Abstract
Background
Serum/plasma albumin is an important and widely used laboratory marker and it is important that we measure albumin correctly without bias. We had indications that the immunoturbidimetric method on Cobas c 501 and the bromocresol purple (BCP) method on Architect 16000 differed, so we decided to study these methods more closely.
Method
A total of 1,951 patient requests with albumin measured with both the Architect BCP and Cobas immunoturbidimetric methods were extracted from the laboratory system. A comparison with fresh plasma samples was also performed that included immunoturbidimetric and BCP methods on Cobas c 501 and analysis of the international protein calibrator ERM‐DA470k/IFCC.
Results
The median difference between the Abbott BCP and Roche immunoturbidimetric methods was 3.3 g/l and the Roche method overestimated ERM‐DA470k/IFCC by 2.2 g/l. The Roche immunoturbidimetric method gave higher values than the Roche BCP method: y = 1.111x – 0.739, R² = 0.971.
Conclusion
The Roche immunoturbidimetric albumin method gives clearly higher values than the Abbott and Roche BCP methods when analyzing fresh patient samples. The differences between the two methods were similar at normal and low albumin levels.
Keywords: albumin, BCP, bromocresol purple, calibration, immunoturbidimetric method, turbidimetry
Introduction
Albumin is the most abundant plasma protein in humans, accounting for 50–60% of the total amount of proteins 1. Serum or plasma albumin is also one of our most frequently requested laboratory tests. Albumin has been extensively studied and over the last 50 years we have seen major advances in our understanding of the different functions of albumin 2.
Albumin is responsible for approximately 70–80% of the colloid osmotic pressure of the blood compartment 3. Many endogenous compounds and drugs are bound to and transported by albumin 2, 4. Albumin also has metabolic, antioxidant, anticoagulant, and acid–base functions 5, 6, 7.
Hyperalbuminemia is mainly due to dehydration 8, while hypoalbuminemia may be caused by a wide range of disorders including decreased synthesis due to malabsorption, malnutrition, malignancies, inflammation, and liver disease or increased losses due to nephrotic syndrome, protein‐losing enteropathy, and burn injuries 2, 7, 9. The serum albumin concentration is often used as a marker for protein status and protein malnutrition 10, 11. Albumin is also widely used as a prognostic marker. Prognostic plasma biomarkers are of considerable clinical value due to their accessibility. Albumin has been shown to be a prognostic marker in various cancers 12, 13, 14. It is also a prognostic marker in end‐stage renal disease, geriatric patients, hip fractures, broad hospital populations, and general populations 15. Albumin was also included in the original APACHE score for risk assessment of intensive care patients 16. National or international cut‐off values are usually applied when using biomarkers for risk assessment and it is thus important that the calibration of the methods used for setting the cut‐off values and the local albumin method do not differ.
Albumin is usually measured with dye‐binding assays such as bromocresol green (BCG) or bromocresol purple (BCP) or with immunological assays. The BCG methods have been reported to overestimate albumin because of interference by acute‐phase proteins 17, 18, 19, while the BCP methods have been reported to have a good agreement with immunological albumin methods 20, 21. The calibrations of current commercial albumin assays are usually traceable to the international protein calibrator European reference material (ERM)‐DA470k/IFCC 22, 23. With the same calibrator, the tests should give similar results. At our laboratory we have two plasma albumin assays: a BCP assay on an Architect 16000 available around the clock and an immunoturbidimetric assay on a Cobas c 501 instrument available during office hours.
We had initial indications that our two albumin methods did not provide the same results. We thus decided to compare the two methods more closely with patient samples to evaluate if there were differences that could have clinical implications. We also wanted to compare the agreement at different albumin concentrations as it has previously been reported that the absolute overestimates by dye methods are greater in the low albumin range, because these patients often have elevation of other proteins that could react with the dye.
Materials and Methods
Samples
Test results were extracted from laboratory information system at the Department of Clinical Chemistry and Pharmacology, Uppsala University Hospital, Uppsala. Only test results for individuals with complete personal numbers were extracted. The results were extracted together with information of age and sex of the patient. The study was approved by the local ethical board (01‐367) and complied with the World Medical Association Declaration of Helsinki regarding ethical conduct of research. The samples were collected in Li‐heparin PST tubes (366567, Becton, Dickinson, and Company; Franklin Lakes; NJ) for both albumin methods. Fresh patient samples were collected at Akademiska University Hospital in Uppsala and sent to Karolinska University Hospital in Stockholm (Beckman Coulter Immage and Roche Diagnostics Modular P) and Falun (Architect c8000) for comparison of albumin results on different instrument platforms.
Albumin Methods
In Uppsala, albumin was measured immunoturbidimetrically with reagent (product number 04469658190) from Roche Diagnostics (Mannheim, Germany) on a Cobas c 501 (Roche Diagnostics). The method is calibrated using CFAS calibrator from Roche Diagnostics, which is traceable to the international protein calibrator ERM‐DA470k/IFCC. The total coefficient of variation (CV) for the immunoturbidimetric albumin method was 2.8% at 24.4 g/l and 2.8% at 48 g/l. Albumin was also measured with BCP reagent (7D54‐21) from Abbott Diagnostics (Abbott Park, IL) on an Architect 16000 (Abbott Laboratories). The method was calibrated using Multiconstituent Calibrator 1E65‐05 from Abbot Laboratories, which is also traceable to ERM‐DA470k/IFCC. The total CV for the BCP method was 0.3% at 39 g/l and 0.4% at 49 g/l.
Albumin was also measured with Roche Diagnostic BCP reagent and Abbot Diagnostics BCP reagent on the Cobas c 501 as a comparison. In the second round, albumin was also measured with fresh patient samples on several instrument platforms with different immunological methods; Beckman Coulter Immage (Stockholm), Roche Diagnostics Modular P (Stockholm), Roche Diagnostics Cobas c 501 (Uppsala), and Abbott Laboratories Architect c 8000 (Falun). The total CVs for albumin were 7% at 26 g/l and 5% at 37 g/l for the Beckman Coulter Immage method, 1.5% at 25 g/l and 1.2% at 43 g/l for the Roche Diagnostics Modular P method and 1.0% at 40.9 g/l for the Architect method (Falun).
Statistical Analysis
Linear regression analysis and median differences were calculated using Excel 2013.
Results
Comparison Between Architect and Cobas Methods for Plasma Albumin
Requests for albumin measured on 1,951 patients assayed both with the Architect BCP and Cobas c501 immunoturbidimetric methods were extracted from the laboratory system during the time period January 1, 2013, to February 10, 2014. The linear regression equation was y = 1.047x + 1.687, R² = 0.930, with the cobas 501 method as the dependent (y) (Fig. 1). The median difference between the two methods was 3.3 g/l.
Figure 1.
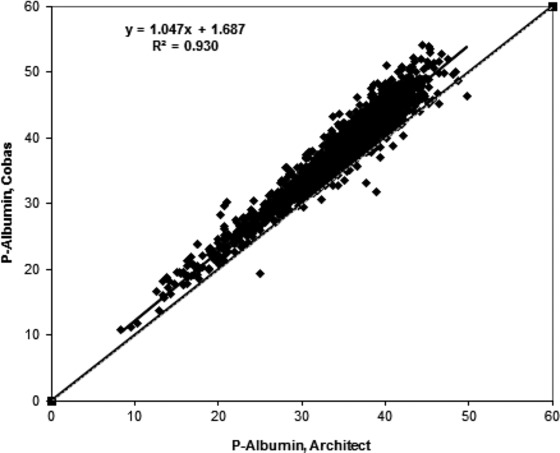
Comparison between Architect and Cobas methods for plasma albumin. The comparison is based on simultaneous requests for both methods (n = 1,951).
Comparison Between Architect BCP (Uppsala) and Immunoturbidimetric Methods on the Cobas Instrument
A total of 43 samples were analyzed on the Cobas instrument with Roche immunoturbidimetric and BCP methods and Abbott BCP method. There was a good agreement between the two BCP methods when analyzed on the Cobas instrument: y = 0.987x – 0.453, R² = 0.996. The Roche immunoturbidimetric method gave higher values than the Roche BCP method with patient samples: y = 1.111x – 0.739, R² = 0.971. The corresponding equation for the internal controls was y = 1.063x – 0.542, R² = 0.991, indicating that the median difference between the two methods when analyzing nine internal laboratory control samples was smaller than for the patient samples (Fig. 2).
Figure 2.
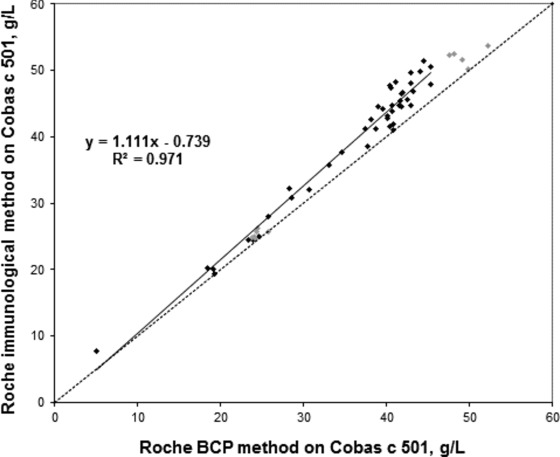
Comparison between the Roche BCP method (x‐axis) and immunological method (y‐axis) on Cobas c 501. Black markers are patient samples and gray markers are internal laboratory controls. The equation presented in the figure is based on the patient samples. The equation for the internal controls was y = 1.063x – 0.542 (R² = 0.991).
Comparison Among Architect BCP (Falun), Immage Immunological (Stockholm), Modular P (Stockholm), and Cobas c 501 Immunoturbidimetric (Uppsala) Methods With Simultaneous Measurement of ERM‐DA470k/IFCC
A total of 20 patient samples were analyzed with immunological methods on Immage, Modular P, and Cobas c501 instruments and with BCP method on Architect c 8000 instrument. Albumin concentrations varied between 13 and 38 g/l in samples analyzed with BCP method on Architect c 8000. Results from Cobas c501 instrument were generally higher than results obtained with other instruments. The mean difference between results obtained with Immage and Architect was 1.2 g/l, between Modular P and Architect 1.4 g/l and between Cobas c501 and Architect 2.5 g/l.
ERM‐DA470k/IFCC has an assigned value of 37.2 g/l. When analyzed with the Cobas immunoturbidimetric assay the result was 39.4 g/l. Architect reported a value of 37.3, Immage 36.6 and Modular P 37.6 g/L (Fig. 3). The equation for the correlation between Architect (Falun) and Cobas c 501 (Uppsala) with 19 patient samples was y = 0.954x + 3.869 (R² = 0.958).
Figure 3.
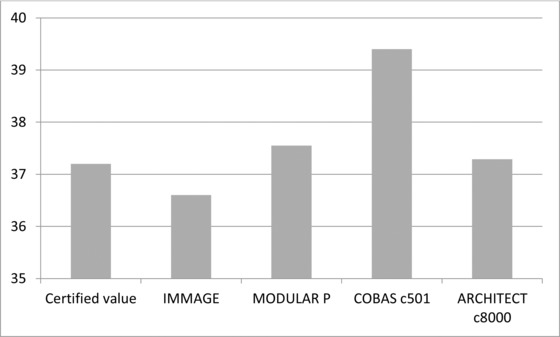
Test results obtained with the international protein calibrator ERM‐DA470k/IFCC analyzed on Immage, Modular P, Cobas c 501, and Architect c8000. The ERM calibrator was run in duplicate on each instrument.
Discussion
The laboratory in Uppsala has two albumin methods, and the BCP method is a STAT test with test turnaround times of approximately 40 min. The immunoturbidimetric method is used in conjunction with our plasma protein electrophoresis and the test turnaround time is usually >24 hours. The number of immunoturbidimetric albumin tests performed is less than 10% of the bromocresol tests performed. We used to consider the immunological method superior to the BCP method especially for patients with low albumin values. This was the reason for having two albumin methods. It is an old saying that dye‐binding assays overestimate albumin in the low range (<25–35 g/l) since patients with low albumin values often have elevated levels of other proteins that could interfere with the dye‐based albumin methods 24, 25. This could lead to misclassification of patients.
The immunoturbidimetric Cobas c 501 method overestimates the albumin values in patient samples by slightly more than 3 g/l in comparison with the Abbott BCP method. The overestimation by the immunoturbidimetric method is supported by the result obtained with the ERM‐DA470k/IFCC calibrator and the results obtained with the Roche BCP method.
When we compare the results obtained with the immunoturbidimetric Roche method and the dye method on the Architect, there is no indication that the BCP method gives higher results than the immunoturbidimetric method at least down to 10 g/l. Less than 0.1% of our albumin requests have albumin values below 10 g/l and when we look at repeated results in the same patient the samples give similar results from day to day, indicating that the results from individual patients can be monitored over time.
Immunological methods have higher reagents costs than dye‐binding assays. Immunological assays thus have to provide higher‐quality test results to motivate the higher costs. Looking at the present data, it could be questioned if the increased cost for the c 501 method could be motivated. To us, it is not acceptable to have two albumin methods that differ by more than 3 g/L.
In conclusion, we here report data strongly indicating that the immunoturbidimetric albumin method on Cobas c 501 is not correctly calibrated according DA470k/IFCC and thus overestimate the albumin concentration in fresh patient plasma samples. The methods differences were smaller for internal controls. The results show the importance of using fresh patient samples for method comparisons.
Conflict of interest
The authors declare that they have no conflicts of interest.
Grant sponsor: Uppsala University Hospital Research Fund.
References
- 1. Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus 2013;11 Suppl 4:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth 2000;85:599–610. [DOI] [PubMed] [Google Scholar]
- 3. Caraceni P, Domenicali M, Tovoli A, et al. Clinical indications for the albumin use: Still a controversial issue. Eur J Intern Med 2013;24:721–728. [DOI] [PubMed] [Google Scholar]
- 4. Chen TA, Tsao YC, Chen A, et al. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol 2009;44:619–625. [DOI] [PubMed] [Google Scholar]
- 5. Fanali G, di Masi A, Trezza V, et al. Human serum albumin: From bench to bedside. Mol Aspects Med 2012;33:209–290. [DOI] [PubMed] [Google Scholar]
- 6. Garcia‐Martinez R, Caraceni P, Bernardi M, et al. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836–1846. [DOI] [PubMed] [Google Scholar]
- 7. Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab 2013;59:945–952. [PubMed] [Google Scholar]
- 8. Holliday MA. Extracellular fluid and its proteins: Dehydration, shock, and recovery. Pediatr Nephrol 1999;13:989–995. [DOI] [PubMed] [Google Scholar]
- 9. Cartotto R, Callum J. A review of the use of human albumin in burn patients. J Burn Care Res 2012;33:702–717 [DOI] [PubMed] [Google Scholar]
- 10. Cabrerizo S, Cuadras D, Gomez‐Busto F, et al. Serum albumin and health in older people: Review and meta‐analysis. Maturitas 2015;81:17–27. [DOI] [PubMed] [Google Scholar]
- 11. Ong C, Han WM, Wong JJ, Lee JH. Nutrition biomarkers and clinical outcomes in critically ill children: A critical appraisal of the literature. Clin Nutr 2014;33:191–197. [DOI] [PubMed] [Google Scholar]
- 12. Fujii T, Sutoh T, Morita H, et al. Serum albumin is superior to prealbumin for predicting short‐term recurrence in patients with operable colorectal cancer. Nutr Cancer 2012;64:1169–1173. [DOI] [PubMed] [Google Scholar]
- 13. Han S, Huang Y, Li Z, Hou H, Wu A. The prognostic role of preoperative serum albumin levels in glioblastoma patients. BMC Cancer 2015;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin MY, Liu WY, Tolan AM, et al. Preoperative serum albumin but not prealbumin is an excellent predictor of postoperative complications and mortality in patients with gastrointestinal cancer. Am Surg 2011;77:1286–1289. [PubMed] [Google Scholar]
- 15. Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol 1997;50:693–703. [DOI] [PubMed] [Google Scholar]
- 16. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 17. Brackeen GL, Dover JS, Long CL. Serum albumin. Differences in assay specificity. Nutr Clin Pract 1989;4:203–205. [DOI] [PubMed] [Google Scholar]
- 18. Speicher CE, Widish JR, Gaudot FJ, Hepler BR. An evaluation of the overestimation of serum albumin by bromcresol green. J Clin Pathol 1978;69:347–350. [DOI] [PubMed] [Google Scholar]
- 19. Gustafsson JE. Improved specificity of serum albumin determination and estimation of "acute phase reactants" by use of the bromcresol green reaction. Clin Chem 1976;22:616–622. [PubMed] [Google Scholar]
- 20. Pinnell AE, Northam BE. New automated dye‐binding method for serum albumin determination with bromcresol purple. Clin Chem 1978;24:80–86. [PubMed] [Google Scholar]
- 21. Wells FE, Addison GM, Postlethwaite RJ. Albumin analysis in serum of haemodialysis patients: Discrepancies between bromocresol purple, bromocresol green and electroimmunoassay. Ann Clin Biochem 1985;22:304–309. [DOI] [PubMed] [Google Scholar]
- 22. Merlini G, Blirup‐Jensen S, Johnson AM, et al. IFCC Committee on Plasma Proteins (C‐PP). Standardizing plasma protein measurements worldwide: A challenging enterprise. Clin Chem Lab Med 2010;48:1567–1575. [DOI] [PubMed] [Google Scholar]
- 23. Zegers I, Keller T, Schreiber W, et al. Characterization of the new serum protein reference material ERM‐DA470k/IFCC: Value assignment by immunoassay. Clin Chem 2010;56:1880–1888. [DOI] [PubMed] [Google Scholar]
- 24. Geen RJ, Day BS, Powers DM. Multilayer‐film bromcresol green method for albumin measurement significantly inaccurate when albumin/globulin ratio is <0.8. Clin Chem 1991;37:767–768. [PubMed] [Google Scholar]
- 25. Webster D, Bignell AHC, Attwood EC. An assessment of the suitability of bromcresol green for the determination of serum albumin. Clin Chem Acta 1974;53:101–108. [DOI] [PubMed] [Google Scholar]


