Abstract
Objective
This meta‐analysis aimed to identify the value of serum YKL‐40 level for the diagnosis of coronary artery disease (CAD).
Methods
Through searching the following electronic databases: the Cochrane Library Database (Issue 12, 2013), Web of Science (1945∼2013), PubMed (1966∼2013), CINAHL (1982∼2013), EMBASE (1980∼2013), and the Chinese Biomedical Database (CBM; 1982∼2013), related articles were determined without any language restrictions. STATA statistical software (Version 12.0, Stata Corporation, College Station, TX) was chosen to deal with statistical data. Standard mean difference (SMD) and its corresponding 95% confidence interval (95% CI) were calculated.
Results
Eleven clinical case‐control studies that recruited 1,175 CAD patients and 1,261 healthy controls were selected for statistical analysis. The main findings of our meta‐analysis showed that serum YKL‐40 level in CAD patients was significantly higher than that in control subjects (SMD = 2.79, 95% CI = 1.73∼3.85, P < 0.001). Ethnicity‐stratified analysis indicated a higher serum YKL‐40 level in CAD patients than control subjects among China, Korea, and Denmark populations (China: SMD = 2.97, 95% CI = 1.21∼4.74, P = 0.001; Korea: SMD = 0.66, 95% CI = 0.17∼1.15, P = 0.008; Denmark: SMD = 1.85, 95% CI = 1.42∼2.29, P < 0.001; respectively), but not in Turkey (SMD = 4.52, 95% CI = –2.87∼11.91, P = 0.231).
Conclusion
The present meta‐analysis suggests that an elevated serum YKL‐40 level may be used as a promising diagnostic tool for early identification of CAD.
Keywords: coronary artery disease, meta‐analysis, YKL‐40
INTRODUCTION
Coronary artery disease (CAD), also known as coronary heart disease (CHD), is the most common type of heart disease and a main reason of heart attacks, which generally develops due to damaged or diseased coronary arteries 1. As a global health problem, cardiovascular disease is still one major cause of disability and mortality worldwide, accounting for 30∼40% of all human deaths among the delayed degenerative diseases, occurring mostly in low‐ and middle‐income countries 2. According to statistics, in the United States, an estimated 386,324 cases died of CAD and approximately 635,000 people suffered from coronary attack in 2009; additionally, about 280,000 patients even have a recurrent attack 3. As we all know, CAD is considered to be a complex and multifactorial disease like the other forms of cancers, which may result from the interaction of genetic and environmental factors 4, 5. To date, evidence demonstrate that obesity is the main risk factor for the development and progress of CAD, while age, sex, physical exercise, hypertension, family history, smoking, alcohol intake, and work stress may also contribute to the susceptibility to CAD 6, 7, 8. In addition, epidemiological studies showed that serum human cartilage glycoprotein‐39 (YKL‐40) level widely correlated with cardiovascular disease and may be a potential indicator to the development and progression of CAD 9, 10.
YKL‐40, also known as chitinase‐3‐like protein 1 (CHI3L1), is a heparin‐binding and chitin‐binding glycoprotein encoded by the CHI3L1 gene in humans, which is assigned to chromosome 1q31‐32, and approximately 40 kDa in size 11, 12. It has been widely suggested that YKL‐40 is a kind of highly conserved protein mainly secreted by various cell‐types including human macrophages, synovial cells, chondrocytes, neutrophils, and vascular smooth muscle cells 13. As an inflammatory glycoprotein, YKL‐40 is involved in endothelial dysfunction by promoting chemotaxis, cells adhesion and migration, reorganization, and extracellular matrix remodeling 14. Elevated concentration of YKL‐40 is also found in plasma or serum in different cancer patients and in patients with inflammatory diseases including breast cancer, colon cancer, rheumatoid arthritis, inflammatory bowel disease, asthma, type 1 diabetes, and type 2 diabetes 15, 16. More significantly, increased YKL‐40 serum level has been correlated with poor prognosis and decreased survival rates of various cancer patients; thus, elevated YKL‐40 serum level may serve as a possible biomarker for the development and progress of cancers and inflammatory diseases 16, 17. Furthermore, experimental investigation shows that serum concentrations of YKL‐40 are elevated in patients with cardiovascular diseases such as acute myocardial infarction (MI), heart failure (HF), and stable CAD 9, 10, 18. Therefore, it is hypothesized that serum YKL‐40 level may be closely associated with the development and prognosis of CAD. Although the exact physiological role of YKL‐40 in CAD is still unclear, accumulating evidence have been showed that elevated serum YKL‐40 level might play an important role in the pathological changes of CAD 19, 20; contradictory results were also reported in many other studies 21, 22. In this regard, it is worth performing a meta‐analysis of all available data to identify the value of serum YKL‐40 level for the diagnosis of CAD.
MATERIALS AND METHODS
Literature Search and Selection Criteria
The identification of related articles was implemented through searching the following electronic databases without any language restrictions: the Cochrane Library Database (Issue 12, 2013), Web of Science (1945∼2013), PubMed (1966∼2013), CINAHL (1982∼2013), EMBASE (1980∼2013), and the Chinese Biomedical Database (CBM; 1982∼2013). We employed a highly sensitive search strategy in combination with the following keywords and Medical Subject Headings (MeSH) terms: (“CHI3L1 protein, human” or “CHI3L1” or “cartilage gp‐39” or “YKL40” or “YKL‐40” or “HCGP39” or “human cartilage glycoprotein‐39” or “human cartilage gp39” or “HC‐gp39” or “38‐kDa heparin‐binding glycoprotein”) and (“myocardial infarction” or “coronary artery disease” or “MI” or “CAD” or “myocardial infarct” or “myocardiac infarction” or “myocardium infarction” or “cardiac infarction” or “coronary heart disease” or “CHD” or “heart infarction” or “acute myocardial infarction” or “AMI”). A manual search on the basis of references provided by the included articles was carried out to acquire other potential articles.
Three types of criteria were established for the selection of eligible studies: 1 must concern the diagnostic value of serum YKL‐40 level for CAD; 2 patients in eligible studies should have either a cardiac history or symptoms sufficient to warrant angiography, and the indications included a history of CAD, clinical evidence of angina pectoris, and suspected chest pain; patients who had organic valvular heart disease, malignancy, chronic kidney and hepatic failure, collagen vascular disease, pulmonary embolism, and infectious diseases must be excluded; 3 enough information on serum YKL‐40 level should be supplied in eligible articles. Article that did not meet the inclusion criteria was excluded. If authors published several studies of the same subjects, either the most recent or largest sample size publication was included.
Data Extraction and Methodological Assessment
Two authors used a standardized form to extract the following data from included studies: language of publication, publication year of article, the first author's surname, geographical location, design of study, total number of cases, sample size, the source of controls, detection method, serum YKL‐40 level, etc.
Two observers separately assessed methodological quality with the use of the Newcastle–Ottawa Scale (NOS) criteria 23. The NOS criteria comprised three aspects: 1 subject selection: 0∼4; 2 comparability of subject: 0∼2; 3 clinical outcome: 0∼3. NOS scores ranged from 0 to 9; and a score with good quality should be ≥ 7.
Statistical Analysis
In order to achieve rigorous statistical analysis, we choose the STATA statistical software (Version 12.0, Stata Corporation, College Station, TX) to deal with statistical data. Standard mean difference (SMD) and its corresponding 95% confidence interval (95% CI) were calculated. The statistical significance of pooled SMDs was evaluated by the Z test. Tests for heterogeneity are commonly used to decide on methods for combining studies and for selection of random‐effect model or fixed‐effects model. Between‐study heterogeneity was assessed by Cochran's Q‐statistic and I 2 tests in our meta‐analysis 24. A P value < 0.05 or I2 > 50% means that these studies were heterogeneous, and then the random‐effect model was employed, otherwise the fixed‐effects model was implemented. We also make use of subgroup analyses to explore reasons for heterogeneity. A sensitivity analysis was implemented for evaluating the influence of single study on the overall estimate. The presence of publication bias was examined by funnel plots and Egger's linear regression test 25.
RESULTS
Baseline Characteristics of Included Studies
A total of 50 articles relevant to the searched keywords were initially identified. We reviewed the titles and abstracts of all the articles and 23 articles were excluded; full texts and data integrity were then reviewed and another 13 articles were finally excluded. Another two studies were also excluded due to lack of data integrity (Fig. 1). Eventually, 11 clinical case‐control studies with a total of 1,175 CAD patients and 1,261 healthy subjects met our inclusion criteria for quantitative data analysis 19, 20, 21, 22, 26, 27, 28, 29, 30, 31, 32. The range of publication years of the eligible studies ranged from 2007 to 2013. The distribution of the number of topic‐related literature in the electronic database over the last decade is presented in Figure 2. Overall, nine studies were conducted among Asians and the other two studies among Caucasians. The method used for this meta‐analysis is enzyme‐linked immunosorbent assay (ELISA), except for one study adopted for the novel method of immunoradiometric assay (IRMA). NOS scores of all included studies were ≥6. Main characteristics and methodological quality of all eligible studies are listed in Table 1.
Figure 1.
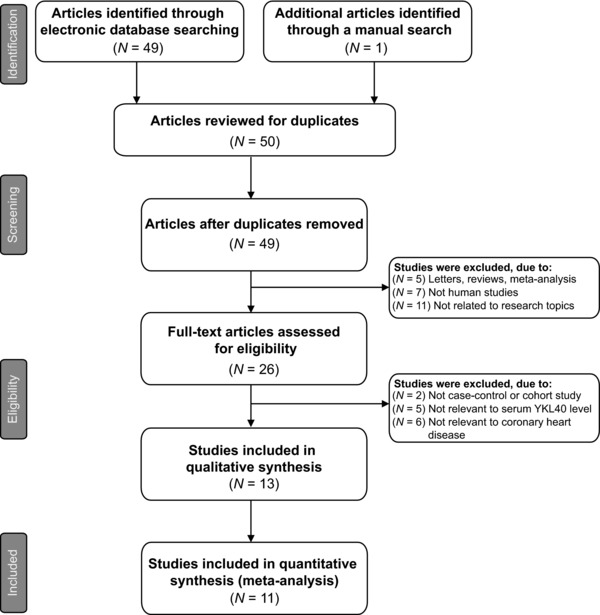
Flow chart of literature search and study selection. Eleven studies were included in this meta‐analysis.
Figure 2.
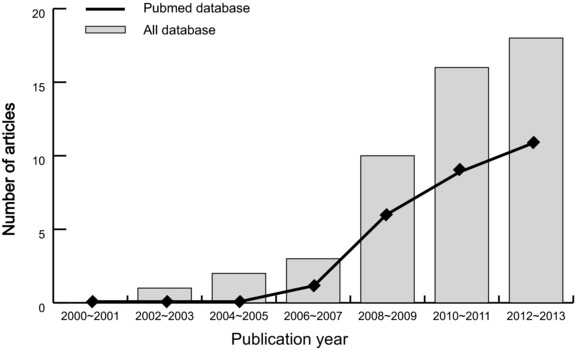
The distribution of the number of topic‐related literature in electronic database over the last decade.
Table 1.
Main Characteristics and Methodological Quality of All Eligible Studies
| Sample size | Gender (M/F) | Age (years) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | Year | Country | Disease | Case | Control | Case | Control | Case | Control | Method | Marker | NOS score |
| Erdogan T 22 | 2013 | Turkey | CAD | 30 | 30 | 17/13 | 19/11 | 61 ± 10 | 58 ± 12 | ELISA | YKL‐40 | 6 |
| Sui X 21 | 2013 | China | CAD | 134 | 112 | 99/35 | 80/32 | 56.7 ± 8.1 | 56.0 ± 8.8 | ELISA | YKL‐40 | 8 |
| Zheng JL 26 | 2012 | China | CAD | 108 | 89 | 58/50 | 48/41 | 66.1 ± 10.4 | 64.4 ± 8.4 | ELISA | YKL‐40 | 8 |
| Zhang SY 27 | 2012 | China | CAD | 40 | 40 | ‐ | ‐ | ‐ | 58.1 ± 9.2 | ELISA | YKL‐40 | 5 |
| Xie F 28 | 2012 | China | CAD | 410 | 442 | 263/147 | 267/175 | 59.8 ± 11.2 | 59.5 ± 11.7 | ELISA | YKL‐40 | 8 |
| Kim HM 29 | 2012 | Korea | CAD | 41 | 29 | 19/22 | 9/20 | 60 ± 5 | 58 ± 6 | IRMA | YKL‐40 | 6 |
| Guo YG 31 | 2011 | China | CAD | 29 | 26 | 19/10 | 16/10 | 63.6 ± 8.9 | 58.2 ± 9.2 | ELISA | YKL‐40 | 6 |
| Mathiasen AB 30 | 2011 | Denmark | CAD | 206 | 245 | 138/68 | 111/134 | 64.8 ± 10.3 | 47.2 ± 17.4 | ELISA | YKL‐40 | 8 |
| Rathcke CN 32 | 2010 | Denmark | CAD | 68 | 175 | 73/102 | 45/23 | 61.0 ± 11.5 | ELISA | YKL‐40 | 7 | |
| Li ZQ 19 | 2009 | China | CAD | 61 | 20 | 38/23 | 13/7 | 65 (35∼84) | 69 (60∼79) | ELISA | YKL‐40 | 7 |
| Kucur M 20 | 2007 | Turkey | CAD | 48 | 53 | 39/9 | 41/12 | 61 ± 10.3 | 55.7 ± 8.2 | ELISA | YKL‐40 | 7 |
M, male; F, female; NOS, Newcastle–Ottawa Scale; CAD, coronary artery disease; ELISA, enzyme‐linked immunosorbent assay; IRMA, immunoradiometric assay; YKL‐40, serum human cartilage glycoprotein‐39.
Quantitative Data Synthesis
The random‐effects model was conducted because significant heterogeneity existed between studies (all P < 0.05). When all the eligible studies were pooled into the meta‐analysis, a significant relationship was observed between the increased serum level of YKL‐40 and the pathogenesis of the CAD than those of control subjects (SMD = 2.79, 95% CI = 1.73∼3.85, P < 0.001; Fig. 3).
Figure 3.
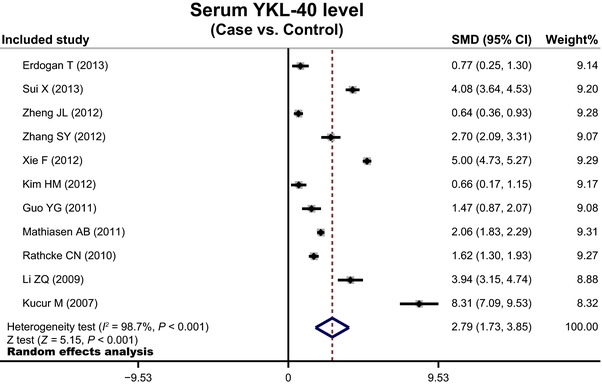
Forest plots for the value of serum YKL‐40 level for the diagnosis of coronary artery disease.
Subgroup analysis was conducted to investigate potential sources of heterogeneity. Country‐stratified analysis findings showed that the elevated serum level of YKL‐40 was significantly related to the development in CAD patients than those of healthy controls among China, Korea, and Denmark populations (China: SMD = 2.97, 95% CI = 1.21∼4.74, P = 0.001; Korea: SMD = 0.66, 95% CI = 0.17∼1.15, P = 0.008; Denmark: SMD = 1.85, 95% CI = 1.42∼2.29, P < 0.001, respectively; Fig. 4). However, we failed to find any association between the increased levels of YKL‐40 and the occurrence of CAD among the populations of Turkey (SMD = 4.52, 95% CI = ‐2.87∼11.91, P = 0.231). We also performed a subgroup analysis by sample size, we observed a significant association between the increased serum YKL‐40 level and the development of CAD among both the small and large sample sizes subgroups (all P < 0.005; Fig. 4).
Figure 4.
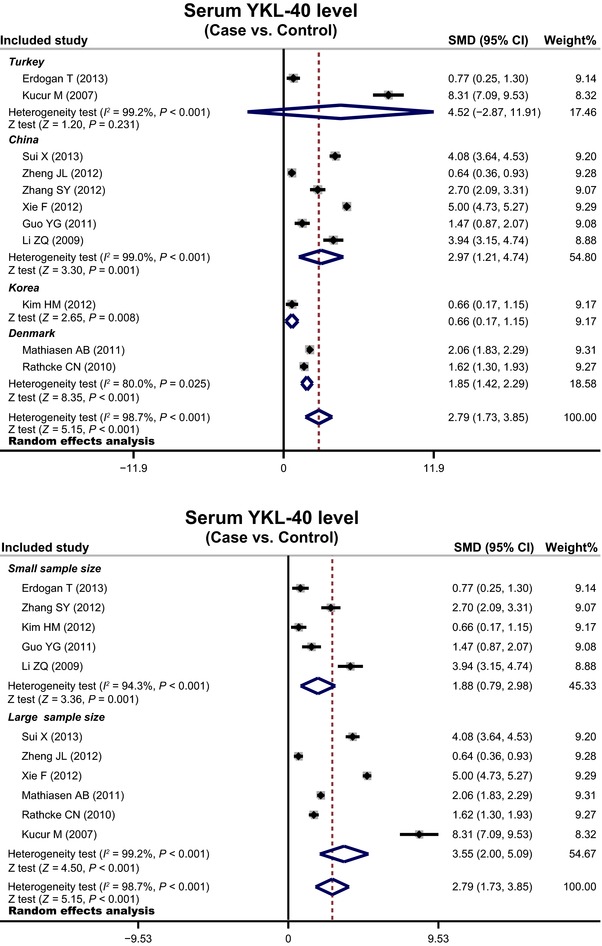
Subgroup analyses by country and sample size for the diagnosis of coronary artery disease.
To assess the influence of each individual study on the pooled odds ratio (ORs), we performed a sensitivity analysis by omitting individual studies. The analysis results indicated that no individual study significantly affects the overall pooled estimates (Fig. 5). Funnel plots did not reveal any evidence of obvious asymmetry in the observation of YKL‐40 levels alteration, and Egger's test also indicated that there was no significant statistical evidence of publication bias for any of the models (P > 0.05; Fig. 6).
Figure 5.
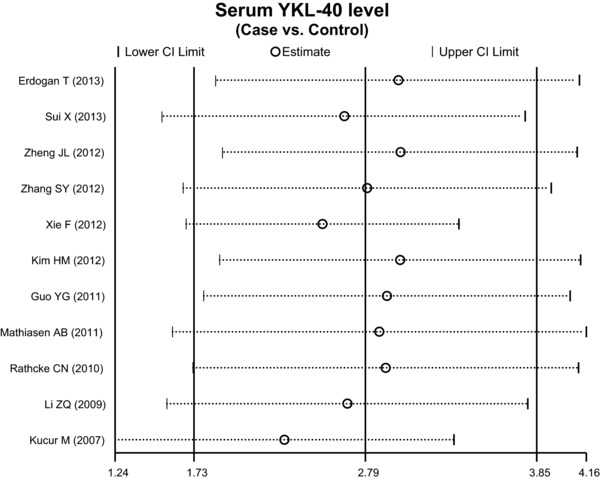
Sensitivity analysis of the summary odds ratio coefficients for the diagnosis of coronary artery disease.
Figure 6.
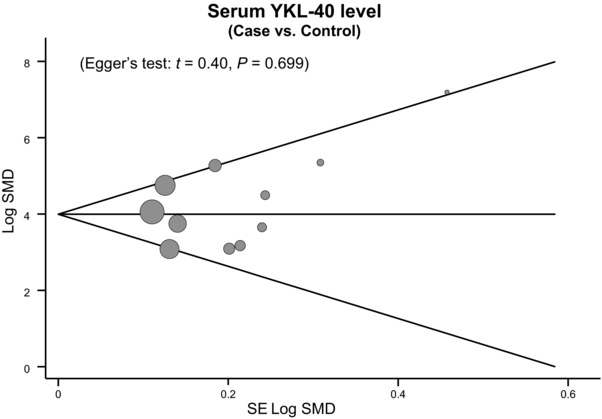
Funnel plot of publication biases for the diagnosis of coronary artery disease.
DISCUSSION
The objective of this meta‐analysis was to investigate the value of serum YKL‐40 level for diagnosis. Our analysis results showed that serum level in patients with CAD was significantly higher than in the control participants, suggesting that this relationship may represent a new opportunity for the possible utility of serum level of YKL‐40 as an inflammatory marker for CAD. It is evident that atherosclerosis, the main reason for CAD, is an inflammatory disease in which the initial process is the active participation of endothelial cells, which may lead to the augmenting monocytes infiltration into the vessel wall, and subsequently, their differentiation and proliferation into lipid‐laden foam cells derived from macrophages 33, 34. Notably, laboratory and prospective clinical studies emphasizing the significant role of inflammation in the pathogenesis of atherosclerosis highlight the critical effects of inflammatory parameters such as YKL‐40 in the diagnosis and prediction of CAD 35, 36. So far, little information is yet available on the correlation of YKL‐40 with atherosclerosis, and there are rare studies focusing on the assessment of this relationship in human with CAD. Exactly, YKL‐40 can be regard as an inflammatory protein because of its secretion by activated macrophages during the later stages of human macrophages differentiation 16. It is noteworthy that macrophages exist in all phases of atherogenesis, and evidence has also demonstrated that the macrophages within atherosclerotic vascular plaques express YKL‐40 35, 37. Therefore, it was plausible to speculate that serum level of YKL‐40 related to the amount of lipid‐loaded macrophages in the atherosclerotic arterial wall has a predictable impact on the presence and extent of CAD. To be specific, the functional role of YKL‐40 in atherogenesis is concentrated in the pathophysiological mechanisms, including inflammatory response, tissue destruction, ongoing fibrosis, smooth muscle proliferation, and migration in the injured vessel wall 10, 16. Especially, inflammatory response with the induced, activated endothelial cells and macrophages may stimulate the migration and abnormal proliferation of vascular smooth muscle cells within the intima, eventually promoting the formation of atherosclerosis plaques 38. In this regard, the participation of YKL‐40 in modulation of extracellular matrix may affect adhesion and migration under the tissue remodeling process that takes place in atherosclerosis. Coincidently, Xie et al. also supported that YKL‐40 was involved in the early stage of atherosclerosis and its progression, and found in their experiment that patients with CAD had higher levels of YKL‐40 than controls, indicating that YKL‐40 may play an important role in the formation of unstable and ruptured atherosclerotic plaques 28. In addition, Kucur and colleagues also insisted the importance of increased level of YKL‐40 in the prediction of atherosclerosis severity and inflammatory response, not only as a quantitative indicator of CAD extent, but also being a marker of CAD presence 20.
Stratified analysis was further performed based on country to evaluate the connection between the serum level of YKL‐40 and CAD. Our results showed that the increased level of YKL‐40 reveals a significant association with the development of CAD among China, Korea, and Denmark populations, whereas no such observation was detected among the populations of Turkey, suggesting that country differences may be the potential heterogeneity resource of this outcome. A possible explanation for the country differences could be the divergence in environments, genetic backgrounds, and risk factors relating to lifestyle among these populations.
The present study has certain limitations that warrant mentioning. First, due to the small number of studies, our results did not include all the data from all trials to examine the relationship of serum YKL‐40 level with the development of CAD. Nevertheless, via systematically integrating results from each study, our meta‐analysis managed to overcome confines of size or scope in individual studies to acquire more reliable and general information on the correlation of YKL‐40 levels and the progression of CAD. A second limitation of our meta‐analysis is the fact that the results of meta‐analysis might slightly lack reliability to some extent since it is a retrospective study, which may induce potential publication bias. Another potential limitation is that our lack of access to the original and detailed information from the studies limited further evaluation of potential interactions between serum YKL‐40 level and CAD. Even though our meta‐analysis has above limitations, this is the first example of meta‐analysis shedding light on the association between serum YKL‐40 level and CAD development. More significantly, our meta‐analysis has clear selection criteria in literature search strategy. In order to achieve strong objectivity, all the research methods were carried out on strict inclusion and exclusion criteria. In addition, according to these rigorous statistical analyses, this undertaken meta‐analysis will lead to a more accurate conclusion.
In conclusion, the present meta‐analysis suggests that an elevated serum YKL‐40 level may be used as a promising diagnostic tool for early identification of CAD. However, given that several limitations existed in our meta‐analysis, further studies with more integral data and larger sample size are required to obtain a more profound statistical analysis with general applicability.
ACKNOWLEDGMENTS
This study is funded by the National Nature Science Foundation of China through the National Outstanding Youth Science Fund (51103059), the National Natural Science Foundation of Jilin Province (20140101054JC and 201115071), and Jilin Industrial Technology Research and Development Project (2013C023‐3). We would like to acknowledge the reviewers for their helpful comments on this article.
Grant sponsor: National Nature Science Foundation of China; Grant number: 51103059; Grant sponsor: National Natural Science Foundation of Jilin Province; Grant numbers: 20140101054JC and 201115071; Grant sponsor: Jilin Industrial Technology Research and Development Project; Grant number: 2013C023‐3.
REFERENCES
- 1. Teramoto T, Sasaki J, Ishibashi S, et al. Coronary artery disease. J Atheroscler Thromb 2014;21:86–92. [DOI] [PubMed] [Google Scholar]
- 2. Gaziano TA, Bitton A, Anand S, Abrahams‐Gessel S, Murphy A. Growing epidemic of coronary heart disease in low‐ and middle‐income countries. Curr Probl Cardiol 2010;35:72–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prins BP, Lagou V, Asselbergs FW, Snieder H, Fu J. Genetics of coronary artery disease: Genome‐wide association studies and beyond. Atherosclerosis 2012;225:1–10. [DOI] [PubMed] [Google Scholar]
- 5. Kivimaki M, Nyberg ST, Batty GD, et al. Job strain as a risk factor for coronary heart disease: A collaborative meta‐analysis of individual participant data. Lancet 2012;380:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arsenault BJ, Rana JS, Lemieux I, et al. Physical inactivity, abdominal obesity and risk of coronary heart disease in apparently healthy men and women. Int J Obes (Lond) 2010;34:340–347. [DOI] [PubMed] [Google Scholar]
- 7. Hvidtfeldt UA, Tolstrup JS, Jakobsen MU, et al. Alcohol intake and risk of coronary heart disease in younger, middle‐aged, and older adults. Circulation 2010;121:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kivimaki M, Nyberg ST, Fransson EI, et al. Associations of job strain and lifestyle risk factors with risk of coronary artery disease: A meta‐analysis of individual participant data. CMAJ 2013;185:763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kastrup J, Johansen JS, Winkel P, et al. High serum YKL‐40 concentration is associated with cardiovascular and all‐cause mortality in patients with stable coronary artery disease. Eur Heart J 2009;30:1066–1072. [DOI] [PubMed] [Google Scholar]
- 10. Rathcke CN, Vestergaard H. YKL‐40—An emerging biomarker in cardiovascular disease and diabetes. Cardiovasc Diabetol 2009;8:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyrgios I, Galli‐Tsinopoulou A, Stylianou C. Ghrelin‐leptin network influences serum chitinase 3‐like protein 1 (YKL‐40) levels in obese prepubertal children. Regul Pept 2013;183C:69–73. [DOI] [PubMed] [Google Scholar]
- 12. Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp‐39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 1997;43:221–225. [DOI] [PubMed] [Google Scholar]
- 13. Shao R, Hamel K, Petersen L, et al. YKL‐40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009;28:4456–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansen JS, Schultz NA, Jensen BV. Plasma YKL‐40: A potential new cancer biomarker? Future Oncol 2009;5:1065–1082. [DOI] [PubMed] [Google Scholar]
- 15. Ober C, Tan Z, Sun Y, et al. Effect of variation in CHI3L1 on serum YKL‐40 level, risk of asthma, and lung function. N Engl J Med 2008;358:1682–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H. YKL‐40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care 2009;32:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johansen JS, Bojesen SE, Mylin AK, et al. Elevated plasma YKL‐40 predicts increased risk of gastrointestinal cancer and decreased survival after any cancer diagnosis in the general population. J Clin Oncol 2009;27:572–578. [DOI] [PubMed] [Google Scholar]
- 18. Nojgaard C, Host NB, Christensen IJ, et al. Serum levels of YKL‐40 increases in patients with acute myocardial infarction. Coron Artery Dis 2008;19:257–263. [DOI] [PubMed] [Google Scholar]
- 19. Li ZQ, Lin AZ. The serum level of Ykl‐40 increases in patients with acute myocardial infarction. Modern Hosp 2009;9:23–25. [Google Scholar]
- 20. Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL‐40 levels in patients with coronary artery disease. Coron Artery Dis 2007;18:391–396. [DOI] [PubMed] [Google Scholar]
- 21. Sui X, Gao C. Association of serum YKL‐40 levels with the presence and severity of coronary artery disease in patients with obstructive sleep apnea syndrome. Clin Invest Med 2013;36:E306–E311. [DOI] [PubMed] [Google Scholar]
- 22. Erdogan T, Kocaman SA, Cetin M, et al. Increased YKL‐40 levels in patients with isolated coronary artery ectasia: An observational study. Anadolu Kardiyol Derg 2013;13:465–470. [DOI] [PubMed] [Google Scholar]
- 23. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 24. Zintzaras E, Ioannidis JP. HEGESMA: Genome search meta‐analysis and heterogeneity testing. Bioinformatics 2005;21:3672–3673. [DOI] [PubMed] [Google Scholar]
- 25. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta‐analysis. JAMA 2006;295:676–680. [DOI] [PubMed] [Google Scholar]
- 26. Zheng JL, Sun Z, Lu L, et al. Association between serum YKL‐40 level and degree of coronary artery disease in patients with type 2 diabetes mellitus. Chin J Arterial Lerosis 2012:727–730. [Google Scholar]
- 27. Zhang SY. Effect of serum YKL‐40 on diabetic patients with coronary heart disease. Int Med Health Guidance News 2012;18:2242–2244. [Google Scholar]
- 28. Xie F, Qian Q, Chen Z, Ma G, Feng Y. Chitinase 3‐like 1 gene‐329G/A polymorphism, plasma concentration and risk of coronary heart disease in a Chinese population. Gene 2012;499:135–138. [DOI] [PubMed] [Google Scholar]
- 29. Kim HM, Lee BW, Song YM, et al. Potential association between coronary artery disease and the inflammatory biomarker YKL‐40 in asymptomatic patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2012;11:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathiasen AB, Harutyunyan MJ, Jorgensen E, et al. Plasma YKL‐40 in relation to the degree of coronary artery disease in patients with stable ischemic heart disease. Scand J Clin Lab Invest 2011;71:439–447. [DOI] [PubMed] [Google Scholar]
- 31. Guo YG, Zhang FF, Qiu CG, Han ZY, Huang ZW. Relationship between the serum level of ykl‐40 of coronary artery disease accompanied with diabetes and lesion of coronary artery. J Practical Med 2011;27:4414–4416. [Google Scholar]
- 32. Rathcke CN, Kjoller E, Fogh‐Andersen N, Zerahn B, Vestergaard H. NT‐proBNP and circulating inflammation markers in prediction of a normal myocardial scintigraphy in patients with symptoms of coronary artery disease. PLoS One 2010;5:e14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lusis AJ, Mar R, Pajukanta P. Genetics of atherosclerosis. Annu Rev Genomics Hum Genet 2004;5:189–218. [DOI] [PubMed] [Google Scholar]
- 35. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rathcke CN, Raymond I, Kistorp C, et al. Low grade inflammation as measured by levels of YKL‐40: Association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol 2010;143:35–42. [DOI] [PubMed] [Google Scholar]
- 37. Kastrup J. Can YKL‐40 be a new inflammatory biomarker in cardiovascular disease? Immunobiology 2012;217:483–491. [DOI] [PubMed] [Google Scholar]
- 38. Insull W, Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am J Med 2009;122:S3–S14. [DOI] [PubMed] [Google Scholar]


