Abstract
Background
Neutrophil gelatinase‐associated lipocalin (NGAL) is a new useful biomarker for the early diagnosis of acute kidney injury. The aim of the study was to compare two analytical methods for measurement of urinary NGAL: enzyme‐linked immunosorbent assay (ELISA) and chemiluminescent microparticle immunoassay (CMIA).
Methods
Two assays were used to measure urinary NGAL: ELISA kit (R&D Systems) and ARCHITECT Urine NGAL (Abbott Laboratories). The study material was the urine obtained from 30 healthy subjects (mean age 56.4 ± 15.2).
Results
The median value and interquantile range of urinary NGAL in the studied group measured by ELISA (R&D Systems) were 3.5 ng/ml (1.2; 6.6) and by CMIA (ARCHITECT Urine NGAL assay, Abbott Diagnostics) were 4.4 ng/ml (1.9; 9.4). Levels of urinary NGAL obtained by CMIA were significantly higher than by ELISA. There was a significant positive correlation between the concentration of urinary NGAL determined by both methods (r = 0.8625, P < 0.01).
Conclusion
The comparison of individual data obtained by ELISA and CMIA should be taken with care. From laboratory's point of view, ELISA is less expensive than CMIA method for the determination of NGAL in urine. However, CMIA method allows rapid determination of urinary NGAL concentration through automated assay.
Keywords: neutrophil gelatinase‐associated lipocalin, lipocalin‐2, acute kidney injury, biomarker, renal diseases, immunoassay
Introduction
Acute kidney injury (AKI) is a common complication in hospitalized patients, particularly intensive care patients. A rapid development of lesions within the kidneys, diagnostic difficulties in detecting early stages of disease, and high mortality of patients encourages the continuous search for new and more sensitive markers of the disease 1, 2, 3, 4. Many studies have reported great importance of neutrophil gelatinase‐associated lipocalin (NGAL) as an early indicator of AKI 2, 3, 4, 5.
Neutrophil gelatinase‐associated lipocalin (NGAL) also known as lipocalin 2, is a protein belonging to the family of lipocalins, present in the organism in the form of monomer, dimer, and the complex covalently bonded with metalloproteinase 9 4, 6. The tertiary structure of NGAL takes β‐barrel shape with interior that binds ligand. Siderophores, molecules produced by microorganisms that bind to iron, are ligand for NGAL. Thanks to this property, NGAL plays bacteriostatic functions. NGAL plays a role in cell proliferation and differentiation as it is involved in iron transportation and regulation of genes dependent on this element 2, 4, 6, 7. NGAL was detected for the first time in the granules of neutrophils. NGAL is also expressed in other cells of the organism, especially in cells of renal tubules of kidney, liver, lung, prostate, stomach, and colon, among others. Increased NGAL expression was observed in sepsis‐induced epithelial tissue damage, ischemia, or inflammation 2, 4, 6. Numerous studies confirm the increase of the concentration of NGAL in serum and urine of patients who experienced the development of AKI 5, 8, 9, 10.
Determination of NGAL is then of growing importance. Hence the question arises: what method should be applied for the determination? Currently, there are few tests for determining NGAL. Two commonly used methods are enzyme‐linked immunoenzymatic assay (ELISA) and chemiluminescence immunoassay (CLIA). The aim of the study was to compare the measurement of urinary NGAL labeled with ELISA assay and commercial chemiluminescent microparticle immunoassay (CMIA) assay using the ARCHITECT platform.
Material and Methods
The tests performed were approved by the Local Bioethics Committee of Warsaw Medical University (No. KB/10/2010). Material used was a urine obtained from 30 healthy subjects aged 20–90 years (56.4 ± 15.2), 16 women and 14 men. The inclusion criteria were no medical history of chronic diseases and written and informed consent to participate in the study. The urine came from the first morning voiding. The collected urine sample was centrifuged for 10 min at 2,000 × g at room temperature and then filtered with a 0.42 μm filter and syringe. The urine was stored at −80°C until performing the assays.
Determination of the Concentration of NGAL in the Urine Using the ARCHITECT Urine NGAL
The first indication of the concentration of NGAL in urine was performed using CMIA method: the ARCHITECT Urine NGAL assay (Abbott Diagnostics, Abbott Park, IL, USA) based on CMIA, using the ARCHITECT i1000 analyzer from Abbott Diagnostics. ARCHITECT Urine NGAL assay uses microparticles coated with NGAL mouse monoclonal anti‐NGAL antibodies and the conjugate of anti‐NGAL antibodies associated with acridine. NGAL assay was performed according to the manufacturer's instructions described in the assay procedure. Prior to the measurement of urinary NGAL concentration, calibration test was carried out in the concentration range of 0‐1,500 ng/ml and control. In accordance with the assay procedure, urine was thawed at room temperature and then stirred before the measurement, in order to obtain a homogeneous sample. Determination of the concentration of NGAL in the sample was based on the measurement of chemiluminescence by optical analyzer ARCHITECT iSystem optics. Sensitivity of the ARCHITECT Urine NGAL assay specified by manufacturer ranged from 1.5 to 3 ng/ml for the limit of quantitation (LOQ) and from 0.7 to 1 ng/ml for the limit of detection.
Determination of the Concentration of Urinary NGAL Using ELISA
Second determination of the NGAL concentration in urine was performed with ELISA. A kit with solid‐phase ELISA “sandwich” ELISA: Human Lipocalin‐2/NGAL Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) with antibody against human NGAL was used for this method. Urine was thawed at room temperature right before the assay performance. An ELISA assay was carried out strictly according to the procedure specified by the manufacturer. The NGAL concentration measurements were performed in two repetitions and against so‐called blind trial. Absorbance measurement was performed at two wavelengths: 450 nm and 540 nm using PowerWave HT Microplate Spectrophotometer from Biotek. The LOQ for ELISA (R&D Systems) is 0.156 ng/ml. Sensitivity specified by manufacturer is 0.04 ng/ml.
Development of Statistical Data
STATISTICA 10.0 and MedCalc 13.0.6 were used for statistical analysis. In order to evaluate the relationship between the results of both methods, Pearson correlation coefficient was used. To verify the existence of significant differences between the values of the concentration of urinary NGAL determined by ELISA and CMIA, Passing–Bablok regression was used. It allowed for comparison of two methods 11. The significance of differences was also evaluated by Wilcoxon's test after verification of the hypothesis of normality by the Shapiro–Wilk test.
Additional statistical method was the analysis of the Bland–Altman plot. It showed the degree of accordance between the two techniques and the dispersion of data. On the x‐axis, the plot describes average values obtained in the first and second methods, while the y‐axis shows difference of values obtained by these methods. In addition to above, Bland–Altman plot also includes the average of the differences between the above methods and this value increased and decreased by 1.96 standard deviation difference.
Results
The results of concentration of urinary NGAL obtained by ELISA (Human Lipocalin‐2/NGAL Quantikine ELISA kit, R&D Systems) and CMIA (ARCHITECT Urine NGAL assay, Abbott Diagnostics) are presented in Table 1.
Table 1.
Characteristics of Determined Parameters
| Urine level of NGAL (CMIA)a (ng/ml) | Urine level of NGAL (ELISA)b (ng/ml) | r | P | |
|---|---|---|---|---|
| N | 30 | 30 | — | — |
| Mean ± SD | 7.0 ± 6.7 | 5.5 ± 6.3 | 0.8625 | <0.01 |
| Median | 4.4 | 3.5 | — | — |
| 95% Cl for the median | 2.3–8.9 | 1.4–6.2 | — | — |
| Interquartile range | 1.9–9.4 | 1.2–6.6 | — | — |
| Min. | 0.2 | 0.4 | — | — |
| Max. | 28.6 | 25.7 | — | — |
NGAL (CMIA)—NGAL determined in urine with ARCHITECT Urine NGAL (Abbott Laboratories).
NGAL (ELISA)—urinary level of NGAL was assessed by Human Lipocalin‐2/NGAL Quantikine ELISA kit (R&D Systems).
Urinary NGAL range obtained was 0.4–25.7 ng/ml for ELISA and 0.2–28.6 ng/ml for CMIA. The median value, interquartile ranges of urinary NGAL values obtained by ELISA and CMIA were 3.5 ng/ml (1.2; 6.6) and 4.4 ng/ml (1.9; 9.4), respectively.
The assessment of the relationship shows a significant positive correlation between the concentration of NGAL in urine as determined by CMIA on the ARCHITECT platform (Abbott Diagnostics) and by ELISA kit (R&D Systems) (r = 0.8625, P < 0.01). Figure 1 shows the results.
Figure 1.
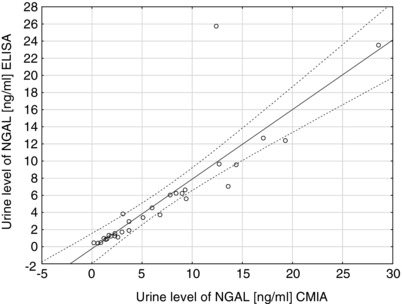
Correlation of urine NGAL level measured by chemiluminescent microparticle immunoassay (CMIA) (Abbott ARCHITECT Urine NGAL) and immunoenzymatic assay ELISA (R&D Systems) (r = 0.8625; P < 0.01).
Statistical analysis of regression by Passing–Bablok method shows no significant deviation from linearity between the results of measurement of urinary NGAL values by ELISA kit (R&D Systems) and CMIA (Abbott Diagnostics) (P = 0.91). Figure 2 shows the results for the comparison.
Figure 2.
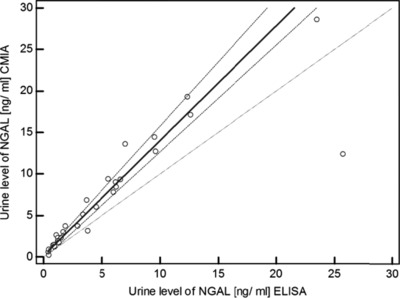
Passing–Bablok regression of urine NGAL level measured by chemiluminescent microparticle immunoassay (CMIA) (Abbott ARCHITECT Urine NGAL) and immunoenzymatic assay ELISA (R&D Systems).
Analysis of regression by Passing–Bablok method showed the dependence shown by the equation: y = 0.1998 + 1.3830x. The interpretation of the graph indicates the existence of a constant, small difference between the concentrations of urinary NGAL measured by CMIA method using platform ARCHITECT (Abbott Diagnostics) and ELISA (R&D Systems). The coefficients of the regression equation are as follows: a = 0.1998 (95% CI: −0.20, 0.34), and b = 1.3830 (95% CI: 1.29, 1.54). The Wilcoxon test showed a statistically significant higher levels of urinary NGAL by CMIA in comparison to ELISA (P = 0.0001).
Comparison of the results obtained by CMIA (Abbott Diagnostics) and ELISA (R&D Systems) using Bland–Altman analysis basing on assessment of differences (Fig. 3) shows that 97% of the values falls within a specified interval (mean ± 1.96 SD).
Figure 3.
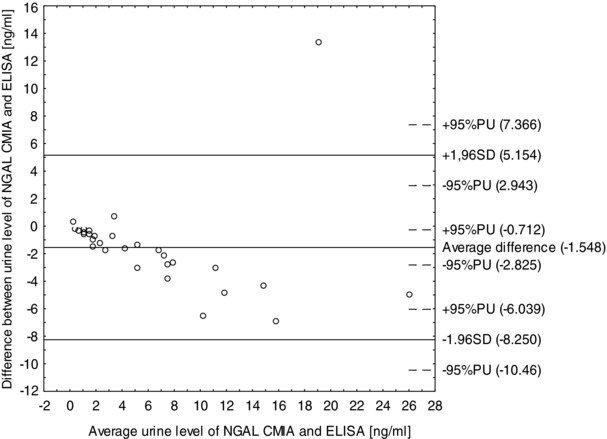
Difference between urine NGAL level measured by chemiluminescent microparticle immunoassay (CMIA) (Abbott ARCHITECT Urine NGAL) and immunoenzymatic assay ELISA (R&D Systems) (ng/ml) versus mean urine NGAL level measured by CMIA method and ELISA method (ng/ml).
Discussion
NGAL expression in various disease states is of the scientists' interest. Studies are conducted to determine the usefulness of the assessment of the level of NGAL in various fields of medicine. Many publications describing NGAL indicate the clinical usefulness of this protein in nephrology. NGAL measurement role is especially important in the diagnosis of AKI 2, 3, 4. Mishra and colleagues showed increased concentrations of NGAL in urine and serum 2 hr after operation when assessing the level of this lipocalin in children undergoing cardiac surgery. This significantly outstripped the increase in serum creatinine (kidney damage indicator). Elevated levels of creatinine were only observed after a few days from the surgery 12. The high prognostic value of NGAL level in the urine in the diagnosis of AKI was also shown by Wagener and colleagues 9. In addition, the results of the studies conducted in last years, indicate the possibility of the use of NGAL determination in patients with chronic kidney disease 13.
However, recently published studies supply information about the predominant secretion of NGAL monomeric form by epithelial cells of injured renal distal tubules, while dimer form of NGAL are released by neutrophils. Several forms of NGAL constitute a problem with differentiation of the origin of NGAL in the urine between the renal or nonrenal source from neutrophils. Nowadays, some limitations are that the existing tests for measuring NGAL do not allow to measure exclusively of renal NGAL 14. Currently, there are few tests for determining of NGAL. The NGAL ARCHITECT platform and commercial ELISA's are available assays that detected predominantly monomeric form of NGAL 14. Referring to study conducted by Kift and colleagues, ELISA (R&D Systems) and ARCHITECT Urine NGAL are characterized by the best testing precision, selectivity, accuracy parallelism, and LOQ from five compared commercial tests 15. ELISA method was used in most studies determining concentration of NGAL in the urine.
An important limitation of ELISA is usage of assay for single measurements of the concentration of NGAL in the urine. Unfortunately, determination of the concentration of urinary NGAL by solid‐phase ELISA kit (R&D Systems) is both time consuming and laborious. Due to the manual execution of the assay, the ELISA is not a standardized method. The disadvantage of ELISA kit from R&D Systems is long time of waiting for the results. Solid‐phase ELISA assay can be used for the determination of NGAL for scientific purposes. Unfortunately, the ELISA has many drawbacks that hinder its use in the routine determination of NGAL in clinical diagnosis 4, 16.
One of the newer methods allowing for the determination of the concentration of urinary NGAL is CMIA used in a commercial assay ARCHITECT Urine NGAL for determining the concentration of NGAL by automated platform ARCHITECT (Abbott Diagnostics). The advantage of CMIA assay is about ten times shorter time of measurement of NGAL in the urine, compared to Human Lipocalin‐2/NGAL Quantikine ELISA kit from R&D Systems. The results of the concentration of NGAL in the urine using the ARCHITECT platform are obtained in about 30 min.
Taking into account the economic aspect, it may be said that the price per test to determine concentrations of one sample is comparable. Higher price of CMIA kit is balanced by the possibility of measuring without repetition. However, a key limitation to CMIA assay applications is the availability and cost of the apparatus.
In this study, a correlation of the concentration of urinary NGAL is measured by ELISA and CMIA using ARCHITECT platform. Correlations between ELISA and CMIA were also confirmed by Grenier and colleagues 17. Basing on the survey, a team of scientists from Abbott Laboratories showed that the test for the determination of NGAL on the ARCHITECT platform is characterized by a good precision and reproducibility and sensitivity. Similar conclusions were presented by Kift and colleagues; they compared five commercial tests for the determination of the concentration of NGAL in the urine 15. Bennett with colleagues demonstrated the importance of NGAL as an early biomarker of AKI in patients after cardiac surgery. They measured concentration of NGAL using the ARCHITECT platform. According to the results obtained by the Bennett's team, increase in urinary NGAL levels correlate with severity of renal injury and may be useful in early detection of pathologies. Also in this study, the correlation of results obtained by ELISA and CMIA was confirmed 18. This confirms the possibility of using the automated assay for clinical CMIA determination of NGAL in the urine, particularly useful in the diagnosis of AKI. Measuring the concentration of NGAL in urine on the ARCHITECT platform may be one of the routine laboratory parameters determined in the assessment of renal function in patients at particular risk for AKI.
A comparison of two methods of ELISA and CMIA showed significant differences between measured concentrations of NGAL in the urine. Statistical analysis of the results showed that the concentrations of NGAL marked by the ARCHITECT Urine NGAL assay are significantly higher than that by ELISA from R&D Systems. This suggests that NGAL concentration results cannot be fully comparable between described methods. The interpretation of these data is not clear. Still accurate statistical procedure to compare methods was not developed. The reasons for the reported differences may result from different measurement technologies, differences in the manufacturer's reagents, patterns, and antibodies.
Both methods can be applied for measuring NGAL concentration in urine but the comparison of data assess by ELISA and CMIA should be taken with care. In spite of approximate average between the two methods, comparing individual results require corrective action. From laboratory's point of view, ELISA is less expensive than CMIA method for determination NGAL in urine. However, CMIA method allows rapid determination of urinary NGAL concentration through automated assay and it may be a convenient parameter for the routine diagnosis of renal diseases in the urine.
Conflict of Interest
The authors report no conflicts of interest.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supplementary Material for Review NK.pdf
Supplementary Material for Review LP.pdf
Supplementary Material for Review EK.pdf
Supplementary Material for Review AWT.pdf
References
- 1. Case J, Khan S, Khalid R, Khan A. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013;2013:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Devarajan P. Review: Neutrophil gelatinase‐associated lipocalin: A troponin‐like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–428. [DOI] [PubMed] [Google Scholar]
- 3. Devarajan P. Neutrophil gelatinase‐associated lipocalin (NGAL): A new marker of kidney disease. Scand J Clin Lab Invest Suppl 2008;241:89–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase‐associated lipocalin (NGAL) as biomarker of acute kidney injury: A review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med 2012;50:1505–1517. [DOI] [PubMed] [Google Scholar]
- 5. Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Goldstein SL urine neutrophil gelatinase‐associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit Care 2007;11:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kowsalya R, Jagatheesh K, Padarthi Pavan Kumar, Elangovan N. A functional approach on neutrophil gelatinase associated lipocalin (NGAL). IJB 2013;2:5. [Google Scholar]
- 7. Schmidt‐Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase‐associated lipocalin. J Am Soc Nephrol 2007;18:407–413. [DOI] [PubMed] [Google Scholar]
- 8. Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, et al. Urinary neutrophil gelatinase‐associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med 2009;47:79–82. [DOI] [PubMed] [Google Scholar]
- 9. Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, et al. Association between increases in urinary neutrophil gelatinase‐associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 2006;105:485–491. [DOI] [PubMed] [Google Scholar]
- 10. Haase‐Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase‐associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann Clin Biochem 2014;51:335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bilic‐Zulle L. Comparison of methods: Passing and Bablok regression. Biochem Med (Zagreb) 2011;21:49–52. [DOI] [PubMed] [Google Scholar]
- 12. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231–1238. [DOI] [PubMed] [Google Scholar]
- 13. Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio RM, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 2009;4:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haase‐Fielitz A1, Haase M, Devarajan P. Neutrophil gelatinase‐associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann Clin Biochem. 2014;51(03):335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kift RL, Messenger MP, Wind TC, Hepburn S, Wilson M, Thompson D, et al. A comparison of the analytical performance of five commercially available assays for neutrophil gelatinase‐associated lipocalin using urine. Ann Clin Biochem 2013;50:236–244. [DOI] [PubMed] [Google Scholar]
- 16. Cavalier E, Bekaert AC, Carlisi A, Legrand D, Krzesinski JM, Delanaye P. Neutrophil gelatinase‐associated lipocalin (NGAL) determined in urine with the Abbott Architect or in plasma with the Biosite Triage? The laboratory's point of view. Clin Chem Lab Med 2011;49:339–341. [DOI] [PubMed] [Google Scholar]
- 17. Grenier FC, Ali S, Syed H, Workman R, Martens F, Liao M, et al. Evaluation of the ARCHITECT urine NGAL assay: Assay performance, specimen handling requirements and biological variability. Clin Biochem 2010;43:615–620. [DOI] [PubMed] [Google Scholar]
- 18. Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol 2008;3:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supplementary Material for Review NK.pdf
Supplementary Material for Review LP.pdf
Supplementary Material for Review EK.pdf
Supplementary Material for Review AWT.pdf


