citric acid | Immediate or modified release | Binkley et al. (2012) |
| Insulin, lactulose | CODES | Multilayer tablet | Polymer layers, pH-based release | Immediate or controlled release | Katsuma et al. (2004) |
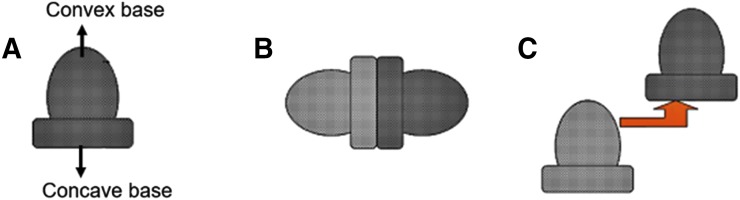
The dome matrix-based drug-releasing devices consist of a dome-shaped swellable matrix module with a convex front and concave base as shown in Fig. 2. These tablets have two configurations: 1) void configuration and 2) piled configuration. The drug release patterns in case of dome-matrix tablets are controlled by the configuration of modules (Hascicek et al., 2011). A higher drug release rate has been observed for the convex front in comparison with the concave base. The dome-matrix tablets showed a higher initial drug release rate compared with conventional tablets. Prolonged delivery of norfloxacin as dome-matrix tablet techniques has also been established in the past (Oliveira et al., 2011), but due to the complexity of these module systems, these tablets are not as popular as other controlled release systems yet. To the best of our knowledge, this technology is still at exploratory level. However, such technologies will have significant impact in treating acute/chronic diseases involving multiple progression pathways, where multiple drugs are needed to block the disease progress.
Fig. 2.
(A) Dome matrix tablet module with void configuration (B) and piled configuration (C). Adapted and modified from Moodley et al. (2012).
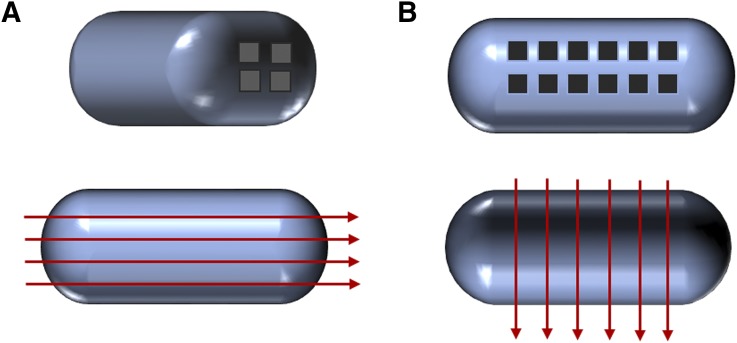
Polymer-based 3D printed tablets have been fabricated to maintain controlled drug release over a particular time period to retain the therapeutic level of the drug intake. The 3D printed tablets can be produced by various methods such as 3D inkjet printing (Kyobula et al., 2017), an extrusion-based (Khaled et al., 2018), or a fused deposition modeling (Sadia et al., 2018). These tablets demonstrate high drug loading and immediate drug release while maintaining the active physical form of the drug. The size, as well as the shape of the contrived tablets can be modified according to the personalized use. These tablets can also be fabricated with a large number of shorter perforated channels with particular width and length to control the drug release as the show in Fig. 3.
Fig. 3.
Image showing perforated channels in 3D channeled tablet: (A) parallel and (B) at right angle to long axis. Adapted and modified from Sadia et al. (2018).
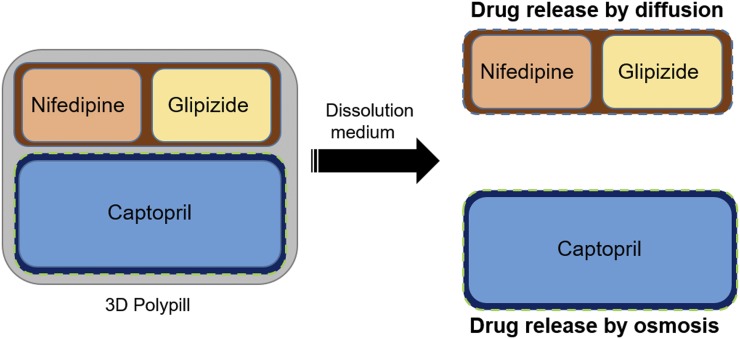
Roberts and coworkers (Khaled et al., 2015) fabricated a 3D polypill with multiple active drug molecules confined in well-defined separated compartments for controlled drug release for the treatment of hypertension in type I diabetic patients. Captopril, glipizide, and nifedipine were used to make a 3D polypill (Fig. 4). The captopril drug release was maintained through an osmotic pump-based mechanism, whereas glipizide and nifedipine showed the Korsmeyer-Peppas release kinetics. The study showed that a 3D polypill was able to deliver all three drugs without any detectable interaction between them.
Fig. 4.
Image showing 3D polypill containing three drugs, namely, captopril, nifedipine, and glipizide. Adapted and modified from Khaled et al. (2015).
The aim of these technologies is to enhance the bioavailability of drug molecules. Many of the above mentioned technologies are under clinical trials, but there are still numerous challenges that need to be tackled to use these technologies in real time. A summary of various controlled release tablet technologies has been done below (Table 1).
Capsules
Capsules are the safest and most acceptable form of oral administration to patients, especially if the drug has a bad taste, odor, or is photosensitive. There are two types of capsules: 1) hard gelatin capsules with solid-fill formulations and 2) soft gelatin capsules with liquid-fill or semisolid-fill formulations, such as vitamin E, cough preparations, etc., with suitable excipients that aid the dosage form. The shape of a capsule makes it easy to swallow, and the lubricant coating is soothing and moisturizing. Like tablets, capsules can also facilitate sustained release with target specificity across the GIT. In this section of the review, we focused on 1) the innovation of the materials used to make capsules and 2) the fillings of drug complex/drug/drug conjugate/micro- or nano-formulation used for better bioavailability of the drug in the body. Scientists have scrutinized the literature for various aspects of capsule formation, such as the use of polymers (Ma, 2014; Lamb et al., 2016), and for the target delivery of drugs and for surgical treatments, such as medical robot capsules (Mapara and Patravale, 2017). A summary of recent developments in capsule technologies is presented in Table 2.
TABLE 2.
Summary of advanced capsule technologies
| Drug | Capsule | Purpose | Advantage | Reference |
|---|---|---|---|---|
| Ga-citrate | Gelatin capsules | To study the gastrointestinal transit time | Extended release | Wagner et al. (2017) |
| Tributyrin | Whey protein and γ-cyclodextrin | To study In vitro release of butyric acid for the treatment of intestinal disorder | Controlled release | Donovan et al. (2017) |
| Sulforhodamine 101 (fluorescent probe as model drug) | Rhamnogalacturonan-I | For the treatment of colonic cancer and better protection of the drug throughout the GIT | Controlled release | Svagan et al. (2016) |
| Ivermectin | PCL elastomers | For extended release of anti-malaria drugs | Controlled and extended release | Bellinger et al. (2016) |
| Veledimex | Gelatin capsules | To regulate gene expression and studying the drug pharmacokinetics | Controlled release | Mulugeta et al. (2018) |
| Paracetamol | Sporopollenin exine capsules (SEC) with carboxymethyl cellulose and epichlorohydrin coating | To enhance bioavailability of paracetamol | Extended release | Alshehri et al. (2016) |
| Curcumin | Mesoporous silica microcapsules using N-hexadecyl palmitate (NHP) in Tween 40 | To improve the stability and gastro-resistant delivery intestinal absorption of curcumin | Controlled release | Kim et al. (2016) |
| Probucol | Chenodeoxycholic acid (CDCA) microcapsules | To improve permeation properties of the drug | Targeted oral delivery | Mooranian et al. (2015) |
| Gliclazide | CDCA and sodium alginate | To enhance delivery of Gliclazide with CDCA in lower intestine with prolong release | Targeted oral delivery | Mathavan et al. (2016) |
| Fluorescent particles | Particle-in-particle | For effective colon cancer treatment | Targeted oral delivery | Ma et al. (2015) |
| Sodium salt of Furosemide | Microwells of poly-l-lactic acid with coating of Eudragit | To deliver the powdered drug to the target site throughout the GIT | Targeted oral delivery | Nielsen et al. (2015) |
| Insulin | Whey protein (WPI), polyglycerol and sodium alginate (SA) or carboxymethylcellulose (CMC) coating | To enhance oral drug delivery | Controlled release | Cardenas-Bailon et al. (2015) |
| Insulin | (PLGA)-lipid –PEG hybrid nanoparticles filled in gelatin capsules with hydro-proxy-propyl methyl cellulose phthalate polymer coating | To enhance bioavailability and protection against acidic environment | Controlled release | Yu et al. (2015) |
| Oxycodone | DETERx microsphere capsules | To enhance the bioavailability specially for patients with dysphagia | Controlled and extended release | Fleming et al. (2016) |
| Budesonide | Eudragit S-100 and L-100 gelatin capsules | To increase the lag time to target colon related diseases | Controlled release | Yehia et al. (2011) |
| Docetaxel | Surfactant based solid dispersions in gelatin capsule | To attain higher solubility and dissolution | Controlled release | Moes et al. (2011) |
Intestinal Patches
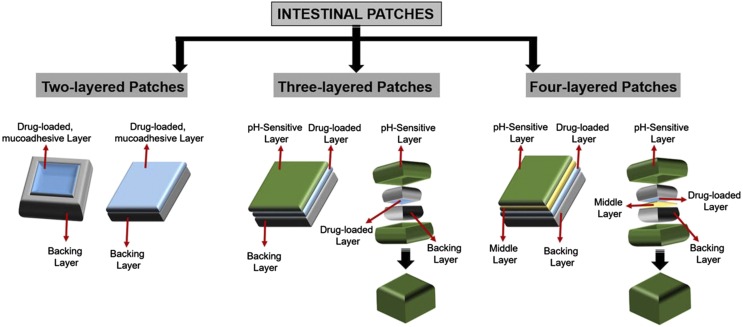
Intestinal patches are millimeter in size, two- to four-layered oral drug delivery devices inspired by transdermal patches. They were invented to enhance bioavailability, decrease, the GIT degradation of drugs, and avoid the painful injection of drugs, such as insulin for diabetes and anticancer drugs for cancer chemotherapy (Tao and Desai, 2005; Wong, 2010; Teutonico and Ponchel, 2011; Banerjee and Mitragotri, 2017; Kirsch et al., 2017) (Fig. 5).
Fig. 5.
Types of intestinal patches. Adapted and modified from Banerjee and Mitragotri (2017).
The intestinal patches are divided into three kinds, depending upon the number of layers used in the design of a patch: 1) two-layered patch, 2) three-layered patch, and 3) four-layered patch (Fig. 5). The two-layered intestinal patches are composed of a backing layer and a drug-loaded mucoadhesive layer. The backing layer generally consists of hydrophobic polymers such as cellulose acetate and its derivatives (Eiamtrakarn et al., 2002). The backing layer supports the unidirectional release of drug toward the intestinal mucosal side. The mucoadhesive layer is generally made up of mucoadhesive polymers, such as chitosan derivatives, alginates, hydroxypropyl cellulose, etc. (Thanou et al., 2001).
The mucoadhesive polymers have been reviewed in detail by other groups (Andrews et al., 2009; Roy et al., 2009). The purpose of a mucoadhesive layer is to ensure the strong attachment toward the intestinal mucosal layer (Banerjee et al., 2016). In three-layered intestinal patches, an additional pH-sensitive layer has been introduced for the protection of the drug layer against the acidic pH of the stomach, whereas the four-layered intestinal patch consists of two separate layers for drug and mucoadhesive polymers (Fig. 5).
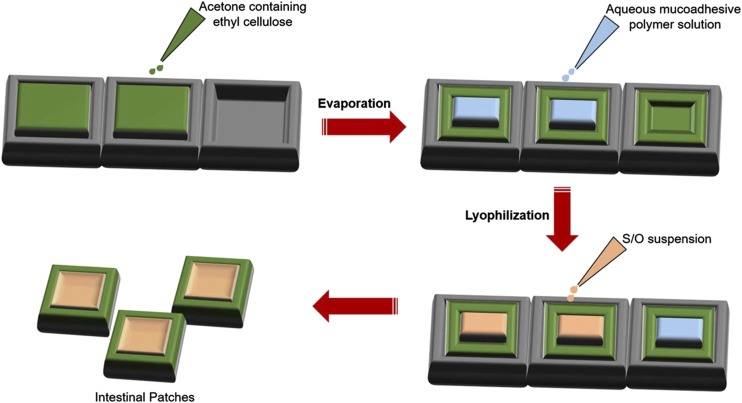
The mucoadhesive intestinal patches are generally prepared by an evaporation-lyophilization method (Fig. 6) (Toorisaka et al., 2012). Generally, the backing layer or drug impermeable layer is prepared with 5% w/w ethyl cellulose in acetone solution. The solution is poured into silicon molds and allowed to evaporate at room temperature. The aqueous mucoadhesive polymer solution is added and lyophilized for 1 day. Furthermore, the suspension containing drug was added into a lyophilized layer.
Fig. 6.
Image showing the process of intestinal patch formation. Adapted and modified from Toorisaka et al. (2012).
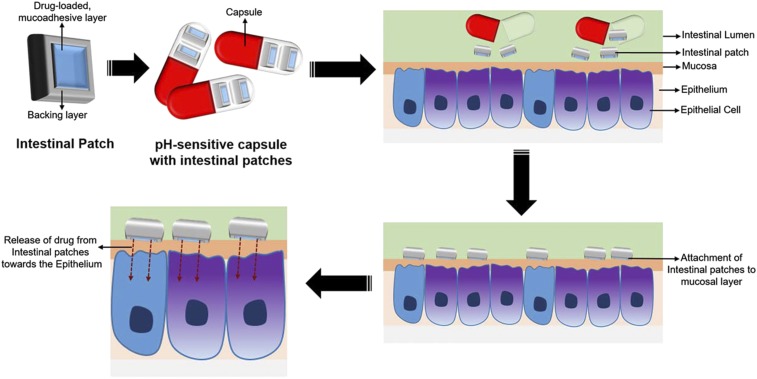
The functioning of a typical intestinal patch is based on the pH conditions in the GIT (Fig. 7). The intestinal patches, with the backing layer and drug-loaded mucoadhesive layer, can be filled in pH-sensitive capsules to protect them from acidic conditions and enzymatic degradation in the stomach for the enhanced intestinal delivery of the drugs. In the intestine, these patches attach to the mucosal layer of the intestine due to presence of the mucoadhesive polymer layer and secure the unidirectional flow of loaded drug molecules toward the intestinal epithelium for adsorption, resulting in better bioavailability of the drug in body (Gupta et al., 2016). Mitragotri and his coworkers (Gupta et al., 2013) reported that the intestinal patches developed in their laboratory as more efficient in the rat intestine in comparison with the Caco-2 monolayer intestinal cells due to absence of mucosa in cell monolayers.
Fig. 7.
Schematic representation of working of an intestinal patch. Adapted and modified from Banerjee and Mitragotri (2017).
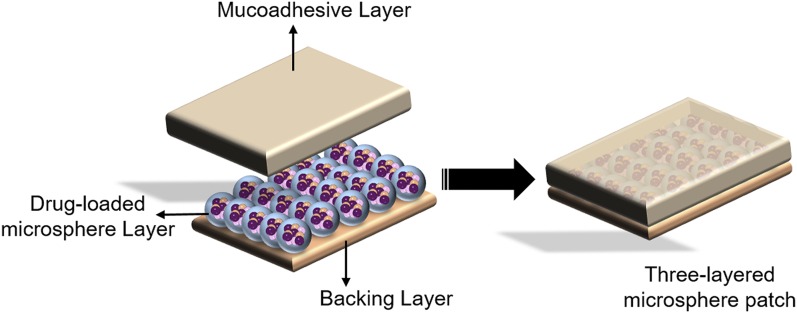
Shen and Mitragotri (2002) introduced the microsphere patches containing three layers: a backing layer, a drug-loaded microsphere layer, and a mucoadhesive layer (Fig. 8). Sulforhodamine B was used as a model drug to be encapsulated into the bovine serum albumin-based microspheres with a diameter of 10–30 µm. It was observed that 95% of loaded sulforhodamine B was released from the mucoadhesive layer into PBS under in vitro release conditions.
Fig. 8.
Image showing a three-layered microsphere patch. Adapted and modified from Shen and Mitragotri (2002).
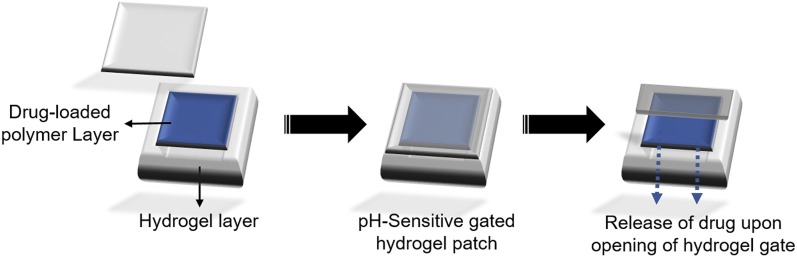
Lee and coworkers (He et al., 2004) introduced pH-sensitive gated hydrogel patches for controlled oral drug delivery. The gated hydrogel patch consisted of two parts: a polymer-based drug reservoir and a pH-sensitive hydrogel gate. The free-radical photo-polymerization reactions were used to prepare the gated hydrogel patch. At appropriate pH conditions, the hydrogel gate swells up to expose the drug matrix for delivery at appropriate position (Fig. 9).
Fig. 9.
Image showing working of a pH-sensitive gated hydrogel patch at appropriate pH. Adapted and modified from He et al., (2004).
Bernkop-Schnürch and coworkers (Bernkop-Schnürch, 2005; Hoyer et al., 2009) developed the intestinal patches for oral delivery of insulin. It was observed that the patches were able to release around 75% of loaded insulin in 6 hours. In comparison with subcutaneous insulin injections, these patches showed 2.2% increase in relative bioavailability. But still, for these patches to be used as oral delivery vehicles of insulin, they will require significant improvements and considerations. Recently, Mitragotri and coworkers (Banerjee et al., 2016, 2017) prepared the insulin-dimethyl palmitoyl ammonio propanesulfonate micropatches for the efficient oral delivery of insulin. Propanesulfonate is considered to enhance paracellular drug uptake due to its involvement in the opening of intestinal tight junctions. The intestinal patches reduced glucose levels in nondiabetic rats, where serial dosing of the patches for every 30 min resulted in 41% lowering of glucose level in 8 hours.
Desai and coworkers (Ainslie et al., 2008) developed bioadhesive patches by using microfabrication techniques such as photolithography and etching. These bioadhesive patches contained multiple reservoirs for controlled drug release. The group had demonstrated the formation of multi-layered devices with up to three different drug layers for simultaneous controlled release in the Caco2 monolayer. These devices have been used for the synergetic effect of both insulin and camptothecin for controlled release in a simultaneous fashion. These devices have been tested in a Caco2 monolayer but are still under improvements and considerations for the intestinal conditions. Further details about similar devices can be found in Chirra and Desai (2012), Fox et al. (2014, 2015), and Nielsen et al. (2018).
Microneedle Patches
The microneedle patches are like robotic pills or capsule-shaped devices containing needles that can be used for enhancing the bioavailability of the biologic drug class like proteins, hormones, growth factors, etc., across the GIT in a safe manner. The microneedle patches are generally fabricated from drug-loaded biocompatible polymers. The microneedle patches come in two types 1) hollow microneedle patch and 2) solid microneedle patch. In both types, microneedles are coated with pH-sensitive layer, which dissolves in appropriate site in GIT to release the drug-containing needles. In the case of a hollow microneedle patch, the drug release occurs due to peristaltic movement in the intestine, whereas, in solid microneedle patches, the microneedles break off from the pill and penetrate the intestinal walls and release the drug.
Rani Therapeutics (https://www.ranitherapeutics.com/) developed many microneedle patches that have the ability to deliver macromolecules like proteins and antibodies. Rani Therapeutics claims its Rani Pill is a low cost, safe, effective, and painless method to deliver various biologic molecules. A swallowable device capsule was designed containing a guide tube for tissue penetrating position, a delivery member, and a release element. The release element was designed to degrade in the intestinal conditions for enhanced absorption and delivery. The technology claims to enhance delivery of poorly absorbed drugs such as parathyroid hormone, interleukin-17, somatostatin, etc. (Imran, 2014).
The ability of these intestinal patches without/with microneedles for the delivery of high-dose drugs or to modulate the release profiles needs to be further investigated.
Ionic Liquids
Ionic liquids are drug salts in the liquid state with low melting points. These liquids have charged particles that offer stronger interactions, thereby resulting in better stability as shown in Fig. 10. As ionic liquids form strong bonds with drug molecules and their salt counterparts, these can be useful in terms of drug repurposing and multiple drug dosing. Ionic liquids exhibited better permeability and bioavailability when acidic and basic drugs were used (Rogers and Seddon, 2003; Shamshina et al., 2015).
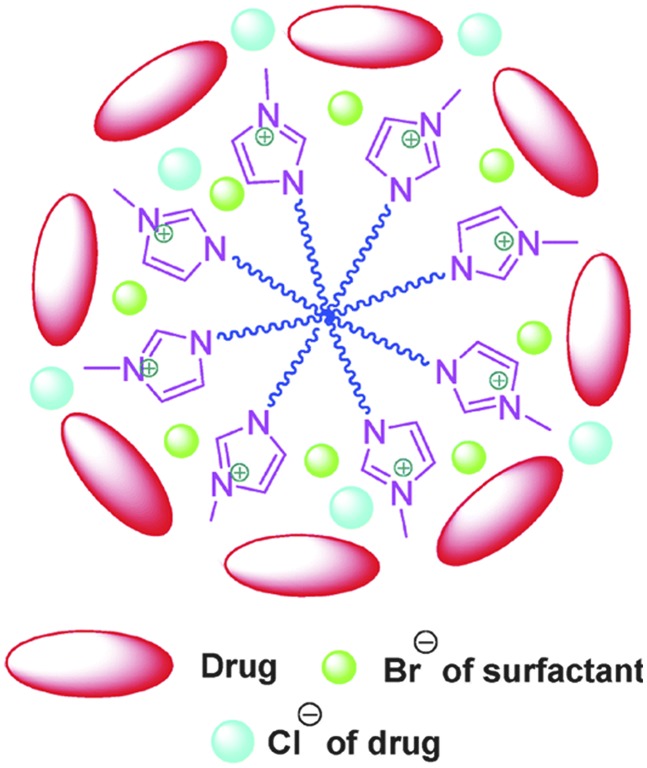
Fig. 10.
Image showing possible adsorption of drug [C14mim] [Br] micelles. Reproduced with permission from Mahajan et al. (2012).
Porter and coworkers (Williams et al., 2014; Sahbaz et al., 2015, 2017; Williams et al., 2018) pioneered ILs for improving the oral delivery of poorly bioavailable drugs. In one of the examples, they demonstrated enhanced solubility of drugs such as danazol and itraconazole using 1-hexyl-3-hexyloxycarbonylpyridinium cation and dicyanamide [N(CN)2] anion, resulting in almost ∼100 times increased solubility. In another study, some weakly basic drugs such as itraconazole, cinnarizine, and halofantrine were converted to lipophilic ionic liquids using their hydrochloride salts and were combined with lipid systems for improved oral delivery (Sahbaz et al., 2015). Similar studies were conducted using various other high/low soluble or high/poor permeable drugs such as tolfenamic acid, meclofenamic acid, diclofenac, ibuprofen, amlodipine fexofenadine, ranitidine, and metformin (Sahbaz et al., 2017; Williams et al., 2018). Ionic liquids resulted in the possibility of higher dose administration in comparison with parent drugs, specially, in self-emulsifying drug delivery systems or using their lipophilic salts like lauryl sulfate, oleate, and docusate as counter ions. Assembling protic ionic liquids of diphenhydramine with commonly used analgesics naproxen and ibuprophen shows low diffusivity, high viscosity, poor ionic conductivity, and good wettability but exhibited low dissolution rates, which were improved with the use of silica mesoporous carriers. These protic ionic liquids carrier composites exhibited rapid dissolution and better bioavailability in capsule forms (Wang et al., 2018a). The water-soluble acidic active pharmaceutical ingredients such as diclofenac, ibuprofen, ketoprofen, naproxen, sulfadiazine, sulfamethoxazole, and tolbutamide were converted into tetrabutylphosphonium ionic liquids and the results were quite encouraging compared with their respective free acid forms (Balk et al., 2015). A library of 36 counter ions as halide salts was prepared to map the pharmaceutical design space for ionic liquid of Selurampanel (Wiest et al., 2017).
Ionic liquids have been used to deliver macromolecules and proteins; like insulin, when mixed with choline and geranate ionic liquid, had shown a 45% decrease in blood glucose levels, even at low insulin doses of 3–10 IU/kg (accompanied by sustained release up to 12 hours) (Banerjee et al., 2018). The concept of vesicle synthesis in an aqueous medium was proposed using ionic liquids where anionic surfactant SDS was used for the cationic drug amitriptyline hydrochloride. These new amitriptyline hydrochloride-SDS ionic liquid had shown improved controlled release of the drug (Zhang et al., 2013).
While ionic liquids are proving beneficial in the effective delivery of a range of drug molecules that otherwise are poorly bioavailable, the safety of such delivery vehicles remains to be established.
Hydrogels
Hydrogels are 3D crosslink of synthetic and natural polymers, which can absorb considerable amounts of water without dissolving. The crosslinking network can be easily achieved via a variety of stimuli like pH sensitivity, stereo complexation, ionic interaction, condensation reaction, maturation, grafting, and enzymatic reaction. This 3D grid gives outstanding mechanical strength and adhesive properties to gels. Use of the biocompatible and biodegradable polymers with a stimulus responsive property offers the hydrogels as an alternate for oral drug delivery, in particular for enhancing the stability of drug in GI tract. Previous authors have endeavored to organize literature on oral administration, like buccal cavity administration (Yadav et al., 2009; Chaturvedi et al., 2013; Aminabhavi et al., 2014; Liu et al., 2017; Fonseca-Santos and Chorilli, 2018).
Chitosan and alginate hydrogels have been extensively used in biomedical applications (Du et al., 2015). For instance, in buccal delivery of lidocaine, chitosan with catechols using genipin cross linker has been tested in vitro and in vivo in rabbits (Xu et al., 2015). The pH-sensitive nanocomposite hydrogel beads of carboxymethyl cellulose and ZnO nanoparticles were prepared using a model drug propranolol and Fe3+ ion as a crosslinking agent (Zare-Akbari et al., 2016). Carboxymethyl cellulose with montmorillonite (modified chitosan with l-valine and phenylboronic acid) in a diabetic rat model showed better insulin levels even after 4 hours of dosing (Li et al., 2017). Alginate acts as a good encapsulating agent along with 3D network provider, e.g., oral delivery of doxorubicin-loaded liposomes for oral cancer (Shtenberg et al., 2018), emodin (Cong et al., 2018), insulin nano-emulsion was coated with alginate/chitosan using CaCl2 as cross linkers (Li et al., 2013).
Hydroxyethyl methacrylate nanogel via emulsion polymerization was developed for the oral delivery of insulin with improved bioavailability that controlled blood glucose levels for up to 12 hours (Wang et al., 2018). Insulin-loaded glucose responsive nanocarriers dispersed in hyaluronic acid hydrogel, offered better protection (almost 50% more protective than without hyaluronic acid gel nanocarriers) from harsh gastric environment in vivo in rats (Li et al., 2016).
To enhance the intestinal absorption of proteins, Sharma and coworkers reported the delivery of insulin-like proteins using modified cyclodextrin as methyl-β-cyclodextrin and further encapsulated in polymethacrylic acid hydrogel (Sajeesh et al., 2010c). N-vinyl pyrrolidone (NVP) merged polymethacrylic acid-chitosan microparticles (Sajeesh and Sharma, 2011) and poly(methacrylic acid)-chitosan-poly(ethylene glycol) hydrogels (Sajeesh et al., 2010) and thiol modified polymethacrylic acid-polyethylene glycol-chitosan [poly(ethylene glycol)]-based hydrogel (Sajeesh et al., 2010). An intricate gel of guar gum, poly(acrylic acid), and cyclodextrin using tetraethyl orthosilicate as a linker has shown promise for the delivery of dexamethasone via oral route (Das and Subuddhi, 2015).
Peppas and coworkers (Betancourt et al., 2010; Koetting and Peppas, 2014; O’Connor et al., 2017) have pioneered to make hydrogels for the delivery of hydrophilic/hydrophobic and small/large molecule drugs incorporating a variety of polymers. The terpolymer hydrogels P[(MAA-co-NVP)-g-EG] composed of methacrylic acid (MAA), NVP, and poly(ethylene glycol) were prepared by varying poly(ethylene glycol) chain lengths, for protein delivery using model proteins such as insulin and porcine growth hormone (O’Connor et al., 2017). Similarly, pH responsive hydrogels such as poly[itaconic acid-grafted-poly(ethylene glycol)] for high isoelectric point biomolecules like salmon calcitonin (Koetting and Peppas, 2014), while poly(methacrylic acid)-grafted-poly(ethylene glycol) (P(MAA-g-EG)) has been used for oral delivery of hematologic factor IX hFIX (Horava et al., 2016). A substantial amount of work has been reported on the use of hydrophobic particle-trapped hydrogels for hydrophobic drug delivery (Schoener et al., 2013; Puranik et al., 2016). Over the years, this work has contributed toward a fundamental understanding of structure-function relationships of hydrogels and their application to drug delivery of diverse physicochemical attributes.
Superporous hydrogels are a new, upcoming field for nonconventional target drug delivery owing to their high swelling and high mechanical strength, making them a good candidate for the oral delivery of protein and peptides (Mastropietro et al., 2012). A summary of recent developments in hydrogel technologies is presented in Table 3.
TABLE 3.
Summary of recent hydrogel technologies applied in drug delivery
| Drug | Hydrogel | Purpose | Reference |
|---|---|---|---|
| Insulin | Methyl β-cyclodextrin in polymethacrylic acid (PMAA) | Improve intestinal transport upon oral delivery | Sajeesh et al. (2010) |
| Insulin | Hydroxyethyl methacrylate (HEMA) | “do” | Wang et al. (2018) |
| Insulin | Carboxymethyl cellulose/poly(acrylic acid) | “do” | Gao et al. (2014) |
| Insulin and porcine growth hormone | Terpolymer hydrogels P((MAA-co-NVP)-g-EG) composed of methacrylic acid (MAA), N-vinyl pyrrolidone (NVP), and poly(ethylene glycol) (PEG) | “do” | O’Connor et al. (2017) |
| Dexamethasone | Guar gum (GG), poly(acrylic acid) (PAA) and cyclodextrin (CD) and tetraethyl orthosilicate as linker | Enhanced drug delivery | Das and Subuddhi (2015) |
| Salmon calcitonin | poly(itaconic acid-grafted-poly(ethylene glycol)) (P(IA-g-EG)) | Improve intestinal transport upon oral delivery | Koetting and Peppas (2014) |
| Hematological factor IX hFIX | poly(methacrylic acid)-grafted-poly(ethylene glycol) (P(MAA-g-EG)) (P(MAA-g-EG)) | Improve intestinal transport upon oral delivery | Horava et al. (2016) |
| Lidocaine | Chitosan with catechols and genipin cross linker | Rapid onset of action via buccal route | Xu et al. (2015) |
| Propranolol | Carboxymethyl cellulose and ZnO nanoparticles using Fe3+ ion as a crosslinking agent | Enhanced drug delivery | Zare-Akbari et al. (2016) |
Nanosystems
Conventional delivery systems release the drug in the body at a specific site (e.g., tablets, capsules, patches, gels in the intestine) for a determined time, from where the drug has to be absorbed, except for the intravenous route. Oral administration remains the preferred route for drug delivery but numerous drugs are poorly bioavailable for reasons such as low mucosal permeability, restricted permeation to a region of the GIT, low solubility or stability of the compound, and elimination-degradation of a fraction of drug without absorption (Tibbitt et al., 2016). Nanoscale drug delivery systems hold the promise of novel clinical interventions because their miniscule size enables them to act in vivo at the subcellular level (Langer and Weissleder, 2015). During the gastrointestinal transit, the entrapped drug is believed to be protected from enzymatic degradation and the gastrointestinal milieu on account of absorption of the intact particulate form. The favored sites for nanoparticulate uptake appear to be the lympho-epithelial M cells of the Peyer’s patches, because it has been shown that microparticles remain in the Peyer’s patches while nanoparticles disseminate systemically (Jani et al., 1990). It is hypothesized that, following the binding of nanoparticles to the apical membrane of M cells, internalization and shuttling to lymphocytes occur, with particle distribution being dependent on particle size and surface charge (Hussain et al., 1997; Delie, 1998). However, only a fraction of the dose is absorbed from passive nanosystems (Lamprou et al., 2013). Receptor-mediated drug delivery using functional nanosystems has been explored over the years to enhance the therapeutic index of drugs (Gref et al., 1994; Peer et al., 2007; Zhu et al., 2012; Mura et al., 2013; Cheng et al., 2015a). Cyclodextrins have been extensively used in drug delivery, in particular for small molecule delivery. The current advancement and potential future of cyclodextrin nanopaticles was recently reviewed elsewhere (Adeoye and Cabral-Marques, 2017). The recent use of receptor-mediated uptake in the GIT presents an exciting opportunity for targeted and enhanced delivery of nanosystems via vitamin B12 (Petrus et al., 2009; Fowler et al., 2013), folate-receptor (Anderson et al., 2001; Roger et al., 2012), neonatal fc receptor (Pridgen et al., 2013), and transferrin receptor (TfR; Amet et al., 2010; Shofner et al., 2010; Du et al., 2013). A summary of recent developments in nanoparticle technologies applied in drug delivery is presented in Table 4.
TABLE 4.
Summary of recent nanoparticle technologies applied in drug delivery
| Drug | Nanosystems | Purpose | Reference |
|---|---|---|---|
| Insulin | l-valine and phenylboronic acid modified chitosan-based multifunctional nanocarrier | To overcome multiple barriers for oral insulin delivery | Li et al. (2017) |
| Insulin | PLGA and Pluronic F68 based nanoparticles | To enhance the bioavailability using negatively changed nanoparticles for better absorption | Czuba et al. (2018) |
| Curcumin | Folic acid conjugated PLGA–PEG copolymer based nanosystem | To carryout site specific release of hydrophobic anti-cancer drugs | Pillai et al. (2015) |
| Curcumin | N-carboxymethyl chitosan based nanoparticles | To enhance the drug delivery to lymphatic system | Baek and Cho (2017) |
| Curcumin | Polyacrylamide-grafted-xanthan gum based nanoparticles | To improve the bioavailability of drug for colon targeting | Mutalik et al. (2016) |
| Paclitaxel and curcumin | Folate conjugated lipid nanoparticles | To co-deliver both drugs to enhance uptake and inhibit multidrug resistant | Liu et al. (2017) |
| Cisplatin prodrug [Asplatin, c,c,t-[PtCl2(NH3)2(OH) (aspirin)] | Cholesterol–asplatin-incorporated mPEG-PLGA nanoparticles | To improve the oral delivery with improved efficacy and reduced toxicity | Cheng et al. (2015) |
| Doxorubicin | HN-1 medicated PEGylated DOX | To improve the penetration efficiency to oral squamous cell carcinoma | Wang et al. (2017b) |
| Triclabendazole | Chitosan 70/5 and Miglyol 812 | To develop oral nanoformulation for anti-parasitic drugs | Real et al. (2018) |
| Cyclosporine A | Lipid nanoparticles | To enhance drug delivery | Guada et al. (2016) |
| Betulinic acid | PLGA based nanoparticles | For improved anti- hepatocellular carcinoma activity than the parent compound | Kumar et al. (2018) |
| Olmesartan medoxomil | Lipid nanoparticles | To enhance oral bioavailability | Kaithwas et al. (2017) |
| Epirubicin | PLGA based nanoparticles with PEG modification | To improve permeability across the ileum of Rodent | Tariq et al. (2016) |
| Scutellarin | Nanosystem based on chitosan derivatives | To enhance oral bioavailability | Wang et al. (2017) |
| Resveratrol | Zein-based nanoparticles | Improved oral bioavailability and anti-inflammatory effects | Penalva et al. (2015) |
| Superoxide dismutase | Zein-alginate nanoparticles | For steady state release of drug to decrease endotoxicity of IP administration | Lee et al. (2016) |
| Quercetin | Modified chitosan and alginate nanoparticles | To reduce blood glucose levels in diabetic rats | Mukhopadhyay et al. (2018) |
| Hesperetin | Nanocrystals of drug using phytantriol and lecithin with Pluronic F-127 or mannitol as surfactant | To improve site targeting using 2D and 3D arrays of nanocrystals | Shete et al. (2015) |
| Paclitaxel | Eudragit-coated nanorods | For better therapeutic response by taking advantage of less macrophagial consumption of the polymer | Yilmaz et al. (2016) |
| Platinum complex drugs | B-lactoglobulin-pectin encapsulated nanoparticles | To protect the drug through GIT using natural coating of pectin | Izadi et al. (2016) |
While promising, the receptor-mediated drug-delivery approaches currently use endogenous ligands to modify the particles that can be outcompeted by the physiologic ligands that are present in high concentrations (Lundquist and Artursson, 2016). To address this limitation, our laboratory recently reported the use of gambogic acid (GA), known for its affinity to transferrin receptors, independent of transferrin binding (Kasibhatla et al., 2005), as a noncompetitive ligand for TfR present in the small intestine (Saini et al., 2016; Ganugula et al., 2017a). The GA modified poly (lactic-co-glycolic acid) nanoparticles demonstrated noncompetitive transport in cellulo and improved oral bioavailability of encapsulated drugs or drug-like compounds (e.g., cyclosporine, curcumin, and insulin) in rodents (Saini et al., 2016; Ganugula et al., 2017a; Kaur et al., 2019). Our laboratory also observed that the ligand-receptor stoichiometry plays an important role in receptor-mediated drug delivery. Optimized ligand-receptor stoichiometry has been achieved through multiple coupling sites in newly formed polyester, which showed enhanced uptake and efficient drug delivery compared with benchmarking control, GA modified poly (lactic-co-glycolic acid) (Ganugula, et al., 2017b). It will be interesting to see if the improved bioavailability will subsequently lead to improved clinical outcomes, in particular chronic conditions on oral dosing.
Exosomes.
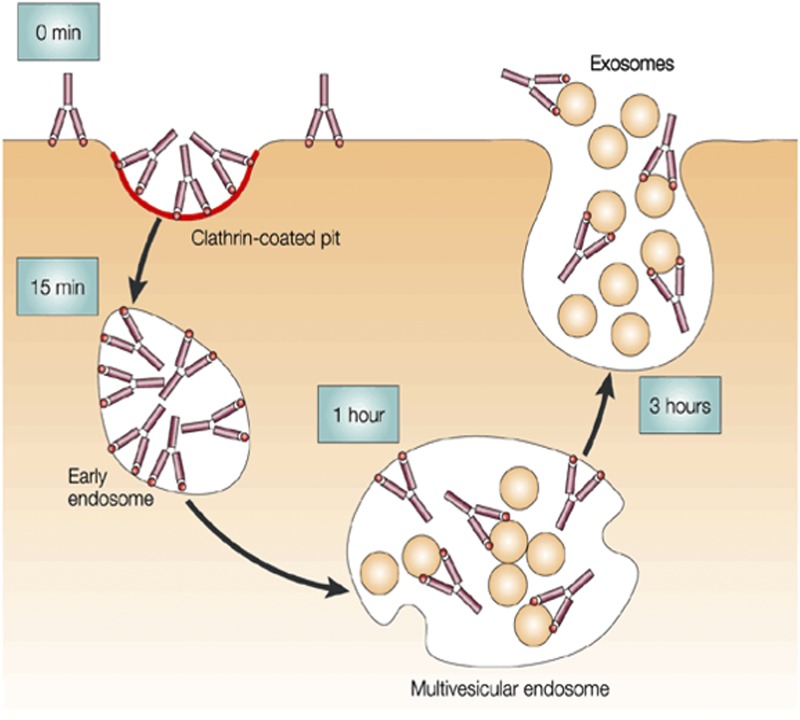
Exosomes are saucer-shaped nanovesicles of biologic origin (Fig. 11) that participate in cell communication and act as cellular couriers to facilitate the transfer of lipids, proteins, and RNAs from one cell to another (Zomer et al., 2010).
Fig. 11.
Schematic representation of exosome formation. Reproduced with permission from Théry et al. (2002).
Exosomes have been explored as nanocarrier to deliver macromolecules such as peptides, proteins, RNAs, etc., across the cell barriers (Alvarez-Erviti et al., 2011; Kamerkar et al., 2017; Wang et al., 2018). In general, exosomes are separated from dead cells and other debris by a series of centrifugations, followed by separation through flotation in sucrose gradients. The exosomes can be derived from bovine milk by removing the milk proteins (caseins) with hydrochloric acid through isoelectric precipitation followed by centrifugation to remove the debris (Yamauchi et al., 2019). As a cellular courier, the milk exosomes have been used as carriers for a variety of macromolecules such as proteins, microRNAs, anticancer drugs, etc. (Munagala et al., 2016; Samuel et al., 2017; Manca et al., 2018). These nanodevices are cost effective, scalable, and biocompatible and show enhanced drug bioavailability and cross species tolerance without any immune response. Moreover, the exosomes can be functionalized for targeted delivery of drugs. The milk exosome has been used to deliver flavonoids, like Anthos, as anticancer molecules for multiple cancer types (Munagala et al., 2017). Along with this, the milk exosomes have been used for the oral drug delivery of paclitaxel to enhance the efficacy and reduce the side effects associated with the drug (Agrawal et al., 2017).
Future Perspectives
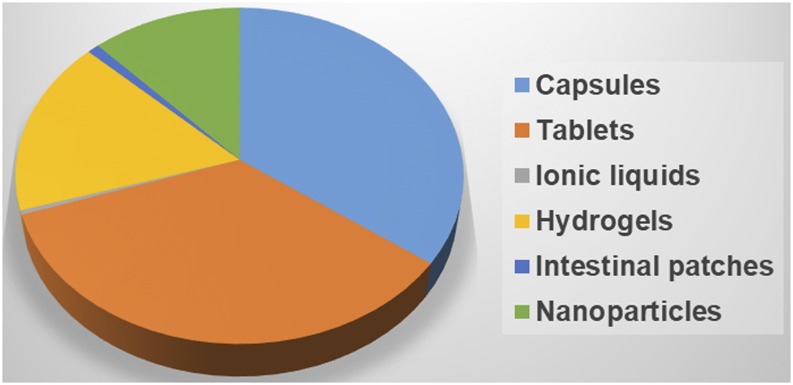
Oral drug delivery technologies have come a long way from simple tablets to the most sophisticated nanoparticle technologies (Fig. 12). These developments are possible due to the better understanding of the intestinal barriers and the possible ports of entry to the systemic circulation via the portal vein and intestinal lymphatics. The significant body of literature describing the efforts of small and large molecule delivery across the intestinal barriers only builds the confidence in nonconventional delivery strategies.
Fig. 12.
Pie chart showing the distribution of patents for various oral drug delivery technologies.
While delivery technology is as important as the active ingredient (drug) itself, it is important to realize that one technology does not fit all. It is very essential to understand the room for improvement when considering delivery technology; equally important is striking a balance between innovation and associated risk to ensure smooth translation. The success eventually lies in simplicity, and the focus should be in optimizing minimum effective therapeutic concentrations aided by delivery technologies. The application of nonconventional oral delivery strategies to repurpose the existing drugs should be reasonably straight forward, as we already understand the pharmacology and safety profiles that can greatly reduce the risk of attrition in drug development during clinical phases. This route of administration does not require the formulation to be sterile, an added advantage to run phase 0/I trials or, for that matter, conducting efficacy studies in higher order species, if adequate safety measures are established.
Abbreviations
- 3D
three-dimensional
- GA
gambogic acid
- GIT
gastrointestinal tract
- MAA
methacrylic acid
- NVP
N-vinyl pyrrolidone
- PTH
parathyroid hormone
- TfR
transferrin receptors
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Kaur, Arora, Ravi Kumar.
Footnotes
This work was supported by National Institutes of Health National Eye Institute [Grant No. R01EY028169 to M.N.V.R.K.].
References
- Adeoye O, Cabral-Marques H. (2017) Cyclodextrin nanosystems in oral drug delivery: a mini review. Int J Pharm 531:521–531. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, et al. (2017) Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 13:1627–1636. [DOI] [PubMed] [Google Scholar]
- Ainslie KM, Kraning CM, Desai TA. (2008) Microfabrication of an asymmetric, multi-layered microdevice for controlled release of orally delivered therapeutics. Lab Chip 8:1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri SM, Al-Lohedan HA, Al-Farraj E, Alhokbany N, Chaudhary AA, Ahamad T. (2016) Macroporous natural capsules extracted from Phoenix dactylifera L. spore and their application in oral drugs delivery. Int J Pharm 504:39–47. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. [DOI] [PubMed] [Google Scholar]
- Amet N, Wang W, Shen WC. (2010) Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release 141:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminabhavi TM, Nadagouda MN, Joshi SD, More UA. (2014) Guar gum as platform for the oral controlled release of therapeutics. Expert Opin Drug Deliv 11:753–766. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Eliot LA, Stevenson BR, Rogers JA. (2001) Formulation and evaluation of a folic acid receptor-targeted oral vancomycin liposomal dosage form. Pharm Res 18:316–322. [DOI] [PubMed] [Google Scholar]
- Andrews GP, Laverty TP, Jones DS. (2009) Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm 71:505–518. [DOI] [PubMed] [Google Scholar]
- Baek JS, Cho CW. (2017) Surface modification of solid lipid nanoparticles for oral delivery of curcumin: improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur J Pharm Biopharm 117:132–140. [DOI] [PubMed] [Google Scholar]
- Bai JPF. (2016) Pharmacodynamics and systems pharmacology approaches to repurposing drugs in the wake of global health burden. J Pharm Sci 105:3007–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk A, Wiest J, Widmer T, Galli B, Holzgrabe U, Meinel L. (2015) Transformation of acidic poorly water soluble drugs into ionic liquids. Eur J Pharm Biopharm 94:73–82. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Ibsen K, Brown T, Chen R, Agatemor C, Mitragotri S. (2018) Ionic liquids for oral insulin delivery. Proc Natl Acad Sci USA 115:7296–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Lee J, Mitragotri S. (2016) Intestinal mucoadhesive devices for oral delivery of insulin. Bioeng Transl Med 1:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Mitragotri S. (2017) Intestinal patch systems for oral drug delivery. Curr Opin Pharmacol 36:58–65. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Wong J, Gogoi R, Brown T, Mitragotri S. (2017) Intestinal micropatches for oral insulin delivery. J Drug Target 25:608–615. [DOI] [PubMed] [Google Scholar]
- Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, Mo S, Lee YL, Mazdiyasni H, Kogan L, et al. (2016) Oral, ultra-long-lasting drug delivery: application toward malaria elimination goals. Sci Transl Med 8:365ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernkop-Schnürch A. (2005) Thiomers: a new generation of mucoadhesive polymers. Adv Drug Deliv Rev 57:1569–1582. [DOI] [PubMed] [Google Scholar]
- Betancourt T, Pardo J, Soo K, Peppas NA. (2010) Characterization of pH-responsive hydrogels of poly(itaconic acid-g-ethylene glycol) prepared by UV-initiated free radical polymerization as biomaterials for oral delivery of bioactive agents. J Biomed Mater Res A 93:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Quinn D, Weiss M, Markabi S, Weidenman M, Edson K, Karlsson G, Pohlmann H, Wigal S. (2003) Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs 5:833–841. [DOI] [PubMed] [Google Scholar]
- Binkley N, Bolognese M, Sidorowicz-Bialynicka A, Vally T, Trout R, Miller C, Buben CE, Gilligan JP, Krause DS, Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) Investigators (2012) A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: the Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial. J Bone Miner Res 27:1821–1829. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Bailón F, Osorio-Revilla G, Gallardo-Velázquez T. (2015) Microencapsulation of insulin using a W/O/W double emulsion followed by complex coacervation to provide protection in the gastrointestinal tract. J Microencapsul 32:308–316. [DOI] [PubMed] [Google Scholar]
- Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, Kusko R, Zeskind B, Risso S, Kagan E, et al. (2018) Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol 175:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi K, Ganguly K, Nadagouda MN, Aminabhavi TM. (2013) Polymeric hydrogels for oral insulin delivery. J Control Release 165:129–138. [DOI] [PubMed] [Google Scholar]
- Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. (2015a) A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov 14:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Shi H, Huang H, Cao Z, Wang J, Liu Y. (2015b) Oral delivery of a platinum anticancer drug using lipid assisted polymeric nanoparticles. Chem Commun (Camb) 51:17536–17539. [DOI] [PubMed] [Google Scholar]
- Chirra HD, Desai TA. (2012) Emerging microtechnologies for the development of oral drug delivery devices. Adv Drug Deliv Rev 64:1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choonara BF, Choonara YE, Kumar P, Bijukumar D, du Toit LC, Pillay V. (2014) A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol Adv 32:1269–1282. [DOI] [PubMed] [Google Scholar]
- Cipolla DC, Gonda I. (2012) Formulation technology to repurpose drugs for inhalation delivery. Drug Discov Today Ther Strat 8:123–130. [Google Scholar]
- Cong Z, Shi Y, Wang Y, Wang Y, Niu J, Chen N, Xue H. (2018) A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int J Biol Macromol 107 (Pt A):855–864. [DOI] [PubMed] [Google Scholar]
- Czuba E, Diop M, Mura C, Schaschkow A, Langlois A, Bietiger W, Neidl R, Virciglio A, Auberval N, Julien-David D, et al. (2018) Oral insulin delivery, the challenge to increase insulin bioavailability: influence of surface charge in nanoparticle system. Int J Pharm 542:47–55. [DOI] [PubMed] [Google Scholar]
- Das S, Subuddhi U. (2015) pH-Responsive guar gum hydrogels for controlled delivery of dexamethasone to the intestine. Int J Biol Macromol 79:856–863. [DOI] [PubMed] [Google Scholar]
- Del Curto MD, Maroni A, Palugan L, Zema L, Gazzaniga A, Sangalli ME. (2011) Oral delivery system for two-pulse colonic release of protein drugs and protease inhibitor/absorption enhancer compounds. J Pharm Sci 100:3251–3259. [DOI] [PubMed] [Google Scholar]
- Delie F. (1998) Evaluation of nano- and microparticle uptake by the gastrointestinal tract. Adv Drug Deliv Rev 34:221–233. [DOI] [PubMed] [Google Scholar]
- Donovan JD, Bauer L, Fahey GC, Jr, Lee Y. (2017) In vitro digestion and fermentation of microencapsulated tributyrin for the delivery of butyrate. J Food Sci 82:1491–1499. [DOI] [PubMed] [Google Scholar]
- Du H, Liu M, Yang X, Zhai G. (2015) The design of pH-sensitive chitosan-based formulations for gastrointestinal delivery. Drug Discov Today 20:1004–1011. [DOI] [PubMed] [Google Scholar]
- Du W, Fan Y, Zheng N, He B, Yuan L, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. (2013) Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials 34:794–806. [DOI] [PubMed] [Google Scholar]
- Eiamtrakarn S, Itoh Y, Kishimoto J, Yoshikawa Y, Shibata N, Murakami M, Takada K. (2002) Gastrointestinal mucoadhesive patch system (GI-MAPS) for oral administration of G-CSF, a model protein. Biomaterials 23:145–152. [DOI] [PubMed] [Google Scholar]
- El-Aassar MR, Hafez EE, El-Deeb NM, Fouda MM. (2014) Microencapsulation of lectin anti-cancer agent and controlled release by alginate beads, biosafety approach. Int J Biol Macromol 69:88–94. [DOI] [PubMed] [Google Scholar]
- Elder DP, Holm R, Diego HL. (2013) Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int J Pharm 453:88–100. [DOI] [PubMed] [Google Scholar]
- Fleming AB, Carlson DR, Varanasi RK, Grima M, Mayock SP, Saim S, Kopecky EA. (2016) Evaluation of an extended-release, abuse-deterrent, microsphere-in-capsule analgesic for the management of patients with Chronic Pain With Dysphagia (CPD). Pain Pract 16:334–344. [DOI] [PubMed] [Google Scholar]
- Fonseca-Santos B, Chorilli M. (2018) An overview of polymeric dosage forms in buccal drug delivery: state of art, design of formulations and their in vivo performance evaluation. Mater Sci Eng C 86:129–143. [DOI] [PubMed] [Google Scholar]
- Fowler R, Vllasaliu D, Trillo FF, Garnett M, Alexander C, Horsley H, Smith B, Whitcombe I, Eaton M, Stolnik S. (2013) Nanoparticle transport in epithelial cells: pathway switching through bioconjugation. Small 9:3282–3294. [DOI] [PubMed] [Google Scholar]
- Fox CB, Chirra HD, Desai TA. (2014) Planar bioadhesive microdevices: a new technology for oral drug delivery. Curr Pharm Biotechnol 15:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CB, Kim J, Le LV, Nemeth CL, Chirra HD, Desai TA. (2015) Micro/nanofabricated platforms for oral drug delivery. J Control Release 219:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganugula R, Arora M, Guada M, Saini P, Kumar MNVR. (2017a) Noncompetitive active transport exploiting intestinal transferrin receptors for oral delivery of proteins by tunable nanoplatform. ACS Macro Lett 6:161–164. [DOI] [PubMed] [Google Scholar]
- Ganugula R, Arora M, Saini P, Guada M, Kumar MNVR. (2017b) Next generation precision-polyesters enabling optimization of ligand-receptor stoichiometry for modular drug delivery. J Am Chem Soc 139:7203–7216. [DOI] [PubMed] [Google Scholar]
- Gao X, Cao Y, Song X, Zhang Z, Zhuang X, He C, Chen X. (2014) Biodegradable, pH-responsive carboxymethyl cellulose/poly(acrylic acid) hydrogels for oral insulin delivery. Macromol Biosci 14:565–575. [DOI] [PubMed] [Google Scholar]
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. (1994) Biodegradable long-circulating polymeric nanospheres. Science 263:1600–1603. [DOI] [PubMed] [Google Scholar]
- Guada M, Lana H, Gil AG, Dios-Viéitez M del C, Blanco-Prieto MJ. (2016) Cyclosporine A lipid nanoparticles for oral administration: pharmacodynamics and safety evaluation. Eur J Pharm Biopharm 101:112–118. [DOI] [PubMed] [Google Scholar]
- Guarascio AJ, Slain D. (2015) Review of the new delayed-release oral tablet and intravenous dosage forms of posaconazole. Pharmacotherapy 35:208–219. [DOI] [PubMed] [Google Scholar]
- Gupta V, Hwang BH, Doshi N, Banerjee A, Anselmo AC, Mitragotri S. (2016) Delivery of exenatide and insulin using mucoadhesive intestinal devices. Ann Biomed Eng 44:1993–2007. [DOI] [PubMed] [Google Scholar]
- Gupta V, Hwang BH, Lee J, Anselmo AC, Doshi N, Mitragotri S. (2013) Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J Control Release 172:753–762. [DOI] [PubMed] [Google Scholar]
- Hascicek C, Rossi A, Colombo P, Massimo G, Strusi OL, Colombo G. (2011) Assemblage of drug release modules: effect of module shape and position in the assembled systems on floating behavior and release rate. Eur J Pharm Biopharm 77:116–121. [DOI] [PubMed] [Google Scholar]
- He H, Cao X, Lee LJ. (2004) Design of a novel hydrogel-based intelligent system for controlled drug release. J Control Release 95:391–402. [DOI] [PubMed] [Google Scholar]
- Hong Y, Liu G, Gu Z. (2016) Recent advances of starch-based excipients used in extended-release tablets: a review. Drug Deliv 23:12–20. [DOI] [PubMed] [Google Scholar]
- Horava SD, Moy KJ, Peppas NA. (2016) Biodegradable hydrophilic carriers for the oral delivery of hematological factor IX for hemophilia B treatment. Int J Pharm 514:220–228. [DOI] [PubMed] [Google Scholar]
- Hoyer H, Greindl M, Bernkop-Schnürch A. (2009) Design and in vivo evaluation of a patch system based on thiolated polymers. J Pharm Sci 98:620–627. [DOI] [PubMed] [Google Scholar]
- Huang L, Injac SG, Cui K, Braun F, Lin Q, Du Y, Zhang H, Kogiso M, Lindsay H, Zhao S, et al. (2018) Systems biology–based drug repositioning identifies digoxin as a potential therapy for groups 3 and 4 medulloblastoma. Sci Transl Med 10: eaat0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain N, Jani PU, Florence AT. (1997) Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm Res 14:613–618. [DOI] [PubMed] [Google Scholar]
- Imran M (2014) inventors. Swallowable drug delivery device and methods of drug delivery. U.S. Patent 8,721,620. Assignee: Rani Therapeutics LLC.
- Izadi Z, Divsalar A, Saboury AA, Sawyer L. (2016) β-Lactoglobulin-pectin nanoparticle-based oral drug delivery system for potential treatment of colon cancer. Chem Biol Drug Des 88:209–216. [DOI] [PubMed] [Google Scholar]
- Jani P, Halbert GW, Langridge J, Florence AT. (1990) Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol 42:821–826. [DOI] [PubMed] [Google Scholar]
- Kaithwas V, Dora CP, Kushwah V, Jain S. (2017) Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf B Biointerfaces 154:10–20. [DOI] [PubMed] [Google Scholar]
- Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasibhatla S, Jessen KA, Maliartchouk S, Wang JY, English NM, Drewe J, Qiu L, Archer SP, Ponce AE, Sirisoma N, et al. (2005) A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc Natl Acad Sci USA 102:12095–12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma M, Watanabe S, Takemura S, Sako K, Sawada T, Masuda Y, Nakamura K, Fukui M, Connor AL, Wilding IR. (2004) Scintigraphic evaluation of a novel colon-targeted delivery system (CODES) in healthy volunteers. J Pharm Sci 93:1287–1299. [DOI] [PubMed] [Google Scholar]
- Kaur G, Arora M, Ganugula R, Kumar MNVR. (2019) Double-headed nanosystems for oral drug delivery. Chem Commun 55:4761–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled SA, Alexander MR, Wildman RD, Wallace MJ, Sharpe S, Yoo J, Roberts CJ. (2018) 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int J Pharm 538:223–230. [DOI] [PubMed] [Google Scholar]
- Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. (2015) 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm 494:643–650. [DOI] [PubMed] [Google Scholar]
- Kim S, Diab R, Joubert O, Canilho N, Pasc A. (2016) Core-shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf B Biointerfaces 140:161–168. [DOI] [PubMed] [Google Scholar]
- Kirsch K, Hanke U, Weitschies W. (2017) An overview of intestinal wafers for oral drug delivery. Eur J Pharm Biopharm 114:135–144. [DOI] [PubMed] [Google Scholar]
- Knipe JM, Chen F, Peppas NA. (2015) Enzymatic biodegradation of hydrogels for protein delivery targeted to the small intestine. Biomacromolecules 16:962–972. [DOI] [PubMed] [Google Scholar]
- Koetting MC, Peppas NA. (2014) pH-Responsive poly(itaconic acid-co-N-vinylpyrrolidone) hydrogels with reduced ionic strength loading solutions offer improved oral delivery potential for high isoelectric point-exhibiting therapeutic proteins. Int J Pharm 471:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Singh AK, Raj V, Rai A, Keshari AK, Kumar D, Maity B, Prakash A, Maiti S, Saha S. (2018) Poly(lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid for improved treatment of hepatic cancer: characterization, in vitro and in vivo evaluations. Int J Nanomedicine 13:975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyobula M, Adedeji A, Alexander MR, Saleh E, Wildman R, Ashcroft I, Gellert PR, Roberts CJ. (2017) 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release 261:207–215. [DOI] [PubMed] [Google Scholar]
- Lamb YN, Garnock-Jones KP, Keam SJ. (2016) Oxycodone DETERx® ER capsules: a review in severe, chronic pain. Drugs 76:1759–1769. [DOI] [PubMed] [Google Scholar]
- Lamprou DA, Venkatpurwar V, Kumar MNVR. (2013) Atomic force microscopy images label-free, drug encapsulated nanoparticles in vivo and detects difference in tissue mechanical properties of treated and untreated: a tip for nanotoxicology. PLoS One 8:e64490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Weissleder R. (2015) Nanotechnology. JAMA 313:135–136. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim YC, Park JH. (2016) Zein-alginate based oral drug delivery systems: protection and release of therapeutic proteins. Int J Pharm 515:300–306. [DOI] [PubMed] [Google Scholar]
- Leonard TW, Lynch J, McKenna MJ, Brayden DJ. (2006) Promoting absorption of drugs in humans using medium-chain fatty acid-based solid dosage forms: GIPET. Expert Opin Drug Deliv 3:685–692. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang G, Yu W, Liu D, Chen H, Liu Y, Huang Q, Tong Z, Yao J, Kong X. (2016) A composite hydrogel system containing glucose-responsive nanocarriers for oral delivery of insulin. Mater Sci Eng C 69:37–45. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang G, Yu W, Liu D, Chen H, Liu Y, Tong Z, Kong X, Yao J. (2017) Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater Sci Eng C 70:278–286. [DOI] [PubMed] [Google Scholar]
- Li X, Qi J, Xie Y, Zhang X, Hu S, Xu Y, Lu Y, Wu W. (2013) Nanoemulsions coated with alginate/chitosan as oral insulin delivery systems: preparation, characterization, and hypoglycemic effect in rats. Int J Nanomedicine 8:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yao W, Rao Y, Lu X, Gao J. (2017) pH-Responsive carriers for oral drug delivery: challenges and opportunities of current platforms. Drug Deliv 24:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist P, Artursson P. (2016) Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev 106 (Pt B):256–276. [DOI] [PubMed] [Google Scholar]
- Ma G. (2014) Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. J Control Release 193:324–340. [DOI] [PubMed] [Google Scholar]
- Ma Y, Fuchs AV, Boase NRB, Rolfe BE, Coombes AGA, Thurecht KJ. (2015) The in vivo fate of nanoparticles and nanoparticle-loaded microcapsules after oral administration in mice: evaluation of their potential for colon-specific delivery. Eur J Pharm Biopharm 94:393–403. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Sharma R, Mahajan RK. (2012) An investigation of drug binding ability of a surface active ionic liquid: micellization, electrochemical, and spectroscopic studies. Langmuir 28:17238–17246.23214438 [Google Scholar]
- Makary P. (2014) Principles of salt formation. UK J Pharm Biosci 2:1–4. [Google Scholar]
- Malewar N, Avachat M, Kulkarni S, Pokharkar V. (2015) Design and evaluation of novel barrier layer technologies for controlling venlafaxine hydrochloride release from tablet dosage form. Pharm Dev Technol 20:588–597. [DOI] [PubMed] [Google Scholar]
- Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, Zempleni J. (2018) Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep 8:11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapara SS, Patravale VB. (2017) Medical capsule robots: a renaissance for diagnostics, drug delivery and surgical treatment. J Control Release 261:337–351. [DOI] [PubMed] [Google Scholar]
- Mastropietro DJ, Omidian H, Park K. (2012) Drug delivery applications for superporous hydrogels. Expert Opin Drug Deliv 9:71–89. [DOI] [PubMed] [Google Scholar]
- Mathavan S, Chen-Tan N, Arfuso F, Al-Salami H. (2016) The role of the bile acid chenodeoxycholic acid in the targeted oral delivery of the anti-diabetic drug gliclazide, and its applications in type 1 diabetes. Artif Cells Nanomed Biotechnol 44:1508–1519. [DOI] [PubMed] [Google Scholar]
- Mignani S, El Kazzouli S, Bousmina M, Majoral JP. (2013) Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv Drug Deliv Rev 65:1316–1330. [DOI] [PubMed] [Google Scholar]
- Moes JJ, Koolen SLW, Huitema ADR, Schellens JHM, Beijnen JH, Nuijen B. (2011) Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int J Pharm 420:244–250. [DOI] [PubMed] [Google Scholar]
- Moodley K, Pillay V, Choonara YE, du Toit LC, Ndesendo VMK, Kumar P, Cooppan S, Bawa P. (2012) Oral drug delivery systems comprising altered geometric configurations for controlled drug delivery. Int J Mol Sci 13:18–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooranian A, Negrulj R, Mikov M, Golocorbin-Kon S, Arfuso F, Al-Salami H. (2015) Novel chenodeoxycholic acid-sodium alginate matrix in the microencapsulation of the potential antidiabetic drug, probucol. An in vitro study. J Microencapsul 32:589–597. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Maity S, Mandal S, Chakraborti AS, Prajapati AK, Kundu PP. (2018) Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr Polym 182:42–51. [DOI] [PubMed] [Google Scholar]
- Mulugeta LY, Yao L, Mould D, Jacobs B, Florian J, Smith B, Sinha V, Barrett JS. (2018) Leveraging big data in pediatric development programs: proceedings from the 2016 American College of Clinical Pharmacology Annual Meeting Symposium. Clin Pharmacol Ther 104:81–87. [DOI] [PubMed] [Google Scholar]
- Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM, Kyakulaga AH, Singh IP, Vadhanam MV, Gupta RC. (2017) Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett 393:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R, Aqil F, Jeyabalan J, Gupta RC. (2016) Bovine milk-derived exosomes for drug delivery. Cancer Lett 371:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura S, Nicolas J, Couvreur P. (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12:991–1003. [DOI] [PubMed] [Google Scholar]
- Mutalik S, Suthar NA, Managuli RS, Shetty PK, Avadhani K, Kalthur G, Kulkarni RV, Thomas R. (2016) Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int J Biol Macromol 86:709–720. [DOI] [PubMed] [Google Scholar]
- Nielsen LH, Keller SS, Boisen A. (2018) Microfabricated devices for oral drug delivery. Lab Chip 18:2348–2358. [DOI] [PubMed] [Google Scholar]
- Nielsen LH, Nagstrup J, Gordon S, Keller SS, Østergaard J, Rades T, Müllertz A, Boisen A. (2015) pH-triggered drug release from biodegradable microwells for oral drug delivery. Biomed Microdevices 17:9958. [DOI] [PubMed] [Google Scholar]
- Nokhodchi A, Raja S, Patel P, Asare-Addo K. (2012) The role of oral controlled release matrix tablets in drug delivery systems. Bioimpacts 2:175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor C, Steichen S, Peppas NA. (2017) Student Award for Outstanding Research Winner in the Undergraduate Category for the 2017 Society for Biomaterials Annual Meeting and Exposition, April 5-8, 2017, Minneapolis, Minnesota: development and characterization of stimuli-responsive hydrogel microcarriers for oral protein delivery. J Biomed Mater Res A 105:1243–1251. [DOI] [PubMed] [Google Scholar]
- Oliveira PR, Bernardi LS, Strusi OL, Mercuri S, Segatto Silva MA, Colombo P, Sonvico F. (2011) Assembled modules technology for site-specific prolonged delivery of norfloxacin. Int J Pharm 405:90–96. [DOI] [PubMed] [Google Scholar]
- Omer ME, Alkatheri A, Yassin AEB, Albogami AM, Al Bekairy AM. (2017) Modified geometry three-layered tablet as a platform for class II drugs zero-order release system. Trop J Pharm Res 16:1773–1778. [Google Scholar]
- Paulmurugan R, Bhethanabotla R, Mishra K, Devulapally R, Foygel K, Sekar TV, Ananta JS, Massoud TF, Joy A. (2016) Folate receptor-targeted polymeric micellar nanocarriers for delivery of orlistat as a repurposed drug against triple-negative breast cancer. Mol Cancer Ther 15:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–760. [DOI] [PubMed] [Google Scholar]
- Penalva R, Esparza I, Larraneta E, González-Navarro CJ, Gamazo C, Irache JM. (2015) Zein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. J Agric Food Chem 63:5603–5611. [DOI] [PubMed] [Google Scholar]
- Peska L, Buza K, Koller J. (2017) Drug-target interaction prediction: a Bayesian ranking approach. Comput Methods Programs Biomed 152:15–21. [DOI] [PubMed] [Google Scholar]
- Petrus AK, Fairchild TJ, Doyle RP. (2009) Traveling the vitamin B12 pathway: oral delivery of protein and peptide drugs. Angew Chem Int Ed Engl 48:1022–1028. [DOI] [PubMed] [Google Scholar]
- Pillai JJ, Thulasidasan AKT, Anto RJ, Devika NC, Ashwanikumar N, Kumar GSV. (2015) Curcumin entrapped folic acid conjugated PLGA–PEG nanoparticles exhibit enhanced anticancer activity by site specific delivery. RSC Adv 5:25518–25524. [Google Scholar]
- Preis M. (2015) Orally disintegrating films and mini-tablets-innovative dosage forms of choice for pediatric use. AAPS PharmSciTech 16:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, Langer R, Farokhzad OC. (2013) Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci Transl Med 5:213ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik AS, Pao LP, White VM, Peppas NA. (2016) Synthesis and characterization of pH-responsive nanoscale hydrogels for oral delivery of hydrophobic therapeutics. Eur J Pharm Biopharm 108:196–213. [DOI] [PubMed] [Google Scholar]
- Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, et al. (2018) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18:41–58. [DOI] [PubMed] [Google Scholar]
- Real D, Hoffmann S, Leonardi D, Salomon C, Goycoolea FM. (2018) Chitosan-based nanodelivery systems applied to the development of novel triclabendazole formulations. PLoS One 13:e0207625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger E, Kalscheuer S, Kirtane A, Guru BR, Grill AE, Whittum-Hudson J, Panyam J. (2012) Folic acid functionalized nanoparticles for enhanced oral drug delivery. Mol Pharm 9:2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Seddon KR. (2003) Chemistry. Ionic liquids--solvents of the future? Science 302:792–793. [DOI] [PubMed] [Google Scholar]
- Roy S, Pal K, Anis A, Pramanik K, Prabhakar B. (2009) Polymers in mucoadhesive drug-delivery systems: a brief note. Des Monomers Polym 12:483–495. [Google Scholar]
- Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. (2018) Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release 269:355–363. [DOI] [PubMed] [Google Scholar]
- Sahbaz Y, Nguyen TH, Ford L, McEvoy CL, Williams HD, Scammells PJ, Porter CJH. (2017) Ionic liquid forms of weakly acidic drugs in oral lipid formulations: preparation, characterization, in vitro digestion, and in vivo absorption studies. Mol Pharm 14:3669–3683. [DOI] [PubMed] [Google Scholar]
- Sahbaz Y, Williams HD, Nguyen TH, Saunders J, Ford L, Charman SA, Scammells PJ, Porter CJH. (2015) Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid based formulations. Mol Pharm 12:1980–1991. [DOI] [PubMed] [Google Scholar]
- Saini P, Ganugula R, Arora M, Kumar MNVR. (2016) The next generation non-competitive active polyester nanosystems for transferrin receptor-mediated peroral transport utilizing gambogic acid as a ligand. Sci Rep 6:29501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajeesh S, Bouchemal K, Marsaud V, Vauthier C, Sharma CP. (2010) Cyclodextrin complexed insulin encapsulated hydrogel microparticles: an oral delivery system for insulin. J Control Release 147:377–384. [DOI] [PubMed] [Google Scholar]
- Sajeesh S, Bouchemal K, Sharma CP, Vauthier C. (2010c) Surface-functionalized polymethacrylic acid based hydrogel microparticles for oral drug delivery. Eur J Pharm Biopharm 74:209–218. [DOI] [PubMed] [Google Scholar]
- Sajeesh S, Sharma CP. (2011) Mucoadhesive hydrogel microparticles based on poly (methacrylic acid-vinyl pyrrolidone)-chitosan for oral drug delivery. Drug Deliv 18:227–235. [DOI] [PubMed] [Google Scholar]
- Sajeesh S, Vauthier C, Gueutin C, Ponchel G, Sharma CP. (2010) Thiol functionalized polymethacrylic acid-based hydrogel microparticles for oral insulin delivery. Acta Biomater 6:3072–3080. [DOI] [PubMed] [Google Scholar]
- Samuel M, Chisanga D, Liem M, Keerthikumar S, Anand S, Ang CS, Adda CG, Versteegen E, Jois M, Mathivanan S. (2017) Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci Rep 7:5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell JW, Bosley J. (2016) When quality beats quantity: decision theory, drug discovery, and the reproducibility crisis. PLoS One 11:e0147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoener CA, Hutson HN, Peppas NA. (2013) pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the oral delivery of chemotherapeutics. J Biomed Mater Res A 101:2229–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serajuddin ATM. (2007) Salt formation to improve drug solubility. Adv Drug Deliv Rev 59:603–616. [DOI] [PubMed] [Google Scholar]
- Shamshina JL, Kelley SP, Gurau G, Rogers RD. (2015) Chemistry: develop ionic liquid drugs. Nature 528:188–189. [DOI] [PubMed] [Google Scholar]
- Shen Z, Mitragotri S. (2002) Intestinal patches for oral drug delivery. Pharm Res 19:391–395. [DOI] [PubMed] [Google Scholar]
- Shete G, Pawar YB, Thanki K, Jain S, Bansal AK. (2015) Oral bioavailability and pharmacodynamic activity of hesperetin nanocrystals generated using a novel bottom-up technology. Mol Pharm 12:1158–1170. [DOI] [PubMed] [Google Scholar]
- Shofner JP, Phillips MA, Peppas NA. (2010) Cellular evaluation of synthesized insulin/transferrin bioconjugates for oral insulin delivery using intelligent complexation hydrogels. Macromol Biosci 10:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtenberg Y, Goldfeder M, Prinz H, Shainsky J, Ghantous Y, Abu El-Naaj I, Schroeder A, Bianco-Peled H. (2018) Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int J Biol Macromol 111:62–69. [DOI] [PubMed] [Google Scholar]
- Silber BM. (2010) Driving drug discovery: the fundamental role of academic labs. Sci Transl Med 2:30cm16. [DOI] [PubMed] [Google Scholar]
- Svagan AJ, Kusic A, De Gobba C, Larsen FH, Sassene P, Zhou Q, van de Weert M, Mullertz A, Jørgensen B, Ulvskov P. (2016) Rhamnogalacturonan-I based microcapsules for targeted drug release. PLoS One 11:e0168050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao SL, Desai TA. (2005) Gastrointestinal patch systems for oral drug delivery. Drug Discov Today 10:909–915. [DOI] [PubMed] [Google Scholar]
- Tariq M, Alam MA, Singh AT, Panda AK, Talegaonkar S. (2016) Surface decorated nanoparticles as surrogate carriers for improved transport and absorption of epirubicin across the gastrointestinal tract: pharmacokinetic and pharmacodynamic investigations. Int J Pharm 501:18–31. [DOI] [PubMed] [Google Scholar]
- Teutonico D, Ponchel G. (2011) Patches for improving gastrointestinal absorption: an overview. Drug Discov Today 16:991–997. [DOI] [PubMed] [Google Scholar]
- Thanou M, Verhoef JC, Junginger HE. (2001) Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev 52:117–126. [DOI] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, Amigorena S. (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579. [DOI] [PubMed] [Google Scholar]
- Tibbitt MW, Dahlman JE, Langer R. (2016) Emerging frontiers in drug delivery. J Am Chem Soc 138:704–717. [DOI] [PubMed] [Google Scholar]
- Toorisaka E, Watanabe K, Ono H, Hirata M, Kamiya N, Goto M. (2012) Intestinal patches with an immobilized solid-in-oil formulation for oral protein delivery. Acta Biomater 8:653–658. [DOI] [PubMed] [Google Scholar]
- Wagner RH, Savir-Baruch B, Halama JR, Venu M, Gabriel MS, Bova D. (2017) Proof of concept: design and initial evaluation of a device to measure gastrointestinal transit time. J Nucl Med Technol 45:230–235. [DOI] [PubMed] [Google Scholar]
- Wang C, Chopade SA, Guo Y, Early JT, Tang B, Wang E, Hillmyer MA, Lodge TP, Sun CC. (2018a) Preparation, characterization, and formulation development of drug-drug protic ionic liquids of diphenhydramine with ibuprofen and naproxen. Mol Pharm 15:4190–4201. [DOI] [PubMed] [Google Scholar]
- Wang J, Tan J, Luo J, Huang P, Zhou W, Chen L, Long L, Zhang LM, Zhu B, Yang L, et al. (2017a) Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J Nanobiotechnology 15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yu J, Kadungure T, Beyene J, Zhang H, Lu Q. (2017b) ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat Commun 9:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cheng D, Liu L, Li X. (2018c) Development of poly(hydroxyethyl methacrylate) nanogel for effective oral insulin delivery. Pharm Dev Technol 23:351–357. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wan G, Li Z, Shi S, Chen B, Li C, Zhang L, Wang Y. (2017b) PEGylated doxorubicin nanoparticles mediated by HN-1 peptide for targeted treatment of oral squamous cell carcinoma. Int J Pharm 525:21–31. [DOI] [PubMed] [Google Scholar]
- Wiest J, Saedtler M, Balk A, Merget B, Widmer T, Bruhn H, Raccuglia M, Walid E, Picard F, Stopper H, et al. (2017) Mapping the pharmaceutical design space by amorphous ionic liquid strategies. J Control Release 268:314–322. [DOI] [PubMed] [Google Scholar]
- Wilding IR, Davis SS, Sparrow RA, Ziemniak JA, Heald DL. (1995) Pharmacoscintigraphic evaluation of a modified release (Geomatrix®) diltiazem formulation. J Control Release 33:89–97. [Google Scholar]
- Williams HD, Ford L, Lim S, Han S, Baumann J, Sullivan H, Vodak D, Igonin A, Benameur H, Pouton CW, et al. (2018) Transformation of biopharmaceutical classification system class I and III drugs into ionic liquids and lipophilic salts for enhanced developability using lipid formulations. J Pharm Sci 107:203–216. [DOI] [PubMed] [Google Scholar]
- Williams HD, Sahbaz Y, Ford L, Nguyen TH, Scammells PJ, Porter CJH. (2014) Ionic liquids provide unique opportunities for oral drug delivery: structure optimization and in vivo evidence of utility. Chem Commun (Camb) 50:1688–1690. [DOI] [PubMed] [Google Scholar]
- Wong TW. (2010) Design of oral insulin delivery systems. J Drug Target 18:79–92. [DOI] [PubMed] [Google Scholar]
- Xu J, Strandman S, Zhu JX, Barralet J, Cerruti M. (2015) Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 37:395–404. [DOI] [PubMed] [Google Scholar]
- Yadav N, Morris G, Harding SE, Ang S, Adams GG. (2009) Various non-injectable delivery systems for the treatment of diabetes mellitus. Endocr Metab Immune Disord Drug Targets 9:1–13. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Shimizu K, Rahman M, Ishikawa H, Takase H, Ugawa S, Okada A, Inoshima Y. (2019) Efficient method for isolation of exosomes from raw bovine milk. Drug Dev Ind Pharm 45:359–364. [DOI] [PubMed] [Google Scholar]
- Yehia SA, Elshafeey AH, Elsayed I. (2011) Pulsatile systems for colon targeting of budesonide: in vitro and in vivo evaluation. Drug Deliv 18:620–630. [DOI] [PubMed] [Google Scholar]
- Yilmaz C, Sarisozen C, Torchilin V, Busnaina A. (2016) Novel nanoprinting for oral delivery of poorly soluble drugs. Methodist DeBakey Cardiovasc J 12:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Li Y, Liu CS, Chen Q, Wang GH, Guo W, Wu XE, Li DH, Wu WD, Chen XD. (2015) Enteric-coated capsules filled with mono-disperse micro-particles containing PLGA-lipid-PEG nanoparticles for oral delivery of insulin. Int J Pharm 484:181–191. [DOI] [PubMed] [Google Scholar]
- Zare-Akbari Z, Farhadnejad H, Furughi-Nia B, Abedin S, Yadollahi M, Khorsand-Ghayeni M. (2016) PH-sensitive bionanocomposite hydrogel beads based on carboxymethyl cellulose/ZnO nanoparticle as drug carrier. Int J Biol Macromol 93 (Pt A):1317–1327. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu J, Tian T, Gao Y, Ji X, Li Z, Luan Y. (2013) Pharmaceutically active ionic liquid self-assembled vesicles for the application as an efficient drug delivery system. ChemPhysChem 14:3454–3457. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, Kirk J, Eppler B, Klinman DM, Sui Y, et al. (2012) Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med 18:1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer Ł, Kasperek R, Poleszak E. (2014) [Modern polymers in matrix tablets technology]. Polim Med 44:189–196. [PubMed] [Google Scholar]
- Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. (2010) Exosomes: fit to deliver small RNA. Commun Integr Biol 3:447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]