Abstract
Pancreatic ductal adenocarcinoma (PDAC), the fourth leading cause of cancer-related death in the United States, is highly aggressive and resistant to both chemo- and radiotherapy. It remains one of the most difficult-to-treat cancers, not only due to its unique pathobiological features such as stroma-rich desmoplastic tumors surrounded by hypovascular and hypoperfused vessels limiting the transport of therapeutic agents, but also due to problematic early detection, which renders most treatment options largely ineffective, resulting in extensive metastasis. To elevate therapeutic effectiveness of treatments and overt their toxicity, significant enthusiasm was generated to exploit new strategies for combating PDAC. Combination therapy targeting different barriers to mitigate delivery issues and reduce tumor recurrence and metastasis has demonstrated optimal outcomes in patients’ survival and quality of life, providing possible approaches to overcome therapeutic challenges. This paper aims to provide an overview of currently explored multimodal therapies using either conventional therapy or nanomedicines along with rationale, up-to-date progress, as well as the key challenges that must be overcome. Understanding the future directions of the field may assist in the successful development of novel treatment strategies for enhancing therapeutic efficacy in PDAC.
Introduction
Pancreatic cancer (PC) remains a treatment-refractory malignancy with a median survival of 5 to 6 months (Rahib et al., 2014). PCs are split into two main groups: endocrine and exocrine tumors. Pancreatic ductal adenocarcinoma (PDAC) is an exocrine tumor, representing over 90% of all pancreatic malignancies (Pelosi et al., 2017). Although PDAC accounts for only about 2% of all cancer cases, it is the fourth leading cause of death due to cancer in the United States. It is estimated that ∼45,750 deaths from this disease will be reported in 2019 (https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf). PDAC is associated with a very poor prognosis, in which mortality closely parallels incidence. The 5-year survival rate has only improved marginally over the past decades and remains as low as 6%, which is perhaps due to the fact that the majority of PDAC cases are diagnosed at late stages with widespread metastases (Hall et al., 2018). Although the evolution of PDAC starts from its earliest nonmalignant precursor lesions, most patients with PDAC are asymptomatic until the disease develops to an advanced stage. The retroperitoneal position of the pancreas and the absence of sensitive and noninvasive biomarkers represent additional hurdles to the imaging, screening, and early detection of PDAC (Kaur et al., 2012). Although surgical resection is considered as the only potentially curative treatment of PDAC, less than 20% of patients are suitable candidates for this procedure since the disease is far too advanced when diagnosed and thus inoperable (Adamska et al., 2017). PDAC remains one of the most difficult-to-treat cancers, owing to its aggressive nature, complex tumor microenvironment, and intrinsic resistance to chemotherapeutics, which renders most treatment options mostly ineffective. Since the 1990s, single-agent gemcitabine, a nucleoside analog of deoxycytidine that blocks DNA replication and several forms of DNA repair, has been the standard of care for patients with advanced PDAC. For the subsequent years, many therapies were investigated to improve chemotherapeutic strategies for PDAC but the rate of successful clinical trials was relatively low (Hall et al., 2018). To date, only two systemic therapies have demonstrated the improved outcomes of the treatment compared with gemcitabine alone, at the expense of increased adverse effects (Conroy et al., 2011). Thus, new more effective and less toxic therapeutic strategies targeting both cancer cells and the tumor microenvironment are urgently needed to combat PDAC. In this context, nanotechnology-based drug delivery platforms can offer the possibilities to achieve efficient tumor targeting, lower drug-related toxicities, and improve clinical outcome (Meng and Nel, 2018). Overall, this review aims to provide a synopsis of currently explored multimodal therapies using either conventional therapy or nanomedicines to treat PDAC, and discusses new challenges, presented along with further considerations, and the crucial roles of interdisciplinary approaches.
Pathophysiology, Diagnosis, and Staging of PDAC
PDAC follows a pathway of progression from normal ductal epithelium to duct lesions and invasive ductal adenocarcinoma. It takes approximately 17 years for PDAC to progress from a tumor-initiating cell to the development of metastatic disease (Yachida et al., 2010). This process is characterized by multiple genetic alterations that trigger the tumor progression cascade, such as activation of oncogenes, of which mutationally activated KRAS plays the utmost vital role in tumor initiation and maintenance during the whole PDAC progression through regulation of cell division, differentiation, and apoptosis (Korc, 2010; Eser et al., 2014). On the other hand, the frequent inactivation of tumor suppressor genes, including p16INK4A/CDKN2A, TP53, and DPC4/SMAD4, also contribute to the deregulation of cell cycle and biologic aggressiveness of PDAC (Zhang et al., 2016a). The CDKN2A gene encodes two different proteins, p16 and p14ARF, which are both cell-cycle regulators. Loss or promoter methylation of the CDKN2A gene markedly promotes cell proliferation and tumor progression. TP53, a cellular stress sensor, is mutated in about 70% of human PDAC (Morton et al., 2010). Its inactivation leads to uncontrolled cell growth and increased cell survival, and in combination with activated KRAS has been shown to drive the genomic instability and tumor metastatic capacity (Bardeesy et al., 2006). The loss or loss of function of the DPC4/SMAD4 tumor suppression gene is another common genetic alteration that results in disruption of the TGF-β pathway, occurs relatively late in pancreatic carcinogenesis, and was found to be associated with poor prognosis in PDAC patients. In addition to the driver mutations in these four genes, high-frequency alterations in wingless-related integration site signaling, chromatin remodeling, Hedgehog signaling, DNA repair, and cell cycle processes were also observed (Witkiewicz et al., 2015). By histologic studies and clinical observations, it is postulated that before the final formation of invasive cancer, there is a stepwise progression of precursor lesions such as intraductal papillary mucinous neoplasms, mucinous cystic neoplasms (Hruban et al., 2004), and pancreatic intraepithelial neoplasia, a most common and well-defined precursor to PDAC (Yonezawa et al., 2008). The progression of pancreatic intraepithelial neoplasia lesions follows a pathway from no dysplasia to moderate dysplasia, to high-grade dysplasia (carcinoma in situ), and to invasive carcinoma, accompanied by increased frequency of multiple genetic alterations (Koorstra et al., 2008; Muniraj et al., 2013; Distler et al., 2014; Kamisawa et al., 2016). Among different types of cancers, PC is recognized as the utmost devastating and difficult-to-treat cancer. There are a number of key features that make it a particularly challenging disease. PC is characterized by significant genomic heterogeneity: recent extensive genetic studies showed an average of 63 genetic aberrations across 12 functional pathways in the majority of PCs (Jones et al., 2008; Cancer Genome Atlas Research Network, 2017). Importantly, the pathway components that may be altered in a specific tumor vary widely, which makes developing tailored therapies targeting specific genes quite challenging. Tumor stroma is another defining hallmark of PDAC, which is considered to be one of the most stroma-rich cancers. Dense fibrotic stroma occupies the majority of the tumor mass and consists of extracellular matrix (ECM) components and non-neoplastic cells including fibroblastic, vascular, and immune cells. The excessive deposition of ECM generates high interstitial fluid pressures that compress blood vessels and cause hypoperfusion, hypovascularity, and hypoxia. This desmoplastic hypovascular tumor microenvironment is now recognized as promoting tumor growth, facilitating its invasive and metastatic potential and immunosuppression, and impairing the delivery of chemotherapeutics (Kaur et al., 2013; Xie and Xie, 2015).
Early and accurate diagnosis of PDAC is crucial for oncologists to determine effective and timely treatment options for patients. Currently, there are no validated early detection strategies for PDAC, even for high-risk patients. PDAC detection and staging is usually based on a combination of imaging techniques (e.g., computed tomography, transabdominal ultrasound, and magnetic resonance imaging), tumor markers (e.g., carbohydrate antigen 19-9, carcinoembryonic antigen, and osteopontin), and clinical presentations (e.g., progressive weight loss, anorexia, and abdominal pain), as well as the gold standard diagnostic tool: biopsy. Staging assessment is based on the extent of invasion into the pancreas and surrounding tissue, presence or absence of spread to lymph nodes, and presence or absence of metastasis. The tumor/node/metastasis staging system serves as a clinically useful tool in prognosis, surveillance, and treatment planning as well as risk stratification in clinical trials for patients with PDAC (Zhang et al., 2016a).
Therapeutic Strategies
Surgical resection with chemotherapy (usually adjuvant) remains the preferable and only potentially curative approach in PDAC clinical management. Sadly, due to the aggressive nature of the disease, late prognosis, and early metastasis, patients suffering from locally advanced or metastatic PDAC at presentation are usually no longer candidates for surgical removal of the tumor (Manji et al., 2017). As a result, the primary goal of clinical management for these patients is to control the disease and improve the quality of life. Gemcitabine (GEM) has been accepted as the standard first-line therapy for patients having advanced PDAC. There is no alternative monotherapeutic regimen proven to be more beneficial compared with GEM in terms of progression-free survival (PFS) and overall survival (OS) (Conroy et al., 2016). However, due to the drawbacks of GEM (instability in plasma leading to extremely short half-life, inefficient cell uptake, and complex intracellular metabolism), and the fast growth of intrinsic and acquired chemo-resistance during treatment, there is an urgent need for new and disruptive strategies for PDAC (Spadi et al., 2016; Cloyd et al., 2017). Combination therapy is emerging at this point with superior cost-to-efficacy value and overall outcomes.
Combination Therapies
Combination therapy has become the major means to combat cancer thanks to its primary advantages of increased efficacy without, or with minimal, addictive toxicities at equal or reduced administrating doses, which in most cases is recommended for patients if available. Ideal combination therapy should address the following aspects: 1) maximization of therapeutic efficacy of each single drug of the combination; 2) minimization of intrinsic and acquired crossresistance of drugs possibly occurring in the treatment; and 3) diminishing overlapping adverse effects for better tolerability. Drug combinations with distinct molecular mechanisms of action exhibit various remarkable improvements in treatment outcomes, ultimately leading to more promising patient compliance. Regarding PDAC, as previously mentioned, GEM is the only approved first-line monotherapy in PDAC; however, unfortunately it still delivers unsatisfactory therapeutic outcomes in prolonging PFS and OS of patients with locally advanced and metastatic PDAC (Hidalgo et al., 2015). As a result, most of the combination regimens tested have centered on GEM. Multiple combination therapies composed of GEM and different cytotoxic and biologic agents have undergone clinical evaluation for patients with various stages of PDAC. The gold standards for evaluating the efficacy of these treatments are PFS and OS (Table 1).
TABLE 1.
Selected Gemcitabine-based combination therapies evaluated in clinical trials
| Treatment | Target | RR |
PFS |
OS |
Reference |
|---|---|---|---|---|---|
| % | mo | mo | |||
| GEM-based combination therapy | |||||
| Capecitabine | DNA | 19.1 vs. 12.4 | 5.3 vs. 3.8 | 7.1 vs. 6.2 | Cunningham et al., 2009 |
| 7.3 vs. 5.9 | — | 8.4 vs. 7.2 | Herrmann et al., 2007 | ||
| Cisplatin | DNA | 26.4 vs. 9.2 | 5.0 vs. 2.0 | 7.5 vs. 5.0 | Colucci et al., 2002 |
| 11.5 vs. 9.0 | 5.3 vs. 3.1 | 7.5 vs. 6.0 | Heinemann et al., 2006 | ||
| 12.9 vs. 10.1 | 3.8 vs. 3.9 | 7.2 vs. 8.3 | Colucci et al., 2010 | ||
| Oxaliplatin | DNA | 26.8 vs. 17.3 | 5.8 vs. 3.7 | 9.0 vs. 7.1 | Louvet et al., 2005 |
| 9.0 vs. 6.0 | 2.7 vs. 2.6 | 5.7 vs. 4.9 | Poplin et al., 2009 | ||
| Irinotecan | Top I | 15.0 vs. 10.0 | 2.8 vs. 2.9 | 6.4 vs. 6.5 | Stathopoulos et al., 2006 |
| 16.1 vs. 4.4 | 3.5 vs. 3.0 | 6.3 vs. 6.6 | Rocha Lima et al., 2004 | ||
| Pemetrexed | Folate metabolism | 14.8 vs. 7.1 | 3.9 vs. 3.3 | 6.2 vs. 6.3 | Oettle et al., 2005 |
| Erlotinib | EGFR | 8.6 vs. 8.0 | 3.8 vs. 3.6 | 6.2 vs. 5.9 | Moore et al., 2007 |
| Cetuximab | EGFR | 12.0 vs. 14.0 | 3.4 vs. 3.0 | 6.3 vs. 5.9 | Philip et al., 2010 |
| Nimotuzumab | EGFR | 8.6 vs. 8.6 | 5.1 vs. 3.4 | 8.6 vs. 6.0 | Schultheis et al., 2017 |
| Bevacizumab | VEGF | 13.0 vs. 10.0 | 3.8 vs. 2.9 | 5.8 vs. 5.9 | Kindler et al., 2010 |
| Ablifercept | VEGF | — | 3.7 vs. 3.7 | 6.5 vs. 7.8 | Rougier et al., 2013 |
| Sunitinib | VEGFR and PDGFR | 7.1 vs. 6.1 | 2.9 vs. 3.3 | 7.6 vs. 9.2 | Bergmann et al., 2015 |
| Axitinib | VEGFR 1-3, c-KIT, and PDGFR | 5.0 vs. 2.0 | 4.4 vs. 4.4 | 8.5 vs. 8.3 | Kindler et al., 2011 |
| 4.9 vs. 1.6 | 4.4 vs. 4.4 | 5.1 vs. 5.4 | Ioka et al., 2015 | ||
| Sorafenib | VEGFR, PDGFR, and Raf | 23.0 vs. 19.0 | 5.7 vs. 3.8 | 9.2 vs. 8.0 | Gonçalves et al., 2012 |
| Vismodegib | SMO | 8.0 vs. 13.0 | 4.0 vs. 2.5 | 6.9 vs. 6.1 | Catenacci et al., 2015 |
| 0 | 3.7 vs. 2.4 | 6.3 vs. 5.4 | Catennaci 2012 | ||
| 21.7 | 2.8 | 5.3 | Kim et al., 2014 | ||
| Trametinib | MEK | 22.0 vs. 18.0 | 3.7 vs. 3.5 | 8.4 vs. 6.7 | Infante et al., 2014 |
| Pimasertib | MEK | 9.1 vs. 9.1 | 3.7 vs. 2.8 | 7.3 vs. 8.3 | Van Cutsem et al., 2018 |
| Rigosertib | PLK1 and PI3K | 19.0 vs. 13.0 | 3.4 vs. 3.4 | 6.1 vs. 6.4 | O’Neil et al., 2015 |
| Tipifarnib | Farnesyltransferase | 6.0% vs. 8.0 | 3.7 vs. 3.6 | 6.4 vs. 6.1 | Van Cutsem et al., 2004 |
| Ganitumab | IGF-1R | 16.0 vs. 10.0 | 3.7 vs. 3.6 | 7.1 vs. 7.2 | Fuchs et al., 2015 |
| Evofosfamide | DNA (hypoxia activated) | 26.0 vs. 12.0 | 5.6 vs. 3.6 | 9.2 vs. 6.9 | Borad et al., 2015 |
| 20.0 vs. 16.0 | 5.5 vs. 3.7 | 8.7 vs. 7.6 | Cutsem et al., 2016 | ||
| GEM + Erlotinib–based combination therapy | |||||
| Oxaliplatin | DNA | 45.0 | 4.8 | 8.4 | Cascinu et al., 2014 |
| 21.0 | 5.2 | 10.5 | Yun et al., 2014 | ||
| Bevacizumab | VEGF | 13.5 vs. 8.6 | 4.6 vs. 3.6 | 7.1 vs. 6.0 | Katopodis et al., 2014 |
| Cixutumumab | IGF-1R | — | 3.6 vs. 3.6 | 7.0 vs. 6.7 | Van Cutsem et al., 2009 |
| GEM + Cisplatin–based combination therapy | |||||
| Sorafenib | VEGFR, PDGFR, and Raf | 3.4 vs. 3.6 | 4.3 vs. 4.5 | 7.5 vs. 8.3 | Philip et al., 2014 |
c-KIT, mast/stem cell growth factor receptor; EGFR, epidermal growth factor receptor; IGF-1R, insulin-like growth factor 1 receptor; MEK, mitogen-activated protein kinase; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; PLK1, polo-like kinase 1; Raf, family of three serine/threonine-specific protein kinases; SMO, smoothened [a class frizzled (class F) G protein-coupled receptor]; Top I, topoisomerase I; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; —, no data available.
Generally, GEM-based combination therapies include previously approved or well-known chemotherapeutic regimens currently being used for PDAC (Teague et al., 2015), such as capecitabine, oxaliplatin, irinotecan, paclitaxel, and molecular targeting agents like erlotinib (an epidermal growth factor inhibitor) (Chiorean and Coveler, 2015; Seicean et al., 2015), sunitinib (a vascular endothelial growth factor receptor inhibitor), pimasertib (a mitogen-activated protein kinase inhibitor), bevacizumab (a vascular endothelial growth factor), vismodegib (a heat shock protein inhibitor), etc. Among the GEM-based molecular targeting combination therapies tested in clinical trials, the only one that received Food and Drug Administration approval is the erlotinib + GEM combination, with improved PFS and OS (although the improvement in OS was merely 2 weeks) (Wang et al., 2015). Combining platinum-based agents with GEM is not as efficient as combining them with 5-fluorouracil (5-FU), since no improvement in primary measures of the trials was demonstrated. As a result, platinum-based agents are mostly used in combination with 5-FU. Despite encouraging outcomes of other GEM-based combination therapies in early phase studies including response rate (RR), adverse effects, and tolerability, most phase III trials have failed to improve the survival benefits and quality of life, with PFS and OS still limited to 5–9 months for locally advanced or metastatic PDAC (Aprile et al., 2017). No statistically significant progress was achieved throughout the clinical trials compared with GEM monotherapy, whereas some results were even inferior to GEM monotherapy. For instance, rigosertib, a small-molecule RAS mimetic and inhibitor of the phosphoinositide 3-kinase and polo-like kinase 1 pathways, showed promising synergistic activity with GEM in preclinical patient-derived xenograft models of PDAC. However, the combination of rigosertib and GEM did not improve the treatment outcome in patients with metastatic pancreatic cancer in a phase II/III trial (NCT01360853). Median OS was 6.1 months for the rigosertib + GEM combination versus 6.4 months for GEM alone (O’Neil et al., 2015). It was suggested that the lack of clinical activity for rigosertib in KRAS mutant PDAC patients could be linked to the inherent heterogeneity of the disease.
Rapid growth of resistance to GEM-based regimens and frequent relapse vastly accelerate the development of non-GEM–based combination therapies as alternatives for PDAC patients who are refractory to GEM-containing regimens. It has been proven that there is low crossresistance between GEM and 5-FU despite the fact that they are both nucleoside analogs. Alternatively, GEM-based therapies can also be given to patients previously treated with fluoropyrimidine-based therapies. Among these therapies, the most impressive and successful one is a multidrug regimen consisting of folinic acid, 5-FU, irinotecan, and oxaliplatin (FOLFIRINOX). Before that, extensive partial combinations of FOLFIRINOX were tested in the clinic, catalyzing the emergence of FOLFIRINOX. The three most representative regimens are the following combinations: 1) 5-FU, folinic acid, and oxaliplatin; 2) 5-FU, folinic acid, and irinotecan; and 3) capecitabine, an oral prodrug of 5-FU, and oxaliplatin (Vaccaro et al., 2011). The superiority of FOLFIRINOX over GEM was recognized in all efficacy parameters, including OS (11.1 vs. 6.8 months), PFS (6.4 vs. 3.3 months), and 1-year survival rate (48.4% vs. 20.6%). Unfortunately, FOLFIRINOX treatment–related side effects also are severe, including fatigue, bone marrow suppression with 45.7% grade 3 or 4 neutropenia, 12.7% diarrhea, and 9.0% sensory neuropathy, which leads to the termination of treatment in one-third of patients due to poor tolerability issues. On the other hand, the quality-of-life measures strongly support FOLFIRINOX for patients at the late stage with definitively less decrease in the health status (Khushman et al., 2015; Rombouts et al., 2016). Consequently, FOLFIRINOX is reserved as a preferred first-line therapy for patients with locally advanced or metastatic PDAC when they have good performance status. In some cases, it is also recommended (Jutric and Melstrom, 2017) for disease down-staging or can be considered as a neoadjuvant regimen for patients with borderline resectable tumors (Paniccia et al., 2014; Hackert et al., 2016; Godhi et al., 2017). In the hope of being beneficial for those unqualified patients, regimens with dose reduction of chemotherapeutics, referred to as modified FOLFIRINOX (mFOLFIRINOX) (Marsh Rde et al., 2015), are widely implemented in the clinic and have demonstrated comparable results (PFS and OS of 6.1 and 10.2 months, respectively) with manageable adverse effects. The recent PRODIGE 24/CCTG PA.6 multicenter randomized phase III clinical trial (NCT01526135) also assessed the benefit of mFOLFIRINOX compared with GEM monotherapy in an adjuvant setting for patients with resected PDAC. Based on reported data, median disease-free survival was 21.6 months for mFOLFIRINOX and 12.8 months for GEM. However, grade 3/4 toxicities were reported in 75.5% of patients receiving mFOLFIRINOX compared with 51.5% of patients receiving GEM (Lee and Park, 2016; Conroy et al., 2018). To further extend the potential of this promising regimen, some innovative combinations with FOLFIRINOX as a chemotherapeutic platform are underway in early phase clinical trials (Chiorean et al., 2016), including molecular targeting therapy (IPI-926 and NCT01383538) (Ko et al., 2016a), immunoradiation therapy (nivolumab with stereotactic body radiation and NCT03563248), redox metabolism therapy (CPI-613, NCT03699319, and NCT01835041), vaccination therapy (ipilimumab and NCT01896869) and tumor-associated macrophage targeting therapy (PF-04136309 and NCT01413022).
Despite success, conventional combination therapies in the treatment of PDAC confer a small advantage over single agent therapy and the survival times of PDAC patients remain unsatisfactory. Chemotherapy regimens are inevitably facing challenges such as poor bioavailability and intrinsic toxicity, compromising their efficacy and further utilization. As such, new strategies that will allow better delivery of chemotherapeutic agents to the tumor while decreasing systemic toxicity are urgently needed. To this end, one promising option is the implementation of nanothechnology-based therapeutic approaches in PDAC. Indeed, the unique characteristics of nanocarriers such as their nanoscale sizes, high surface-to-volume ratios, high loading capacity, and favorable drug release profiles make them suitable for delivering chemotherapeutic drugs to the target tumor tissue (Au et al., 2016; Shi et al., 2017). The physicochemical characteristics of nanocarriers can be readily adjusted to facilitate the delivery of a variety of therapeutic agents including small molecular drugs, biomacromolecules, and inorganic nanoparticles. These nanomedicines can be surface functionalized to present targeting ligands to a receptor of interest in order to home them at the desired site. Combining drugs in one delivery carrier is another advantageous strategy for controlling the pharmacokinetics and co-delivery of the desired drug ratio in vivo, and a variety of nanoscale carriers have been investigated in terms of their ability to deliver multiple drugs (Hu et al., 2016; Zhang et al., 2016b). Several nanomedicines have been approved for clinical use in cancer treatment, including PDAC, and many others in clinical development have demonstrated great promise.
Nanomedicines in PDAC Treatment Protocols
Currently, two nanotechnology-based therapeutics, nanoparticle albumin-bound paclitaxel [nab-paclitaxel (nab-PTX) or abraxane] and liposomal formulation of irinotecan (onivyde) have been approved for PDAC treatment in combination with other chemotherapeutic agents (Zhang et al., 2016b; Shi et al., 2017). Taxanes are useful components in the systemic treatment of many cancers; however, unfortunately, it had exhibited very few noticeable benefits as single or combination therapies in PDAC until the emergence of nab-PTX. The encapsulation of paclitaxel into the human serum albumin–based drug delivery system allows for enhanced delivery of paclitaxel to the tumor, consequently leading to increased bioavailability and alleviated toxicity to normal tissue compared with cremophor-based paclitaxel formulation (Kim, 2017). In the combination regimen with GEM, it demonstrated significant improvements in most of the clinical outcome parameters (RR: 23% vs. 7%; PFS: 5.5 months vs. 3.7 months; OS: 8.5 and 6.7 months) compared with GEM alone. The number of patients with serious adverse events was similar in the two treatment groups (Von Hoff et al., 2013). These results have led to the approval of nab-PTX by the Food and Drug Administration as a first-line combination therapy with GEM for patients with locally advanced and metastatic PDAC. Targeting tumor stromal cells and modulation of the tumor microenvironment by nab-PTX have been proposed as contributing factors underlying the therapeutic activity of nab-PTX in this combination (Fig. 1). A clinical study that evaluated the effects of pretreatment with the nab-PTX and GEM combination in patients with operable PDAC showed that improved outcomes of this treatment were in good correlation with reduced tumor stiffness as determined by endoscopic ultrasound elastography (Alvarez et al., 2013). Moreover, in marked contrast with tumors exposed preoperatively to conventional chemoradiation, studied for comparison, less abundant collagen matrix and decreased number of tumor-associated fibroblasts around tumor glands were detected in the resected tumor tissues from patients treated with nab-PTX and GEM (Alvarez et al., 2013). Although the number of patients in this study was small, the reported data support the hypothesis that the therapeutic benefits of nab-PTX can be associated with stromal distortion. In addition, active transport mechanisms mediated by albumin cellular receptors may be responsible for the increased intratumoral concentrations of paclitaxel. Other possible mechanisms, like inactivation of GEM catabolizing enzymes, are still under investigation. Since the regulatory approval of the nab-PTX/GEM regimen in 2013, this combination has been intensely evaluated in multiple clinical trials in conjunction with a variety of other drugs targeting cancer stem-like cells, tumor microenvironment, kinase and signaling pathways, and immunotherapy (Tables 2 and 3).
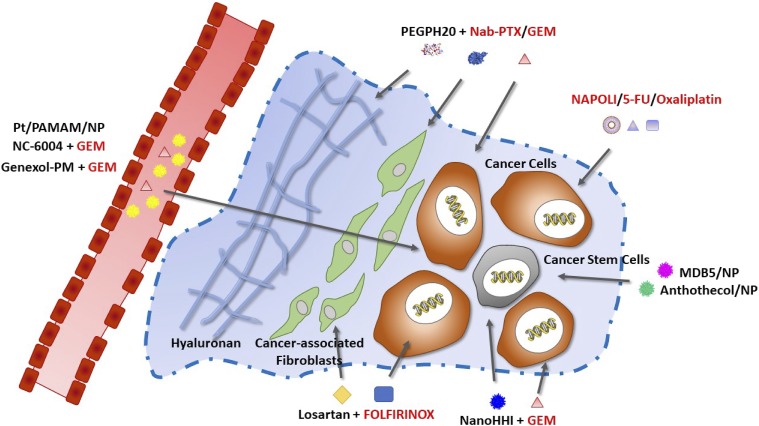
Fig. 1.
Targets and therapeutic approaches including conventional and nanotechnology-based drugs that have been approved by the Food and Drug Administration (red) or in pre-clinical or clinical evaluation (black) for PDAC treatment.
TABLE 2.
Nab-PTX/GEM–based combination therapy in clinical trials (completed)
| Regimen | Target | RR |
PFS |
OS |
Reference |
|---|---|---|---|---|---|
| % | mo | mo | |||
| PCSC targeting | |||||
| Vantictumab | WNT | 53.0 | 7.2 | — | Messersmith et al., 2016 |
| Ipafricept | WNT | 29.0 | 3.9 | — | Weekes et al., 2016 |
| Tarextumab | Notch 2/3 | 19.1 vs. 31.8 | 3.7 vs. 5.5 | 6.4 vs. 7.9 | O’Reilly et al., 2017 |
| Vismodegib | SMO | 43.0 | 5.5 | 10 | Jesus-Acosta et al., 2014 |
| Sonidegib | SMO | 39.0 | — | — | Lee et al., 2017 |
| VERSUS-4718 | FAK | — | — | — | |
| Kinase or signaling pathway targeting | |||||
| LCL161 | Apoptosis proteins | — | — | — | |
| Istiratumab | IGF-1R/ErbB3 | — | — | — | Ko et al., 2016b |
| Erlotinib | EGFR | 46 | 5.3 | 9.3 | Leichman et al., 2012 |
| Aptorsen | HSP27 (NF-κB) | 18.0 vs. 18.0 | 2.7 vs. 3.8 | 5.3 vs. 6.9 | Ko et al., 2017 |
| Momelotinib | JAK | 28 | — | — | Ng et al., 2019 |
| Itacitinib | JAK | 24 | — | — | Beatty et al., 2018 |
| Other targets | |||||
| PEGPH20 | HA | 45.0 vs. 31.0 | 11.5 vs. 8.5 | — | Hingorani et al., 2018 |
| Evofosfamide | DNA (hypoxia-activated) | 53.0 | — | — | Borad et al., 2016 |
ErbB3, receptor tyrosine-protein kinase (also known as human epidermal growth factor receptor 3); EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; HA, hyaluronic acid; HSP27, heat shock protein 27 (also known as heat shock protein beta-1); IGF-1R, insulin-like growth factor 1 receptor; JAK, Janus kinase; NF-κB, nuclear factor kappa B; Notch 2/3, neurogenic locus notch homolog protein 2/3; SMO, smoothened; WNT, wingless-related integration site; —, no data available.
TABLE 3.
Nab-PTX/GEM–based combination therapy in clinical trials (ongoing)
| Regimen | Target | Clinic Trial | Phase | Status |
|---|---|---|---|---|
| PCSC targeting | ||||
| Sonidegib | SMO | NCT02358161 | I/II | U |
| NCT01431794 | I/II | ANR | ||
| Napabucasin | STAT3 | NCT02993731 | III | R |
| Kinase or signaling pathway targeting | ||||
| Afatinib | ErbB2 and EGFR | NCT02975141 | I | ANR |
| Ficlatuzumab | HGF | NCT03316599 | I | R |
| FG-3019 | CTGF | NCT02210559 | I/II | ANR |
| Ibrutinib | BTK | NCT02562898 | I/II | ANR |
| NCT02436668 | III | ANR | ||
| Adavosertib | WEE1 | NCT02194829 | I/II | ANR |
| Nintedanib | VEGFR, FGFR, and PDGFR | NCT02902484 | I/II | R |
| Ceritinib + cisplatin | ALK | NCT02227940 | I | R |
| BYL-719 | PI3K | NCT02155088 | I | ANR |
| TGR-1202 | PI3K-δ | NCT02574663 | I | ANR |
| 9-ING-41 | GSK-3β | NCT03678883 | I/II | NR |
| Ulixertinib | ERK | NCT02608229 | I | R |
| Cisplatin + BGB324 | AXL | NCT03649321 | I/II | NR |
| Olaratumab | PDGFR-α | NCT03086369 | I/II | R |
| Immune system targeting | ||||
| Tocilizumab | IL-6R | NCT02767557 | II | R |
| Indoximod | IDO | NCT02077881 | I/II | ANR |
| ALT-803 | IL-15 superagonist | NCT02559674 | I | ANR |
| Selicrelumab | CD40 antigen stimulants | NCT02588443 | I | R |
| Durvalumab + tremelimumab | PD-1L + CTLA-4 | NCT02879318 | II | ANR |
| Nivolumab + paricalcitol + cisplatin | PD-1 | NCT02754726 | II | R |
| Oleclumab + durvalumab | PD-1 + PD-L1 | NCT03611556 | I/II | R |
| Cabiralizumab + nivolumab | CSF1R + PD-L1 | NCT03336216 | II | R |
| BMS-813160 + nivolumab | CCR2/5 + PD-L1 | NCT03184870 | I/II | R |
| APX005M + nivolumab | CD40 agonist + PD-L1 | NCT03214250 | I/II | R |
| BMS-813160 + nivolumab | CCR2/5 + PD-L1 | NCT03496662 | I/II | R |
| Pembrolizumab + paricalcitol | PD-1 + VD receptor activator | NCT02930902 | I | R |
| Other targets | ||||
| Nal-Iri/5-FU + folinic acid | DNA and Top I | NCT03703063 | I | R |
| Enzalutamide | AR antagonist | NCT02138383 | I | ANR |
| CPI-613 | TCA cycle | NCT03435289 | I | R |
| SGT-53 | p53 gene | NCT02340117 | II | R |
| ARQ-761 | NQO1 | NCT02514031 | I | R |
| Selinexor | SINE | NCT02178436 | I/II | ANR |
| Hydroxychloroquine | TLR | NCT01978184 | II | ANR |
| PEGPH20 | HA | NCT02487277 | II | R |
| NCT02715804 | III | R | ||
| PEGPH20 + rivaroxaban | HA and Factor Xa | NCT02921022 | U | R |
| ensituximab | MUC5AC | NCT01834235 | I/II | ANR |
| VCN-01 | pRB | NCT02045602 | I | R |
ALK, anaplastic lymphoma kinase; ANR, active, not recruiting; AR, androgen receptor; AXL, cell surface receptor tyrosine kinase, part of the TAM family of kinases; BTK, Bruton’s tyrosine kinase; CCR2/5, C-C chemokine receptor type 2/5; CD40, cluster of differentiation 40; CSF1R, colony stimulating factor 1 receptor; CTGF, connective tissue growth factor; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGFR, epidermal growth factor receptor; ErbB2, receptor tyrosine-protein kinase (also known as human epidermal growth factor receptor 2); ERK, extracellular signal-regulated kinase; Factor Xa, activated form of Stuart-Prower factor; FGFR, fibroblast growth factor receptor; GSK-3β, glycogen synthase kinase 3β; HA, hyaluronic acid; HGF, hepatocyte growth factor; IDO, indoleamine-pyrrole 2,3-dioxygenase; IL-15, interleukin-15; IL-6R, interleukin 6 receptor; MUC5AC, mucin 5AC (a protein that in humans is encoded by the MUC5AC gene); NQO1, NADPH dehydrogenase (quinone) 1; NR, not recruiting; P53, transformation-related protein 53 (any isoform of a protein encoded by homologous genes in various organisms); PD-1, programmed cell death protein; PD-1L, programmed cell death protein ligand; PDGFR, platelet-derived growth factor receptors; PDGFR-α, platelet-derived growth factor receptor α; PI3K, phosphoinositide 3-kinase; pRB, phosphorylated retinoblastoma protein; R, recruiting; SINE, short interspersed nuclear element; SMO, smoothened [a class frizzled (Class F) G protein-coupled receptor]; STAT3, signal transducer and activator of transcription 3 (a transcription factor); TCA cycle, tricarboxylic acid cycle; TLR, Toll-like receptor; Top I, topoisomerase I; U, unknown; VEGFR, vascular endothelial growth factor receptor; WEE1, nuclear kinase belonging to the Ser/Thr family of protein kinases.
Liposomal formulation of irinotecan is another nanomedicine that was approved for use in a combination regimen with 5-FU and folinic acid as a second-line therapy for patients with metastatic PDAC who progressed after gemcitabine-based chemotherapy (Ko, 2016; Kipps et al., 2017; Wang-Gillam et al., 2017). Based on the results of a pivotal NAPOLI-1 trial, the combination treatment of liposomal irinotecan with 5-FU and folinic acid demonstrated enhanced RR (16% vs. 1%), prolonged PFS (2.3 months vs. 1.4 months, demonstrated as time to treatment failure), and OS (6.1 months vs. 4.2 months) compared with the 5-FU/folinic acid regimen with a manageable safety profile (Wang-Gillam et al., 2016). No significant OS advantage was observed between patients assigned to liposomal irinotecan monotherapy and those allocated to the 5-FU/folinic acid regimen. Quality-of-life measures did not differ substantially from baseline in any treatment group. The observed excellent performance of liposomal irinotecan led to initiation of clinical studies evaluating the safety, tolerability, and preliminary efficacy of this nanomedicine in combination with other anticancer therapies (Chen et al., 2015; Wang-Gillam et al., 2016; Glassman et al., 2018). For example, the combination of liposomal irinotecan, 5-FU/folinic acid, and oxaliplatin, which mimics the FOLFORINOX regimen (Rahman et al., 2017), is currently being explored for patients with advanced PDAC who have not received prior chemotherapy or as a preoperative regimen in resectable pancreatic cancer (NCT03528785, NCT02551991, NCT03487016, and NCT03483038). Other liposomal irinotecan plus 5-FU/folinic acid–based therapies under investigation include combinations with BAX2398 (NCT02697058), eryaspase (NCT03665441), cabiralizumab + nivolumab (NCT03336216), bermekimab (NCT03207724), and rucaparib (NCT03337087).
Similar to conventional chemotherapy, treatment regimens involving nanomedicines only modestly improve the overall survival of patients. As our understanding of the complex tumor microenvironment and molecular landscape of PDAC continues to improve, substantial effort is being undertaken in the development of targeted multiagent therapeutic strategies and drug delivery systems that might improve the effectiveness of PDAC treatment. The novel approaches employing combination therapies that are discussed in following section may provide a means of overcoming pathophysiological barriers in patients with PDAC, thus reducing therapeutic resistance.
Targeting Desmoplastic Stromal Barriers
The resistance of human PDAC to systemic therapies is unusual compared with other solid carcinomas, and the observed lack of survival benefit might at least partly evolve from the predominant desmoplastic stroma reaction and the pronounced hypovascularity that impede efficient drug delivery to the tumor cells. Thus, normalization of the tumor microenvironment represents a promising strategy in order to improve the penetration and efficacy of systemic chemotherapeutics and/or nanomedicines and may provide important therapeutic outcomes. Such strategies are especially attractive because these pathophysiological barriers cannot be overcome through nanomedicine design alone. One of the approaches used to address the stroma is to target the noncellular components. In this setting, one promising drug is a (polyethylene glycol, PEG) PEGylated version of Halozyme’s proprietary recombinant human hyaluronidase enzyme, rHuPH20 (PEGPH20) that is able to degrade hyaluronan, which is an abundant component of the ECM in pancreatic stroma. Preclinical studies in a genetically engineered mouse model of PDAC have demonstrated that enzymatic depletion of hyaluronan by PEGPH20 induced the re-expansion of tumor blood vessels as well as an unexpected selective change in tumor endothelial ultrastructure and macromolecular permeability, which resulted in increasing the intratumoral delivery of two chemotherapeutic agents, doxorubicin and GEM. These changes in tumor vasculature and drug delivery also prolonged the survival of the mouse model treated with the PEGPH20 and GEM combination compared with GEM monotherapy (Jacobetz et al., 2013). In a phase Ib study (NCT01453153) in patients with stage IV untreated pancreatic cancer, the PEGPH20 plus GEM combination was well tolerated and showed promising clinical activity, particularly in patients with tumors expressing high hyaluronic acid levels (Hingorani et al., 2016). Consistent with preclinical studies, the imaging analyses in selected patients demonstrated a rapid increase in tumor perfusion within 24 hours after PEGPH20 dosing, which resulted in higher intratumoral concentration of subsequently administered chemotherapy. The promising results of this trial warranted follow-up studies combining PEGPH20 with other agents. A combination of PEGPH20 plus GEM/nab-PTX was evaluated versus GEM/nab-PTX in an open-label randomized phase 2 trial (HALO 2020) in patents with untreated metastatic PDAC (Hingorani et al., 2018). The study results demonstrated an overall improvement in PFS for patients receiving PEGPH20 plus GEM/nab-PTX. With the limited number of patients, the greatest treatment benefit with the PEGPH20 plus GEM/nab-PTX treatment was observed in patients with high-hyaluronan tumors (median PFS of 9.2 month and overall RR of 71%) compared with the GEM/nab-PTX control arm (median PFS of 4.3 months and overall RR of 29%). Currently, PEGPH20 is under investigation in the phase III randomized HALO-109-301 trial given in combination with GEM/nab-PTX (NCT02715804) in a biomarker-selected patient population with high hyaluronic acid levels (Fig. 1). The limitations of this therapy include thromboembolic events; therefore, anticoagulation is now administered in conjunction with PEGPH20 in active clinical trials (Hingorani et al., 2015; Adiseshaiah et al., 2016; Meng and Nel, 2018). Collagen is another ECM component that contributes to solid stress and vessel compression in tumors. Jain and his colleagues (Chauhan et al., 2013) have demonstrated that the angiotensin inhibitor losartan reduces stromal collagen and hyaluronan production, which is associated with decreased activity of cancer-associated fibroblasts. Consequently, losartan reduces solid stress in tumors, resulting in increased vascular perfusion and drug and oxygen delivery and potentiates chemotherapy in PC models. Losartan also increased the efficacy of the liposomal formulation of doxorubicin, Doxil (Diop-Frimpong et al., 2011). Interestingly, the administration of losartan or Doxil alone did not affect the growth of pancreatic tumors, but the tumors were significantly smaller in mice treated with the losartan plus Doxil combination. These observations are in good agreement with previously reported results of clinical evaluation of liposomal doxorubicin in patients with unresectable PDAC where no complete or partial responses were seen (Halford et al., 2001). Thus, losartan shows potential to enhance the intratumoral penetration and efficacy of small and large therapeutics in patients with PDAC. In parallel, retrospective clinical data suggested that patients with PDAC receiving GEM monotherapy and treated with angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors to manage hypertension survived approximately 6 months longer than those who were only on GEM therapy (Nakai et al., 2010). Collectively, these findings formed the basis of an ongoing phase II clinical trial investigating the combination of losartan and FOLFIRINOX and then chemoradiotherapy in unresectable locally advanced PDAC (NCT01821729) (Fig. 1).
Hedgehog signaling has been shown to be active in pancreatic carcinogenesis (Bailey et al., 2008) and its inhibition caused tumor growth suppression associated with reduction in the expression of Hedgehog target genes in the stroma compared with control-treated xenograft tumors (Feldmann et al., 2008; Yauch et al., 2008; Mills et al., 2013). Preclinical studies in genetically engineered mouse models have demonstrated that inhibition of Hedgehog pathway signaling transiently increases intratumoral vessel diameter, which results in stromal disruption and enhanced drug delivery (Olive et al., 2009). Unfortunately, a recent phase II trial (NCT01130142) of GEM plus the sonic Hedgehog inhibitor (HHI) saridegib versus GEM plus placebo in previously untreated patients with metastatic PDAC resulted in a disappointing outcome. The trial had to be halted due to progressive disease and decreased median OS in patients treated with GEM and saridegib (Richards et al., 2012). Furthermore, recently reported data on the clinical evaluation of vismodegib (another smoothened inhibitor) and a GEM combination failed to demonstrate its benefits over GEM (Catenacci et al., 2015). The reasons for the failure of those drugs in the clinical setting are not entirely clear. Perhaps the stroma is contributing to some tumor control; therefore, permanent stroma depletion may also increase the opportunity for cancer cells to metastasize and alleviate stress associated with hypoxia (Özdemir et al., 2014). These results emphasize the need to invest more effort in the identification of markers of sensitivity during patient selection as well as in the development of rational therapeutic combinations and dosing regimens. Overall, pharmacologic inhibition of tumor stroma in combination with chemotherapy is a promising strategy as a means to potentiate delivery of therapeutics to the tumor. However, the ECM is a complex network having both pro- and antitumor effects and a deeper knowledge of the interactions between stroma and cancer cells is required to translate this strategy into clinical success.
Normalization of tumor vasculature using antiangiogenic agents is another approach that enhances extravasation of therapeutic agents at tumor sites. The tumor vasculature is abnormal in the architecture and morphology of the vascular wall. Tumor vessels are often tortuous and irregular, with poorly aligned defective endothelial cells, detached pericytes, and lack of a smooth muscle layer (Whipple and Korc, 2008). The leaky nature of tumor vasculature coupled with a dysfunctional lymphatic system forms the physiologic basis of the enhanced permeability and retention effect, which is beneficial to nanocarriers in order to extravasate from vessels and accumulate in tumors (Duncan, 2006). However, the low tumor perfusion, unevenly distributed blood flow, and heterogeneous vessel permeability hinder the homogeneous distribution and deep penetration of the drugs and nanomedicines throughout the tumor (Jain, 2012; Miao et al., 2015). Correction of excessive angiogenesis signaling to repair abnormalities in vascular structure and function may enhance drug delivery, thereby increasing chemotherapy activity. The vascular-normalization concept was evaluated in several clinical trials, in which vascular endothelial growth factor–targeted agents (bevacizumab, axitinib, and aflibercept) were added to GEM in the treatment of patients with advanced-stage PDAC (Kindler et al., 2010; Rougier et al., 2013; Tian et al., 2013; Sahora et al., 2014). The addition of an agent against vascular endothelial growth factor receptors to GEM-based chemotherapy resulted in higher overall RR but no survival advantage. It is possible that the inhibition of vascular endothelial growth factor receptors was quickly overwhelmed by the signaling forces coming from the other major pathways (e.g., epidermal growth factor receptors). Therefore, it is conceivable that simultaneous targeting of multiple pathways may prevent the rapid emergence of resistance. As an additional consideration, the chemotherapeutics should be administered during the window of normalization to obtain improved delivery, since vascular normalization is a transient process (Goel et al., 2011).
With respect to the nanocarrier systems, it is well established that the size of the nanomaterial affects the kinetics and extent of tumor accumulation and penetration. Kataoka and colleagues (Cabral et al., 2011) compared the tumor deposition of drug-loaded polymeric micelles of different sizes (30–100 nm) that exhibited similar circulation profiles and comparable tumor accumulation. It was found that the micelles with diameters above 50 nm mostly accumulated at the periphery of tumors and were not able to efficiently penetrate into the interstitium of poorly permeable, hypovascular tumors such as the human pancreatic cancer BxPC3 model; consequently, this resulted in very limited antitumor activity (Choi et al., 2007, 2011; Longmire et al., 2008; Cabral et al., 2011). Conversely, very small nanoparticles with size below 10 nm are often associated with a shorter blood half-life and fast clearance through renal filtration. In general, the vasculature of pancreatic tumors is moderately permeable, with pore sizes in the range of 50–60 nm (Chauhan and Jain, 2013). Thus, a size range of 15–50 nm would be preferable in order to exploit the leakiness of the tumor vasculature as far as transport is concerned. Consistent with this concept, cisplatin-incorporated polymeric micelles composed of polyethylene glycol/poly(glutamic acid) block copolymers with a size of about 30 nm (under the development name NC-6004; NanoCarrier Co., Ltd.) were designed to provide sustained release of cisplatin and utilize the enhanced permeability and retention effect to target the release of platinum to tumors (Nishiyama et al., 2003). Preclinical studies have indicated that NC-6004 exhibited prolonged blood circulation, preferential distribution to tumors, significantly lowered toxicity levels (compared with cisplatin at equivalent doses), and increased antitumor activity (Uchino et al., 2005; Cabral et al., 2011; Baba et al., 2012). These characteristics have been demonstrated in both a phase I study, where NC-6004 was used as monotherapy for patients with solid tumors, and a phase Ib/II study, where NC-6004 was used in combination with GEM in patients with pancreatic cancer (Plummer et al., 2011; Doi et al., 2017; Subbiah et al., 2018). Based on these results, a phase III trial is currently ongoing in Asia on the combination therapy of NC-6004 and GEM versus GEM monotherapy in the treatment of patients with locally advanced or metastatic PDAC (NCT02043288) (Fig. 1). Another nanomedicine, Genexol-PM, a 20–50 nm polyethylene glycol/poly(D,L-lactide) copolymer micellar formulation of paclitaxel, was evaluated as monotherapy in treatment-naive advanced PDAC patients in a single-arm phase II study and demonstrated a median OS (6.5 months) and other efficacy parameters preferable to those seen historically with GEM (Saif et al., 2010). The formulation is currently under evaluation in combination with GEM in patients with recurrent and metastatic PDAC (NCT02739633) (Fig. 1).
The contradictory size requirements for prolonged blood circulation and improved tumor penetration have aided the development of multistage systems that could maintain relatively large sizes during blood circulation but convert to smaller particles possessing favorable tissue penetration and diffusivity once accumulated at the tumor site (Wong et al., 2011; Sunoqrot et al., 2014). For example, Li et al. (2016) fabricated pH-sensitive nanoparticles through amphiphilic polymer–directed assembly of platinum prodrug-conjugated polyamidoamine (PAMAM) dendrimers. At physiologic pH, these hybrids had a size of around 100 nm, which favored long blood circulation and enhanced tumor accumulation. Then, the acidic tumor microenvironment triggered the release of small PAMAM prodrugs with sizes of approximately 5 nm, which enabled deep and uniform tumor penetration. Finally, the PAMAM prodrugs were rapidly reduced in the reductive cytosol to release active and potent cisplatin. A proof-of-principle in vivo efficacy study conducted in a BxPC-3 human pancreatic tumor model demonstrated that delivery of cisplatin prodrug via a multistage delivery system resulted in superior tumor shrinkage and prolonged survival time compared with free cisplatin or PAMAM prodrugs loaded in similarly sized, pH-stable nanoparticles. Moreover, intravital confocal laser scanning microscopy analyses confirmed that the released PAMAM enables efficient extravasation and penetration into deep tumor space (Fig. 1). These results emphasize the importance of size on the interstitial diffusivity of particles and suggest that tumor microenvironment–based size switching is a viable strategy for improving drug penetration and therapeutic efficacy, especially in tumors with poor permeability.
Targeting Cancer Stem Cells
As previously mentioned, current PDAC treatments ultimately fail, partially owing to the late diagnosis and dismal prognosis, but also as a result of tumor relapse at local or distant locations after treatment. Recent evidence has demonstrated that a small subpopulation of tumor cells (1%–5%) with extremely high tumorigenic potential, pancreatic cancer stem cells (PCSCs), are responsible for the disease progression, its resistance to chemotherapy and radiation, and driving relapse after treatment in PDAC patients (Hidalgo, 2010; Lonardo et al., 2010; Zhan et al., 2015; Batlle and Clevers, 2017). Moreover, when PCSCs are identified in surgical samples from patients with resectable disease, they confer a shorter survival rate (Rasheed et al., 2010). Of note, conventional therapies have been found to enrich stem cell fraction in tumors (Batlle and Clevers, 2017). Indeed, several studies have demonstrated that monotherapy with GEM increases the relative proportion of PCSCs (Hermann et al., 2007; Li et al., 2011; Zhang et al., 2016c). For example, Hermann et al. (2007) reported that PCSCs were enriched by >2 times in the orthotopic L3.6pl pancreatic tumor model following 3-week GEM treatment. Thus, strategies aimed at specific targeting and eradication of PCSCs may have important clinical implications. One of the most promising approaches to targeting and depleting this cell population is certainly the inhibition of stem cell–associated pathways (e.g., wingless-related integration site, Hedgehog, mTOR, and Notch), and multiple preclinical studies have provided compelling rationale for several phase I and II trials (Tables 2 and 3). However, according to the reported data, these trials have shown very limited positive outcomes with low responses and comparable rates in both PFS and OS. Given these results and our current understanding that all signaling pathways—including those used by PCSCs—function as a coordinated network, it is possible that PCSCs are heterogeneous and able to escape inhibition of an individual signaling pathway (Nimmakayala et al., 2019). It is likely that PCSC targeting will require the design of mechanism-based combination regimens. Funding from the preclinical studies suggest that this hypothesis is worthy of further exploration. For example, Heeschen and colleagues (Mueller et al., 2009) have shown that neither cyclopamine (a Hedgehog pathway inhibitor) nor rapamycin (an mTOR inhibitor) alone or as supplements to GEM chemotherapy were capable of effectively diminishing the PCSC pool. Only combined inhibition of these two pathways together with GEM, resulted in the desired targeting of the PCSCs. Importantly, administration of this triple combination in mice with established patient-derived pancreatic tumors was reasonably tolerated and translated into significantly prolonged long-term survival. Despite the great efficacy of this combination exhibited in the preclinical setting, the most concerns in the clinic are still the drug-related side effects. In this context, utilization of drug delivery systems to overcome some limitations related to drug solubility, stability as well as mitigating off-target toxicity might be beneficial at maximizing drug potentials in eliminating PCSCs. However, the number of reports on nanocarriers as a drug delivery system targeting PCSCs in PDAC is rather limited. For instance, Hedgehog pathway inhibitor 1 (HPI1) is a potent HHI based on in vitro evaluation. To overcome the limitations related to HPI1 poor aqueous solubility, its nanoparticle-based formulation (Nano-HHI) was developed using polyethylene glycol/poly(lactic-co-glycolic acid) copolymers. Nano-HHI particles of approximately 60 nm in size formed stable aqueous dispersions, and improved systemic bioavailability compared with the parent compound. Nano-HHI in combination with GEM suppressed tumor growth in orthotopic Pa03C pancreatic xenografts compared with GEM alone. Importantly, Nano-HHI, either as a single agent or in combination therapy arms, can cause a marked decrease in PCSCs within the tumor, suggesting its potential for further translational development (Fig. 1). Most recently, Mahato and coworkers (Kumar et al., 2017) designed and evaluated a new analog of vismodegib, MDB5, which was shown to be more potent in depleting PCSCs. In a pancreatic tumor mouse model, treatment with MDB5 containing nanoparticles resulted in significant inhibition of tumor growth and was well tolerated. These results suggest that nanoformulations of MDB5 can be further explored as a platform for monotherapy and/or combination therapy. Drug repurposing for oncology indication has recently been realized due to existing preclinical and clinical data and fast Food and Drug Administration approval (Fig. 1). In this context, anthothecol, an antimalarial compound, and its nanoparticle-based formulation were shown to inhibit cell proliferation and colony formation, and induced apoptosis in PCSCs and cancer cell lines, but appeared to have little effect on nonmalignant pancreatic ductal cells. It was found that anthothecol inhibits the Hedgehog signaling pathway by disrupting binding of Gli to DNA, thus acting as a Gli inhibitor (Fig. 1). The other formulations of agents that are effective against PCSCs require further careful evaluation in relevant PDAC animal models to confirm whether their use could potentially improve the treatment outcomes and reduce recurrence of the disease. Other intriguing possibilities include immunotherapy directed against PCSC markers; however, a cautious approach is required because many of these markers can be found on normal stem cells in the hematopoietic system (Batlle and Clevers, 2017). Furthermore, regeneration of the PCSC pool by plasticity of non-PCSCs upon treatment cessation presents another challenge in therapy design targeting. The development of anti-PCSC therapies is in a relatively early stage, and along with the great promise of drug delivery technologies many issues remain unresolved or unknown; therefore, more basic and applied research is needed to translate these therapeutic designs into clinical approaches (Zhao et al., 2013).
Closing Remarks
PDAC remains one of the most devastating cancers with a dismal response to the existing therapies. Despite intensive research over the past decades, the development of effective chemotherapeutic treatments has been slow, with only modest improvement in patients’ survival. Intrinsic and acquired drug resistance extremely shortens the period of effectiveness of drugs, whereas drugs proven to be efficient in other type of cancers (e.g., taxanes and anthracyclines) fail in treating PDAC, indicating that PDAC possesses uniquely challenging characteristics that are not yet completely understood. Combination therapies demonstrate improved outcomes in patients’ survival and quality of life; nevertheless, the overall improvement is still marginal, especially for patients diagnosed with late stages of the disease. With a more comprehensive understanding of the physiology and pathology of PDAC, the expansion of treatment beyond conventional chemotherapies to specific and targeted strategies including nanomedicine-based drug delivery platforms holds great therapeutic promise in combating PDAC. Since a proper drug combination can target multiple pathways, promote synergistic drug action, and suppress the development of drug resistance, the use of multiple therapeutic agents in a treatment regimen has become the primary strategy in treating PDAC. However, this remains a challenging task due to the possible cumulative toxicities as well as the differences in pharmacokinetics and biodistribution profiles that result in inconsistent drug uptake and suboptimal combinatorial effects at the sites of action, which are sensitive to both dosing and scheduling of multiple drugs. Indeed, nanomaterials offer several advantages as therapeutic tools due to design flexibility and small size, and can be engineered to interact with specific biologic components in targeted diseased tissues. Such carriers can simultaneously allow mixing different drugs in one carrier particle, control drug retention, and reconcile the pharmacokinetics, ratiometric delivery of drug combination, and sequential drug release, which are important determinants for better tailored combinatorial regimens in cancer treatment. This, in principle, can open very broad possibilities in designing synergistic drug combinations that could affect multiple aberrant pathways in PDAC (Meng et al., 2015; Lu et al., 2017). The selected studies described herein clearly emphasize that nanomedicines can improve the therapeutic responses observed with standard therapies and achieve accelerated clinical translation. However, the complexity of these systems compared with conventional small molecule drugs and intricate structural properties demands careful engineering of the nanocarriers to achieve the desired effect. Challenges exist in terms of efficient delivery of the cargo to the tumor as well as clearance of the nanomaterials once they have accomplished their mission in vivo. Moving forward, further thorough characterization and understanding of nanomaterial interactions with biologic milieus that drive both their intended action and possible toxicological responses and immunogenicity will be critical in the design and optimization of future cancer nanomedicines and will help in eventually taking them from bench to bedside.
Acknowledgments
We acknowledge the support of the National Institutes of Health National Institute of General Medical Sciences.
Abbreviations
- 5-FU
5-fluorouracil
- ECM
extracellular matrix
- FOLFIRINOX
folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin
- GEM
gemcitabine
- HHI
Hedgehog inhibitor
- mFOLFIRINOX
modified folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin
- nab-PTX
nanoparticle albumin-bound paclitaxel
- OS
overall survival
- PAMAM
polyamidoamine
- PC
pancreatic cancer
- PCSC
pancreatic cancer stem cell
- PDAC
pancreatic ductal adenocarcinoma
- PEGPH20
(polyethylene glycol, PEG) PEGylated version of Halozyme’s proprietary recombinant human hyaluronidase enzyme, rHuPH20
- PFS
progression-free survival
- RR
response rate
Authorship Contributions
Participated in research design: Lei, Xi, Bronich.
Wrote or contributed to the writing of the manuscript: Lei, Xi, Batra, Bronich.
Footnotes
This work was partly supported by an Institutional Development Award from the National Institutes of Health National Institute of General Medical Sciences [Grant P30GM127200 to T.K.B.].
References
- Adamska A, Domenichini A, Falasca M. (2017) Pancreatic ductal adenocarcinoma: current and evolving therapies. Int J Mol Sci 18:E1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adiseshaiah PP, Crist RM, Hook SS, McNeil SE. (2016) Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol 13:750–765. [DOI] [PubMed] [Google Scholar]
- Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, et al. (2013) Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer 109:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile G, Negri FV, Giuliani F, De Carlo E, Melisi D, Simionato F, Silvestris N, Brunetti O, Leone F, Marino D, et al. (2017) Second-line chemotherapy for advanced pancreatic cancer: which is the best option? Crit Rev Oncol Hematol 115:1–12. [DOI] [PubMed] [Google Scholar]
- Au M, Emeto TI, Power J, Vangaveti VN, Lai HC. (2016) Emerging therapeutic potential of nanoparticles in pancreatic cancer: a systematic review of clinical trials. Biomedicines 4:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Matsumoto Y, Kashio A, Cabral H, Nishiyama N, Kataoka K, Yamasoba T. (2012) Micellization of cisplatin (NC-6004) reduces its ototoxicity in guinea pigs. J Control Release 157:112–117. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. (2008) Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 14:5995–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et al. (2006) Both p16Ink4a and the p19Arf-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci USA 103:5947–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Clevers H. (2017) Cancer stem cells revisited. Nat Med 23:1124–1134. [DOI] [PubMed] [Google Scholar]
- Beatty GL, Shahda S, Beck T, Uppal N, Cohen SJ, Donehower R, Gabayan AE, Assad A, Switzky J, Zhen H, et al. (2018) A phase Ib/II study of the JAK1 inhibitor, itacitinib, plus nab-paclitaxel and gemcitabine in advanced solid tumors. Oncologist 23:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann L, Maute L, Heil G, Rüssel J, Weidmann E, Köberle D, Fuxius S, Weigang-Köhler K, Aulitzky WE, Wörmann B, et al. (2015) A prospective randomised phase-II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Eur J Cancer 51:27–36. [DOI] [PubMed] [Google Scholar]
- Borad MJ, Kwak EL, Wang-Gillam A, Ibrahim A, Aldridge J, Olszanski AJ. (2016) Evofosfamide combined with gemcitabine/nab-paclitaxel in patients with previously untreated locally advanced or metastatic pancreatic adenocarcinoma (PAC): results of a phase I trial. J Clin Oncol 34 (Suppl 15):4114. [Google Scholar]
- Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J, Jr, Chiorean EG, Rosen PJ, et al. (2015) Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 33:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, et al. (2011) Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol 6:815–823. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2017) Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32:185–203.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascinu S, Berardi R, Sobrero A, Bidoli P, Labianca R, Siena S, Ferrari D, Barni S, Aitini E, Zagonel V, et al. Italian Group for the Study of Digestive Tract Cancer (GISCAD) (2014) Sorafenib does not improve efficacy of chemotherapy in advanced pancreatic cancer: a GISCAD randomized phase II study. Dig Liver Dis 46:182–186. [DOI] [PubMed] [Google Scholar]
- Catenacci DVT, Bahary N, Edelman MJ, Nattam SR, Marsh RW, Wallace AKA, Cohen DJ, Stiff PJ, Sleckman BG, Thomas SP, Lenz H, Henderson L, Zagaya C, Vannier M, Karrison T, Stadler WM, Kindler HL. (2012) A phase IB/randomized phase II study of gemcitabine (G) plus placebo (P) or vismodegib (V), a hedgehog (Hh) pathway inhibitor, in patients (pts) with metastatic pancreatic cancer (PC): Interim analysis of a University of Chicago phase II consortium study. J Clin Oncol 30 (Suppl 15):4022. [Google Scholar]
- Catenacci DVT, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, et al. (2015) Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol 33:4284–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Jain RK. (2013) Strategies for advancing cancer nanomedicine. Nat Mater 12:958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popović Z, Huang P, Bawendi MG, Boucher Y, Jain RK. (2013) Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4:2516–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LT, Von Hoff DD, Li CP, Wang-Gillam A, Bodoky G, Dean AP, Shan YS, Jameson GS, Macarulla T, Lee KH, et al. (2015) Expanded analyses of napoli-1: phase 3 study of MM-398 (nal-IRI), with or without 5-fluorouracil and leucovorin, versus 5-fluorouracil and leucovorin, in metastatic pancreatic cancer (mPAC) previously treated with gemcitabine-based therapy. J Clin Oncol 33 (Suppl 3):234. [Google Scholar]
- Chiorean EG, Coveler AL. (2015) Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther 9:3529–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorean EG, Von Hoff DD, Tabernero J, El-Maraghi R, Wee Ma W, Reni M, Harris M, Whorf R, Liu H, Shiansong Li J, et al. (2016) Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer 115:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Zuckerman JE, Webster P, Davis ME. (2011) Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci USA 108:6656–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. (2007) Renal clearance of quantum dots. Nat Biotechnol 25:1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd JM, Katz MH, Prakash L, Varadhachary GR, Wolff RA, Shroff RT, Javle M, Fogelman D, Overman M, Crane CH, et al. (2017) Preoperative therapy and pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg 21:164–174. [DOI] [PubMed] [Google Scholar]
- Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E, Lopez M. (2002) Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer 94:902–910. [PubMed] [Google Scholar]
- Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolò M, et al. Gruppo Oncologico Italia Meridionale (GOIM) Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD) Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC) (2010) Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 28:1645–1651. [DOI] [PubMed] [Google Scholar]
- Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. (2016) Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer 57:10–22. [DOI] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. Groupe Tumeurs Digestives of Unicancer. PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. [DOI] [PubMed] [Google Scholar]
- Conroy T, Hammel P, Hebbar M, Abdelghani MB, Wei AC, Raoul JL, Chone L, Francois E, Artru P, Biagi JJ, et al. (2018) Unicancer GI PRODIGE 24/CCTG PA.6 trial: a multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J Clin Oncol 36 (Suppl 18):LBA4001. [Google Scholar]
- Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. (2009) Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 27:5513–5518. [DOI] [PubMed] [Google Scholar]
- Cutsem EV, Lenz HJ, Furuse J, Tabernero J, Heinemann V, Ioka T, Bazin I, Ueno M, Csõszi T, Wasan H, et al. (2016) Evofosfamide (TH-302) in combination with gemcitabine in previously untreated patients with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma: primary analysis of the randomized, double-blind phase III MAESTRO study. J Clin Oncol 34 (Suppl 4):193. [Google Scholar]
- Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. (2011) Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA 108:2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler M, Aust D, Weitz J, Pilarsky C, Grützmann R. (2014) Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int 2014:474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Hamaguchi T, Shitara K, Iwasa S, Shimada Y, Harada M, Naito K, Hayashi N, Masada A, Ohtsu A. (2017) NC-6004 Phase I study in combination with gemcitabine for advanced solid tumors and population PK/PD analysis. Cancer Chemother Pharmacol 79:569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. (2006) Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 6:688–701. [DOI] [PubMed] [Google Scholar]
- Eser S, Schnieke A, Schneider G, Saur D. (2014) Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer 111:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann G, Habbe N, Dhara S, Bisht S, Alvarez H, Fendrich V, Beaty R, Mullendore M, Karikari C, Bardeesy N, et al. (2008) Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut 57:1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, Szczylik C, Moore MJ, Peeters M, Bodoky G, et al. (2015) A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol 26:921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman DC, Palmaira RL, Covington CM, Desai AM, Ku GY, Li J, Harding JJ, Varghese AM, O’Reilly EM, Yu KH. (2018) Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 18:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godhi SA, Parasar K, Saluja S, Mishra P. (2017) “Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer.” Ann Surg 265:E73. [DOI] [PubMed] [Google Scholar]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91:1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X, et al. (2012) BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol 23:2799–2805. [DOI] [PubMed] [Google Scholar]
- Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, Strobel O, Jäger D, Ulrich A, Büchler MW. (2016) Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg 264:457–463. [DOI] [PubMed] [Google Scholar]
- Halford S, Yip D, Karapetis CS, Strickland AH, Steger A, Khawaja HT, Harper PG. (2001) A phase II study evaluating the tolerability and efficacy of CAELYX (liposomal doxorubicin, Doxil) in the treatment of unresectable pancreatic carcinoma. Ann Oncol 12:1399–1402. [DOI] [PubMed] [Google Scholar]
- Hall BR, Cannon A, Atri P, Wichman CS, Smith LM, Ganti AK, Are C, Sasson AR, Kumar S, Batra SK. (2018) Advanced pancreatic cancer: a meta-analysis of clinical trials over thirty years. Oncotarget 9:19396–19405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B, et al. (2006) Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 24:3946–3952. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1:313–323. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B, et al. Swiss Group for Clinical Cancer Research. Central European Cooperative Oncology Group (2007) Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 25:2212–2217. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. (2010) Pancreatic cancer. N Engl J Med 362:1605–1617. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. (2015) Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 15:8–18. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R, et al. (2016) Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 22:2848–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Harris WP, Hendifar AE, Bullock AJ, Wu XW, Huang Y, Jiang P. (2015) High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: interim results of a randomized phase II study. J Clin Oncol 33 (Suppl 15):4006. [Google Scholar]
- Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, et al. (2018) HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol 36:359–366. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, et al. (2004) An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 28:977–987. [DOI] [PubMed] [Google Scholar]
- Hu Q, Sun W, Wang C, Gu Z. (2016) Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv Drug Deliv Rev 98:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K, et al. (2014) A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer 50:2072–2081. [DOI] [PubMed] [Google Scholar]
- Ioka T, Okusaka T, Ohkawa S, Boku N, Sawaki A, Fujii Y, Kamei Y, Takahashi S, Namazu K, Umeyama Y, et al. (2015) Efficacy and safety of axitinib in combination with gemcitabine in advanced pancreatic cancer: subgroup analyses by region, including Japan, from the global randomized phase III trial. Jpn J Clin Oncol 45:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et al. (2013) Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. (2012) Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev 64 (Suppl):353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus-Acosta AD, O’Dwyer PJ, Ramanathan RK, Von Hoff DD, Maitra A, Rasheed ZA, Zheng L, Rajeshkumar NV, Le DT, Hoering A, et al. (2014) A phase II study of vismodegib, a hedgehog (Hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). J Clin Oncol 32 (Suppl 3):257. [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutric Z, Melstrom LG. (2017) New treatment options and management considerations in borderline resectable pancreatic cancer. Oncology (Williston Park) 31:443–452. [PubMed] [Google Scholar]
- Kamisawa T, Wood LD, Itoi T, Takaori K. (2016) Pancreatic cancer. Lancet 388:73–85. [DOI] [PubMed] [Google Scholar]
- Katopodis O, Souglakos J, Stathopoulos E, Christopoulou A, Kontopodis E, Kotsakis A, Kalbakis K, Kentepozidis N, Polyzos A, Hatzidaki D, et al. (2014) Frontline treatment with gemcitabine, oxaliplatin and erlotinib for the treatment of advanced or metastatic pancreatic cancer: a multicenter phase II study of the Hellenic Oncology Research Group (HORG). Cancer Chemother Pharmacol 74:333–340. [DOI] [PubMed] [Google Scholar]
- Kaur S, Baine MJ, Jain M, Sasson AR, Batra SK. (2012) Early diagnosis of pancreatic cancer: challenges and new developments. Biomarkers Med 6:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. (2013) Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol 10:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khushman M, Dempsey N, Maldonado JC, Loaiza-Bonilla A, Velez M, Carcas L, Dammrich D, Hurtado-Cordovi J, Parajuli R, Pollack T, et al. (2015) Full dose neoadjuvant FOLFIRINOX is associated with prolonged survival in patients with locally advanced pancreatic adenocarcinoma. Pancreatology 15:667–673. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al. (2014) Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res 20:5937–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. (2017) nab-Paclitaxel for the treatment of pancreatic cancer. Cancer Manag Res 9:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, et al. (2011) Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 12:256–262. [DOI] [PubMed] [Google Scholar]
- Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, et al. (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28:3617–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps E, Young K, Starling N. (2017) Liposomal irinotecan in gemcitabine-refractory metastatic pancreatic cancer: efficacy, safety and place in therapy. Ther Adv Med Oncol 9:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AH. (2016) Nanomedicine developments in the treatment of metastatic pancreatic cancer: focus on nanoliposomal irinotecan. Int J Nanomedicine 11:1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV, et al. (2016a) A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 45:370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AH, Murphy PB, Peyton JD, Shipley DL, Al-Hazzouri A, Rodriguez FA, Womack MS, IV, Xiong HQ, Waterhouse DM, Tempero MA, et al. (2017) A randomized, double-blinded, phase II trial of gemcitabine and nab-paclitaxel plus apatorsen or placebo in patients with metastatic pancreatic cancer: the RAINIER trial. Oncologist 22:1427–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AH, Murray J, Horgan KE, Dauer J, Curley M, Baum J, Louis CU, Lugovskoy A. (2016b) A multicenter phase II study of istiratumab (MM-141) plus nab-paclitaxel (A) and gemcitabine (G) in metastatic pancreatic cancer (MPC). J Clin Oncol 34 (Suppl 4):481. [Google Scholar]
- Koorstra JB, Feldmann G, Habbe N, Maitra A. (2008) Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs). Langenbecks Arch Surg 393:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M. (2010) Driver mutations: a roadmap for getting close and personal in pancreatic cancer. Cancer Biol Ther 10:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Chaudhary AK, Dong Y, Zhong HA, Mondal G, Lin F, Kumar V, Mahato RI. (2017) Design, synthesis and biological evaluation of novel hedgehog inhibitors for treating pancreatic cancer. Sci Rep 7:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Park SW. (2016) Systemic chemotherapy in advanced pancreatic cancer. Gut Liver 10:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Molenaar RJ, Klaassen R, Bijlsma MF, Weterman MJ, Richel DJ, Wymenga M, van Laarhoven HWM, Wilmink JW. (2017) A Phase I study of LDE225 in combination with gemcitabine and nab-paclitaxel in patients with metastasized pancreatic cancer. Ann Oncol 28:761–768.28057664 [Google Scholar]
- Leichman LP, O’Neill BH, Berlin J, Weekes CD, Ames P, McKinley M, Davies AM, Cohen SJ, Academic Gastrointestinal Cancer Consortium (AGICC) (2012) A phase IB study of erlotinib in combination with gemcitabine and nab-paclitaxel in patients with previously untreated advanced pancreatic cancer: an Academic GI Cancer Consortium (AGICC) study. J Clin Oncol 30 (Suppl 15):4052. [DOI] [PubMed] [Google Scholar]
- Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. (2011) c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141:2218–2227.e5. [DOI] [PubMed] [Google Scholar]
- Li HJ, Du JZ, Du XJ, Xu CF, Sun CY, Wang HX, Cao ZT, Yang XZ, Zhu YH, Nie S, et al. (2016) Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc Natl Acad Sci USA 113:4164–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo E, Hermann PC, Heeschen C. (2010) Pancreatic cancer stem cells—update and future perspectives. Mol Oncol 4:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmire M, Choyke PL, Kobayashi H. (2008) Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 3:703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, et al. GERCOR. GISCAD (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509–3516. [DOI] [PubMed] [Google Scholar]
- Lu J, Liu X, Liao YP, Salazar F, Sun B, Jiang W, Chang CH, Jiang J, Wang X, Wu AM, et al. (2017) Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun 8:1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji GA, Olive KP, Saenger YM, Oberstein P. (2017) Current and emerging therapies in metastatic pancreatic cancer. Clin Cancer Res 23:1670–1678. [DOI] [PubMed] [Google Scholar]
- Marsh Rde W, Talamonti MS, Katz MH, Herman JM. (2015) Pancreatic cancer and FOLFIRINOX: a new era and new questions. Cancer Med 4:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Nel AE. (2018) Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Adv Drug Deliv Rev 130:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, Ji Z, Chang CH, Nel AE. (2015) Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 9:3540–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith W, Cohen S, Shahda S, Lenz HJ, Weekes C, Dotan E, Denlinger C, O’Neil B, Kapoun A, Zhang C, et al. (2016) Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with nab-paclitaxel (Nab-P) and gemcitabine (G) in patients (pts) with previously untreated stage IV pancreatic cancer (PC). Ann Oncol 27 (Suppl 6):677. [Google Scholar]
- Miao L, Lin CM, Huang L. (2015) Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J Control Release 219:192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills LD, Zhang Y, Marler RJ, Herreros-Villanueva M, Zhang L, Almada LL, Couch F, Wetmore C, Pasca di Magliano M, Fernandez-Zapico ME. (2013) Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation. J Biol Chem 288:11786–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. National Cancer Institute of Canada Clinical Trials Group (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966. [DOI] [PubMed] [Google Scholar]
- Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG, et al. (2010) Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA 107:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M, Bartenstein P, D’Haese JG, et al. (2009) Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology 137:1102–1113. [DOI] [PubMed] [Google Scholar]
- Muniraj T, Jamidar PA, Aslanian HR. (2013) Pancreatic cancer: a comprehensive review and update. Dis Mon 59:368–402. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Isayama H, Ijichi H, Sasaki T, Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y, et al. (2010) Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer 103:1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K, Hendifar A, Starodub A, Chaves J, Yang Y, Koh B, Barbie D, Hahn WC, Fuchs CS. (2019) Phase 1 dose-escalation study of momelotinib, a Janus kinase 1/2 inhibitor, combined with gemcitabine and nab-paclitaxel in patients with previously untreated metastatic pancreatic ductal adenocarcinoma. Invest New Drugs 37:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmakayala RK, Batra SK, Ponnusamy MP. (2019) Unraveling the journey of cancer stem cells from origin to metastasis. Biochim Biophys Acta Rev Cancer 1871:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, Nishio K, Matsumura Y, Kataoka K. (2003) Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res 63:8977–8983. [PubMed] [Google Scholar]
- Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, Zimmermann A, John W, Von Hoff D, Arning M, et al. (2005) A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 16:1639–1645. [DOI] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324:1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil BH, Scott AJ, Ma WW, Cohen SJ, Leichman L, Aisner DL, Menter AR, Tejani MA, Cho JK, Granfortuna J, et al. (2015) A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer [published correction appears in (2016) 27:1180]. Ann Oncol 26:1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly EM, Sahai V, Bendell JC, Bullock AJ, LoConte NK, Hatoum H, Ritch PS, Hool H, Leach JW, Sanchez J, et al. (2017) Results of a randomized phase II trial of an anti-notch 2/3, tarextumab (OMP-59R5, TRXT, anti-Notch2/3), in combination with nab-paclitaxel and gemcitabine (Nab-P+Gem) in patients (pts) with untreated metastatic pancreatic cancer (mPC). J Clin Oncol 35 (Suppl 4):279. [Google Scholar]
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25:719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniccia A, Edil BH, Schulick RD, Byers JT, Meguid C, Gajdos C, McCarter MD. (2014) Neoadjuvant FOLFIRINOX application in borderline resectable pancreatic adenocarcinoma: a retrospective cohort study. Medicine (Baltimore) 93:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E, Castelli G, Testa U. (2017) Pancreatic cancer: molecular characterization, clonal evolution and cancer stem cells. Biomedicines 5:E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, et al. (2010) Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 28:3605–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti JK, et al. (2014) Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer 120:2980–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer R, Wilson RH, Calvert H, Boddy AV, Griffin M, Sludden J, Tilby MJ, Eatock M, Pearson DG, Ottley CJ, et al. (2011) A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br J Cancer 104:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P, et al. (2009) Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 27:3778–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913–2921. [DOI] [PubMed] [Google Scholar]
- Rahman FAU, Ali S, Saif MW. (2017) Update on the role of nanoliposomal irinotecan in the treatment of metastatic pancreatic cancer. Therap Adv Gastroenterol 10:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, et al. (2010) Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst 102:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Stephenson J, Wolpin BM, Becerra C, Hamm JT, Messersmith WA, Devens S, Cushing J, Schmalbach T, Fuchs CS. (2012) A phase Ib trial of IPI-926, a hedgehog pathway inhibitor, plus gemcitabine in patients with metastatic pancreatic cancer. J Clin Oncol 30 (Suppl 4):213. [Google Scholar]
- Rocha Lima CM, Green MR, Rotche R, Miller WH, Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. (2004) Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 22:3776–3783. [DOI] [PubMed] [Google Scholar]
- Rombouts SJ, Walma MS, Vogel JA, van Rijssen LB, Wilmink JW, Mohammad NH, van Santvoort HC, Molenaar IQ, Besselink MG. (2016) Systematic review of resection rates and clinical outcomes after FOLFIRINOX-based treatment in patients with locally advanced pancreatic cancer. Ann Surg Oncol 23:4352–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA. (2013) Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer 49:2633–2642. [DOI] [PubMed] [Google Scholar]
- Sahora K, Schindl M, Kuehrer I, Eisenhut A, Werba G, Brostjan C, Telek B, Ba’ssalamah A, Stift J, Schoppmann SF, et al. (2014) A phase II trial of two durations of bevacizumab added to neoadjuvant gemcitabine for borderline and locally advanced pancreatic cancer. Anticancer Res 34:2377–2384. [PubMed] [Google Scholar]
- Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J, Rowen E, Yu J, Kerr RO. (2010) Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest 28:186–194. [DOI] [PubMed] [Google Scholar]
- Schultheis B, Reuter D, Ebert MP, Siveke J, Kerkhoff A, Berdel WE, Hofheinz R, Behringer DM, Schmidt WE, Goker E, et al. (2017) Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann Oncol 28:2429–2435. [DOI] [PubMed] [Google Scholar]
- Seicean A, Petrusel L, Seicean R. (2015) New targeted therapies in pancreatic cancer. World J Gastroenterol 21:6127–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Kantoff PW, Wooster R, Farokhzad OC. (2017) Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17:20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadi R, Brusa F, Ponzetti A, Chiappino I, Birocco N, Ciuffreda L, Satolli MA. (2016) Current therapeutic strategies for advanced pancreatic cancer: a review for clinicians. World J Clin Oncol 7:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, Potamianou A, Ziras N, Boukovinas J, Varthalitis J, et al. (2006) A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer 95:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah V, Grilley-Olson JE, Combest AJ, Sharma N, Tran RH, Bobe I, Osada A, Takahashi K, Balkissoon J, Camp A, et al. (2018) Phase Ib/II trial of NC-6004 (nanoparticle cisplatin) plus gemcitabine in patients with advanced solid tumors. Clin Cancer Res 24:43–51. [DOI] [PubMed] [Google Scholar]
- Sunoqrot S, Bugno J, Lantvit D, Burdette JE, Hong S. (2014) Prolonged blood circulation and enhanced tumor accumulation of folate-targeted dendrimer-polymer hybrid nanoparticles. J Control Release 191:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague A, Lim KH, Wang-Gillam A. (2015) Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol 7:68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Ding W, Kim S, Xu X, Pan M, Chen S. (2013) Efficacy and safety profile of combining agents against epidermal growth factor receptor or vascular endothelium growth factor receptor with gemcitabine-based chemotherapy in patients with advanced pancreatic cancer: a meta-analysis. Pancreatology 13:415–422. [DOI] [PubMed] [Google Scholar]
- Uchino H, Matsumura Y, Negishi T, Koizumi F, Hayashi T, Honda T, Nishiyama N, Kataoka K, Naito S, Kakizoe T. (2005) Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer 93:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro V, Sperduti I, Milella M. (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 365:768–769, author reply 769. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Hidalgo M, Canon JL, Macarulla T, Bazin I, Poddubskaya E, Manojlovic N, Radenkovic D, Verslype C, Raymond E, et al. (2018) Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int J Cancer 143:2053–2064. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, et al. (2004) Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 22:1430–1438. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, et al. (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27:2231–2237. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Wu CY, Yeh YC, Shyr YM, Wu YY, Kuo CY, Hung YP, Chen MH, Lee WP, Luo JC, et al. (2015) Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget 6:18162–18173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et al. NAPOLI-1 Study Group (2016) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387:545–557. [DOI] [PubMed] [Google Scholar]
- Wang-Gillam A, Von Hoff D, Siveke J, Hubner R, Belanger B, Pipas JM, Chen LT. (2017) Nanoliposomal irinotecan in the clinical practice guideline for metastatic pancreatic cancer: applicability to clinical situations. J Clin Oncol 35:689–690. [DOI] [PubMed] [Google Scholar]
- Weekes C, Berlin J, Lenz HJ, O’Neil B, Messersmith W, Cohen S, Dendinger C, Shahda S, Kapoun A, Zhang C, et al. (2016) Phase 1b study of WNT inhibitor ipafricept (IPA, decoy receptor for WNT ligands) with nab-paclitaxel (Nab-P) and gemcitabine (G) in patients (pts) with previously untreated stage IV pancreatic cancer (PC). Ann Oncol 27 (Suppl 6):367. [Google Scholar]
- Whipple C, Korc M. (2008) Targeting angiogenesis in pancreatic cancer: rationale and pitfalls. Langenbecks Arch Surg 393:901–910. [DOI] [PubMed] [Google Scholar]
- Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, et al. (2015) Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 6:6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. (2011) Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci USA 108:2426–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D, Xie K. (2015) Pancreatic cancer stromal biology and therapy. Genes Dis 2:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. (2010) Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467:1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. (2008) A paracrine requirement for hedgehog signalling in cancer. Nature 455:406–410. [DOI] [PubMed] [Google Scholar]
- Yonezawa S, Higashi M, Yamada N, Goto M. (2008) Precursor lesions of pancreatic cancer. Gut Liver 2:137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Kim HJ, Kim KH, Kim SH, Kim HJ, Lee SC, Bae SB, Kim CK, Lee N, Lee KT, et al. (2014) A phase II study of gemcitabine, oxaliplatin, and erlotinib (GEMOX-T) combination chemotherapy in previously untreated patients with locally advanced unresectable or metastatic pancreatic cancer. J Clin Oncol 32 (Suppl 15):e15260. [Google Scholar]
- Zhan HX, Xu JW, Wu D, Zhang TP, Hu SY. (2015) Pancreatic cancer stem cells: new insight into a stubborn disease. Cancer Lett 357:429–437. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, Li J, Huang K. (2016a) Pancreatic cancer epidemiology, detection, and management. Gastroenterology Res Pract 2016:8962321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. (2016b) Nanomedicine of synergistic drug combinations for cancer therapy—strategies and perspectives. J Control Release 240:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Duan Q, Zhao H, Liu T, Wu H, Shen Q, Wang C, Yin T. (2016c) Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-κB/STAT3 signaling cascade. Cancer Lett 382:53–63. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Alakhova DY, Kabanov AV. (2013) Can nanomedicines kill cancer stem cells? Adv Drug Deliv Rev 65:1763–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]