Abstract
Background
VTCN1, a T‐cell regulator, belongs to the immunoglobulin superfamily. It is more highly expressed in tumor tissues than in normal tissues, which suggests that it could serve as a tumor‐related agent. We hypothesize the gene variants for this coinhibitory molecule may be associated with the risk of breast cancer, given such gene polymorphisms could affect its related gene expression.
Methods
Genotypes of the VTCN1 gene variants (rs10754339, rs10801935, and rs3738414) were analyzed in 566 patients with breast cancer and 400 age‐frequency‐matched controls.
Results
Compared with the major allele, the minor alleles of rs10754339, rs10801935, and rs3738414 did modulate the risk of breast cancer with ORs (95% CI) of 1.42 (1.07–1.89), 1.39 (1.10–1.77), and 0.81 (0.67–0.99), respectively. Those with the rs10754339 genotype AG and rs10801935 AC genotype had significantly increased risks when compared with their major genotypes. However, in rs3738414, the AA genotype had a marginally significant decreased risk compared with its wild genotype. In the haplotype‐based analysis, the GCG allele was associated with significantly increased risk (OR: 1.56, 95% CI: 1.09–2.22) based on the AAG reference. Further analyses of the haplotype pairs showed GCG carriers had a significantly increased risk.
Conclusions
In this study, the VTCN1 genetic variants (rs10754339, rs10801935, and rs3738414) indicate they could be connected with the risk of breast cancer, which in turn provides indirect evidence that T‐cell immunity could be involved in the development of breast cancer.
Keywords: T cells, VTCN1, breast cancer, polymorphisms
Abbreviations
- APC
antigen‐presenting cells
- CI
confidence interval
- OR
odds ratio
- PCR‐RFLP
polymerase chain reaction‐ restriction fragment length polymorphism
- SNPs
single nucleotide polymorphisms
- TCR
T‐cell receptors
- VTCN1
V‐set domain containing T‐cell activation inhibitor 1
INTRODUCTION
Breast cancer has become one of the main public health issues for women in Taiwan as well as those in Western nations. Risk factors for breast cancer include both nonmodifiable factors such as genetics and age, and modifiable factors such as lifestyle and the environment 1. Though a simple cause‐and‐effect relationship between immunity of a living body and cancer remains elusive, the fact that a deficient immune system contributes to the development of cancer is indisputable 2.
T lymphocytes, which are the key players in the adaptive immune system which eradicates cancer cells in a living organism, are able to recognize and specifically respond to an immense variety of foreign and native antigens on the surface of cancer cells. To ensure an appropriate T‐cell response, which is essential to eliminate pathogens and to maintain self‐tolerance, T‐cell activation is finely regulated by the antigen dependent and antigen independent signaling pathways. The second signal is delivered by comodulatory molecules of the B7/CD28 family including VTCN1 which plays a negative regulating role in contrast to the other B7 family members. As a matter of fact, in the laboratory, VTCN1 inhibited T‐cell activation through restraining IL‐2 production and arresting the cell cycles of CD4+ and CD8+ cells 3. Clinically, VTCN1 protein has been reported to be over‐expressed in many kinds of cancers, including lung 4, gastric 5, ovarian 6, and breast cancer 7, which strongly suggests VTCN1 protein may be promoting cancer cell development by helping cancer cells escape from being tracked down by T‐cells. And even though the overexpression of VTCN1 protein in patients with metastatic breast cancer has been fully documented 7, evidence on the relationship between VTCN1 and the risk of breast cancer is still limited. Many researchers have shown that certain single nucleotide polymorphisms (SNPs) of VTCN1 can alter protein expression and/or function, which makes the investigation of the potential relationship between these SNPs and the risk of breast cancer possible 8. Therefore, in this article, a hospital‐based control study, we explore the relationship and interaction between SNPs of VTCN1 and the resulting susceptibility to breast cancer.
MATERIALS AND METHODS
Subjects
The study was conducted at the Kaohsiung Medical University under a project approved by the Ethics Committee of Kaohsiung Medical University Hospital. Informed consent, as well as demographic information including age, age of menopause commencement, and other related information were obtained from participants by questionnaires at the time of blood sampling. A total of 566 breast cancer patients and 400 controls were enrolled from Kaohsiung Medical University Hospital, Kaohsiung, Taiwan in this study. Women who had a first diagnosis of histopathologically confirmed breast cancer, from whom blood samples were available, were selected as test cases. None of the cases had a strong family or early onset breast cancer (under 35 years of age) history. Females, without current or previous history of breast cancer, were recruited as control subjects when they conducted their routine annual general health check‐ups in Kaohsiung Medical University Hospital. Women with benign breast tumors, mastitis, benign calcification, other malignant diseases such as lung or liver cancer, or autoimmune diseases, which might be related to polymorphisms of VTCN1, were excluded from both the case and control groups. Detailed clinical information about the study subjects will be described in a latter section and listed in Table 1. However, a brief description is as follows: The present study is based on 566 women with newly diagnosed breast cancer without any substantial therapy and 400 age‐frequency‐matched subjects without a significant history of breast disease serving as the control subjects, ranging in age from 39 to 80 years with the mean age of 50.8 ± 10.4 years and 42 to 65 years with the mean age of 51.8 ± 6.4 years, respectively. The study has been approved by the ethical committee of Kaohsiung Medical University and informed consent was obtained from all participants.
Table 1.
General characteristics of patients with breast cancer and controls
| Parameters | Patients (N = 566) | Controls (N = 400) | P‐value |
|---|---|---|---|
| Ages (yr, mean ± SD) | 50.8 ± 10.4 | 51.8 ± 6.4 | >0.05 |
| Menopausal status, N (%) | |||
| Premenopausal | 278 (49.1%) | 200 (50%) | >0.05 |
| Postmenopausal | 288 (50.9%) | 200 (50%) | |
| Cancer sites, N (%) | |||
| Left breast | 294 (51.9%) | – | |
| Right breast | 164 (46.7%) | – | |
| Bi‐sites | 8 (1.4%) | – | |
| Pathological diagnosis, N (%) | |||
| Ductal carcinoma | 500 (88.3%) | – | |
| Lobular carcinoma | 28 (5.0%) | – | |
| Both | 4 (0.7%) | – | |
| Histology differentiation, N (%) | |||
| Well | 44 (7.8%) | – | |
| Moderate | 372 (65.7%) | – | |
| Poor | 126 (22.3%) | – | |
| Unknown | 24 (4.2%) | – | |
| Tumor sizes (cm), N (%) | |||
| ≤2 | 320 (56.5%) | – | |
| >2—5 | 180 (31.8%) | – | |
| >5 | 28 (5%) | – | |
| Lymph‐node metastasis | |||
| Positive | 202 (35.7%) | – | |
| Negative | 364 (64.3%) | – | |
| Stages for TNM system, N (%) | |||
| In situ | 32 (5.7%) | – | |
| Stage I | 236 (41.7%) | – | |
| Stage II | 170 (30.0%) | – | |
| Stage III | 118 (20.8%) | – | |
| Stage IV | 10 (1.8%) | – | |
| Estrogen Receptor, N (%) | |||
| Positive | 378 (66.8%) | – | |
| Negative | 186 (32.9%) | – | |
| Unknown | 2 (0.3%) | – | |
| Progesterone Receptor, N (%) | |||
| Positive | 322 (56.9%) | – | |
| Negative | 242 (42.8%) | – | |
| Unknown | 2 (0.3%) | – | |
| HER‐2/neu, N (%) | |||
| Positive | 150 (26.5%) | – | |
| Negative | 410 (72.4%) | – | |
| Unknown | 6 (1.1%) | – | |
P‐value < 0.05: significant
Genotyping for single nucleotide polymorphism (SNP)
Blood samples from a total of 966 subjects were drawn into sterile tubes containing sodium EDTA. Leukocyte DNA was extracted from the buffy coat by using a modified phenol‐chloroform extraction method. The obtained DNA was resolved in a Tris‐EDTA buffer and stored at 4°C. The VTCN1 (rs3738414, rs10801935, and rs10754339) SNPs were determined by the polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP) (MJ Research PTC‐200 Peltier Thermal Cycle, Waltham, MA). The PCR‐RFLP assay consisted of two primers for PCR amplification of the sequence of the study and one restriction enzyme for the assay. For SNP rs3738414 (G > A), the G allele has no BtsI cleavage site, whereas the PCR product is cleaved into two fragments of 142 and 277 bp in the presence of A allele. For SNP rs10801935 (A > C), the A allele has no SalI cleavage site, whereas the PCR product is cleaved into two fragments of 226 and 240 bp in the presence of C allele. For SNP rs10754339 (A > G), the G allele has no MscI cleavage site, whereas the PCR product is cleaved into two fragments of 104 and 237 bp in the presence of A allele. The genotypes of the PCR products were confirmed by the DNA sequence analysis. In each experiment, DNA samples from the subjects, together with two or three previously sequenced DNA samples serving as quality controls (one for each genotype) to validate genotyping procedures, were concomitantly amplified by the PCR‐RFLP.
Statistical methods
Data were analyzed by SPSS 14.0 statistical package for Windows (SPSS Inc.). In brief, the unconditional logistic regression model was performed to calculate the odds ratios (OR) with their associated 95% confidence intervals (95% CI) between genotypes and alleles with and without breast cancer. For each SNP, the odds ratio and 95% CI were calculated for each genotype and then for combinations of genotypes. In addition, the haplotype‐based analysis was performed by software, named PLINK (ver. 1.07) 9, which is an open‐source whole genome association analysis toolset (http://pngu.mgh.harvard.edu/∼purcell/plink/), where the haplotype frequency of the combination which is less than 0.01 was neglected in the analysis. A two‐tailed P‐value of < 0.05 was considered to be statistically significant. The statistical power was evaluated by the software named power analysis and sample size (PASS) when the null hypothesis was rejected.
RESULTS
General clinical characteristics of the cases and controls
A total of 566 cases and 400 controls were enrolled in this study. There were nonsignificant differences in age distribution and menopausal status frequency between those two groups (Table 1). Moreover, clinical characteristics such as cancer site, pathology, and clinical stage distribution were similar to those described in the Taiwan Cancer Registry Annual Report 10. Other noteworthy information about the clinical characteristics as listed are as follows: (1) Up to 88% of the cases had ductal carcinoma according to their pathological diagnosis; (2) Around 66% of cases had moderate histological differentiation; (3) A tumor size of less than 2 cm was nearly 57% of the cases; (4) Fewer than two thirds of the cases had lymph‐node involvement; (5) Clinical classification of all of the cases was carcinoma in situ (5.7%), stage I (41.7%), stage II (30%), stage III (20.8%), and stage IV (1.8%) according to the TNM system; (6) The percentages of estrogen receptor positive, progesterone receptor positive, and HER‐2/neu positive were 66.8%, 56.9%, and 72.4%, respectively.
Independent effects of VTCN1 gene polymorphisms
The genotype and the allele frequencies of the three different VTCN1 gene polymorphisms in the cases and controls are listed in Tables 2 and 3, respectively. The results showed that all the allele distributions in rs10754339, rs10801935, and rs3738414 demonstrated statistically significant differences, the details of which are shown in the Tables. However, only certain genotype distributions were statistically significantly different in the cases and the controls (Table 2). For rs10754339, compared with the AA genotype, the AG genotype showed a significantly increased risk of breast cancer (OR = 1.49 with 95% CI = 1.07–2.04, P = 0.015), and the statistical power was 0.64. The rs10801935 AC genotype was associated with a significantly increased risk of breast cancer compared with the AA genotype (OR = 1.75 with 95% CI = 1.31–2.34, P < 0.001), and the statistical power was 0.94. In rs3738414, the AA genotype showed a significantly decreased risk of breast cancer compared with the GG genotype (OR = 0.58 with 95% CI = 0.35–0.98, P = 0.043), and the statistical power was 0.51.
Table 2.
Frequencies and Odds Ratios (OR) of the Genotypic Polymorphisms for VTCN1 5′‐UTR A > G (rs3738414), VTCN1 3′‐UTR A > G (rs10754339), and VTCN1 Intron1 A > C (rs10801935) Genes in Patients and Controls
| Polymorphisms | Patients (N = 566) | Controls (N = 400) | OR (95% CI) | P‐value | Power |
|---|---|---|---|---|---|
| VTCN1 3′‐UTR A > G (rs10754339) | |||||
| Allele frequency | |||||
| A | 978 (86.4%) | 720 (90.0%) | Reference | – | 0.67 |
| G | 154(13.6%) | 80 (10.0%) | 1.42 (1.07–1.89) | 0.017 | |
| Genotype frequency | |||||
| A/A | 420 (74.2%) | 324 (81.0%) | Reference | – | 0.64 |
| A/G | 138 (24.4%) | 72 (18.0%) | 1.49 (1.07–2.04) | 0.015 | |
| G/G | 8 (1.41%) | 4 (1.0%) | 1.54 (0.48–5.82) | 0.482 | |
| VTCN1 intron1 A > C (rs10801935) | |||||
| Allele frequency | |||||
| A | 898 (79.3%) | 674 (84.2%) | Reference | – | |
| C | 234 (20.7%) | 126 (15.8%) | 1.39 (1.10–1.77) | 0.006 | 0.78 |
| Genotype frequency | |||||
| A/A | 350 (61.8%) | 290 (72.5%) | Reference | – | 0.94 |
| A/C | 198 (35.0%) | 94 (23.5%) | 1.75 (1.31–2.34) | >0.001 | |
| C/C | 18 (3.2%) | 16 (4.0%) | 0.93 (0.47–1.88) | 0.84 | |
| VTCN1 5′‐UTR G > A (rs3738414) | |||||
| Allele frequency | |||||
| G | 814 (71.9%) | 540 (73.0%) | Reference | – | 0.55 |
| A | 318 (28.1%) | 260 (27.0%) | 0.81 (0.67–0.99) | 0.037 | |
| Genotype frequency | |||||
| G/G | 280 (49.5%) | 174 (43.5%) | Reference | – | 0.51 |
| G/A | 254 (44.1%) | 192 (48.0%) | 0.82 (0.63–1.07) | 0.149 | |
| A/A | 32 (5.6%) | 34 (8.5%) | 0.58 (0.35–0.98) | 0.043 | |
Table 3.
VTCN1 Haplotype Frequencies in Patients and Controls
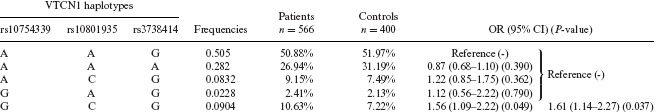
|
Haplotype‐based analysis
The association between VTCN1 SNPs and the risk of breast cancer was further investigated by PLINK software. The detailed results are shown in Tables 3 and 4. Possible haplotypes were then reconstructed; the reconstructed ones with frequencies ≥ 1% are shown in Table 3. The most frequently occurring haplotype in the cases and controls was AAG (rs10754339A, rs10801935A, rs3738414G) (50.5%). Compared with the AAG haplotype, only the GCG haplotype showed a significantly increased risk in breast cancer (OR = 1.56 with 95% CI = 1.09–2.22, P = 0.049). However, the other haplotypes were not significantly associated with the risk of breast cancer development (Table 3). Therefore, we combined all the other haplotypes in a reference group, and the result indicated that the GCG haplotype also showed a significantly increased risk of breast cancer (OR = 1.61 with 95% CI = 1.14–2.27, P = 0.037). Furthermore, by using haplotype‐based pair analysis, shown in Table 4, the results showed that the GCG haplotype had a significantly higher risk than the combination of the other genotypes (OR = 1.57 with 95% CI = 1.11–2.22, P = 0.012) and the GCG diplotype also showed an increased risk, though it was not significant (OR = 3.01 with 95% CI = 0.65–14.6, P = 0.156). As a group, the GCG carriers had a significantly increased risk of breast cancer development (OR = 1.62 with 95% CI = 1.15–2.27, P = 0.006), and the statistical power was 0.79.
Table 4.
Haplotype Pair Frequency of VTCN1 in Patients and Controls
| Haplotype/haplotype | Patients (N = 566) | Controls (N = 400) | OR (95% CI) (P‐value) | Power |
|---|---|---|---|---|
| Others/others | 444 (78.5%) | 342(85.5%) | Reference (‐) | 0.79 |
| GCG/others | 114 (20.1%) | 56 (14.0%) | 1.57 (1.11–2.22) (0.012) | |
| GCG/GCG | 8 (1.4%) | 2 (0.5%) | 3.01 (0.65–14.6) (0.156) | |
| GCG carriers | 122 (21.5%) | 58 (14.5%) | 1.62 (1.15–2.27) (0.006) |
DISCUSSION
Under general conditions, antigen‐presenting cells (APCs), which scavenge tumor cell debris and migrate to lymphoid tissues, can interact with CD4+ and CD8+ T‐cells to induce activation of T‐cells capable of recognizing tumor‐specific or tumor‐associated antigens. The T‐cell activation requires two signals: one through recognition of antigen peptide‐major histocompatibility complexes by the T‐cell receptors (TCR), and the other via costimularoty receptors binding to their ligands on the same APC delivered by a group of costimulatory molecules, functioning either for positive or negative regulation, which belong to the B7 family 11, 12, 13, 14. The downgrading of tumor‐specific T‐cell response by abusing the inhibitory signaling pathway with induction of T‐cell anergy or apoptosis through aberrant tumor costimulatory molecules expression may represent a possible immune escape mechanism; as a result, the risk of tumor development is enhanced 15, 16.
VTCN1, a newly identified member of the B7 family, may negatively regulate T‐cell immunity by restraining T‐cell proliferation, cytokine secretion, and the development of cytotoxicity 17, 18. Therefore, given such a coinhibitory signal to T‐cell activation property, overexpression or aberrant functioning of VTCN1 may be an important tactic employed by tumor cells to avoid tumor‐specific T‐cell attack. Clinically, a higher expression of the VTCN1 protein was found in several tumors, such as lung cancer 5, ovarian cancer 6, renal cell carcinoma 19, and breast cancer 7. Furthermore, previous reports 7 revealed that a higher expression of the VTCN1 protein could be associated with decreasing the number of tumor infiltrating lymphocytes and preventing tumor cells from apoptosis in breast cancer. Thus, it is reasonable to infer that the expression of VTCN1 could modify the susceptibility to breast cancer in early cancer development, which is the goal of this study. Since it is impossible to evaluate the expression of VTCN1 for the general population to test the abovementioned speculation, genotyping of VTCN1 polymorphisms could be a good alternative, given such polymorphisms could affect the normal function of VTCN1 including mRNA translation and stability, etc.
The human VTCN1 gene is located on chromosome 1p11.1, and consists of six exons and five introns 20. Three potentially functional polymorphisms of VTCN1—rs10754339, rs10801935, and re3738414—are located in 3′‐UTR, which could affect the efficiency of VTCN1 synthesis; intron 1, which could cause the alteration of the pre‐mRNA secondary structure; and 5′‐UTR, which could influence splicing of the primary transcript with production of stable mRNA, respectively. Impaired immunity, especially in T‐cells, which can easily induce cancer in an animal model, has been recognized for a long time. Clearly, weakened immunity plays a promoter role in carcinogenicity. However, it is impossible to survey the status of immunity longitudinally in a population to test this theory on humans. In order to evaluate the risk of previous incapacitated T‐cell cytotoxic ability on the development of breast cancer, we utilized 3 SNPs of the VTCN1 genes as proxies, given that the polymorphisms of these locations might eventually affect the capacity of VTCN1, as mentioned above, and lessen the cytotoxic capacity of T‐cells to remove cancer cells. The evidence in this study revealed that the SNPs located on DNA were related to the risk of breast cancer, which suggested tumor‐specific T‐cells played an important role in breast cancer development.
The evidence relating to VTCN1 as a risk factor in breast cancer is still limited. The only similar report was published by Zhang et al. 8. Compared with their study results, the univariate analysis for the risk of these SNPs is consistent, but not in the haplotype analysis, which is confusing to us. Even though we used different packages for the haplotype analysis, both teams used the same criteria in the subsequent analysis, which still could not explain the differences. Furthermore, owing to the strong relationship between VTCN1 and the clinical development of breast cancer, we scrutinized the clinical characteristics of the cases between the two studies, and found there were no statistical differences in these parameters that could explain the discrepancies in the haplotype analysis. Therefore, we speculate those inconsistencies of the haplotype analysis could result from selection bias, especially in the recruitment of the controls. Even though the sample sizes of the controls for both studies were greater than 400, it may still represent only a moderate sample size for haplotype analysis, a kind of high order gene–gene interaction, if the frequency of the mutant is much less than those of the wild type. A large‐scale population‐based case‐control study may reveal the true facts for us.
The limitations of this study should be noted: First, immunity does not play an initiating role in cancer development, which differentiates it from other environmental factors such as smoking, or drinking, etc. Second, deviation from the Hardy–Weinberg equilibrium suggests that this polymorphism might be under selective pressure. The genotype distributions of rs3738414 were not in Hardy–Weinberg equilibrium among the cases and controls (data not shown). Third, we did not evaluate the interaction of genotypes and environmental factors such as diet, passive smoking, and other possible lifestyle risk factors. It is possible, therefore, that environmental exposure might bias the estimation of risk associated with VTCN1 polymorphisms. Fourth, although case‐control studies have well documented weaknesses compared to a longitudinal cohort study, including measuring exposure (recall bias) and temporal relationships between cause and effect, these were not important factors in this study. However the possibility of a selection bias in a small study, particularly when the control group was recruited from the same hospital rather than from the broader community, might have distorted the strength of the associations observed above. A larger scale population‐based case‐control study is required to confirm our findings.
In conclusion, our results indicate that the frequencies of the VTCN1 polymorphisms were significantly different between breast cancer cases and the controls, which suggests that immunity may be involved in breast cancer development, especially in the progression and metastasis stages. A larger‐scale study deserves to be launched to confirm these findings and to explore the relationship between specific environmental factors such as a drinking habit or other particular exposures with the polymorphisms of VTCN1 on breast cancer development.
CONFLICT OF INTEREST
All of the authors declare that they have no conflict of interest.
Grant sponsor: National Science Council, Taiwan
REFERENCES
- 1. McTiernan A. Behavioral risk factors in breast cancer: Can risk be modified? Oncologist 2003;8(4):326–334. [DOI] [PubMed] [Google Scholar]
- 2. Desmetz C, Cortijo C, Mangé A, et al. Humoral response to cancer as a tool for biomarker discovery. J Proteomics 2009;72(6):982–988. [DOI] [PubMed] [Google Scholar]
- 3. Sica GL, Choi IH, Zhu G, et al. B7‐H4, a molecule of the B7 family, Negatively regulates T cell immunity. Immunity 2003;18(6):849–861. [DOI] [PubMed] [Google Scholar]
- 4. Chen C, Qu QX, Shen Y, et al. Induced expression of B7‐H4 on the surface of lung cancer cell by the tumor‐associated macrophages: A potential mechanism of immune escape. Cancer Lett 2012; 317(1):99–105. [DOI] [PubMed] [Google Scholar]
- 5. Arigami T, Uenosono Y, Hirata M, et al. Expression of B7‐H4 in blood of patients with gastric cancer predicts tumor progression and prognosis. J Surg Oncol 2010;102(7):748–752. [DOI] [PubMed] [Google Scholar]
- 6. Simon I, Katsaros D, Rigault de la Longrais I, et al. B7‐H4 is over‐expressed in early‐stage ovarian cancer and is independent of CA125 expression. Gynecol Oncol 2007;106(2):334–341. [DOI] [PubMed] [Google Scholar]
- 7. Tringler B, Zhuo S, Pilkington G, et al. B7‐H4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res 2005;11(5):1842–1848. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Zhang M, Jiang W, et al. B7‐H4 gene polymorphisms are associated with sporadic breast cancer in a Chinese Han population. BMJ Cancer 2009;9(11):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Purcell S, Neale B, Todd‐Brown K, et al. PLINK: A toolset for whole‐genome association and population‐based linkage analysis. Am J Hum Genet 2007; 81(9):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. 15. Bureau of Health Promotion, Department of Health, Executive Yuan, Taiwan. Cancer registry annual report, 2008, TAIWAN. 2011, Republic of China, pp. 568.
- 11. Dong C, Nurieva RI, Prasad D. Immune regulation by novel costimulatory molecules. Immunol Res 2003;28(1):39–48. [DOI] [PubMed] [Google Scholar]
- 12. Flies DB, Chen L. The new B7s: Playing a pivotal role in tumor immunity. J Immunother 2007;30(3):251–260. [DOI] [PubMed] [Google Scholar]
- 13. Andrei I, Chapoval, Jian Ni, et al. B7‐H3: A costimulatroy molecule for T cell activation and IFN‐γproduction. Nat Immunol 2001;2(3):269–274. [DOI] [PubMed] [Google Scholar]
- 14. Loos M, Hedderich DM, Freiss H, et al. B7‐H3 and its role in antitumor immunity. Clin Dev Immunol 2010;2010(11):683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flies DB, Chen L. The new B7s: Playing a pivotal role in tumor immunity. J Immunother 2007;30(3):251–260. [DOI] [PubMed] [Google Scholar]
- 16. Weth R, Biburger M, Wels W. Bispecific costimulatory molecules for activation of tumor‐killing lymphocytes. Cancer Cell Int 2004; 4(Suppl 1):S1(http://www.cancerci.com/content/4/S1/S1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qian Y, Shen L, Xu C, et al. Development of a novel monoclonal antibody to B7‐H4: Characterization and biological activity. Eur J Med Res 2011;16(7):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue Q, Luan XY, Gu YZ, et al. The negative co‐signaling molecule b7‐h4 is expressed by human bone marrow‐derived mesenchymal stem cells and mediates its T‐cell modulatory activity. Stem Cells Dev 2010;19(1):27–38. [DOI] [PubMed] [Google Scholar]
- 19. Krambeck AI, Thompson RH, Dong H, et al. B7‐H4 expression in renal cell carcinoma and tumor vasculature: Associations with cancer progression and survival. Proc Natl Acad Sci U S A 2006;103(27):10391–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi IH, Zhu G, Sica GL, et al. Genomic organization and expression analysis of B7‐H4, an immune inhibitory molecule of the B7 family. J Immunol 2003;171(9):1650–1654. [DOI] [PubMed] [Google Scholar]


