Abstract
Objective
Takayasu's arteritis (TAK) is a chronic, large‐vessel vasculitis. Vitamin D, as a steroidal hormone, has recently been shown to have immunoregulatory and immunosuppressive effects. Low vitamin D levels are demonstrated in various autoimmune disorders. The aim of this study is to investigate vitamin D levels in patients with TAK. A comprehensive review of vitamin D levels in systemic vasculitides (SVs) is also performed.
Methods
The study included 36 patients with TAK, 28 patients with Behçet's disease (BD) as disease control and 30 sex‐matched healthy controls. Plasma 25‐hydroxy vitamin D (25(OH) vit D) levels were measured with high‐performance liquid chromatography. “Deficiency” was defined as 25(OH) vit D levels below 25 nmol/l and “insufficiency” as below 50 nmol/l.
Results
Plasma 25(OH) vit D levels were significantly lower in TAK patients (16.93 ± 10.62 nmol/l) than healthy controls (64.63 ± 21.82 nmol/l). Vitamin D level in BD patients (38.8 ± 20.9 nmol/l) is lower than healthy controls but higher than TAK patients. The frequency of vitamin D deficiency was 83.3% in patients with TAK compared to 3.3% in healthy controls. Plasma 25(OH) vit D levels were same between clinically active and inactive patients. In literature review, very few studies were found to investigate vitamin D in SVs.
Conclusion
We observed a high prevalence of vitamin D deficiency in patients with TAK. As various immune effects of vitamin D on mononuclear cells and arterial endothelium is shown, vitamin D deficiency can be a predisposing factor for immune activation in SV. We therefore suggest monitorization and replacement of vitamin D status in all TAK and other SV patients.
Keywords: Takayasu's arteritis, vitamin D deficiency, vasculitis
BACKGROUND
Takayasu's arteritis (TAK) is a rare, chronic, large‐vessel vasculitis that predominantly affects aorta, its major branches, and the pulmonary arteries 1. Various signs and symptoms such as constitutional features (fever, malaise, anorexia, weight loss), extremity pain, claudication, light headedness, bruits, absent or diminished pulses, and loss of blood pressure can be present according to the vessel involvement 2. The etiology of TAK is still unknown, but infectious agents 3 and genetic factors are implicated 4, 5.
Vitamin D is a steroidal hormone regulating calcium homeostasis, bone formation, and resorption via interaction with parathyroid glands, kidneys, and intestines. Vitamin D shows its biological effect by binding to its intracellular vitamin D receptor (VDR), which is widely distributed in the body, including the cells of the immune system 6. Expression of VDR is shown on monocytes, macrophages, dendritic cells, NK cells, and T and B lymphocytes. With various effects on cell proliferation and differentiation, vitamin D is a potential candidate for immune system regulation 7.
Vitamin D deficiency is very common in patients with various autoimmune and other chronic diseases including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and type 2 diabetes (T2DM) 8, 9, 10. However, studies are very limited in systemic vasculitides (SVs). The aim of this study is to investigate vitamin D levels in patients with TAK. We also performed a comprehensive review of the literature on vitamin D in SVs.
METHODS
The study included 36 patients (age: 40.83 ± 11.57 years, F/M: 33/3) with TAK, 28 (42.1 ± 9.8, F/M: 24/4) patients with Behçet's disease (BD) as disease control, and 30 age‐ and sex‐matched healthy control subjects (age: 38.67 ± 7.35 years, F/M: 27/3) between February 2012 and May 2012. Patients with TAK fulfilled the criteria of American College of Rheumatology (ACR) 11. According to the angiographic classification, 47.2% of the study group had type I disease, 47.2% had type V disease, 2.7% had type IV disease, and 2.7% had type IIb disease 12. Refractory disease for TAK is defined as recently published by the Turkish Takayasu Group, “angiographic or clinical progression despite treatment” or any of the following characteristics: corticosteroid dose >7.5 mg/day after 6 months of treatment despite the administration of conventional immunosuppresives (methotrexate, azathioprine, leflunomide, or cyclophosphamide), new surgery due to persistent disease activity, frequent attacks (>3/year), or mortality associated with disease activity 4. Thirty‐two patients (88.8%) were on oral methyl‐prednisolone therapy. As additional immunosuppression, 20 (55.6%) patients were on azathioprine, 12 (33.3%) were on methotrexate, and 1 (8.3%) were on leflunomide therapy.
After collecting blood samples, plasma 25‐hydroxy vitamin D (25(OH) vit D) levels were measured with high‐performance liquid chromatography (HPLC; Spectra System, GmbH, Munich, Germany). According to the reference range of the test kit, “deficiency” was defined as 25(OH) vit D level below 25 nmol/l and “insufficiency” as below 50 nmol/l, and the recommended level of 25(OH) vit D was above 100 nmol/l 13. The study was performed according to the Declaration of Helsinki. All subjects gave informed consent before participation.
Statistical data were performed with Statistical Package for the Social Sciences 16.0 (SPSS, Chicago, IL) program. Results were expressed as means and standard deviations. The independent samples t‐test and chi‐square test were used for comparisons of data. Pearson correlation test was used for analyzing correlations.
The literature search for vitamin D in vasculitis was performed with keywords “vitamin D” and “vasculitis” in PubMed, and 52 references were found. A cross‐check was also done with keywords for each specific vasculitis, namely, leucocytoclastic vasculitis, Henoch–Schonlein purpura, polyarteritis nodosa, Wegener's granulomatosis Granulomatosis with Polyangitis (GPA), Churg–Strauss syndrome Eosinophilic granulomatosis with polyangiitis (EPA), TAK, giant‐cell arteritis, BD, and Kawasaki's disease. When case reports, reviews, and studies on vitamin D replacement for corticosteroid‐induced osteoporosis were excluded from the analysis, 16 studies were selected for further review.
RESULTS
The mean disease duration of TAK patients was 5.37 ± 4.9 years. Mean erythrocyte sedimentation rate was 23.31 ± 16.24 mm/h, mean C‐reactive protein level was 8.41 ± 12.27 mg/l. Plasma 25(OH) vit D were significantly lower in TAK patients (16.93 ± 10.62 nmol/l) compared to healthy controls (64.63 ± 21.82 nmol/l; P = 0.001; Fig. 1). The frequency of vitamin D deficiency was 83.3% (n = 30) in patients with TAK versus 3.3% (n = 1) in healthy controls (P = 0.001). Plasma 25(OH) vit D were significantly lower in BD patients (38.8 ± 20.9 nmol/l) compared to healthy controls (64.63 ± 21.82 nmol/l; P = 0.001), but higher than patients with TAK (P<0.001 for both). The frequency of vitamin D deficiency was 46.5% (n = 13) in patients with BD. This rate is higher than healthy controls but lower than TAK (P < 0.001 for both).
Figure 1.
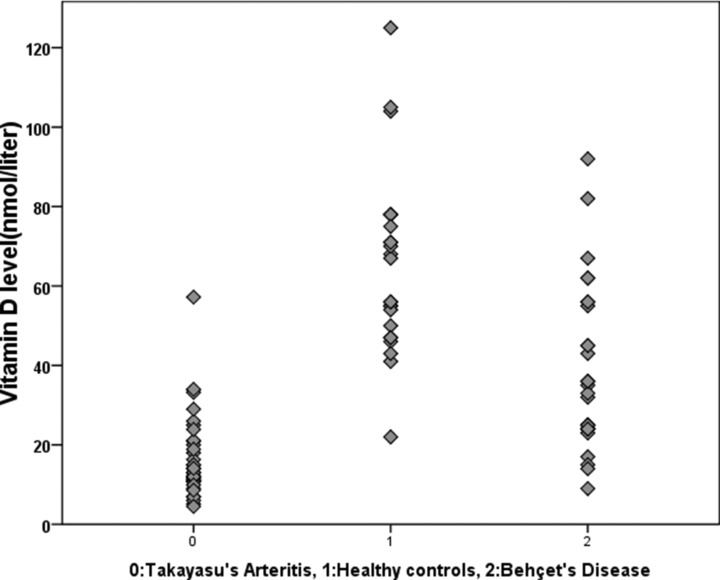
Vitamin D levels in patients with Takayasu's arteritis, Behçet's disease, and healthy controls.
Serum calcium, phosphate, parathyroid hormone, and alkalene phosphatase levels of all patients and healthy controls were in normal ranges. Ten (27.8%) patients with TAK were clinically active according to physician's global assessment. Plasma 25(OH) vit D levels were similar between active and inactive patients (P = 0.75). Similarly, there was no significant difference between male (n = 3, 100%) and female (n = 27, 81%) patients in terms of vitamin D deficiency. No association was present between vitamin D levels and acute‐phase reactants. Similarly, no effect of treatment or disease subtypes on vitamin D levels was observed. No differences were also present between the subgroups of patients with TAK according to age of onset, presence of surgery, or refractory disease (Table 1). Twelve (33.3%) patients were taking oral calcium carbonate (1,200 mg/day) and oral vitamin D3 (400 IU/day). No difference was also present between the patients taking oral vitamin D replacement or not (P = 0.89).
Table 1.
Vitamin D Deficiency in Subgroups of Patients With Takayasu's Arteritis
| Age at onset | Surgery | Refractory disease | |||||
|---|---|---|---|---|---|---|---|
| ≤40 (n = 24) | >40 (n = 12) | Present (n = 15) | Not present (n = 21) | Refractory (n = 10) | Not refractory (n = 26) | ||
| Vitamin D deficiency | n | 20 | 10 | 13 | 17 | 8 | 22 |
| % | 83.3 | 83.3 | 86.6 | 80.9 | 80 | 84.6 | |
| P value | 0.691 | 0.507 | 0.544 | ||||
DISCUSSION AND LITERATURE REVIEW
We observed significantly lower serum 25(OH) vit D levels among TAK patients compared to healthy controls, with a high prevalence of vitamin D deficiency in our study. High prevalence of vitamin D deficiency was reported before also in rheumatological diseases such as BD and ankylosing spondylitis from Turkey, having sunshine along four seasons 14, 15. In the present study, we also found increased vitamin D deficiency in BD. However, to our knowledge, this is the first study that investigates vitamin D levels in patients with TAK.
The etiology of TAK is still unknown, but infectious agents 3, 16 and genetic factors 4, 5 are implicated in the pathogenesis 17, 18. Immunohistochemical studies from the aortic tissue of patients with TAK showed infiltrations by macrophages, T cells (CD4+, CD8+, and γδ+), NK cells, and neutrophils 19. CD4+ T cells initiate and maintain granuloma formation by releasing interferon‐γ (IFNγ) 20, and granulomatous inflammation strongly depends on T‐helper type 1 (Th1) and recently described Th17‐type immune patterns 21, 22.
1,25(OH)2D3, the biologically active form of vitamin D, inhibits T lymphocyte proliferation 23, 24, particularly of the Th1 arm 25, 26. Addition of 1,25(OH)2D3 to peripheral blood mononuclear cells (PBMCs) decreases secretion of IL‐2 and IFNγ, and upregulates IL‐5 and IL‐10 production, leading to a Th2‐resembling response 27. Vitamin D deficiency also leads to an impairment of regulatory T and dendritic cells 28, 29. Addition of 1,25(OH)2D3 was shown to inhibit the expression of IL‐6, an important cytokine that stimulates Th17 cells and is upregulated in large‐vessel vasculitides. With these data accumulating on its immunosuppressive role, replacement or even supraphysiological doses of vitamin D supplementation is recently suggested for all autoimmune diseases 30, 31.
Although we demonstrated low vitamin D levels in our study, in contrast to some other studies, such as in SLE 32, we observed no association with disease activity or vitamin D replacement. Similar results are also reported in the literature for BD. Still, these results should be interpreted with caution, as our active patient subset was small and replacement therapy is only cross‐sectionally questioned in our population.
Limitations of our study were its small sample size, the lack of data regarding sun exposure, use of sunscreen and style of clothing. However, we studied vitamin D levels in winter season (similar to our previous study in BD) 15 when sun exposure was lowest in Istanbul, Turkey, which might decrease the influence of sun exposure to our results.
Literature Review
In the literature review, no study was observed to investigate vitamin D status in patients with large‐vessel vasculitides (TAK and giant‐cell arteritis). In an early study, Chesney et al. have demonstrated the importance of vitamin D deficiency in children receiving glucocorticoids for diseases including vasculitides 33. In an epidemiological survey and meta‐analysis, the incidence of granulomatosis with polyangiitis (Wegener's) and eosinophilic polyangiitis (Churg–Strauss) is shown to be increased with latitude and decreased ambient UV radiation, suggesting a role of vitamin D deficiency in Anti‐neutrophil cytoplasmic antibody ANCA‐associated vasculitides (AAV) 30. However, no studies on vitamin D levels in AAV are published. Vitamin D levels and associated mechanisms are studied mostly in BD 10, 14, 34, 35, 36, 37, 38, 39, 40 Five of these studies demonstrated decreased vitamin D levels in patients with BD, not associated with disease activity 10, 14, 37, 39, 40. In other studies, in vitro modulating effects of vitamin D on toll‐like receptor expression of monocytes, number of T‐regulatory cells, IFNγ/IL‐4 ratio, and Th1/Th17 responses were demonstrated in BD 10, 34, 38. Another study showed ameliorating effects of vitamin D on a Herpes simplex induced Behcet's‐like animal model 35 . Association of VDR gene polymorphisms and vitamin D family genes with BD is also demonstrated 36, 41. In the only other study measuring vitamin D levels in vasculitides, Terrier et al. reported that in chronic hepatitis C infection, patients with vitamin D deficiency had more vasculitis, especially purpura and elevated cyroglobulinemia levels 42.
In vitro effects of vitamin D on vascular endothelium are also studied for clarifying vasculitic mechanisms. Active vitamin D is shown to inhibit VCAM‐1 and IL‐8 expression from human coronary arterial cells (HCAC) 43. Vitamin D also significantly inhibited TNF‐α‐induced (where TNF is tumor necrosis factor) NF‐κB (where NF is nuclear factor) activation and E‐selectin expression in HCAC 44 . Similar results are observed in a chronic kidney disease like environment and calcitriol normalized the expression, secretion, and activity of eNOS, RAGE and IL‐6 45. As an implication for clinical effects on vascular endothelium, vitamin D replacement is shown to improve endothelial functions, measured with flow‐mediated dilatation, in patients with BD 15.
In conclusion, we observed a high prevalence of vitamin D deficiency in patients with TAK. As vitamin D plays an important role in regulating immune system, vitamin D deficiency can be a predisposing factor for immune activation and we suggest the monitorization of vitamin D status and replacement in TAK patients with vitamin D deficiency. Further studies in other SVs are also necessary.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1. Alibaz‐Oner F, Aydin SZ, Direskeneli H. Advances in the diagnosis, assessment and outcome of Takayasu's arteritis. Clin Rheumatol 2013;32(5):541–546. [DOI] [PubMed] [Google Scholar]
- 2. Bicakcigil M, Aksu K, Kamali S, et al. Takayasu's arteritis in Turkey—Clinical and angiographic features of 248 patients. Clin Exp Rheumatol 2009;27(1 Suppl 52):S59–S64. [PubMed] [Google Scholar]
- 3. Kallenberg CG, Tadema H. Vasculitis and infections: Contribution to the issue of autoimmunity reviews devoted to “Autoimmunity and infection.” Autoimmun Rev 2008;8:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sahin Z, Bıcakcıgil M, Aksu K, et al. Takayasu's arteritis is associated with HLA‐B*52, but not with HLA‐B*51, in Turkey. Arthritis Res Ther 2012;14(1):R27–R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saruhan‐Direskeneli G, Hughes T, Aksu K, et al. Identification of multiple genetic susceptibility loci in Takayasu arteritis. Am J Hum Genet 2013;93(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann Rheum Dis 2007;66(9):1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005;26(5):662–687. [DOI] [PubMed] [Google Scholar]
- 8. Orbach H, Zandman‐Goddard G, Amital H, et al. Novel biomarkers in autoimmune diseases: Prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci 2007;1109:385–400. [DOI] [PubMed] [Google Scholar]
- 9. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80(6 Suppl):1678S–1688S. [DOI] [PubMed] [Google Scholar]
- 10. Hamzaoui K, Ben Dhifallah I, Karray E, Sassi FH, Hamzaoui A. Vitamin D modulates peripheral immunity in patients with Behçet's disease. Clin Exp Rheumatol 2010;28(4 Suppl 60):S50–S57. [PubMed] [Google Scholar]
- 11. Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol 1996;54:141–147. [DOI] [PubMed] [Google Scholar]
- 12. Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: New classification. Int J Cardiol 1996;54(Suppl):155–163. [DOI] [PubMed] [Google Scholar]
- 13. Bischoff‐Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson‐Hughes B. Estimation of optimal serum concentrations of 25‐hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28. [DOI] [PubMed] [Google Scholar]
- 14. Erten S, Kucuksahin O, Sahin A, Altunoglu A, Akyol M, Koca C. Decreased plasma vitamin D levels in patients with undifferentiated spondyloarthritis and ankylosing spondylitis. Intern Med 2013;52(3):339–344. [DOI] [PubMed] [Google Scholar]
- 15. Can M, Gunes M, Haliloglu OA, et al. Effect of vitamin D deficiency and replacement on endothelial functions in Behçet's disease. Clin Exp Rheumatol 2012;30(3 Suppl 72):S57–S61. [PubMed] [Google Scholar]
- 16. Kallenberg CG, Tadema H. Vasculitis and infections: Contribution to the issue of autoimmunity reviews devoted to “Autoimmunity and infection.” Autoimmun Rev 2008;8:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noris M. Pathogenesis of Takayasu's arteritis. J Nephrol 2001;14:506–513. [PubMed] [Google Scholar]
- 18. Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu's arteritis: A 2011 update. Autoimmun Rev 2011;11(1):61–67. [DOI] [PubMed] [Google Scholar]
- 19. Seko Y, Minota S, Kawasaki A, et al. Perforinsecreting killer cell infiltration and expression of a 65‐kd heat‐shock protein in aortic tissue of patients with Takayasu's arteritis. J Clin Invest 1994;93:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner AD, Björnsson J, Bartley GB, Goronzy JJ, Weyand CM. Interferon‐gamma producing T cells in giant cell vasculitis represent a minority of tissue‐infiltrating cells and are located distant from the site of pathology. Am J Pathol 1996;148:1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 21. Sneller MC. Granuloma formation, implications for the pathogenesis of vasculitis. Cleve Clin J Med 2002;69(Suppl 2):SII40–SII43. [DOI] [PubMed] [Google Scholar]
- 22. Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T‐cell responses in giant cell arteritis. Circulation 2010;121:906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25‐Dihydroxyvitamin D3 inhibits antigen‐induced T cell activation. J Immunol 1984;133:1748–1754. [PubMed] [Google Scholar]
- 24. Lemire JM. Immunomodulatory role of 1,25‐dihydroxyvitamin D3. J Cell Biochem 1992;49:26–31. [DOI] [PubMed] [Google Scholar]
- 25. Mattner F, Smiroldo S, Galbiati F, et al. Inhibition of Th1 development and treatment of chronic‐relapsing experimental allergic encephalomyelitis by a non‐hypercalcemic analogue of 1,25‐dihydroxyvitamin D(3). Eur J Immunol 2000;30:498–508. [DOI] [PubMed] [Google Scholar]
- 26. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25‐dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 2001;167:4974–4980. [DOI] [PubMed] [Google Scholar]
- 27. Van Etten E, Mathieu C. Immunoregulation by 1,25‐dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol 2005;97:93–101. [DOI] [PubMed] [Google Scholar]
- 28. Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev 2012;12(2):127–136. [DOI] [PubMed] [Google Scholar]
- 29. Ben‐Zvi I, Aranow C, Mackay M, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One 2010;5(2):e9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gatenby PA, Lucas RM, Engelsen O, Ponsonby AL, Clements M. Antineutrophil cytoplasmic antibody associated vasculitides: Could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Rheum 2009;61(10):1417–1424. [DOI] [PubMed] [Google Scholar]
- 31. Allen AC, Kelly S, Basdeo SA, et al. A pilot study of the immunological effects of high‐dose vitamin D in healthy volunteers. Mult Scler 2012;18(12):1797–1800. [DOI] [PubMed] [Google Scholar]
- 32. Mok CC, Birmingham DJ, Ho LY, Hebert LA, Song H, Rovin BH. Vitamin D deficiency as marker for disease activity and damage in systemic lupus erythematosus: A comparison with anti‐dsDNA and anti‐C1q. Lupus 2012;21(1):36–42. [DOI] [PubMed] [Google Scholar]
- 33. Chesney RW, Mazess RB, Hamstra AJ, DeLuca HF, O’ Reagan S. Reduction of serum‐1, 25 dihydroxyvitamin‐D3 in children receiving glucocorticoids. Lancet 1978;2(8100):1123–1125. [DOI] [PubMed] [Google Scholar]
- 34. Tian Y, Wang C, Ye Z, Xiao X, Kijlstra A, Yang P. Effect of 1,25‐dihydroxyvitamin D3 on Th17 and Th1 response in patients with Behçet's disease. Invest Ophthalmol Vis Sci 2012;53(10):6434–6441. [DOI] [PubMed] [Google Scholar]
- 35. Choi B, Lee ES, Sohn S. Vitamin D3 ameliorates herpes simplex virus‐induced Behçet's disease‐like inflammation in a mouse model through down‐regulation of Toll‐like receptors. Clin Exp Rheumatol 2011;29(4 Suppl 67):S13–S19. [PubMed] [Google Scholar]
- 36. Karray EF, Ben Dhifallah I, Ben Abdelghani K, et al. Associations of vitamin D receptor gene polymorphisms FokI and BsmI with susceptibility to rheumatoid arthritis and Behçet's disease in Tunisians. Joint Bone Spine 2012;79(2):144–148. [DOI] [PubMed] [Google Scholar]
- 37. Karatay S, Yildirim K, Karakuzu A, et al. Vitamin D status in patients with Behcet's Disease. Clinics (Sao Paulo) 2011;66(5):721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Do JE, Kwon SY, Park S, Lee ES. Effects of vitamin D on expression of Toll‐like receptors of monocytes from patients with Behcet's disease. Rheumatology (Oxford) 2008;47(6):840–848. [DOI] [PubMed] [Google Scholar]
- 39. Faezi ST, Ansari N, Paragomi P, Akhlaghi M, Ghanavat M, Davatchi F. Vitamin D deficiency in patients with Behcet's disease. J Diabetes Metab Disord 2014;13(1):18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khabbazi A, Rashtchizadeh N, Ghorbanihaghjo A, et al. The status of serum vitamin D in patients with active Behcet's disease compared with controls. Int J Rheum Dis 2014;17(4):430–434. [DOI] [PubMed] [Google Scholar]
- 41. Fang J, Hou S, Xiang Q, et al. Polymorphisms in genetics of vitamin D metabolism confer susceptibility to ocular Behçet disease in a Chinese Han population. Am J Ophthalmol 2014;157(2):488–494. [DOI] [PubMed] [Google Scholar]
- 42. Terrier B, Jehan F, Munteanu M, et al. Low 25‐hydroxyvitamin D serum levels correlate with the presence of extra‐hepatic manifestations in chronic hepatitis C virus infection. Rheumatology (Oxford) 2012;51(11):2083–2090. [DOI] [PubMed] [Google Scholar]
- 43. Kudo K, Hasegawa S, Suzuki Y, et al. 1α,25‐Dihydroxyvitamin D(3) inhibits vascular cellular adhesion molecule‐1 expression and interleukin‐8 production in human coronary arterial endothelial cells. J Steroid Biochem Mol Biol 2012;132(3–5):290–294. [DOI] [PubMed] [Google Scholar]
- 44. Suzuki Y, Ichiyama T, Ohsaki A, Hasegawa S, Shiraishi M, Furukawa S. Anti‐inflammatory effect of 1alpha,25‐dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J Steroid Biochem Mol Biol 2009;113(1–2):134–138. [DOI] [PubMed] [Google Scholar]
- 45. Talmor‐Barkan Y, Bernheim J, Green J, Benchetrit S, Rashid G. Calcitriol counteracts endothelial cell pro‐inflammatory processes in a chronic kidney disease‐like environment. J Steroid Biochem Mol Biol 2011;124(1–2):19–24. [DOI] [PubMed] [Google Scholar]


