Abstract
Background
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the second leading cause of cancer‐related deaths worldwide. The poor prognosis of HCC is mainly because of its discovery at advanced stages. Because chronic hepatitis B (CHB) accounts for 50–80% HCC occurrence worldwide, and immunity is regarded as an emerging hallmark of cancer, we investigated the predictive role of peripheral immune cells in HCC incidence in CHB patients.
Methods
This investigation collected and analyzed data from 89 CHB patients, 94 primary HCC patients with hepatitis B virus (HBV), 81 primary HCC patients without HBV, 69 normal healthy patients, and 257 CHB patients with at least 3‐year regular followup.
Results
The results demonstrated that CHB and primary HCC patients had different concentrations of lymphocytes, neutrophils, and monocytes in their peripheral circulation. Further study showed that the peripheral lymphocyte concentration was an independent prognostic factor for HCC incidence in CHB patients during the 3 years of followup. Finally, a predictive HCC incidence model with an AUROC (area under the receiver operating characteristic) of 0.832 was constructed based on the peripheral lymphocyte concentration, serum alpha‐fetoprotein (AFP) concentration, and cirrhosis status of CHB patients.
Conclusions
The peripheral lymphocyte concentration was an independent prognostic factor for HCC incidence in CHB patients, and a more accurate predictive model based on peripheral lymphocytes, serum AFP, and cirrhosis status was constructed.
Keywords: CHB, HCC, immune cells, lymphocytes
INTRODUCTION
Chronic hepatitis B (CHB) is a hepatitis B virus (HBV) infection that induces chronic inflammatory disease. The immune status of infected patient plays an important role in the outcome of an HBV infection. Normally, an HBV infection in an immune‐competent adult is cured after acute hepatitis. Less than 5% of adult infections do not completely clear the virus in a timely manner and develop CHB 1, whereas approximately 90% of neonate infections develop CHB 1, 2. Approximately 350 million people are chronically infected with HBV worldwide, 75% of them are Asian 3. The deadly complication of CHB is hepatocellular carcinoma (HCC) 4.
HCC ranks as the fifth most prevalent cancer and the second leading cause of cancer‐related deaths worldwide 5. The high mortality rate of HCC is mostly because of its discovery at advanced stages. As CHB accounts for 50–80% of the HCC incidence worldwide 6, it is important to screen and provide early detection of HCC in CHB patients. Until now, many predictive models have been suggested to forecast HCC incidence in CHB patients based on their clinical characteristics. These characteristics include gender, age, drinking, smoking, biochemistry factors, including alanine aminotransferase (ALT), alpha‐fetoprotein (AFP), albumin and bilirubin, or virus‐related characteristics, such as hepatitis B surface antigen (HBsAg) concentration, HBV e antigen (HBeAg) concentration, and HBV DNA concentration 7, 8, 9, 10, 11. However, no predictive model involving patient immune status related markers has been proposed.
The role of immunity in cancer development and progression has been well established and is even regarded as an emerging hallmark of cancer 12, 13. HCC is not excluded from this dogma. Many immune cells and cytokines have been reported to be involved in HCC progression 14, 15, 16. Interestingly, reduced cytotoxic immune cells, such as CD8‐positive T cells and natural killer cells, or increased immune suppressive cells, such as regular T cells, have been reported in CHB patients by many researchers 17, 18, 19. It is highly possible that the immune status of CHB patients may play a very important role in HCC incidence, but no predictive model has yet been constructed. One of reasons for the lack of a predictive model may be that it is both laborious and costly to examine the immune status of most CHB patients, especially detecting the number of CD8‐positive T cells, natural killer cells, or regulatory T cells and their related functions. Thus, it is essential to identify the immune status of CHB patients in a much more straightforward way.
Hematology tests are routinely used in clinical practice, and counting leukocytes is a major part of these tests. Information on peripheral leukocyte subpopulations can be easily and accurately acquired with the development of technology. The subpopulations of peripheral leukocyte, neutrophil, lymphocyte, and monocyte constitute the first defense against cancer 13, 20. Therefore, the prognostic value of peripheral leukocytes and their respective subpopulations in the HCC development of CHB patients was explored in this investigation. The concentrations of peripheral leukocyte, lymphocyte, neutrophil, and monocyte in CHB patients and primary HCC patients with or without HBV were first compared. Results demonstrated that CHB patients and primary HCC patients had different concentrations of lymphocyte, neutrophil, and monocyte in their peripheral circulation. Furthermore, we investigated whether their peripheral concentrations could predict HCC incidence in CHB patients. By combining data from two centers, we found that the peripheral lymphocyte concentration was an independent prognostic factor for HCC incidence in CHB patients during the 3 years of followup. Finally, we built and compared a series of models to predict HCC incidence in CHB patients.
MATERIALS AND METHODS
Ethics Statement
This study was approved by the research ethics boards at Sun Yat‐Sen Memorial Hospital affiliated with Sun‐Yat‐Sen University and Ditan Hospital affiliated with Capital Medical University. Because this study was retrospective and based on routine clinical examination, the data were analyzed anonymously, and informed consent was waived.
Study Population and Procedure
Data from two populations were collected for this study. The first population included 89 CHB patients, 94 primary HCC patients with HBV, 81 primary HCC patients without HBV, and 69 normal healthy controls at the Sun Yat‐Sen Memorial Hospital, China, from January 2009 to December 2012. The second population included 257 CHB patients with regular followup for at least 3 years at the Sun Yat‐Sen Memorial Hospital and Beijing Ditan Hospital. Among these patients, 30 CHB patients were from the Sun Yat‐Sen Memorial Hospital and 16 of them developed HCC by the following visit; 227 CHB patients were from the Beijing Ditan Hospital, and 61 of them developed HCC during the 3‐year follow‐up period.
The exclusion criteria for this study were the following: patients with HIV, hepatitis C or hepatitis D coinfections, any other coinfections, tumor history; patients without complete pathology information, or an age less than 18 years. Data, including serum ALT, total bilirubin (TBIL), gamma glutamyl transpeptidase (γ‐GGT), serum alkaline phosphatase (ALP), albumin, AFP concentration, HBV DNA concentration, qualitative HBsAg, HBeAg, HBeAb, HBcAb, hematological tests, and cirrhosis status, were collected and analyzed. All tests were performed in the clinical laboratory of the Sun Yat‐Sen Memorial Hospital or Beijing Ditan Hospital with current empirical methods. HCC diagnosis was confirmed by liver ultrasonography and/or computed tomography. Data for healthy normal controls were collected from comparable aged and gendered healthy people from a normal physical examination with negative HBsAg and no reported disease in the Sun Yat‐Sen Memorial Hospital.
Statistical Analysis
All statistical analysis was performed with SPSS version 16.0 or Stata version 10.0 with two‐tailed tests, and significance was defined as P‐values <0.05. Continuous variables were compared with a one‐way ANOVA expressed and reported as the mean ± SD. Category variables were compared using the chi‐square (χ 2) test. The cumulative HCC incidence was estimated with the Kaplan–Meier method, and the hazard ratio (HR) and independent prognostic factors were calculated with multivariate Cox regression analysis. The receiver operating characteristic (ROC) curves were used to compare the accuracy of the prediction of HCC incidence in CHB patients with Stata version 10.0.
RESULTS
Primary HCC Patients With HBV Possessed Relatively Lower Peripheral Lymphocyte and Monocyte Concentrations and Higher Peripheral Neutrophil Concentrations Compared With CHB Patients
Data from 89 CHB patients, 94 primary HCC patients with HBV, 81 primary HCC patients without HBV before treatment, and 69 HBsAg‐negative normal healthy controls were collected at the Sun Yat‐Sen Memorial Hospital (see Table 1 for the clinical characteristics of CHB patients and primary HCC patients with HBV). Peripheral leukocyte, lymphocyte, neutrophil, and monocyte concentrations were compared among these four groups (Fig. 1). The results demonstrated that no significant differences in total leukocyte concentration were detected among normal healthy controls, CHB patients, and primary HCC patients with HBV (6.56 ± 2.77 × 109/l for primary HCC with HBV, 6.30 ± 3.12 × 109/l for CHB, and 6.27 ± 1.11 × 109/l for normal healthy controls, P > 0.05; Fig. 1A). The total leukocyte concentration in primary HCC patients without HBV was significantly higher compared with the other groups (8.11 ± 3.48 × 109/l, P < 0.001; Fig. 1A). The peripheral lymphocyte concentration in primary HCC patients with HBV was significantly lower than the concentration in the other groups (1.41 ± 0.59 × 109/l for HCC with HBV, 1.78 ± 0.61 × 109/l for CHB, 1.65 ± 0.59 × 109/l for HCC without HBV, and 2.24 ± 0.58 × 109/l for normal healthy controls, P < 0.01; Fig. 1B). The peripheral lymphocyte concentration in CHB patients and primary HCC patients without HBV was lower compared with normal healthy controls (P < 0.001, Fig. 1B). No significant differences in the peripheral lymphocyte concentrations were detected between CHB patients and primary HCC patients without HBV (P = 0.125, Fig. 1B). Primary HCC patients with HBV had a relatively lower monocyte concentration than CHB patients and primary HCC patients without HBV (0.42 ± 0.26 × 109/l for HCC with HBV, 0.51 ± 0.43 × 109/l for CHB, and 0.60 ± 0.31 × 109/l for HCC without HBV, P < 0.05; Fig. 1D). Normal healthy controls possessed the lowest peripheral monocyte concentration among the four groups (0.27 ± 0.07 × 109/l, P < 0.01; Fig. 1D). No significant differences in the peripheral monocyte concentrations were found between CHB patients and primary HCC patients without HBV (P = 0.069, Fig. 1D). On the contrary, the peripheral neutrophil concentration in HCC patients was elevated compared with CHB patients and normal healthy controls (4.54 ± 2.21 × 109/l for HCC with HBV, 5.55 ± 3.09 × 109/l for HCC without HBV, 3.78 ± 2.65 × 109/l for CHB, and 3.55 ± 0.82 × 109/l for normal healthy controls, P < 0.05; Fig. 1C). Primary HCC without HBV had a higher peripheral neutrophil concentration than patients with HBV (P = 0.006); however, no significant differences were detected between CHB patients and normal healthy controls (P = 0.547; Fig. 1C). Meanwhile, the serum concentrations of HBV DNA, ALT, TBIL, γ‐GGT, ALP, albumin, AFP, and peripheral platelets (PLT) were compared among these four groups, respectively. The results demonstrated that compared with CHB patients, primary HCC patients with HBV possessed a relatively reduced circulation of HBV DNA, lower serum ALT and TBIL concentrations, and higher serum ALP and AFP concentrations (P < 0.05; Fig. 1E–L).
Table 1.
Clinical Characteristics of CHB‐Related Populations
| Population 1 | Population 2 | ||||
|---|---|---|---|---|---|
| CHB | HCC with HBV | CHB | |||
| Clinical characteristic | n = 183 | n = 89 | n = 94 | P‐value* | n = 257 |
| Agea (years) | 51 (20–80) | 50 (20–80) | 52 (23–79) | 0.141 | 46 (19–80) |
| Gender | 0.702 | ||||
| Female | 45 | 23 | 22 | 81 | |
| Male | 138 | 66 | 72 | 176 | |
| HBeAgb | 0.015 | ||||
| Negative | 126 | 56 | 70 | 129 | |
| Positive | 51 | 33 | 18 | 111 | |
| HBeAbb | 0.009 | ||||
| Negative | 68 | 43 | 25 | 109 | |
| Positive | 107 | 46 | 61 | 131 | |
| Cirrhosis | 0.001 | ||||
| Negative | 115 | 70 | 45 | 136 | |
| Positive | 78 | 29 | 49 | 121 | |
*Compared between CHB group and HCC with HBV group in population 1.
All were χ2 test, but “age” was t‐test.
Characterized only known sample.
Figure 1.
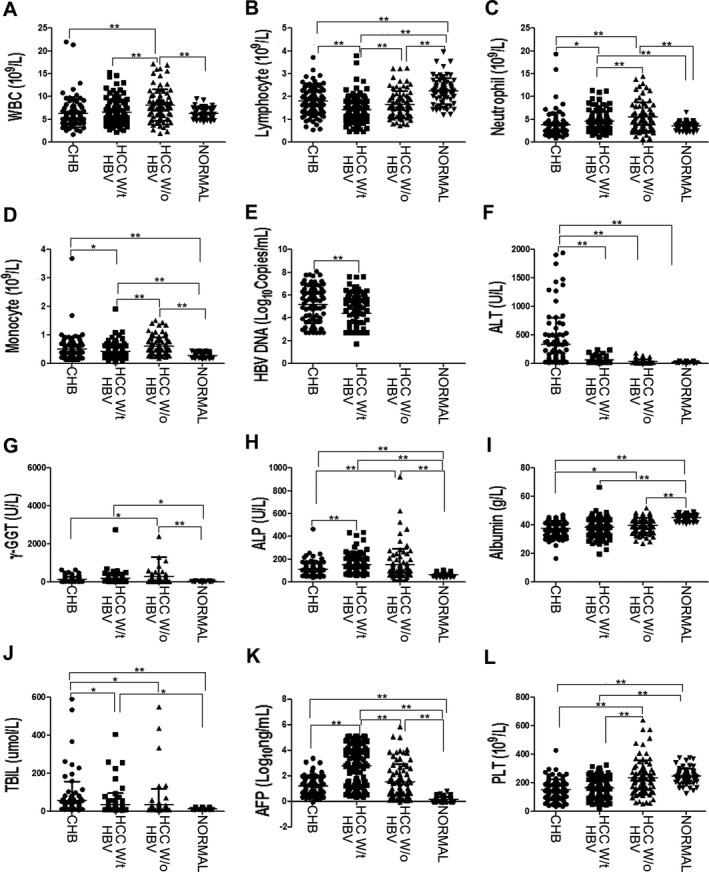
Comparison of different concentrations of the indicated parameters in four groups. (A) Comparison of peripheral leukocyte (WBC) frequency in four groups. (B) Comparison of peripheral lymphocyte frequency in four groups. (C) Comparison of peripheral neutrophil frequency in four groups. (D) Comparison of peripheral monocyte frequency in four groups. (E) Comparison of peripheral HBV DNA concentration in four groups. (F) Comparison of serum ALT concentration in four groups. (G) Comparison of serum γ‐GGT concentration in four groups. (H) Comparison of serum ALPconcentration in four groups. (I) Comparison of serum albumin concentration in four groups. (J) Comparison of serum TBIL concentration in four groups. (K) Comparison of serum AFP concentration in four groups. (L) Comparison of peripheral platelets (PLT) frequency in four groups. HCC W/t HBV, primary HCC patients with HBV; HCC w/o HBV, primary HCC patients without HBV; Normal, normal healthy controls. *P < 0.05 and **P < 0.01.
Peripheral Lymphocyte and Monocyte Concentrations in CHB Patients Correlated With HCC Incidence
The above‐mentioned results demonstrated that CHB and primary HCC patients with HBV had significantly different peripheral neutrophil, lymphocyte, and monocyte concentrations, which have been reported to constitute the first defense barrier against cancer 13, 14, 19, 20. As HCC patients have also been reported to possess impaired immune functions, tumor cells or their microenvironment could impair immune function 21, 22, 23. Another cohort of CHB patient data with at least 3 years of regular followup was collected to investigate the chronological sequence between HCC incidence and peripheral neutrophil, lymphocyte, and monocyte concentration alterations. The data from the Sun Yat‐Sen Memorial Hospital and Beijing Ditan Hospital (see Table 1 for the clinical characteristics) were collected to avoid accidental errors from one study center. All the investigated parameters were divided into two groups according to their median values. The values that were less or equal to the median were termed the low group, and the values that were greater than the median values were classified as the high group. HBeAg, HBeAb, and cirrhosis were classified as negative or positive. For age, those patients less than 50 years were classified as the young group, and patients older than 50 years were classified as the old group. The results of Kaplan–Meier analysis demonstrated that the lower peripheral lymphocyte and monocyte concentration groups had higher HCC incidence during the 3 years of followup (P < 0.001 for lymphocyte concentrations and P = 0.01 for monocyte concentrations), but no significant differences were detected between the low and high peripheral neutrophil groups (Fig. 2). Elevated serum ALT, TBIL, ALP, and AFP concentrations as well as cirrhosis and older age were also risk factors for HCC incidence during the 3 years of followup (Fig. 2).
Figure 2.
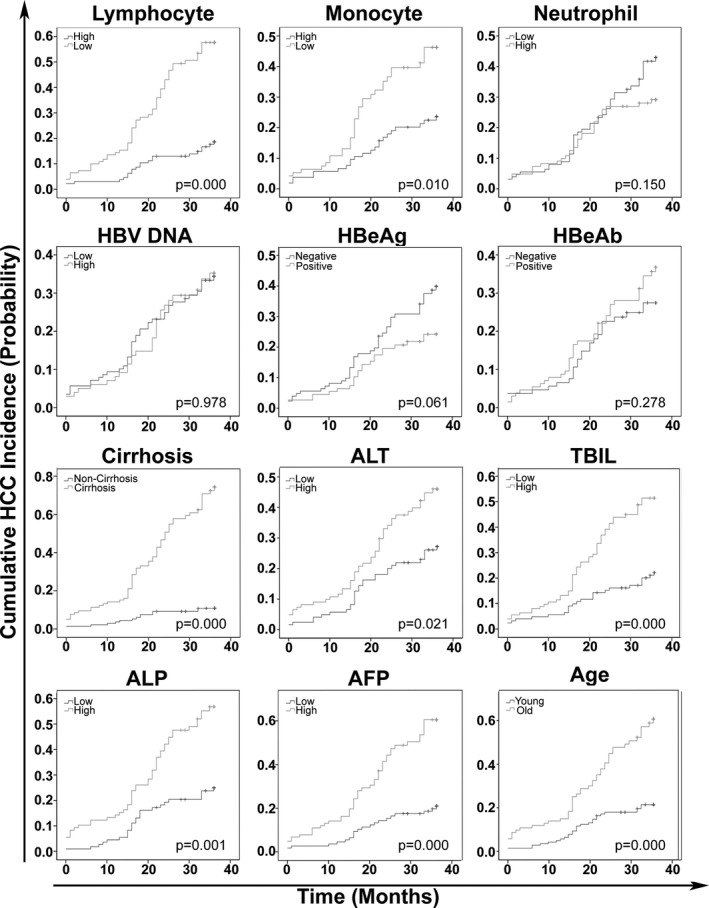
Prognosis of cumulative HCC incidence in CHB patients.
Peripheral Lymphocyte Concentration, Serum AFP Concentration, and Cirrhosis Status Were Independent Prognostic Factors for HCC Incidence in CHB Patients
Univariate and multivariate Cox regression analyses were performed with the risk parameters that correlated with HCC incidence in CHB patients to explore which elements were independent prognostic factors for HCC incidence in CHB patients. The results demonstrated that the peripheral lymphocyte concentration (HR: 2.036), serum AFP concentration (HR: 2.096) and cirrhosis status (HR: 4.603) were independent prognostic factors for HCC incidence in CHB patients during the 3 years of followup (Table 2).
Table 2.
Univariate and Multivariate Cox Regression Analyses of Different Predictive Factors for HCC Incidence in CHB Patients
| Multivariate | ||||
|---|---|---|---|---|
| Variable | Univariate P‐value | HR | 95% CI | P‐value |
| Gender | 0.224 | |||
| Age | 0.000 | 1.099 | 0.583–2.071 | 0.771 |
| HBeAg | 0.061 | |||
| HBeAb | 0.278 | |||
| HBV DNA | 0.978 | |||
| ALT | 0.021 | 0.768 | 0.412–1.432 | 0.406 |
| Cirrhosis | 0.000 | 4.603 | 1.942–10.91 | 0.001 |
| TBIL | 0.000 | 1.172 | 0.597–2.303 | 0.644 |
| ALP | 0.001 | 1.503 | 0.843–2.680 | 0.168 |
| AFP | 0.000 | 2.096 | 1.142–3.844 | 0.017 |
| Lymphocyte | 0.000 | 2.036 | 1.043–3.972 | 0.037 |
| Neutrophil | 0.150 | |||
| Monocyte | 0.010 | 1.563 | 0.865–2.827 | 0.139 |
Predictive Model for HCC Incidence in CHB Patients
To better forecast HCC occurrence in CHB patients, seven models were constructed based on the peripheral lymphocyte, serum AFP, serum ALT, serum TBIL, and serum ALP concentrations and cirrhosis status of CHB patients according to their respective weighted regression coefficients (see below).
Model 1 = 1.527 × cirrhosis + 0.74 × log AFP − 0.71 × lymphocyte
Model 2 = 1.527 × cirrhosis − 0.71 × lymphocyte
Model 3 = 0.74 × log AFP − 0.71 × lymphocyte
Model 4 = 1.527 × cirrhosis + 0.74 × log AFP
Model 5 = 1.527 × cirrhosis + 0.74 × log AFP + 0.159 × log TBIL
Model 6 = 1.527 × cirrhosis + 0.74 × log AFP + 0.407 × log ALP
Model 7 = 1.527 × cirrhosis + 0.74 × log AFP + 0.264 × log ALT
The value for AFP was the patient's serum AFP concentration (ng/ml). The value for TBIL was the patient's serum TBIL concentration (μmol/l). The value for ALT was the patient's serum ALT concentration (U/l). The value for ALP was the patient's serum ALP concentration (U/l). The value for lymphocyte was the patient's peripheral lymphocyte concentration (×109/l). The value for cirrhosis was defined as no cirrhosis for 1 and cirrhosis for 2.
The sensitivity and specificity of these models and their respective independent prognostic markers were compared and analyzed with ROC analysis in Stata version 10. The results demonstrated that Model 1, which combined patients’ peripheral lymphocyte concentration, serum AFP concentration, and cirrhosis status, produced the highest prediction accuracy for HCC incidence in CHB patients (0.832) during the 3 years of followup (P < 0.01, Fig. 3).
Figure 3.
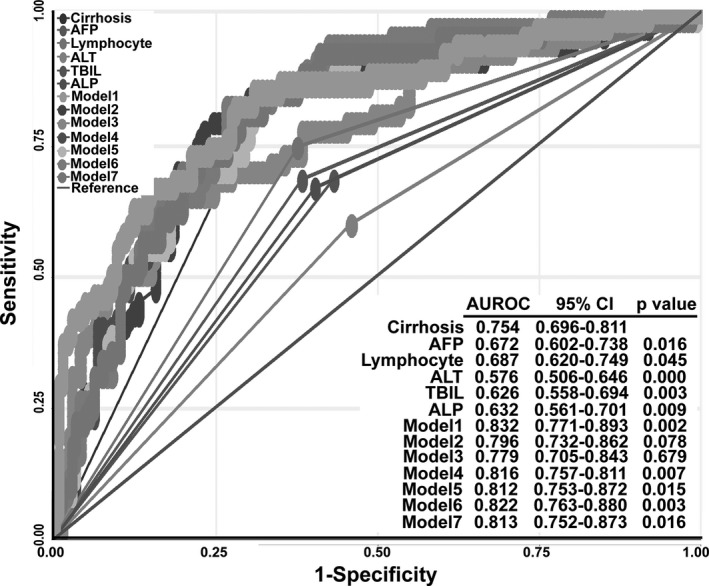
HCC incidence of different predictive models compared with cirrhosis. AUROC, area under the ROC.
DISCUSSION
Impaired immunity in CHB and HCC has been reported by many investigators. For example, the high expression of Tim3 correlated with CD8+ T‐cell depletion and natural killer cell dysfunction in CHB patients 18, and HBcAg induced the expression of interleukin 10, which is one of the most important immune suppression cytokines 24. High frequencies of regulatory T cells and myeloid‐derived suppressor cells have been associated with impaired T cells function in HCC patients 25. Impairment of the functions of natural killer cells and dendritic cells has been reported in advanced HCC 23. Although immunity in HCC and CHB has been studied by many scientists, few of the results were applied to the clinical practice, especially the prediction of HCC occurrence in CHB patients.
Therefore, we explored whether the immune status of CHB patients could predict HCC incidence by investigating the circulating immune cells in a straightforward approach. Although most of the concentrations of peripheral lymphocyte, neutrophil, and monocyte were still within the normal range, the concentrations were different between CHB and primary HCC patients with HBV in the first set of population data. Primary HCC patients with HBV possessed reduced lymphocyte concentrations compared with CHB patients, primary HCC patients without HBV, and normal healthy controls. Decreased cytotoxic lymphocytes and increased regulatory T cells have been reported in CHB patients and HCC patients 25. However, in this study, we found that total peripheral lymphocytes were largely decreased in CHB and HCC patients with HBV compared with healthy controls, which may contribute to the low percentage of regulatory T cells and the relatively high percentage of cytotoxic cells in the periphery. Notably, we cannot exclude the possibility that other types of immune cell populations declined in this process. Monocyte is the precursor to antigen‐presenting cells, which patrol the blood to sense, capture, and present non‐self‐antigens to the immune system and help sustain homeostasis 26. Because both the HBV virus and tumor cells are considered “outside invaders” to the immune system, it was reasonable that we observed significantly elevated peripheral monocyte concentrations in CHB and primary HCC patients compared with normal healthy controls.
Furthermore, we explored whether the reduced lymphocyte concentrations could predict the incidence of HCC in CHB patients. To mitigate the limitations associated with a localized data source, we combined and analyzed longitudinal data both from the Sun Yat‐Sen Memorial Hospital and Beijing Ditan Hospital. The result demonstrated that CHB patients with lower peripheral lymphocyte or monocyte concentrations were more likely to develop HCC during the 3 years of followup. We observed significantly different concentrations of peripheral neutrophil between CHB and primary HCC patients with HBV in the first cohort, but in the second cohort we did not find any significant differences in HCC incidence between the low and high neutrophil concentration groups, which suggested that peripheral neutrophil concentration changes might occur later than peripheral lymphocyte and monocyte concentration alterations.
Then, we continued to study whether peripheral lymphocyte or monocyte concentrations were independent prognostic parameters for HCC incidence in CHB patients. Compared with other HCC occurrence predictive factors, we found that the peripheral lymphocyte concentration, serum AFP concentration, and cirrhosis status in CHB patients were three independent prognostic factors for HCC incidence during the 3 years of followup. To find more sensitive and specific predictive methods for HCC incidence in CHB patients, we constructed a series of models based on these three independent prognostic parameters and other elements. In this investigation, we found that combining the peripheral lymphocyte concentration, serum AFP concentration, and cirrhosis status could produce a more accurate prediction of HCC incidence in CHB patients compared with any other parameter‐based prediction models. This model may help clinically predict HCC incidence in CHB patients in a more straightforward way and benefit most CHB patients.
As the population we studied represented hospital patients and almost half of the studied population were cirrhosis patients, this model may be well suited for the prediction of HCC development in serious CHB patients. Notably, the prediction model had the shortcoming of a limited population in terms of number and population source. As we acquired data from the hospital, CHB carriers rarely went to hospital and were not included in this investigation. Furthermore, we only collected data from two hospitals in mainland China; thus, further investigation is necessary to apply this practice in other regions outside of China.
ACKNOWLEDGMENTS
This study was supported by grants offered to Chaohui Duan by the Natural Science Foundation of Guangdong Province and the Science and Technology Project and Social Development Plan of Guangdong Province in China.
Grant sponsor: Natural Science Foundation of Guangdong Province, China; Grant number: S2013010014007; Grant sponsor: Science and Technology Project and Social Development Plan of Guangdong Province, China; Grant number: 2010B030700007.
REFERENCES
- 1. Fattovich G. Natural history of hepatitis B. J Hepatol 2003;39(Suppl 1):S50–S58. [DOI] [PubMed] [Google Scholar]
- 2. Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol 2003;39(Suppl 1):S64–S69. [DOI] [PubMed] [Google Scholar]
- 3. El‐Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;14:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liaw YF. Natural history of chronic hepatitis B virus infection and long‐term outcome under treatment. Liver Int 2009;29(Suppl 1):100–107. [DOI] [PubMed] [Google Scholar]
- 5. Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 6. Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 2010;15(Suppl. 4):5–13. [DOI] [PubMed] [Google Scholar]
- 7. Wen CP, Lin J, Yang YC, et al. Hepatocellular carcinoma risk prediction model for the general population: The predictive power of transaminases. J Natl Cancer Inst 2012;104:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010;28:1660–1665. [DOI] [PubMed] [Google Scholar]
- 9. Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002;347:168–174. [DOI] [PubMed] [Google Scholar]
- 10. Lu SN, Wang JH, Liu SL, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high‐risk for hepatocellular carcinoma. Cancer 2006;107:2212–2222. [DOI] [PubMed] [Google Scholar]
- 11. Lee MH, Yang HI, Liu J, et al. Prediction models of long‐term cirrhosis and HCC risk in chronic hepatitis B patients: Risk scores integrating host and virus profiles. Hepatology 2013;58:546–554. [DOI] [PubMed] [Google Scholar]
- 12. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 13. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235–271. [DOI] [PubMed] [Google Scholar]
- 14. Pedroza‐Gonzalez A, Verohef C, Ijzermans JN, et al. Activated tumor‐infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology 2013;57:183–194. [DOI] [PubMed] [Google Scholar]
- 15. Flecken T, Spangenberg HC, Thimme R. Immunobiology of hepatocellular carcinoma. Langenbecks Arch Surg 2012;397:673–680. [DOI] [PubMed] [Google Scholar]
- 16. Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD‐L1. J Exp Med 2009;206:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Das A, Hoare M, Davies N, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 2008;205:2111–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ju Y, Hou N, Meng J, et al. T cell immunoglobulin‐ and mucin‐domain‐containing molecule‐3 (Tim‐3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol 2010;52:322–329. [DOI] [PubMed] [Google Scholar]
- 19. Stoop JN, van der Molen RG, Baan CC, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005;41:771–778. [DOI] [PubMed] [Google Scholar]
- 20. Mattarollo SR, Smyth MJ. A novel axis of innate immunity in cancer. Nat Immunol 2010;11:981–982. [DOI] [PubMed] [Google Scholar]
- 21. Mamessier E, Pradel LC, Thibult ML, et al. Peripheral blood NK cells from breast cancer patients are tumor‐induced composite subsets. J Immunol 2013;190:2424–2436. [DOI] [PubMed] [Google Scholar]
- 22. Laurindo MF, Thies FG, Perez EC, Novaes e Brito RR, Mariano M, Popi AF. B16 melanoma cells increase B‐1 cell survival, IL‐10 production and radioresistance in vitro. Immunobiology 2013;218:609–619. [DOI] [PubMed] [Google Scholar]
- 23. Jinushi M, Takehara T, Tatsumi T, et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I‐related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005;43:1013–1020. [DOI] [PubMed] [Google Scholar]
- 24. Li J, Wu W, Peng G, et al. HBcAg induces interleukin‐10 production, inhibiting HBcAg‐specific Th17 responses in chronic hepatitis B patients. Immunol Cell Biol 2010;88:834–841. [DOI] [PubMed] [Google Scholar]
- 25. Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA‐4(+)Foxp3(+) T regulatory cells and myeloid‐derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T‐cell functionality. Cancer Res 2013;73:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]


