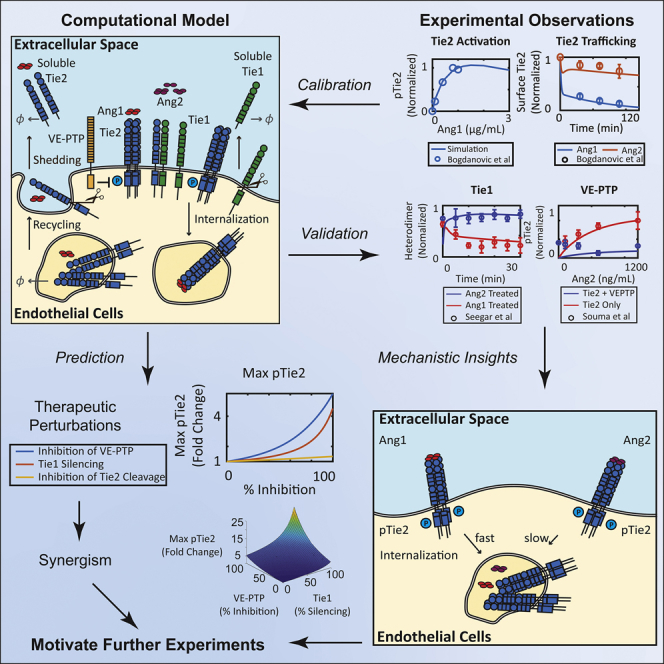
Summary
The angiopoietin-Tie signaling pathway is an important vascular signaling pathway involved in angiogenesis, vascular stability, and quiescence. Dysregulation in the pathway is linked to the impairments in vascular function associated with many diseases, including cancer, ocular diseases, systemic inflammation, and cardiovascular diseases. The present study uses a computational signaling pathway model validated against experimental data to quantitatively study various mechanistic aspects of the angiopoietin-Tie signaling pathway, including receptor activation, trafficking, turnover, and molecular mechanisms of its regulation. The model provides mechanistic insights into the controversial role of Ang2 and its regulators vascular endothelial protein tyrosine phosphatase (VE-PTP) and Tie1 and predicts synergistic effects of inhibition of VE-PTP, Tie1, and Tie2 cleavage on enhancing the vascular protective actions of Tie2.
Subject Areas: Biological Sciences, Cell Biology, Bioinformatics
Graphical Abstract
Highlights
-
•
Mechanistically detailed computational model of the angiopoietin-Tie pathway
-
•
Receptor internalization dynamics modulate effectiveness of VE-PTP
-
•
Inhibition of VE-PTP, Tie1, and Tie2 shedding synergistically enhances Tie2 activation
-
•
Tie1 silencing modulates the effectiveness of inhibiting VE-PTP and Tie2 shedding
Biological Sciences; Cell Biology; Bioinformatics
Introduction
The angiopoietin (Ang)-Tie signaling pathway is an important signaling pathway involved in vascular development, angiogenesis, and remodeling, as well as in the regulation of vascular permeability, homeostasis, quiescence, and stability (Augustin et al., 2009, Eklund and Saharinen, 2013, Fukuhara et al., 2010, Takakura et al., 1998). Over the past decade, the angiopoietin-Tie signaling pathway has been the subject of a tremendous amount of research in search of therapeutic opportunities for many diseases (Saharinen et al., 2017). In the cardiovascular system, dysregulation in the angiopoietin-Tie signaling axis has been linked to vascular impairment, ischemia/reperfusion injury, development of atherosclerotic plaques, as well as peripheral arterial disease (Findley et al., 2008, Fujisawa et al., 2017, Venkat et al., 2017, Woo et al., 2011). In ocular diseases including diabetic macular edema and wet age-related macular degeneration, angiopoietin-Tie signaling is crucial in regulating retinal and choroidal neovascularization and vascular leakage (Khalaf et al., 2017, Shen et al., 2014). The role of Tie2 in cancer, as well as in inflammation, is also being actively explored (Huang et al., 2010, Milam and Parikh, 2015, Parikh, 2017). Recently, angiopoietin-1 (Ang1) has also been studied as a neuroprotective agent that inhibits neural apoptosis (Yin et al., 2019).
The angiopoietin family of proteins consists of Ang1, Ang2, Ang3, and Ang4 that bind to their receptor Tie2. Ang1 and Ang2 have been identified as the main ligands for Tie2 (Davis et al., 1996, Maisonpierre et al., 1997). Ang3 and Ang4 are mouse and human orthologs that are less well studied with unclear biological functions (Fagiani and Christofori, 2013, Lee et al., 2004). Ang1, the natural agonist of Tie2, binds and activates Tie2 to promote vascular stability and is secreted by quiescent, mature vessels (Thurston and Daly, 2012). Ang2 has historically been considered an antagonist that competitively inhibits Ang1 binding and is usually secreted in diseased or remodeling vessels (Maisonpierre et al., 1997, Thurston and Daly, 2012). Recent discoveries that Ang2 can act as agonist under certain conditions suggest that Ang2's role is highly context dependent (Kim et al., 2000, Thurston and Daly, 2012, Yuan et al., 2009). Native Ang1 exists in highly oligomerized forms, forming tetrameric or higher-order multimeric structures, whereas native Ang2 exists in lower oligomeric forms, forming mainly dimers, trimers, or tetramers (Kim et al., 2005). Ligand binding and ligand-specific responses of Tie2, including its trafficking and activation, have been observed to be influenced by the ligand oligomerization state, with the tetrameric form being the lowest oligomeric form of ligand required for Tie2 activation (Kim et al., 2005, Pietila et al., 2012). Activation of Tie2 by either Ang1 or Ang2 results in receptor internalization and release of ligands (Bogdanovic et al., 2006). Vascular endothelial protein tyrosine phosphatase (VE-PTP) regulates the activation of Tie2 by catalyzing its dephosphorylation (Fachinger et al., 1999).
Tie1 has been historically considered an orphan receptor, with no identified natural ligand. A recent study has identified leukocyte cell-derived chemotaxin 2 as a functional ligand of Tie1, modulating its interaction with Tie2 (Xu et al., 2019). The role of Tie1 in the regulation of the angiopoietin-Tie signaling axis is controversial and not completely understood. Korhonen et al. reported that Tie1 is required for the agonistic activity of Ang2 on Tie2 (Korhonen et al., 2016). Seegar et al. proposed that Tie1 negatively regulates Tie2 activation by pre-forming heterodimers with Tie2 and that the functional difference between Ang1 and Ang2 can be explained by the ability of Ang1 to dissociate Tie2 from the Tie1-Tie2 dimer (Seegar et al., 2010). Recent studies by Savant et al. and La Porta et al. proposed that Tie1 can both reduce and sustain Tie2 activation depending on the subpopulation of the cell and time (La Porta et al., 2018, Savant et al., 2015). Both Tie1 and Tie2 extracellular domains are known to be cleaved from the cell surface constitutively and in response to certain growth factors and cytokines (Findley et al., 2007, Marron et al., 2007). Tie1 has been shown to co-localize with Tie2 at cell-cell contacts (Korhonen et al., 2016, Mirando et al., 2019). The exact mechanisms of action of how Tie1 interacts with and affects Tie2 signaling remain elusive. The complex effects of Tie1 on the surface presentation of Tie2, the subcellular localization of Tie2, and downstream Tie2 signaling require understanding the signaling at a network level. Computational modeling allows us to test the effects of different perturbations in silico under different assumptions about the molecular mechanisms and motivates further experimental studies to elucidate the role of Tie1 and Tie2 in the entire network.
Because of the central role of Tie2 activation in promoting vascular stability, therapeutic drugs that target the angiopoietin-Tie signaling axis to enhance Tie2 activation have been extensively explored in ischemic vascular diseases, various types of cancer, ocular diseases, as well as in inflammation. Therapeutics targeting the angiopoietin-Tie signaling axis are reviewed in Saharinen et al. (Saharinen et al., 2017). Clinical development of angiopoietin-Tie-targeting therapeutics has been met with many obstacles, partly due to the complexity and limited understanding of this signaling pathway. Development of MEDI3617, a selective Ang2 antibody, was halted at phase I because of limited clinical activity (Hyman et al., 2018). Nesvacumab, an Ang2-targeting antibody, was stopped at phase II after failing to provide enough evidence to warrant phase III study (Papadopoulos et al., 2016). Therapies targeting the angiopoietin-Tie2 pathway that are currently in clinical development include AKB-9778, a small molecule inhibitor of VE-PTP (Campochiaro et al., 2016, Shen et al., 2014); trebananib, a peptibody that inhibits the binding of both Ang1 and Ang2 to Tie2 (Vergote et al., 2019); vanucizumab, a bispecific antibody against both vascular endothelial growth factor (VEGF) and Ang2 (Hidalgo et al., 2018); and faricimab, a bispecific antibody that shares the same targets (VEGF and Ang2) (Sahni et al., 2019). Therapies targeting the angiopoietin-Tie pathway in preclinical development include cartilage oligomeric matrix protein-Ang1 (Cho et al., 2004, Ryu et al., 2015), an Ang1 variant that has stronger agonistic activity than native Ang1; Ang2-binding Tie2-activating antibody (Han et al., 2016), which converts Ang2 to a Tie2 agonist; VA1 (Anisimov et al., 2013), a VEGF- and Ang1-mimetic that binds and activates both VEGF receptor VEGFR2 and Tie2; and AXT107, a small peptide that enhances Tie2 activation and works by modulating the integrin/Tie2 interaction (Mirando et al., 2019). There remains a need to discover and to quantitatively understand the molecular strategies targeting the angiopoietin-Tie axis that promote Tie2 activation and enhance the vascular protective function of endothelial cells in the disease settings. The present study uses a computational model to study the network of signaling events in the angiopoietin-Tie signaling axis and to quantitatively characterize the effects of different perturbations on the system and their potential synergism or antagonism.
Computational modeling of vascular signaling allows the complex integration of signaling pathways, their regulatory mechanisms, and crosstalk to quantitatively investigate the intricate interplays of molecular mechanisms and effects of perturbations in the signaling pathways involved in angiogenesis and vascular leakage. Previously, we have used computational modeling to study other major vascular signaling pathways, namely the VEGF signaling pathway and the hepatocyte growth factor (HGF) signaling pathway, along with their downstream signaling, regulation, and crosstalk with integrins (Bazzazi et al., 2018a, Bazzazi et al., 2018b, Jafarnejad et al., 2019). These computational studies have helped us quantitatively predict and understand the molecular mechanisms of how perturbations in these signaling pathways potentially affect the signaling outcomes. This study aims to use a similar approach to gain a quantitative understanding of various molecular aspects of the angiopoietin-Tie signaling pathway on a network level.
The present study uses a computational signaling pathway model validated against experimental data to quantitatively study various mechanistic aspects of the angiopoietin-Tie signaling pathway, including receptor activation, trafficking, turnover, and molecular mechanisms of its regulation. The calibrated model captures and reproduces experimental results reported from independent sources and provides mechanistic insights into the controversial roles of Ang2 and its regulators VE-PTP and Tie1. The model quantitatively predicts the synergistic effects of inhibition of VE-PTP, Tie1, and Tie2 cleavage on enhancing vascular protective actions of Tie2.
Results
A Computational Model Calibrated to Experimental Data Captures and Reproduces Experimental Results
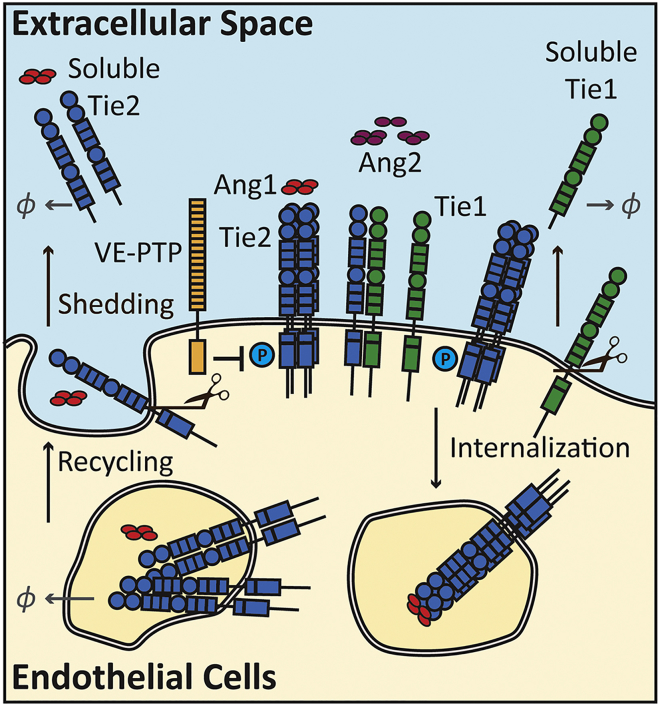
The computational model of the angiopoietin-Tie signaling pathway in the present study includes detailed mass actions of the molecular mechanisms for the ligand and receptor interactions (see Transparent Methods). The reaction rules are summarized in Figure 1.
Figure 1.
Diagram of Model Reaction Rules
Ang1 (tetrameric) and Ang2 (dimeric, trimeric, and tetrameric) induce oligomerization, phosphorylation, and internalization of Tie2. Orphan receptor Tie1 binds and forms heterodimers with receptor Tie2. Internalized receptors are recycled back to the cell surface or degraded. Receptors Tie1 and Tie2 get cleaved from the surface and form soluble receptors Tie1 and Tie2. Soluble Tie2 binds to both ligands Ang1 and Ang2. See also Table S2.
Ang1 is assumed to be in tetrameric form, whereas Ang2 varies between dimeric, trimeric, and tetrameric forms. The model further assumes that the oligomerized ligands, after binding to the receptor, induce fast oligomerization of the receptors. Tetramerized Tie2 receptors bound to either Ang1 or Ang2 can auto-phosphorylate and activate. On the cell surface, VE-PTP is assumed to catalyze dephosphorylation with Michaelis-Menten kinetics, where VE-PTP binds to and dissociates from phosphorylated Tie2 at constant rates, and once associated with Tie2, VE-PTP catalyzes the dephosphorylation reaction at a constant rate. Upon activation, the receptors are internalized, and the ligands are released back to the surface of the cell. Receptor Tie1 is assumed to not be able to bind to any ligand but can heterodimerize with receptor Tie2. Tie1-Tie2 heterodimers can bind to both Ang1 and Ang2, and Ang1 can induce the dissociation of Tie2 from the Tie1-Tie2 heterodimers. Furthermore, both receptors Tie1 and Tie2 are constitutively shed from the surface, turning into soluble Tie1 and soluble Tie2, respectively. Soluble Tie2 can bind to ligands and oligomerize in the extracellular space but is unable to phosphorylate or internalize. Internalized receptors and soluble receptors are degraded by first-order elimination.
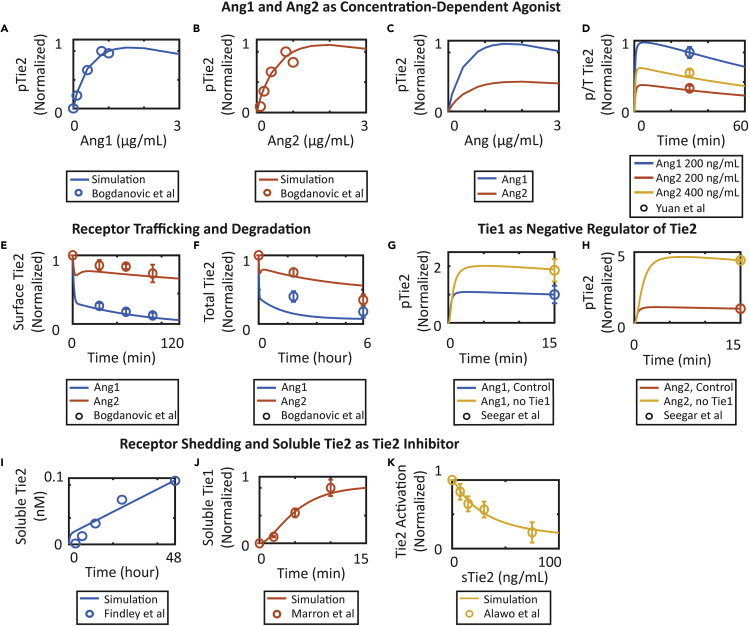
The calibrated model was able to capture and reproduce experimental results reported from independent sources. Consistent with the studies by Bogdanovic et al. and Yuan et al., the model shows that in endothelial cells, both ligands Ang1 and Ang2 activate receptor Tie2 in a ligand-dependent manner, with Ang2 being a weaker agonist than Ang1 (Bogdanovic et al., 2006, Yuan et al., 2009) (Figures 2A–2D). In addition, the model also captures the different receptor internalization rates when induced by Ang1 and Ang2, which affect the overall degradation of Tie2 (Bogdanovic et al., 2006) (Figures 2E and 2F). Furthermore, the model is able to capture that silencing receptor Tie1 enhances both Ang1- and Ang2-induced Tie2 activation at 15 min following angiopoietin treatment in endothelial cells as reported in the study by Seegar et al. (Figures 2G and 2H) (Seegar et al., 2010). Consistent with experiments by Findley et al. and Marron et al., the model reproduces the constitutive extracellular domain shedding of receptors Tie2 and Tie1 from the cell surface, producing soluble receptors in the extracellular space (Figures 2I and 2J) (Findley et al., 2007, Marron et al., 2007). Finally, the calibrated model reproduces the concentration-dependent inhibition effect of soluble Tie2 treatment on Tie2 activation by acting as a ligand trap as reported in Alawo et al. (Figure 2K) (Alawo et al., 2017). The parameters, observables, reactions, and the data used in the calibration are tabulated in Tables S1, S2, S3, and S4.
Figure 2.
Model Calibration Using Global Optimization
(A and B) The concentration-dependent agonistic activity of (A) Ang1 and (B) Ang2.
(C and D) Ang2 functions as partial agonist compared to Ang1.
(E and F) Ang1 and Ang2 induce different receptor trafficking and turnover of Tie2.
(G and H) Silencing orphan receptor Tie1 enhances Ang1- and Ang2-induced Tie2 activation.
(I and J) Receptors Tie2 and Tie1 are constitutively shed from the cell surface and produce soluble receptors.
(K) Soluble Tie2 induces concentration-dependent inhibition of Tie2 activation. Where available, data are presented as mean +/− standard error of the mean (SEM).
See also Table S4.
The uncertainty of parameter estimations using global optimization due to measurement errors in the dataset was assessed using the bootstrap method (Endo et al., 2015, St John and Doyle, 2013). The distributions of optimal parameters obtained using 50 re-sampled datasets are visualized in the violin plot in Figure S1A. Local sensitivity analysis was performed on all optimal parameter sets obtained using re-sampled datasets to assess the identifiability of the parameters. We assumed that a parameter is identifiable if 95% of the local sensitivity distribution maintains a consistent sign, as in previous studies (St John and Doyle, 2013). Of all fitted parameters, the internalization rates of phosphorylated Tie2 (kintang1ptie, kintang2ptie) and the degradation rates of soluble receptors (kdegstie1, kdegstie2) were unidentifiable (Figure S1B).
VE-PTP Dephosphorylates Ang2-Activated Tie2 More Effectively Than Ang1-Activated Tie2
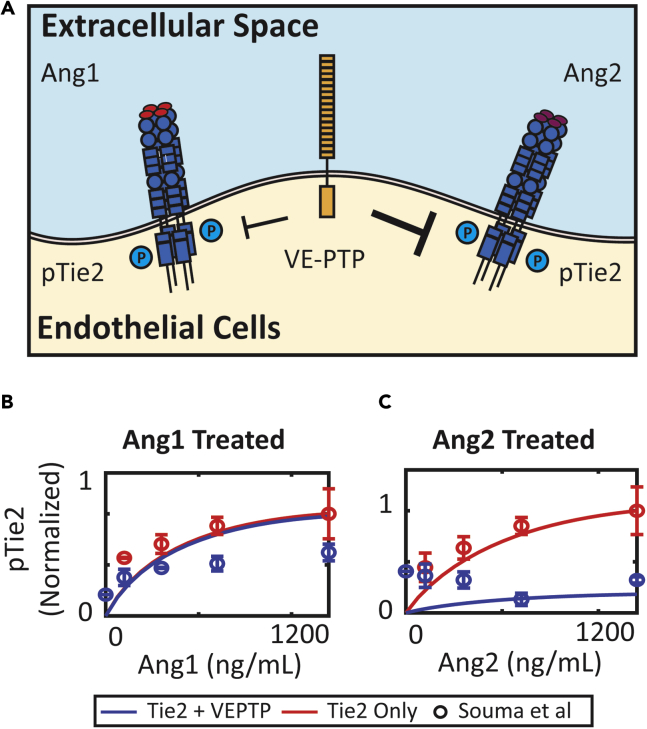
Ang2 acts as a Tie2 agonist in lymphatic endothelial cells, whereas in blood endothelial cells, Ang2 acts mostly as an antagonist. A recent study by Souma et al. proposed that VE-PTP controls the context-dependent function of Ang2 by differentially regulating Ang1- and Ang2-activated Tie2 (Souma et al., 2018) (Figure 3A). Souma et al. demonstrated in Tie2- and VE-PTP-transfected cells that VE-PTP can abolish Ang2-induced Tie2 activation but is less effective in dephosphorylating Ang1-induced phosphorylated Tie2. The model did not assume different reactions or reaction rates for the dephosphorylation of Ang1- or Ang2-activated Tie2 by VE-PTP and was not calibrated to these data. Simulations of knocking down VE-PTP predicted that adding VE-PTP to Ang2-activated Tie2 reduced Tie2 phosphorylation by 90% but has little to no effect on Ang1-activated Tie2 (Figures 3B and 3C), consistent with the data reported by Souma et al.
Figure 3.
VE-PTP Reduces Ang2-Induced Activation of Tie2, but Not Ang1
(A) VE-PTP is more effective in regulating Ang2-activated Tie2 compared to Ang1-activated Tie2.
(B) Simulation of concentration-dependent activation of Tie2 with VE-PTP by Ang1 and concentration-dependent activation of Tie2 without VE-PTP by Ang1 compared to experimental data by Souma et al.
(C) Simulation of concentration-dependent activation of Tie2 with VE-PTP by Ang2 and concentration-dependent activation of Tie2 without VE-PTP by Ang2 compared to experimental data by Souma et al. Data are presented as mean ± SEM.
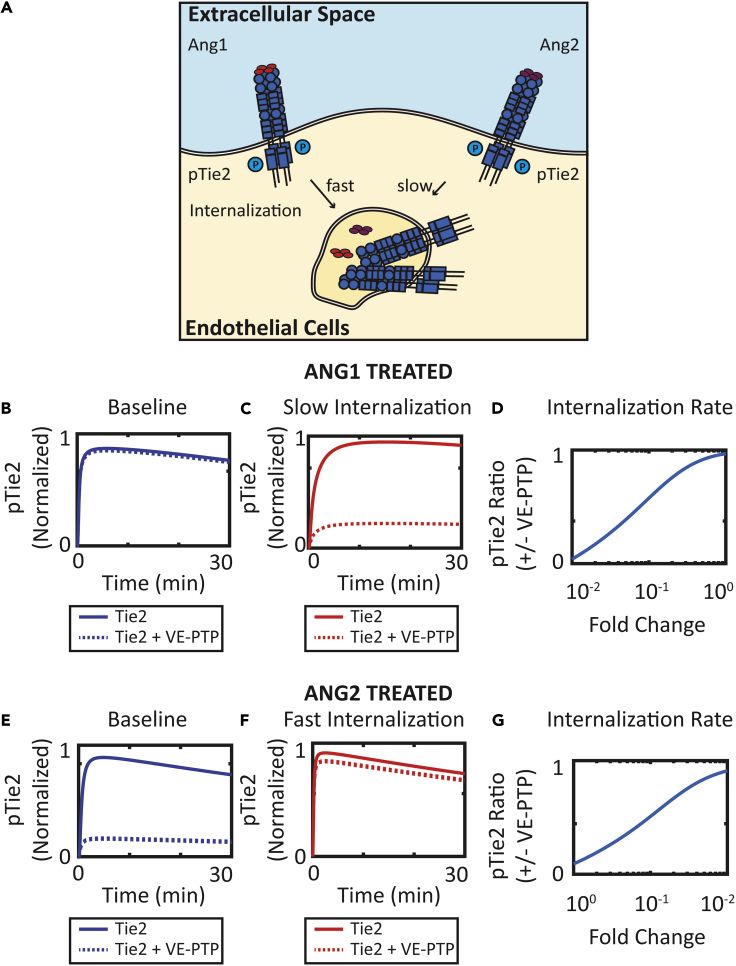
Simulations were then performed to investigate the molecular mechanisms of how VE-PTP differentially regulates Ang1- and Ang2-activated Tie2. We hypothesize that the internalization rates of Ang1- and Ang2-activated Tie2 modulate the responsiveness of Tie2 activation to VE-PTP dephosphorylation (Figure 4A). To understand how internalization rates affect the action of VE-PTP, the internalization rate of Ang1-bound Tie2 was varied from its baseline value to one-hundredth of the baseline value to investigate the effect of internalization rate on the effectiveness of VE-PTP. At baseline value for Ang1-activated Tie2 (fast internalization), VE-PTP has little effect on phosphorylated Tie2 when VE-PTP is added (Figure 4B). When the internalization rate is decreased to one-hundredth of its baseline value, VE-PTP reduces phosphorylated Tie2 by 80% (Figure 4C). The ratio of phosphorylated Tie2 at 30 min in the presence of VE-PTP and absence of VE-PTP increases as the internalization rate of Ang1-bound Tie2 increases from one-hundredth of its baseline value to the baseline value (Figure 4D). In Ang2-treated cells, the ratio of phosphorylated Tie2 in the presence of VE-PTP and absence of Tie2 also increases as the internalization rate of Ang2-bound Tie2 increases. At the baseline internalization rate, VE-PTP reduces phosphorylated Tie2 by 80% (Figure 4E). Increasing the internalization rate by 100-fold decreases the effectiveness of VE-PTP (Figure 4F). The ratio of phosphorylated Tie2 at 30 min in the presence of VE-PTP and absence of VE-PTP also increases as the internalization rate of Ang2-bound Tie2 increases from its baseline value to 100 times the baseline value (Figure 4G).
Figure 4.
Effect of Internalization Rate on the Effectiveness of VE-PTP
(A) Ang1 induces faster internalization of Tie2 compared to Ang2.
(B and C) Simulation of phosphorylated Tie2 over time following Ang1 treatment with and without VE-PTP at (B) baseline internalization rate and (C) reduced internalization rate.
(D) The ratio of phosphorylated Tie2 at 30 min with and without VE-PTP increases as internalization rate increases following Ang1 treatment.
(E and F) Simulation of phosphorylated Tie2 over time following Ang1 treatment with and without VE-PTP at (E) baseline internalization rate and (F) increased internalization rate.
(G) The ratio of phosphorylated Tie2 at 30 min with and without VE-PTP increases as internalization rate increases following Ang2 treatment.
Tie2 Phosphorylation Is Sensitive to Parameters for Tie2 Cleavage, VE-PTP Action, and Tie1 Expression
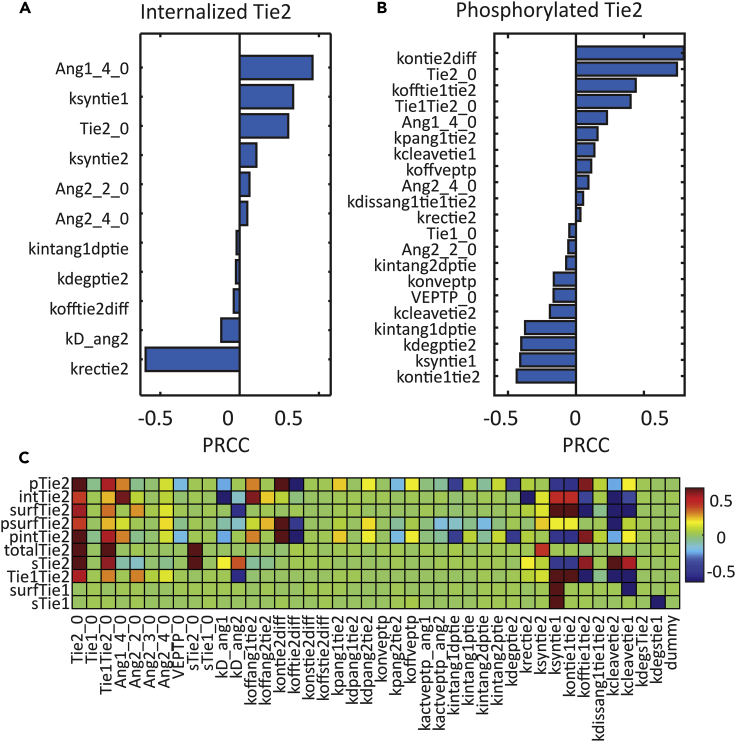
We performed global sensitivity analysis using Latin hypercube sampling and partial rank correlation coefficient (PRCC) (Marino et al., 2008) to look at the effect of changes in parameters on some outputs of the model, most importantly, on the activation of Tie2 (phosphorylated Tie2 at 30 min) and trafficking of Tie2 (internalized Tie2 at 30 min). Statistically significant parameters (Bonferroni corrected p value < 0.01) with positive and negative PRCC values (from high to low) for internalized Tie2 and phosphorylated Tie2 are shown in Figures 5A and 5B. The PRCC values for all parameters and more outputs from the model are shown in Figure 5C. A table with all parameters, their names, and description, along with their best-fit values are included in the Supplementary Data file (Table S1).
Figure 5.
Global Parameter Sensitivity Analysis
(A) Sensitive parameters for internalized Tie2 at 30 min post-stimulation and their partial rank correlation coefficient (PRCC) values.
(B) Sensitive parameters for phosphorylated Tie2 at 30 min post-stimulation and their PRCC values.
(C) PRCC values for all parameters on the observables of the model. Tables with all parameters (Table S1) and all observables (Table S3) are included in the Supplemental Information.
See also Figure S1.
Internalized Tie2 at 30 min is sensitive to the initial concentration of Tie2 (Tie2_0) and the synthesis rate of Tie2 (ksyntie2). Internalized Tie2 concentration is especially sensitive to parameters involved in the trafficking and turnover of Tie2 (krectie2, kdegptie2), as well as to the initial concentrations of the ligands (Ang1_4_0, Ang2_2_0, and Ang2_4_0), and the dissociation rate constant of Ang2 (kD_ang2) (Figure 5A). Phosphorylated Tie2 at 30 min is sensitive to the initial phosphorylation rate (kpang1tie2), initial concentration of Tie2 (Tie2_0), and trafficking parameters, including the internalization rate of phosphorylated Tie2 (kintang1dptie2, kintang2dptie2), recycling rate (krectie2), and degradation rate of phosphorylated Tie2 (kdegptie2). Initial concentrations of tetrameric ligands (Ang1_4_0, Ang2_4_0) have a positive correlation with Tie2 activation while the initial concentration of ligands in lower oligomeric forms (Ang2_2_0) has a negative PRCC value (Figure 5B).
Noticeably, Tie2 activation is found to be sensitive to parameters for various regulation mechanisms in the angiopoietin-Tie signaling axis, including VE-PTP and Tie1 and Tie2 cleavage. Phosphorylated Tie2 is sensitive to parameters for VE-PTP action, including the initial concentration of VE-PTP (VEPTP_0) and the binding rate of VE-PTP to activated Tie2 (konveptp), with both of them having negative PRCC values. The dissociation rate of VE-PTP binding has a negative PRCC value, suggesting inhibiting VE-PTP enhances Tie2 activation. Tie2 activation is also sensitive to Tie1-related parameters. The synthesis rate of Tie1 (ksyntie1), initial concentration of Tie1 (Tie1_0), and association rate of Tie1:Tie2 binding (kontie1tie2) have negative PRCC values, whereas the cleavage rate of Tie1 (kcleavetie1), dissociation rate of Tie1:Tie2 heterodimer (kofftie1tie2), as well as the Ang1-induced dissociation rate of Tie1:Tie2 (kdissang1tie1tie2) have positive PRCC values with phosphorylated Tie2, suggesting that heterodimer formation with Tie1 inhibits Tie2 activation. Finally, phosphorylated Tie2 at 30 min is also sensitive to Tie2 cleavage rate (kcleavtie2), suggesting that cleavage of Tie2 inhibits Tie2 activation (Figure 5B).
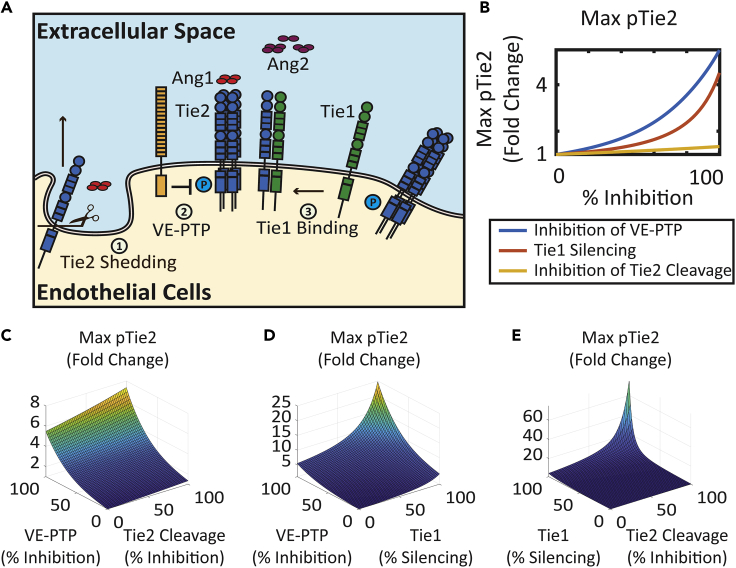
Inhibition of Tie2 Cleavage, Inhibition of VE-PTP, and Silencing of Tie1 Enhance Activation of Tie2
The global sensitivity analysis allows us to find potential perturbations of the system that enhance the activation of Tie2. The global sensitivity analysis suggested the potential Tie2 activation-enhancing effects of inhibition of Tie2 cleavage and concentration of VE-PTP and Tie1 (Figure 6A). The model is used to quantitatively study the effect of these perturbations and their synergistic effects on the enhancement of Tie2 phosphorylation.
Figure 6.
Inhibition of Tie2 Shedding, VE-PTP, and Tie1 Rescues Ang2-Induced Tie2 Activation
(A) Diagram of regulation mechanisms of Tie2 signaling: Tie2 shedding, VE-PTP action, and Tie1 binding.
(B) Effect of inhibition of VE-PTP, Tie1, and Tie2 cleavage on maximum phosphorylated Tie2 for 30 min post-stimulation.
(C) Effect of combination of inhibition of VE-PTP and Tie2 cleavage on maximum phosphorylated Tie2 for 30 min post-stimulation.
(D) Effect of combination of inhibition of VE-PTP and Tie1 on maximum phosphorylated Tie2 for 30 min post-stimulation.
(E) Effect of combination of inhibition of Tie1 and Tie2 cleavage on maximum phosphorylated Tie2 for 30 min post-stimulation.
See also Figure S2.
Assuming a baseline simulation of Tie2 activation by 200 ng/mL of Ang2, the model predicts that the inhibition of Tie2 cleavage enhances maximal Tie2 phosphorylation by up to 1.34-fold, where complete inhibition of VE-PTP enhances maximal Tie2 phosphorylation by up to 5.5-fold, and Tie1 silencing enhanced Tie2 phosphorylation by up to 4.5-fold (Figure 6B). The combination of the different inhibition mechanisms demonstrated synergy in promoting activation of Tie2 (Figures 6C–6E). Combination of VE-PTP inhibition and inhibition of Tie2 cleavage enhances maximal Tie2 phosphorylation by Ang2 by up to 7.4-fold (Figure 6C). Although Tie1 silencing by itself has a moderate effect on enhancing Tie2 activation, Tie1 silencing significantly potentiates the responsiveness of Tie2 activation to both the inhibition of Tie2 cleavage and the inhibition of VE-PTP. With complete inhibition of Tie1, VE-PTP inhibition increases Tie2 phosphorylation by up to 25-fold (Figure 6D), and inhibition of Tie2 shedding increases Tie2 phosphorylation by 75-fold (Figure 6E). This further suggests the important role of the orphan receptor Tie1 in regulating Tie2 activation and its responsiveness to other regulatory mechanisms in the angiopoietin-Tie signaling axis.
Effect of inhibition of VE-PTP, Tie1 concentration, and Tie2 cleavage on Ang1-treated Tie2 is presented in Figure S2. Inhibition of VE-PTP has little effect on enhancing Ang1-treated Tie2 activation because VE-PTP is less effective in regulating Ang1-activated Tie2. Combinations of VE-PTP inhibition with Tie1 silencing and with Tie2 cleavage inhibition are dominated by the effect of Tie1 silencing and Tie2 cleavage inhibition, respectively (Figures S2C and S2D). Combination of inhibition of Tie1 and Tie2 cleavage demonstrated synergy in enhancing Ang1-treated Tie2 activation (Figure S2E).
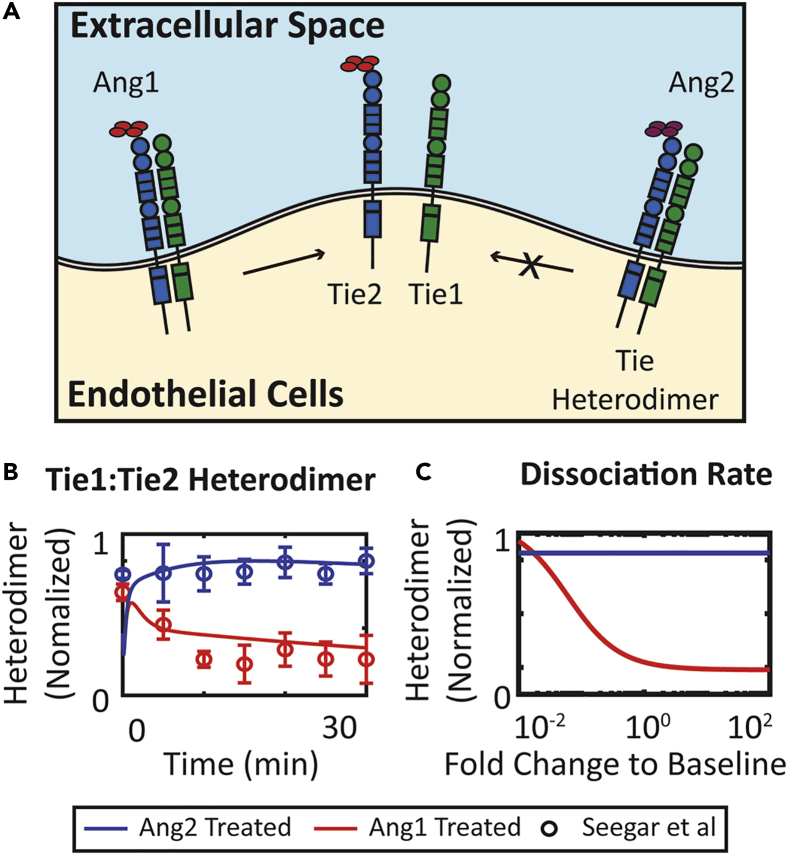
The Model Captures and Reproduces the Dissociation of Tie1:Tie2 Heterodimer by Ang1
To obtain a quantitative understanding of the molecular mechanism behind the inhibitory effect of Tie1, we compared our model prediction of dissociation of Tie1:Tie2 heterodimers with experimental data. Seegar et al. reported that Ang1, but not Ang2, induces the dissociation of Tie2 from Tie1:Tie2 heterodimer (Figure 7A) (Seegar et al., 2010). The model is able to capture and reproduce the dissociation of the heterodimer by Ang1 (Figure 7B). The model assumes that Ang1-bound heterodimers dissociate at a rate characterized by parameter kdissang1tie1tie2. This parameter was varied by 100-fold below or above its baseline value to understand the effect of changing the dissociation rate of Ang1-bound heterodimer on the concentration of heterodimer at 30 min. The dissociation rate does not affect Ang2-treated heterodimer. Heterodimer concentration at 30 min decreases as the dissociation rate increases for Ang1-treated endothelial cells (Figure 7C). The model also predicts that in addition to modulating ligand responsiveness, Tie1 is also predicted to modulate the responsiveness of Tie2 to other regulatory mechanisms including VE-PTP and Tie2 shedding (Figures 6D and 6E).
Figure 7.
Ang1 Dissociates Tie2 from Tie1:Tie2 Heterodimer
(A) Ang1, but not Ang2, stimulates dissociation of Tie1:Tie2 heterodimer.
(B) The concentration of Tie1:Tie2 heterodimer over time following Ang2 treatment and Ang1 treatment compared to experimental results by Seegar et al.
(C) Heterodimer concentration at 30 min decreases as kdissang1tie1tie2 increases following Ang1 treatment but does not change following Ang2 treatment. Data are presented as mean ± SEM.
See also Figure S3.
Tie2 Activation Is Enhanced by Tie1 Cleavage and Regulated by Tie2 Cleavage
To understand the effect of extracellular domain cleavage of Tie1 and Tie2 on Tie2 signaling, the cleavage rates of Tie1 (kcleavagetie1) and Tie2 (kcleavagetie2) were varied 100-fold below or above their baseline values. Phosphorylated Tie2 at 30 min decreases as the Tie2 cleavage rate increases and increases as the Tie1 cleavage rate increases, suggesting that the molecular balance between surface Tie1 and Tie2 controls the activity of Tie2 (Figure S3A). Increasing the Tie2 cleavage rate by 100-fold diminishes phosphorylated Tie2 by more than 99%, whereas decreasing it by 100-fold increases phosphorylated Tie2 by 20% (Figure S3B). Increasing the Tie1 cleavage rate by 100-fold, on the other hand, enhances phosphorylated Tie2 by up to 6 times (Figure S3C).
Discussion
The angiopoietin-Tie signaling axis is tightly regulated by the complex interplay of molecular balance between the ligands, receptor availability, trafficking, degradation, and various regulatory mechanisms including VE-PTP binding, Tie1 binding, and receptor ectodomain cleavage. The computational model calibrated against experimental data provides a platform for quantitative investigation of these regulation mechanisms, and the effects of perturbations or therapeutics targeting these mechanisms. The model also suggests further experiments to build a better understanding of the complex signaling events in the angiopoietin-Tie signaling axis.
Receptor Trafficking Kinetics Affects VE-PTP Efficacy, Causing It to Differentially Regulate Ang1- and Ang2-Activated Tie2
The activity of VE-PTP regulates the phosphorylation of Tie2 and its downstream signaling. Ang2 functions as an agonist in lymphatic endothelial cells in the absence of VE-PTP and as an antagonist in blood endothelial cells in the presence of VE-PTP. Souma et al. suggested that VE-PTP plays a role in determining the context-dependent function of Ang2 by abrogating Ang2-activated Tie2 in blood endothelial cells (Souma et al., 2018). Inhibiting VE-PTP would, therefore, convert Ang2 to an agonist, stabilizing the vasculature through activation of Tie2 (Shen et al., 2014).
The mechanism of how VE-PTP differentially regulates Ang1- and Ang2-activated Tie2 is not clear. VE-PTP regulates Tie2 by directly associating with Tie2 and catalyzing its dephosphorylation (Winderlich et al., 2009). Ang1 and Ang2 share the same binding domains and have similar kinetics of binding to Tie2 (Davis et al., 1996, Fiedler et al., 2003, Maisonpierre et al., 1997). The major differences between the two ligands are their oligomerization states and the receptor internalization kinetics that they induce. Ang1 induces higher internalization rates of Tie2 compared to Ang2 (Bogdanovic et al., 2006). Our simulation indeed demonstrated the role of internalization rates in modulating the efficacy of VE-PTP by showing that decreasing the internalization rate of Ang1-activated Tie2 increases the VE-PTP efficacy and that increasing the internalization rate of Ang2-activated Tie2 has the reverse effect (Figure 4). Based on these results, we propose that the different trafficking kinetics induced by Ang1 and Ang2 can explain the difference in VE-PTP efficacy. We hypothesize that experiments simultaneously examining the internalization of Tie2 and effect of VE-PTP would help further elucidate the molecular mechanism of VE-PTP's action and that therapeutically increasing the internalization rate of Ang2-activated Tie2 may enhance the agonistic function of Ang2.
The Orphan Receptor Tie1 Regulates Tie2 Responsiveness and Ligand Activity by Forming Heterodimers That Can Dissociate When Bound to Ang1
Tie1 is an orphan receptor with no identified ligand (Korhonen et al., 2016, La Porta et al., 2018, Mueller and Kontos, 2016, Savant et al., 2015, Seegar et al., 2010, Woo et al., 2011). Tie1 interacts with the angiopoietin-Tie signaling axis by forming a heterodimer with Tie2 (Leppanen et al., 2017). The role of Tie1 in regulating Tie2 signaling is contradictory. Some studies suggested that Tie1 is required for the agonistic activity of the angiopoietins (D'Amico et al., 2014, Korhonen et al., 2016), whereas others have suggested that Tie1 acts as an inhibitor of Tie2 activation (Marron et al., 2007, Seegar et al., 2010, Yuan et al., 2007). Savant et al. proposed that Tie1 exerts context-dependent functions according to endothelial cell subpopulations: in tip cells, Tie1 counter-regulates Tie2 by limiting its surface presentations, whereas, in stalk cells, Tie1 sustains Tie2 by co-localizing with Tie2 (Savant et al., 2015). A subsequent study by La Porta et al. further reported the effect of Tie1 deletion on vessel stabilization through angiopoietin-Tie signaling (La Porta et al., 2018). The Tie2-sustaining and angiopoietin-potentiating role of Tie1 can be partly explained by its co-localization with Tie2 at cell junctions (Leppanen et al., 2017). Savant et al. have also suggested that Tie1:Tie2 heterodimers co-localize at cell-cell contacts, thereby serving as a reservoir for sustained Tie2 signaling (Savant et al., 2015), in agreement with previous studies (Hansen et al., 2010, Saharinen et al., 2005). The present model, without considering the junctional localization of the receptors, more closely models the tip cell population discussed in Savant et al., and therefore, focuses on investigating the molecular mechanisms of Tie1 as an inhibitor of Tie2. We acknowledge that the molecular mechanisms of the Tie2-enhancing activity of Tie1 need to be further investigated in subsequent studies where junctional localization is considered.
The model assumes that Tie1 forms heterodimers with Tie2, limiting access of Tie2 to the ligands and effectively inhibiting Tie2 activation. This effect of Tie1 is consistent with and calibrated to the study reported by Seegar et al. (Seegar et al., 2010). In their study, Seegar et al. showed using a fluorescence resonance energy transfer assay that Tie1 and Tie2 can pre-form heterodimers in the absence of ligand, and Ang1 is able to dissociate Tie2 from heterodimers upon binding, distinguishing it from Ang2, which cannot induce dissociation of the heterodimer (Figure 7). Korhonen et al., on the other hand, showed that the interaction of Tie1 and Tie2 increased after ligand stimulation and that Tie1:Tie2 heterodimers are observed at cell-cell contacts (Korhonen et al., 2016). The enhancement of heterodimer formation induced by ligand potentially contributes to the Tie2-sustaining function of Tie1. The model, without implementing junctional localization, is not yet able to reproduce this phenomenon at its current stage. In both cases, Tie1 receptors play an important role in the regulation of Tie2 activation by Ang2. Indeed, Song et al. reported that compared to blood endothelial cells, lymphatic endothelial cells have a reduced level of expression of Tie1, suggesting that the limited regulation by Tie1 might be another factor contributing to the agonistic activity of Ang2 in lymphatic endothelial cells in addition to the lack of VE-PTP expression there (Song et al., 2012). In addition, Tie1 has been demonstrated to affect the localization and, therefore, the internalization rate of Tie2 (Korhonen et al., 2016, Savant et al., 2015, Seegar et al., 2010). Our model predicts that the internalization rate of Tie2 modulates the responsiveness of Tie2 to regulation by VE-PTP (Figure 4), further complicating the regulation of Tie2 activation by Ang2. Our hypothesis that the context-dependent function of Ang2 in blood endothelial cells and in lymphatic endothelial cells is controlled jointly by VE-PTP and Tie1 can be readily tested by further experiments in both cell types.
Extracellular Domain Cleavage of Tie1 and Tie2 Modulates Angiopoietin-Tie Signaling
Cleavage of Tie2 from the cell surface is an important regulatory mechanism in the angiopoietin-Tie signaling pathway (Reusch et al., 2001). In addition to limiting the availability of receptors, the product of the extracellular domain shedding, soluble Tie2, also acts as a ligand trap by binding to the ligands in the extracellular space (Alawo et al., 2017). Soluble Tie2 is found to be elevated in many microvasculature-related diseases, including inflammation (Parikh, 2017), systemic sclerosis (Moritz et al., 2017), congestive heart failure (Chong et al., 2004), and peripheral arterial disease (Findley et al., 2008). In pathological angiogenesis, Tie2 signaling is not only attenuated by the antagonistic activity of Ang2, but VEGF also induces extracellular domain cleavage of Tie2 in a phosphoinositide-3 kinase/Akt-dependent manner, further diminishing the activity of Tie2 (Findley et al., 2007). In vitro, soluble Tie2 represses Tie2 signaling in a concentration-dependent manner (Alawo et al., 2017), consistent with the clinical observation that, among patients with peripheral arterial disease, the plasma level of soluble Tie2 distinguishes patients with intermittent claudication and those with critical limb ischemia, the more severe manifestation (Findley et al., 2008). In accordance with experimental findings, the model predicts the concentration-dependent inhibition of Tie2 activation by soluble Tie2 (Figure 2K) and the inhibitory effect of increasing the extracellular domain shedding rate of Tie2 (Figure S3A).
The orphan receptor Tie1 also undergoes extracellular domain cleavage. This process has been observed to be mediated by protein kinase C and metalloproteases (McCarthy et al., 1999, Yabkowitz et al., 1997). Phorbol ester and VEGF also stimulate extracellular domain cleavage of Tie1 (Marron et al., 2007). Marron et al. further proposed that the proteolytic cleavage of Tie1 serves the role of modulating ligand responsiveness of Tie2 (Marron et al., 2007). Model simulations have indeed shown that Tie2 activation can be enhanced by increasing the extracellular domain shedding rate of Tie1 (Figure S3A) and that Tie1 silencing can modulate Tie2 responsiveness to other regulatory mechanisms such as VE-PTP inhibition and inhibition of Tie2 cleavage (Figures 6D and 6E).
In conclusion, the present study uses a computational model of the angiopoietin-Tie signaling pathway to investigate the major aspects of the molecular mechanisms of Tie2 signaling and its regulation in endothelial cells. The calibrated model captured and explained multiple experimental observations of the Tie2 signaling pathway, including the action of regulator VE-PTP and the controversial role of Tie1 and Ang2. The model predicted the synergistic effects of inhibition of VE-PTP, Tie1, and Tie2 cleavage on enhancing the vascular protective actions of Tie2. Simulations showed the role of internalization rate in determining the differential efficacy of VE-PTP in regulating Ang1- and Ang2-activated Tie2 and explored the role of extracellular domain shedding of Tie1 and Tie2 in Tie2 activation. In addition, the model suggested the role of Tie1 silencing in the responsiveness of Tie2 to VE-PTP inhibition and inhibition of Tie2 cleavage. The model also motivates further experimental studies to investigate the molecular mechanisms of VE-PTP and Tie1 and to validate the potential synergism predicted by the model.
Overall, the present computational model of the angiopoietin-Tie pathway allows us to make perturbations on many isolated aspects of the molecular mechanisms in the complex reaction network formed by the singling pathway and to quantitatively study the effects of these perturbations that would be otherwise difficult to achieve experimentally. The model serves to further our understanding of the angiopoietin-Tie2 pathway, suggests additional experiments that can further elucidate the molecular mechanisms, and provides a platform for testing and quantitatively studying the signaling pathway, therapeutics targeting the pathway, and crosstalk with other cellular signaling pathways at a molecular level.
Limitations of the Study
An important limitation of the model is that it does not take into account the subcellular localization of the Tie2 receptors to cell-cell junctions and cell-matrix contacts, as observed in confluent endothelial cells. Tie2 receptors at cell-ECM contacts and at cell-cell junctions have different effects on downstream signaling through extracellular signal-regulated kinases (ERK), Akt/endothelial nitric oxide synthase (eNOS), and Dok-R pathways (Fukuhara et al., 2008, Saharinen et al., 2008). Tie1 and integrins can also affect Tie2 signaling by affecting the localization and internalization of Tie2 (Korhonen et al., 2016, Mirando et al., 2019, Savant et al., 2015, Seegar et al., 2010).
In addition, the role of Tie1 in Tie2 activation and its exact molecular mechanisms of action remain controversial. The present model mostly considers the function of Tie1 as a regulator of the surface presentation of Tie2. This inhibitory role of Tie1 has been observed in a number of previous studies (Hansen et al., 2010, Marron et al., 2007, Savant et al., 2015, Seegar et al., 2010), whereas the Tie2-enhancing activities of Tie1 have also been observed (Kim et al., 2016, Korhonen et al., 2016, Mueller and Kontos, 2016, Savant et al., 2015). Studies have also suggested a potential dual function of Tie1 where it regulates the presentation of Tie2 at the cell surface but sustains Tie2 signaling by co-localizing with it at cell-cell junction (La Porta et al., 2018, Savant et al., 2015). We acknowledge that the results of the model are based on the assumption of Tie1 as an inhibitor of Tie2 and that the present model cannot fully recapitulate the experimental findings on the role of Tie1 because of lack of sufficient experimental data on the junctional localization of Tie2. Future studies should consider the molecular mechanisms by which Tie1 enhances and sustains Tie2 signaling when such experimental data are available. The current model also motivates additional experimental studies into the molecular mechanisms of Tie1 to further elucidate its role.
The present model also does not consider the signaling of receptors and action of VE-PTP in endosomal compartments, which has been observed in many receptor tyrosine kinases, because of limitation of experimental data (Weddell and Imoukhuede, 2017). Additionally, the downstream signaling of Tie2 is not considered. Tie2 signaling shares many downstream signaling features with other signaling pathways, including the VEGF signaling pathway through Akt/eNOS (Bazzazi et al., 2018a), and the HGF/Met signaling pathway through Akt (Jafarnejad et al., 2019). VEGF also affects Tie2 signaling by inducing the release of Ang2 stored in Weibel-Palade bodies (Matsushita et al., 2005) and stimulating the extracellular domain cleavage of Tie2 (Findley et al., 2007). A recent study demonstrated that targeting microRNA-15a and microRNA-16 enhanced Tie2 signaling and improved angiogenesis and perfusion (Besnier et al., 2019), suggesting that the present model can further integrate with computational models of microRNAs to elucidate the intricate interplay between microRNAs, VEGF pathway, and angiopoietin-Tie pathway (Zhao and Popel, 2015, Zhao et al., 2019). The current model provides a platform for further analysis of Tie2 localization and crosstalk with integrin and other signaling pathways to be explored in subsequent studies.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors thank Dr. Adam Mirando for constructive comments. This work was supported by the National Institutes of Health grants R01HL101200, R01CA138264, and R01EY028996 (A.S.P.). Part of this research project was conducted using computational resources (and/or scientific computing services) at the Maryland Advanced Research Computing Center (MARCC).
Author Contributions
Y.Z. and A.S.P. conceived and designed the study. C.D.K. and B.H.A. provided critical input into the study. Y.Z. implemented the model in BioNetGen, performed the computer simulations, analyzed the data, and drafted the manuscript. Y.Z., C.D.K., B.H.A., and A.S.P. participated in writing and editing the manuscript.
Declaration of Interests
C.D.K. serves on the Scientific Advisory Board for Aerpio Pharmaceuticals. The other authors declare no competing interests.
Published: October 25, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.10.006.
Data and Code Availability
The model reaction network, as an SBML file, is available in the Supplemental Files (Data S1). All parameter values, datasets, and reactions used in the model are included in the Supplemental Information.
Supplemental Information
References
- Alawo D.O.A., Tahir T.A., Fischer M., Bates D.G., Amirova S.R., Brindle N.P.J. Regulation of angiopoietin signalling by soluble Tie2 ectodomain and engineered ligand trap. Sci. Rep. 2017;7:3658. doi: 10.1038/s41598-017-03981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov A., Tvorogov D., Alitalo A., Leppanen V.M., An Y., Han E.C., Orsenigo F., Gaal E.I., Holopainen T., Koh Y.J. Vascular endothelial growth factor-angiopoietin chimera with improved properties for therapeutic angiogenesis. Circulation. 2013;127:424–434. doi: 10.1161/CIRCULATIONAHA.112.127472. [DOI] [PubMed] [Google Scholar]
- Augustin H.G., Koh G.Y., Thurston G., Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Bazzazi H., Zhang Y., Jafarnejad M., Isenberg J.S., Annex B.H., Popel A.S. Computer simulation of TSP1 inhibition of VEGF-Akt-eNOS: an angiogenesis triple threat. Front. Physiol. 2018;9:644. doi: 10.3389/fphys.2018.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzazi H., Zhang Y., Jafarnejad M., Popel A.S. Computational modeling of synergistic interaction between alphaVbeta3 integrin and VEGFR2 in endothelial cells: implications for the mechanism of action of angiogenesis-modulating integrin-binding peptides. J. Theor. Biol. 2018;455:212–221. doi: 10.1016/j.jtbi.2018.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnier M., Shantikumar S., Anwar M., Dixit P., Chamorro-Jorganes A., Sweaad W., Sala-Newby G., Madeddu P., Thomas A.C., Howard L. miR-15a/-16 inhibit angiogenesis by targeting the Tie2 coding sequence: therapeutic potential of a miR-15a/16 decoy system in limb ischemia. Mol. Ther. Nucleic Acids. 2019;17:49–62. doi: 10.1016/j.omtn.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic E., Nguyen V.P., Dumont D.J. Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J. Cell Sci. 2006;119:3551–3560. doi: 10.1242/jcs.03077. [DOI] [PubMed] [Google Scholar]
- Campochiaro P.A., Khanani A., Singer M., Patel S., Boyer D., Dugel P., Kherani S., Withers B., Gambino L., Peters K. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology. 2016;123:1722–1730. doi: 10.1016/j.ophtha.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Cho C.H., Kammerer R.A., Lee H.J., Steinmetz M.O., Ryu Y.S., Lee S.H., Yasunaga K., Kim K.T., Kim I., Choi H.H. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc. Natl. Acad. Sci. U S A. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A.Y., Caine G.J., Freestone B., Blann A.D., Lip G.Y. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J. Am. Coll. Cardiol. 2004;43:423–428. doi: 10.1016/j.jacc.2003.08.042. [DOI] [PubMed] [Google Scholar]
- D'Amico G., Korhonen E.A., Anisimov A., Zarkada G., Holopainen T., Hagerling R., Kiefer F., Eklund L., Sormunen R., Elamaa H. Tie1 deletion inhibits tumor growth and improves angiopoietin antagonist therapy. J. Clin. Invest. 2014;124:824–834. doi: 10.1172/JCI68897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Aldrich T.H., Jones P.F., Acheson A., Compton D.L., Jain V., Ryan T.E., Bruno J., Radziejewski C., Maisonpierre P.C. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Eklund L., Saharinen P. Angiopoietin signaling in the vasculature. Exp. Cell Res. 2013;319:1271–1280. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Endo T., Watanabe T., Yamamoto A. Confidence interval estimation by bootstrap method for uncertainty quantification using random sampling method. J. Nucl. Sci. Technol. 2015;52:993–999. [Google Scholar]
- Fachinger G., Deutsch U., Risau W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene. 1999;18:5948–5953. doi: 10.1038/sj.onc.1202992. [DOI] [PubMed] [Google Scholar]
- Fagiani E., Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Fiedler U., Krissl T., Koidl S., Weiss C., Koblizek T., Deutsch U., Martiny-Baron G., Marme D., Augustin H.G. Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J. Biol. Chem. 2003;278:1721–1727. doi: 10.1074/jbc.M208550200. [DOI] [PubMed] [Google Scholar]
- Findley C.M., Cudmore M.J., Ahmed A., Kontos C.D. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb. Vasc. Biol. 2007;27:2619–2626. doi: 10.1161/ATVBAHA.107.150482. [DOI] [PubMed] [Google Scholar]
- Findley C.M., Mitchell R.G., Duscha B.D., Annex B.H., Kontos C.D. Plasma levels of soluble Tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J. Am. Coll. Cardiol. 2008;52:387–393. doi: 10.1016/j.jacc.2008.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Wang K., Niu X.L., Egginton S., Ahmad S., Hewett P., Kontos C.D., Ahmed A. Angiopoietin-1 promotes atherosclerosis by increasing the proportion of circulating Gr1+ monocytes. Cardiovasc. Res. 2017;113:81–89. doi: 10.1093/cvr/cvw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Sako K., Minami T., Noda K., Kim H.Z., Kodama T., Shibuya M., Takakura N., Koh G.Y., Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Sako K., Noda K., Zhang J., Minami M., Mochizuki N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol. Histopathol. 2010;25:387–396. doi: 10.14670/HH-25.387. [DOI] [PubMed] [Google Scholar]
- Han S., Lee S.J., Kim K.E., Lee H.S., Oh N., Park I., Ko E., Oh S.J., Lee Y.S., Kim D. Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci. Transl. Med. 2016;8:335ra355. doi: 10.1126/scitranslmed.aad9260. [DOI] [PubMed] [Google Scholar]
- Hansen T.M., Singh H., Tahir T.A., Brindle N.P.J. Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell. Signal. 2010;22:527–532. doi: 10.1016/j.cellsig.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M., Martinez-Garcia M., Le Tourneau C., Massard C., Garralda E., Boni V., Taus A., Albanell J., Sablin M.P., Alt M. First-in-human phase I study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clin. Cancer Res. 2018;24:1536–1545. doi: 10.1158/1078-0432.CCR-17-1588. [DOI] [PubMed] [Google Scholar]
- Huang H., Bhat A., Woodnutt G., Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat. Rev. Cancer. 2010;10:575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- Hyman D.M., Rizvi N., Natale R., Armstrong D.K., Birrer M., Recht L., Dotan E., Makker V., Kaley T., Kuruvilla D. Phase I study of MEDI3617, a selective angiopoietin-2 inhibitor alone and combined with carboplatin/paclitaxel, paclitaxel, or bevacizumab for advanced solid tumors. Clin. Cancer Res. 2018;24:2749–2757. doi: 10.1158/1078-0432.CCR-17-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarnejad M., Sové R.J., Danilova L., Mirando A.C., Zhang Y., Yarchoan M., Tran P.T., Pandey N.B., Fertig E.J., Popel A.S. Mechanistically detailed systems biology modeling of the HGF/Met pathway in hepatocellular carcinoma. NPJ Syst. Biol. Appl. 2019;5:29. doi: 10.1038/s41540-019-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf N., Helmy H., Labib H., Fahmy I., El Hamid M.A., Moemen L. Role of angiopoietins and tie-2 in diabetic retinopathy. Electron. Physician. 2017;9:5031–5035. doi: 10.19082/5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Kim J.H., Moon S.O., Kwak H.J., Kim N.G., Koh G.Y. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- Kim K.T., Choi H.H., Steinmetz M.O., Maco B., Kammerer R.A., Ahn S.Y., Kim H.Z., Lee G.M., Koh G.Y. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J. Biol. Chem. 2005;280:20126–20131. doi: 10.1074/jbc.M500292200. [DOI] [PubMed] [Google Scholar]
- Kim M., Allen B., Korhonen E.A., Nitschke M., Yang H.W., Baluk P., Saharinen P., Alitalo K., Daly C., Thurston G. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Invest. 2016;126:3511–3525. doi: 10.1172/JCI84871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen E.A., Lampinen A., Giri H., Anisimov A., Kim M., Allen B., Fang S., D'Amico G., Sipila T.J., Lohela M. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J. Clin. Invest. 2016;126:3495–3510. doi: 10.1172/JCI84923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Porta S., Roth L., Singhal M., Mogler C., Spegg C., Schieb B., Qu X., Adams R.H., Baldwin H.S., Savant S. Endothelial Tie1-mediated angiogenesis and vascular abnormalization promote tumor progression and metastasis. J. Clin. Invest. 2018;128:834–845. doi: 10.1172/JCI94674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Cho C.H., Hwang S.J., Choi H.H., Kim K.T., Ahn S.Y., Kim J.H., Oh J.L., Lee G.M., Koh G.Y. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004;18:1200–1208. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- Leppanen V.M., Saharinen P., Alitalo K. Structural basis of Tie2 activation and Tie2/Tie1 heterodimerization. Proc. Natl. Acad. Sci. U S A. 2017;114:4376–4381. doi: 10.1073/pnas.1616166114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P.C., Suri C., Jones P.F., Bartunkova S., Wiegand S.J., Radziejewski C., Compton D., McClain J., Aldrich T.H., Papadopoulos N. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Marino S., Hogue I.B., Ray C.J., Kirschner D.E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 2008;254:178–196. doi: 10.1016/j.jtbi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron M.B., Singh H., Tahir T.A., Kavumkal J., Kim H.Z., Koh G.Y., Brindle N.P. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J. Biol. Chem. 2007;282:30509–30517. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Yamakuchi M., Morrell C.N., Ozaki M., O'Rourke B., Irani K., Lowenstein C.J. Vascular endothelial growth factor regulation of Weibel-Palade-body exocytosis. Blood. 2005;105:207–214. doi: 10.1182/blood-2004-04-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.J., Burrows R., Bell S.C., Christie G., Bell P.R., Brindle N.P. Potential roles of metalloprotease mediated ectodomain cleavage in signaling by the endothelial receptor tyrosine kinase Tie-1. Lab. Invest. 1999;79:889–895. [PubMed] [Google Scholar]
- Milam K.E., Parikh S.M. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers. 2015;3:e957508. doi: 10.4161/21688362.2014.957508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirando A.C., Shen J., Silva R.L.E., Chu Z., Sass N.C., Lorenc V.E., Green J.J., Campochiaro P.A., Popel A.S., Pandey N.B. A collagen IV-derived peptide disrupts alpha5beta1 integrin and potentiates Ang2/Tie2 signaling. JCI Insight. 2019;4:e122043. doi: 10.1172/jci.insight.122043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz F., Schniering J., Distler J.H.W., Gay R.E., Gay S., Distler O., Maurer B. Tie2 as a novel key factor of microangiopathy in systemic sclerosis. Arthritis Res. Ther. 2017;19:105. doi: 10.1186/s13075-017-1304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.B., Kontos C.D. Tie1: an orphan receptor provides context for angiopoietin-2/Tie2 signaling. J. Clin. Invest. 2016;126:3188–3191. doi: 10.1172/JCI89963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos K.P., Kelley R.K., Tolcher A.W., Razak A.R., Van Loon K., Patnaik A., Bedard P.L., Alfaro A.A., Beeram M., Adriaens L. A phase I first-in-human study of nesvacumab (REGN910), a fully human anti-angiopoietin-2 (Ang2) monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer Res. 2016;22:1348–1355. doi: 10.1158/1078-0432.CCR-15-1221. [DOI] [PubMed] [Google Scholar]
- Parikh S.M. The angiopoietin-tie2 signaling axis in systemic inflammation. J. Am. Soc. Nephrol. 2017;28:1973–1982. doi: 10.1681/ASN.2017010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietila R., Natynki M., Tammela T., Kangas J., Pulkki K.H., Limaye N., Vikkula M., Koh G.Y., Saharinen P., Alitalo K. Ligand oligomerization state controls Tie2 receptor trafficking and angiopoietin-2-specific responses. J. Cell Sci. 2012;125:2212–2223. doi: 10.1242/jcs.098020. [DOI] [PubMed] [Google Scholar]
- Reusch P., Barleon B., Weindel K., Martiny-Baron G., Godde A., Siemeister G., Marme D. Identification of a soluble form of the angiopoietin receptor TIE-2 released from endothelial cells and present in human blood. Angiogenesis. 2001;4:123–131. doi: 10.1023/a:1012226627813. [DOI] [PubMed] [Google Scholar]
- Ryu J.K., Kim W.J., Koh Y.J., Piao S., Jin H.R., Lee S.W., Choi M.J., Shin H.Y., Kwon M.H., Jung K. Designed angiopoietin-1 variant, COMP-angiopoietin-1, rescues erectile function through healthy cavernous angiogenesis in a hypercholesterolemic mouse. Sci. Rep. 2015;5:9222. doi: 10.1038/srep09222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P., Kerkela K., Ekman N., Marron M., Brindle N., Lee G.M., Augustin H., Koh G.Y., Alitalo K. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J. Cell Biol. 2005;169:239–243. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P., Eklund L., Miettinen J., Wirkkala R., Anisimov A., Winderlich M., Nottebaum A., Vestweber D., Deutsch U., Koh G.Y. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- Saharinen P., Eklund L., Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017;16:635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- Sahni J., Patel S.S., Dugel P.U., Khanani A.M., Jhaveri C.D., Wykoff C.C., Hershberger V.S., Pauly-Evers M., Sadikhov S., Szczesny P. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with Faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126:1155–1170. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- Savant S., La Porta S., Budnik A., Busch K., Hu J., Tisch N., Korn C., Valls A.F., Benest A.V., Terhardt D. The orphan receptor Tie1 controls angiogenesis and vascular remodeling by differentially regulating Tie2 in tip and stalk cells. Cell Rep. 2015;12:1761–1773. doi: 10.1016/j.celrep.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegar T.C., Eller B., Tzvetkova-Robev D., Kolev M.V., Henderson S.C., Nikolov D.B., Barton W.A. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol. Cell. 2010;37:643–655. doi: 10.1016/j.molcel.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Frye M., Lee B.L., Reinardy J.L., McClung J.M., Ding K., Kojima M., Xia H., Seidel C., Lima e Silva R. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J. Clin. Invest. 2014;124:4564–4576. doi: 10.1172/JCI74527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.H., Kim K.L., Lee K.A., Suh W. Tie1 regulates the Tie2 agonistic role of angiopoietin-2 in human lymphatic endothelial cells. Biochem. Biophys. Res. Commun. 2012;419:281–286. doi: 10.1016/j.bbrc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Souma T., Thomson B.R., Heinen S., Carota I.A., Yamaguchi S., Onay T., Liu P., Ghosh A.K., Li C., Eremina V. Context-dependent functions of angiopoietin 2 are determined by the endothelial phosphatase VEPTP. Proc. Natl. Acad. Sci. U S A. 2018;115:1298–1303. doi: 10.1073/pnas.1714446115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John P.C., Doyle F.J., 3rd Estimating confidence intervals in predicted responses for oscillatory biological models. BMC Syst. Biol. 2013;7:71. doi: 10.1186/1752-0509-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura N., Huang X.L., Naruse T., Hamaguchi I., Dumont D.J., Yancopoulos G.D., Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- Thurston G., Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect. Med. 2012;2:a006550. doi: 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P., Chopp M., Chen J. Blood-brain barrier disruption, vascular impairment, and ischemia/reperfusion damage in diabetic stroke. J. Am. Heart Assoc. 2017;6:e005819. doi: 10.1161/JAHA.117.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote I., Scambia G., O'Malley D.M., Van Calster B., Park S.Y., Del Campo J.M., Meier W., Bamias A., Colombo N., Wenham R.M. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:862–876. doi: 10.1016/S1470-2045(19)30178-0. [DOI] [PubMed] [Google Scholar]
- Weddell J.C., Imoukhuede P.I. Integrative meta-modeling identifies endocytic vesicles, late endosome and the nucleus as the cellular compartments primarily directing RTK signaling. Integr. Biol. (Camb.) 2017;9:464–484. doi: 10.1039/c7ib00011a. [DOI] [PubMed] [Google Scholar]
- Winderlich M., Keller L., Cagna G., Broermann A., Kamenyeva O., Kiefer F., Deutsch U., Nottebaum A.F., Vestweber D. VE-PTP controls blood vessel development by balancing Tie-2 activity. J. Cell Biol. 2009;185:657–671. doi: 10.1083/jcb.200811159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K.V., Qu X., Babaev V.R., Linton M.F., Guzman R.J., Fazio S., Baldwin H.S. Tie1 attenuation reduces murine atherosclerosis in a dose-dependent and shear stress-specific manner. J. Clin. Invest. 2011;121:1624–1635. doi: 10.1172/JCI42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Xu H.H., Lin Y., Sun X., Wang L.J., Fang Z.P., Su X.H., Liang X.J., Hu Y., Liu Z.M. LECT2, a ligand for Tie1, plays a crucial role in liver fibrogenesis. Cell. 2019;178:1478–1492.e20. doi: 10.1016/j.cell.2019.07.021. [DOI] [PubMed] [Google Scholar]
- Yabkowitz R., Meyer S., Yanagihara D., Brankow D., Staley T., Elliott G., Hu S., Ratzkin B. Regulation of tie receptor expression on human endothelial cells by protein kinase C-mediated release of soluble tie. Blood. 1997;90:706–715. [PubMed] [Google Scholar]
- Yin J., Gong G., Liu X. Angiopoietin: a novel neuroprotective/neurotrophic agent. Neuroscience. 2019;411:177–184. doi: 10.1016/j.neuroscience.2019.05.038. [DOI] [PubMed] [Google Scholar]
- Yuan H.T., Venkatesha S., Chan B., Deutsch U., Mammoto T., Sukhatme V.P., Woolf A.S., Karumanchi S.A. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 2007;21:3171–3183. doi: 10.1096/fj.07-8487com. [DOI] [PubMed] [Google Scholar]
- Yuan H.T., Khankin E.V., Karumanchi S.A., Parikh S.M. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol. Cell Biol. 2009;29:2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Popel A.S. Computational model of MicroRNA control of HIF-VEGF pathway: insights into the pathophysiology of ischemic vascular disease and cancer. PLoS Comput. Biol. 2015;11:e1004612. doi: 10.1371/journal.pcbi.1004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhang Y., Popel A.S. Mechanistic computational models of MicroRNA-mediated signaling networks in human diseases. Int. J. Mol. Sci. 2019;20:421. doi: 10.3390/ijms20020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The model reaction network, as an SBML file, is available in the Supplemental Files (Data S1). All parameter values, datasets, and reactions used in the model are included in the Supplemental Information.