Abstract
Background
Soluble urokinase plasminogen activator receptor (suPAR) has been studied in a variety of diseases. The aim of the study is to investigate the levels of suPAR in neonates with sepsis.
Methods
The infants enrolled to this prospective study were classified into four groups. Group 1, 2, and 3 were referred as the patient groups (40 infants), and group 4 was referred as control group (26 infants). Blood samples for whole blood count, C‐reactive protein (CRP), suPAR and blood culture were obtained before initiating antimicrobial therapy, and two further samples were obtained on day 3 and at the end of the treatment for CRP and suPAR.
Results
The mean gestational ages of patient and control groups was similar. The median level of initial suPAR was 18.8 ng/mL (range 6.8–30.1 ng/mL) in the patient groups, and 6.0 ng/mL (range 3.7–10.8 ng/mL) in the control group (P < 0.001). A significant decrease in suPAR level was observed from the inclusion to the third day and end of the treatment (P < 0.001). The area under the curve (AUC) for suPAR is 0.959 (95% Cl: 0.919–0.999) and for CRP is 0.782 (95% Cl: 0.669–0.895). At a cut‐off value of 11.3 ng/mL for suPAR the specificity was 100%, and the sensitivity was 82.5%. There was a positive correlation between laboratory values of CRP and suPAR (r: 0.359, P = 0.003).
Conclusion
This is the first study that investigated the levels of suPAR in neonates and our results demonstrate that suPAR is a powerful marker of inflammation in infants with sepsis.
Keywords: soluble urokinase plasminogen activator receptor, neonate, sepsis, marker, urokinase plasminogen activator receptor
INTRODUCTION
Neonatal sepsis is a severe disease condition with high risks of morbidity and mortality. Early diagnosis and treatment are crucial to improve disease outcome. Diagnosis of sepsis may be difficult due to often nonspecific and subtle clinical presentation especially at the onset of infection, and can easily be attributed to other common noninfectious causes. Initiation of antibiotic treatment to infants who have nonspecific findings is usually unavoidable until the result of blood culture is obtained. Blood culture is the gold standard diagnostic method, but is also fraught with difficulties 1, 2, 3.
Several hematological tests such as total leukocyte count, total neutrophil count, immature neutrophil count, immature/total neutrophil ratio, morphological, and degenerative changes in neutrophils have been used for the early and reliable diagnosis of neonatal sepsis. The nonspecific nature of these tests has directed clinicians to search for more specific laboratory tests 3, 4.
The urokinase plasminogen activator receptor (uPAR) is expressed on most leucocytes including neutrophils, lymphocytes, monocytes, and macrophages which are crucially important in the pathogenesis of sepsis. The interaction of uPAR with its ligand, the urokinase plasminogen activator (uPA), results in numerous immunologic events including cell migration, adhesion, proliferation, and fibrinolysis. After cleavage from the cell surface, the soluble form of uPAR, namely, soluble urokinase plasminogen activator receptor (suPAR), can be found in the blood and other organic fluids in all individuals 5, 6, 7, 8, 9.
suPAR has been studied in a variety of diseases. Recently, suPAR has been suggested as a novel prognostic marker to identify high‐risk patients, and may be useful for the clinical management of serious infectious diseases 1, 6, 10, 11, 12, 13.
The association between sepsis and serum levels of suPAR has not been investigated in neonates. This is the first study that investigated the levels of suPAR in neonates with sepsis.
MATERIALS AND METHODS
Forty newborn infants who were diagnosed as having clinical suspected neonatal late‐onset sepsis in the neonatal intensive care unit of Ankara University Faculty of Medicine between March 2010 and March 2012 were included in this prospective study. Twenty six infants with prematurity or indirect hyperbilirubinemia or hypoglycemia, who had no signs of clinical and laboratory infection were selected as the control group. Informed consents were obtained from the parents for patients and control subjects.
The infants were classified into four groups according to the criteria defined by Gitto et al. 14: group 1 (high probable sepsis), group 2 (probable sepsis), group 3 (possible sepsis), and group 4 (no sepsis, control group). Group 1, 2, and 3 were referred as the patient groups. Table 1 lists the criteria for classifying the study groups. Exclusion criteria included administration of antibiotic treatment before the study entry and refusal of parental consent.
Table 1.
Criteria for defining the sepsis 14
| Groups | Criteria |
|---|---|
| Group 1 | At least 3 sepsis‐related clinical signsa |
| High probable sepsis | CRP > 1 mg per 100 ml |
| At least two other altered serum parameters in addition to CRPb | |
| Blood culture; positive or negative | |
| Group 2 | Less than three sepsis‐related clinical signsa |
| Probable sepsis | CRP > 1 mg per 100 ml |
| At least two other altered serum parameters in addition to CRPb | |
| Blood culture; negative | |
| Group 3 | Less than three sepsis‐related clinical signsa |
| Possible sepsis | CRP < 1 mg per 100 ml |
| Less than 2 other altered serum parameters in addition to CRPb | |
| Blood culture; negative | |
| Group 4 | No sepsis‐related clinical signsa |
| No sepsis | CRP < 1 mg per 100 ml |
| No altered serum parameters | |
| Blood culture; negative |
CRP: C‐reactive protein.
Sepsis‐related clinical signs: temperature instability, apnea, need for supplemented oxygen, need for ventilation, tachycardia/bradycardia, hypotension, feeding intolerance, abdominal distension, necrotizing enterocolitis.
Serum parameters other than CRP: white blood cell count, absolute neutrophil count, platelet count.
At the time of diagnosis of late‐onset sepsis, blood samples for whole blood count, C‐reactive protein (CRP), suPAR, and blood culture were obtained from neonates before initiating antimicrobial therapy. Also cerebrospinal fluid and urine cultures were obtained from these infants. Two further samples were obtained on day 3 and at the end of the treatment for CRP and suPAR in the patient group. The whole blood count, CRP, and only one blood sample for suPAR were obtained from each control subject.
Blood samples for suPAR measurement were obtained from participating subjects into tubes containing EDTA. Cells were removed after centrifugation at 3,000 rpm for 10 min within 1 hr, and the supernatants were stored at −70°C until used. Plasma suPAR concentrations were analyzed using a commercially available enzyme immunoassay (suPARnosticTM, Virogates, Copenhagen, Denmark) according to the manufacturer's instructions; the lower detection limit was 0.1 ng/ml. The assay is a double monoclonal antibody sandwich assay that measures all circulating suPAR, including full‐length and cleaved forms of the receptor.
Infants with suspected sepsis were initiated vancomycine plus aminoglicozide empirically. If meningitis was present the treatment was initiated as vancomycine plus seftazidime/meropenem. Neonates who had positive blood and/or CSF culture and antibiotic susceptibility results were treated with antibiotics according to antibiotic susceptibility of the specific bacteria. Antibiotic treatments lasted for 7–21 days.
The study was approved by the Ethics Committee of Ankara University Faculty of Medicine, and was funded by Ankara University Research Funding Center (10B3330012). The study has been registered at www.clinicaltrials.gov, as NCT01294865.
A comparison between the groups was performed using the t‐test and/or Mann–Whitney U‐test for nonparametric continuous variables in independent‐samples and chi‐squared or Fisher's exact tests as appropriate for categorical variables. The data were presented as mean ± standard deviation, and/or median (minimum‐maximum) for continuous variables, in addition, percentages and distribution of frequency for categorical variables. Comparisons between control group and patient groups were done by Kruskall Wallis tests after adjustment for multiple comparisons. Concentrations of CRP and suPAR were correlated according to Spearman's rank of order. Receiver operator curve (ROC) analysis was done to discriminate infants with sepsis. Sensitivity and specificity were calculated according to ROC curve. P < 0.05 was regarded as significant. All analyses were carried out using Statistical Package for Social Sciences (SPSS) version 15 for Windows.
RESULTS
A total of 66 newborn infants were enrolled in the study (40 infants in patient groups, 26 infants in the control group). There were 13 infants in group 1, 11 infants in group 2, and 16 infants in group 3. There were 26 infants in group 4 as the control group.
The mean gestational ages of patient and control groups was similar (P = 0.071), whereas the mean birth weight of patient groups (Group 1, 2, and 3) was lower than the control group (P = 0.013). The mean gestational age and birth weight of group 1, 2, and 3 were similar. There were no differences between the groups with respect to gender, mode of delivery, Apgar scores at 1 and 5 min. The demographic characteristics of the patient and control groups are shown in Table 2.
Table 2.
The demographic characteristics of study group
| Group 1 | Group 2 | Group 3 | Group 4 | |||
|---|---|---|---|---|---|---|
| (n = 13) | (n = 11) | (n = 16) | P | (n = 26) | P | |
| Gestational age (weeks)a | 31.8 ± 4.2 | 33.6 ± 5.3 | 30.7 ± 2.4 | 0.64 | 33 ± 2.4 | 0.07 |
| Birth Weight (g)a | 1689.6 ± 914.5 | 1758.8 ± 923.3 | 1408.1 ± 600 | 0.61 | 1983.8 ± 535.5 | 0.013 |
| Male gender, n (%) | 7 (54) | 5 (46) | 7 (44) | 0.85 | 21 (62) | 0.84 |
| Ceserean section, n (%) | 10 (77) | 8 (73) | 15 (94) | 0.3 | 17 (81) | 0.11 |
| Apgar 1′b | 7 | 7 | 7 | 0.42 | 7 | 0.27 |
| Apgar 5′b | 9 | 9 | 8.5 | 0.65 | 9 | 0.18 |
Data are reported as mean ± SD.
Data are reported as median.
Ten (77%) of 13 infants in group1 had positive blood culture (Klebsiella pneumonia, n = 6, Escherichia Coli, n = 2, Enterobacter aerogenes, n = 1, Staphylococcus aureus, n = 1). Although one of the patients with positive blood culture had the highest suPAR level, the suPAR levels of patients with positive and negative blood culture did not differ. Four infants from all patient groups had meningitis.
Table 3 shows the initial leukocyte count, absolute neutrophil count, CRP, and suPAR levels of the study group. The initial mean leukocyte counts were 13 237 ± 10 389, 14 300 ± 9874, 10 756 ± 8331, and 10 977 ± 3059 mm−3 in group 1, 2, 3, and 4, respectively. There was no difference between the groups in terms of mean leukocyte count. The absolute neutrophil counts were similar in the groups. The initial mean platelet counts were lower than the control group in patient groups (P = 0.014), whereas there was no statistically significant difference in platelet counts of group 1, 2, and 3.
Table 3.
The initial leukocyte count, absolute neutrophil count, C‐reactive protein (CRP), and soluble urokinase plasminogen activator receptor (suPAR) levels of sudy group
| Group 1 (n = 13) | Group 2 (n = 11) | Group 3 (n = 16) | P | Group 4 (n = 26) | P | |
|---|---|---|---|---|---|---|
| Leukocyte count (mm−3)a | 13237 ± 10389 | 14300 ± 9874 | 10756 ± 8331 | 0.92 | 10977 ± 3059 | 0.47 |
| Absolute neutrophil count (mm−3)a | 7838 ± 7093 | 9440 ± 7792 | 6584 ± 6291 | 0.92 | 7499 ± 2418 | 0.4 |
| Platelet count (mm−3)a | 226770 ± 111853 | 197273 ± 129396 | 232313 ± 120044 | 0.56 | 272885 ± 64468 | 0.014 |
| CRP (mg/dl)a | 6.4 ± 3.3 | 5.2 ± 2.1 | 0.24 ± 0.2 | 0.000 | 0.26 ± 0.2 | 0.000 |
| suPAR (ng/ml)a | 18.6 ± 7.2 | 14.7 ± 6.7 | 18.4 ± 5.2 | 0.56 | 6.3 ± 1.8 | 0.000 |
All data are reported as mean ± SD.
The mean CRP levels of the patient groups were significantly higher than the control group (P < 0.001). Also the CRP levels of group 1 and 2 were higher than the CRP levels of group 3 (P < 0.001).
The plasma level of suPAR was measurable in all samples. The median level of initial suPAR was 18.8 ng/ml (range 6.8–30.1 ng/ml) in the patient groups, and 6.0 ng/ml (range 3.7–10.8 ng/ml) in the control group. The mean suPAR levels were significantly higher in the patient groups than the control group (P < 0.001). But there were no difference between group 1, 2, and 3 (P = 0.56).
A significant decrease in suPAR level was observed from the inclusion (median = 18.8 ng/ml, IQR: 6.8–30.1 ng/ml) to the sample at the third day of the treatment (median = 9.3 ng/ml, IQR: 3.6–16.1 ng/ml) and to the sample at the end of the treatment (median = 6.5 ng/ml, IQR: 1.8–12.1 ng/ml) in the patient groups (P = 0.000) (Fig. 1).
Figure 1.
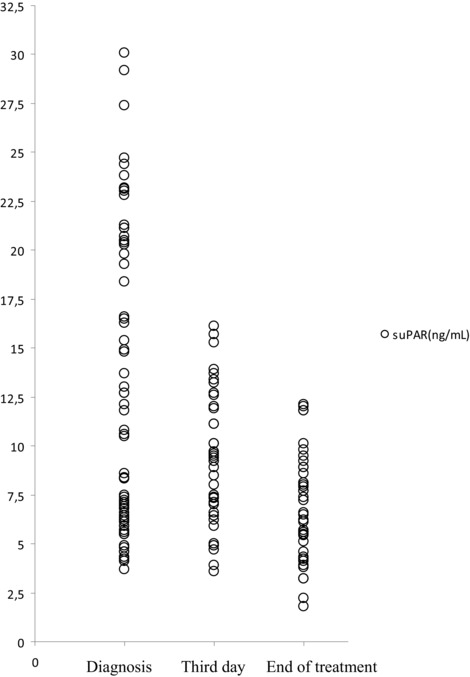
Figure showing the soluble urokinase plasminogen activator receptor (suPAR) levels measured at diagnosis and trends of levels at the third day and end of the treatment.
ROC analysis of initial leukocyte count, absolute neutrophil count, CRP, and suPAR levels at diagnosis to discriminate among infants with sepsis or not is shown in Figure 2. The area under the curve (AUC) for suPAR is 0.959 (95% Cl: 0.919–0.999) and for CRP is 0.782 (95% Cl: 0.669–0.895). At a cut‐off value of 11.3 ng/ml for suPAR the specificity was 100% and the sensitivity was 82.5%. There was a positive correlation between laboratory values of CRP and suPAR (r: 0.359, P = 0.003).
Figure 2.
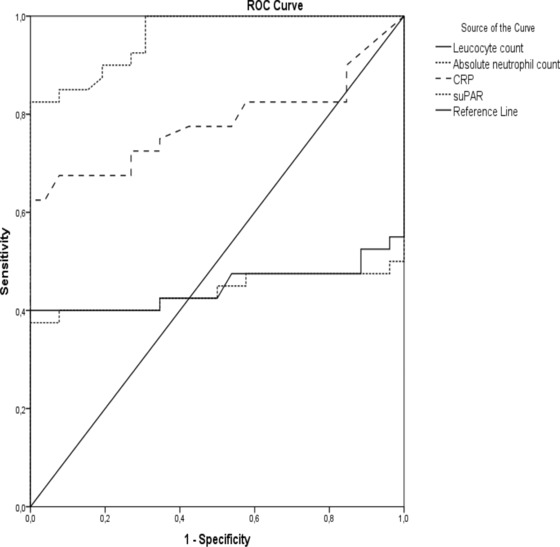
ROC analysis for initial leukocyte count, absolute neutrophil count, C‐reactive protein (CRP) and soluble urokinase plasminogen activator receptor (suPAR) levels at diagnosis.
Six (15%) of 40 infants died in the patient groups. Two of them were in group 1, three of them were in group 2, and one of them was in group 3. Only one of the patient who was in group 1 died due to infectious causes. None of the infants died in the control group.
DISCUSSION
To our knowledge, this is the first study that reports suPAR concentrations and examines the association between sepsis and suPAR in neonates. We found the suPAR levels to be highly and significantly elevated among neonates with sepsis.
Several studies indicate that an elevated suPAR level in plasma is associated with a negative outcome in critically ill patients with systemic inflammatory response syndrome, bacteremia, sepsis, and septic shock. It has been shown that suPAR has a role in the early risk assessment of patients with sepsis and predicts mortality in these patients 9, 11, 15, 16, 17. However, studies have also shown that suPAR did not appear to be superior to other biomarkers like CRP and procalcitonin (PCT), in diagnosing sepsis 17, 18. Also serum uPAR and suPAR levels have been found to be elevated and of prognostic value for survival in patients suffering from different malignant diseases such as ovarian and colorectal cancers 19, 20.
Both early and late‐onset infections remain important causes of neonatal morbidity and mortality. Early clinical features of infection are, however, often subtle, nonspecific, and difficult to recognize 1, 21, 22, 23. The usefulness of conventional hematologic tests in assisting frontline neonatologists to differentiate between infected and noninfected infants is limited. Not only does the total white cell count exhibit a wide range of normality, machine measurements of neutrophil counts are inaccurate in the presence of nucleated red blood cells, and assessment of neutrophil band forms is subjective and requires an experienced hematologist to review the blood film 1. The use of blood culture, the ‘‘gold standard’’ for diagnosis of bacteremia, is also fraught with difficulties 24. Hence, it is important to diagnose neonatal sepsis in a rapid and accurate way. But it is unlikely that a single infection marker would possess all the characteristics of an ‘‘ideal’’ infection marker.
In this study, we found significantly higher levels of plasma suPAR levels in patients with sepsis compared to infants without sepsis. We measured suPAR plasma concentrations at the diagnosis of sepsis before the treatment started, at the third day and end of the treatment. The effective treatment of sepsis resulted in a decrease in suPAR levels during treatment and after full recovery as demonstrated in other clinical trials 6, 25. These data suggest that sequential suPAR levels may be of use in following the acute response to treatment in sepsis.
CRP is the most commonly used acute‐phase reactant in neonates 26. In the present study it has been demonstrated that suPAR plasma concentrations are correlated with serum CRP levels.
In healthy adults, the median value of suPAR has been cited as 1.5 ng/ml 27 or 2.5 ng/ml 13, depending on the assay. In our study, we used the latter assay, and the median value of suPAR in controls was 6.0 ng/ml. We found a normalization of the suPAR levels at the end of the treatment in patient group (median suPAR: 6.5 ng/ml). Yılmaz et al. reported a cut‐off value of 2.8 ng/ml with a sensitivity of 92% and specificity of 85% 9. In an another study the cut‐off value of 5.5 ng/ml has been found to have a sensitivity of 75% and specificity of 72% 28. Several studies in adults reported that values greater than 10 ng/ml may be predictive of death 12. We have found that a cut‐off value of 11.3 ng/ml has a sensitivity of 82.5% and specificity of 100% for diagnosing sepsis. We did not evaluate whether the suPAR levels predict mortality, because only one patient died of infection. The limitation of our study is the small number of infants in the control group to give a reference value for suPAR in neonates.
As a result, plasma levels of suPAR are increased in infants with sepsis. Our results suggest that suPAR is a powerful marker of inflammation in infants with sepsis but does not appear to be superior to CRP, concordant with previous studies. The independent diagnostic and predictive value of suPAR needs further study to determine whether this biomarker could be used to diagnose sepsis and follow the response to treatment in neonates.
Grant sponsor: Ankara University Research Funding Center; Grant number: 10B3330012.
The study has been registered at www.clinicaltrials.gov, as NCT01294865.
REFERENCES
- 1. Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opin Pediatr 2006;18:125–131. [DOI] [PubMed] [Google Scholar]
- 2. Ng PC. Clinical trials for evaluating diagnostic markers of infection in neonates. Biol Neonate 2005;87:111–112. [DOI] [PubMed] [Google Scholar]
- 3. Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. WB Saunders Company, Philadephia; 2001. [Google Scholar]
- 4. Arnon S, Litmanovitz I. Diagnostic tests in neonatal sepsis. Curr Opin Infect Dis 2008;21:223–227. [DOI] [PubMed] [Google Scholar]
- 5. Thunø M, Macho B, Eugen‐Olsen J. suPAR: The molecular crystal ball. Dis Markers 2009;27:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eugen‐Olsen J, Gustafson P, Sidenius N, et al. The serum level of soluble urokinase receptor is elevated in tuberculosis patients and predicts mortality during treatment: A community study from Guinea‐Bissau. Int J Tuberc Lung Dis 2002;6:686–692. [PubMed] [Google Scholar]
- 7. Andersen O, Eugen‐Olsen J, Kofoed K, Iversen J, Haugaard SB. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV‐infected patients receiving highly active antiretroviral therapy. J Med Virol 2008;80:209–216. [DOI] [PubMed] [Google Scholar]
- 8. Juffermans NP, Dekkers PE, Verbon A, Speelman P, van Deventer SJ, van der Poll T. Concurrent upregulation of urokinase plasminogen activator receptor and CD11b during tuberculosis and experimental endotoxemia. Infect Immun 2001;69:5182–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yilmaz G, Köksal I, Karahan SC, Mentese A. The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in systemic inflammatory response syndrome. Clin Biochem 2011;44:1227–1230. [DOI] [PubMed] [Google Scholar]
- 10. Sidenius N, Sier CF, Ullum H, et al. Serum level of soluble urokinase‐type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood 2000;96:4091–4095. [PubMed] [Google Scholar]
- 11. Wittenhagen P, Kronborg G, Weis N, et al. The plasma level of soluble urokinase receptor is elevated in patients with Streptococcus pneumoniae bacteraemia and predicts mortality. Clin Microbiol Infect 2004;10:409–415. [DOI] [PubMed] [Google Scholar]
- 12. Donadello K, Scolletta S, Covajes C, Vincent JL. suPAR as a prognostic biomarker in sepsis. BMC Med 2012;10:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gustafsson A, Ljunggren L, Bodelsson M, Berkestedt I. The Prognostic Value of suPAR Compared to Other Inflammatory Markers in Patients with Severe Sepsis. Biomark Insights 2012;7:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gitto E, Karbownik M, Reiter RJ, et al. Effects of melatonin treatment in septic newborns. Pediatr Res 2001;50:756–760. [DOI] [PubMed] [Google Scholar]
- 15. Huttunen R, Syrjänen J, Vuento R, et al. Plasma level of soluble urokinase‐type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: A prospective cohort study. J Intern Med 2011;270:32–40. [DOI] [PubMed] [Google Scholar]
- 16. Mölkänen T, Ruotsalainen E, Thorball CW, Järvinen A. Elevated soluble urokinase plasminogen activator receptor (suPAR) predicts mortality in Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2011;30:1417–1424. [DOI] [PubMed] [Google Scholar]
- 17. Koch A, Voigt S, Kruschinski C, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 2011;15:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kofoed K, Andersen O, Kronborg G, et al. Use of plasma C‐reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase‐type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells‐1 in combination to diagnose infections: A prospective study. Crit Care 2007;11(2):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sier CF, Stephens R, Bizik J, et al. The level of urokinase‐type plasminogen activator receptor is increased in serum of ovarian cancer patients. Cancer Res 1998;58:1843–1849. [PubMed] [Google Scholar]
- 20. Stephens RW, Nielsen HJ, Christensen IJ, et al. Plasma urokinase receptor levels in patients with colorectal cancer: Relationship to prognosis. J Natl Cancer Inst 1999;91:869–874. [DOI] [PubMed] [Google Scholar]
- 21. Stoll BJ, Hansen NI, Higgins RD, et al. National Institute of Child Health and Human Development. Very low birth weight preterm infants with early onset neonatal sepsis: The predominance of gram‐negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J 2005;24:635–639. [DOI] [PubMed] [Google Scholar]
- 22. Stoll BJ, Hansen NI, Adams‐Chapman I, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low‐birth‐weight infants with neonatal infection. JAMA 2004;292:2357–2365. [DOI] [PubMed] [Google Scholar]
- 23. Stoll BJ, Hansen N, Fanaroff AA, et al. Late‐onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285–291. [DOI] [PubMed] [Google Scholar]
- 24. Polin RA. The “ins and outs” of neonatal sepsis. J Pediatr 2003;143:3–4. [DOI] [PubMed] [Google Scholar]
- 25. Ostrowski SR, Katzenstein TL, Piironen T, Gerstoft J, Pedersen BK, Ullum H. Soluble urokinase receptor levels in plasma during 5 years of highly active antiretroviral therapy in HIV‐1‐infected patients. J Acquir Immune Defic Syndr 2004;35:337–342. [DOI] [PubMed] [Google Scholar]
- 26. Couto RC, Barbosa JA, Pedrosa TM, Biscione FM. C‐reactive protein‐guided approach may shorten length of antimicrobial treatment of culture‐proven late‐onset sepsis: an intervention study. Braz J Infect Dis 2007;11:240–245. [DOI] [PubMed] [Google Scholar]
- 27. Stephens RW, Pedersen AN, Nielsen HJ, et al. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin Chem 1997;43:1868–1876. [PubMed] [Google Scholar]
- 28. Donadello K, Covajes C, Scolletta S, et al. Clinical value of suPAR, a new biomarker. Intensive Care Med 2011;37: S199 (abst). [Google Scholar]


