Abstract
Background
We investigated the prevalence and risk factors for vitamin D deficiency in Korean patients with anemia.
Methods
We included 200 anemic patients and 300 controls. Anemia was defined according to the WHO criteria. Serum 25‐hydroxyvitamin D [25(OH)D] was measured using an electrochemiluminescence immunoassay. We compared serum 25(OH)D levels based on the presence and subtypes of anemia.
Results
We found that 91% (182/200) and 87.3% (262/300) of patients exhibited 25(OH)D inadequacies (<20 ng/ml) in the anemic (median hemoglobin (Hb), 9.6 g/dl) and control groups (median Hb 13.8 g/dl), respectively. The prevalence of 25(OH)D deficiency (<12 ng/ml) was significantly higher in the anemic group than in the control group (52.5% (105/200) vs. 25% (75/300), P < 0.0001), with an odds ratio of 3.316 (95% CI, 2.265–4.854; P < 0.0001). The prevalence of 25(OH)D deficiency was not different among anemia subtypes. Female gender and high C‐reactive protein (CRP) were associated with vitamin D deficiency in anemic group.
Conclusions
This study demonstrates that vitamin D deficiency is associated with anemia. Therefore, the measurement of serum 25(OH)D levels and appropriate vitamin D supplementation should be considered in anemic patients, particularly in females and patients with high CRP level.
Keywords: anemia, vitamin D deficiency, 25‐hydroxyvitamin D [25(OH)D]
INTRODUCTION
The classic role of vitamin D is to regulate calcium and phosphorus homeostasis in bone and mineral metabolism. The major circulating form of vitamin D, 25‐hydroxyvitamin D [25(OH)D], is produced via hydroxylation of vitamin D that is either ingested or synthesized in the liver. Biologically active 1,25‐dihydroxyvitamin D [1,25(OH)2D] is then formed via a second hydroxylation of 25(OH)D in the kidney. Serum 25(OH)D is a more accurate marker for evaluation of vitamin D status than 1,25(OH)2D due to its longer half‐life, more limited dietary intake, greater concentration, and ease of measurement 1.
Recently, vitamin D has been suggested to have an additional role in nonskeletal functions including cellular proliferation and differentiation, muscle function, immunity, and erythropoiesis. Vitamin D deficiency has also been shown to be associated with disease processes such as diabetes mellitus, chronic kidney disease (CKD), hypertension, cancer, aortic aneurysms, and anemia 2, 3, 4, 5, 6.
Several studies have reported a role for vitamin D in erythropoiesis due to its presence in the bone marrow 7, 8. Levels of 1,25(OH)2D in the bone marrow are several hundred fold higher than in the plasma 9. Furthermore, vitamin D deficiency is associated with various types of anemia including iron deficiency anemia (IDA), anemia of CKD (ACKD), and anemia of inflammation (AI). In patients with CKD not requiring dialysis, lower 25(OH)D levels are associated with lower hemoglobin (Hb) concentrations 10. In hemodialysis patients, vitamin D repletion has resulted in dose reductions of erythrocyte‐stimulating agents and increased reticulocytosis 11, 12, 13. Vitamin D deficiency is also independently associated with anemia in end‐stage heart failure 14. Other studies have shown that vitamin D deficiency has a high prevalence in children with IDA 15, in elderly patients with AI 6, and in cardiac surgical patients with ACKD 16.
Both vitamin D deficiency and anemia are very common health problems in Korean patients 17, 18. According to the Fourth Korea National Health And Nutrition Examination Survey (KNHANES IV) of 2008, vitamin D inadequacy with serum 25(OH)D levels <20 ng/ml was found in 47.3% of males and 64.5% of females 17. The prevalence of anemia in Korea was reported to be 9.0% in patients >10 years old, and rose rapidly with advancing age 18.
In this study, we investigated the prevalence of vitamin D deficiency in Korean patients with anemia. In addition, although vitamin D deficiency is an independent risk factor for anemia, not all anemic patients have vitamin D deficiency. Therefore, we also analyzed risk factors associated with vitamin D deficiency state among anemic patients.
MATERIALS AND METHODS
Study Subjects and Data Collection
The study subjects included 200 Korean patients (median age, 66 years; range, 19–91 years) diagnosed with anemia based on the World Health Organization (WHO) criteria and referred for anemia workup testing to our laboratory between September 2011 and August 2012. Three hundred nonanemic controls (median age, 65 years; range, 23–91 years) were also enrolled from health promotion center. After obtaining written informed consent, blood samples were drawn. Serum 25(OH)D was measured using a COBAS E411 analyzer (Roche Diagnostics, Indianapolis, IN) with an electrochemiluminescence immunoassay according to the manufacturer's instructions. We collected clinical and laboratory data including complete blood count (CBC), ferritin, serum iron, total iron‐binding capacity (TIBC), unsaturated iron‐binding capacity (UIBC), transferrin saturation, folate, vitamin B12, creatinine (Cr), estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease (MDRD) formula, and C‐reactive protein (CRP). This study was approved by the Institutional Review Board of the Konyang University Hospital.
Definitions of Anemia and Vitamin D Deficiency
Anemia was defined according to the WHO criteria as having an Hb level of <13 g/dl in men and <12 g/dl in women. Anemia subtypes were determined based on clinical and laboratory data. IDA was diagnosed when serum ferritin was <30 ng/ml and transferrin saturation was <16%. ACKD was defined as an eGFR <60 ml/min/1.73 m2. AI was defined as serum iron levels <60 μg/dl in the absence of iron deficiency, or ferritin >100 ng/ml. The remaining patients were categorized as having unexplained anemia. A deficiency of 25(OH)D was defined as <12 ng/ml, and inadequacy was defined as <20 ng/ml based on North American Institute of Medicine 19.
Statistical Analyses
Statistical analyses were carried out using PASW 22 software (IBM Corporation, Somers, NY). Mann–Whitney U tests were used to compare the median values of serum 25(OH)D between anemic patients and the control group. For the analysis of serum 25(OH)D status based on anemia subtypes, Kruskal–Wallis tests were used. Multivariable logistic regression was performed to evaluate associations between risk factors and serum 25(OH)D status in anemic patients. P‐values <0.05 were considered to be statistically significant.
RESULTS
Baseline Characteristics of Patients
Baseline characteristics of the patients are shown in Table 1. A total of 200 anemic patients (86 males and 114 females) and 300 controls (118 males and 182 females) were included in this study. The distributions of age (P = 0.12) and gender (P = 0.17) were not different between groups. Sixty (30%) anemic patients were diagnosed with IDA, while sixty‐three (31.5%) and sixty (30%) were diagnosed with ACD and AI, respectively. The median Hb level was 9.6 g/dl (range, 5.5–12.3 g/dl) in anemic patients and 13.8 g/dl (range, 12.0–17.2 g/dl) in controls (P < 0.0001).
Table 1.
Clinical Characteristics and Laboratory Data for Korean Patients With Anemia
| Characteristics | N = 200 |
|---|---|
| Age (years) | 62.96 ± 16.43 |
| Gender (male:female) | 86:114 |
| Hb (g/dl) | 9.38 ± 1.61 |
| Hematocrit (%) | 28.18 ± 5.31 |
| Mean corpuscular volume (fl) | 87.96 ± 11.01 |
| White blood cells (/mm3) | 7.57 ± 5.09 |
| Platelets (/mm3) | 248.5 ± 98.45 |
| Ferritin (ng/ml) | 265.9 ± 388.1 |
| Iron (μg/dl) | 40.25 ± 31.34 |
| TIBC (μg/dl) | 272.9 ± 100.5 |
| Transferrin saturation (%) | 16.4 ± 14.09 |
| Creatinine (mg/dl) | 1.58 ± 2.11 |
| eGFR (ml/min/1.73 m2) | 68.14 ± 32.48 |
| CRP (mg/dl) | 5.24 ± 8.57 |
| IDA (n (%)) | 60 (30%) |
| ACD (n (%)) | 123 (61.5%) |
| ACKD (n (%)) | 63 (31.5%) |
| AI (n (%)) | 60 (30%) |
| Unexplained anemia (n (%)) | 17 (8.5%) |
Descriptive data are expressed as means ± SDs or numbers (%).
ACD, anemia of chronic disease.
Serum 25(OH)D Levels and the Prevalence of Vitamin D Deficiency
The median serum level of 25(OH)D was 11.21 ng/ml in anemic patients and 16.35 ng/ml in controls (P < 0.0001). The prevalence of 25(OH)D inadequacy was 91% (182/200) in the anemic group and 87.3% (262/300) in the control group (P = 0.247). Serum 25(OH)D deficiency was significantly more common in the anemic group than in the control group (52.5% (105/200) vs. 25% (75/300), P < 0.0001). The odds ratio for 25(OH)D deficiency in anemic patients was 3.316 (95% CI, 2.265–4.854; P < 0.0001). The levels of 25(OH)D in 18 anemic patients (9%) were below the minimum detection limit of 3.0 ng/ml. Figure 1 shows the distribution of 25(OH)D deficiencies in anemic patients stratified by age and gender. More than 60% of anemic patients <50 years of age exhibited vitamin D deficiencies. Additionally, nearly half of the patients ≥60 years of age had vitamin D deficiencies. The distribution of vitamin D levels was different according to gender (P < 0.0001). The median 25(OH)D levels in anemic patients and controls were 16.24 and 17.31 ng/ml in males, and 9.61 and 13.3 ng/ml in females, respectively. Vitamin D deficiencies were significantly more common in both male and female anemic patients than in controls; 34.9 versus 18.6% in male (P = 0.0097) and 66.7 versus 29.1% in female (P < 0.0001).
Figure 1.
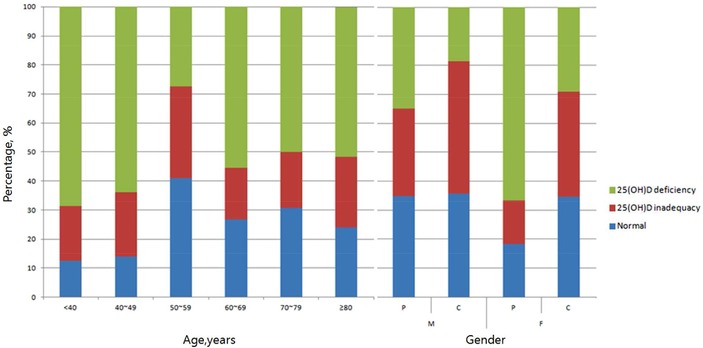
The distribution of vitamin D status according to age or gender in Korean anemic patients. P, patients; C, controls; M, male; F, female.
Association of Vitamin D Deficiency With Anemia Subtypes
Table 2 depicts the prevalence of vitamin D deficiency with respect to anemia subtype as well as in controls. The distribution of 25(OH)D levels (P = 0.169) and the prevalence of 25(OH)D deficiency (P = 0.730) were not different among anemia subtypes.
Table 2.
The Distribution of Vitamin D Deficiency Among Anemia Subtypes and the Control Group
| 25(OH)D level (ng/ml) | Anemic group (n = 200) | IDA (n = 60) | ACKD (n = 63) | AI (n = 60) | Unexplained anemia (n = 17) | Control group (n = 300) |
|---|---|---|---|---|---|---|
| ≥20 (n (%)) | 51 (26) | 14 (23) | 16 (25) | 15 (25) | 6 (35) | 105 (35) |
| 13–19 (n (%)) | 43 (22) | 14 (23) | 11 (18) | 15 (25) | 3 (18) | 120 (40) |
| <12 (n (%)) | 106 (53) | 32 (53) | 36 (57) | 30 (50) | 8 (47) | 75 (25) |
| Median value | 11.21 | 11.9 | 9.8 | 11.8 | 13.5 | 16.4 |
n, number; ACD, anemia of chronic disease.
Risk Factors for Vitamin D Deficiency in Anemic Patients
Table 3 shows the adjusted odds ratios for vitamin D deficiency according to variable factors in anemic patients. Female gender and high CRP were statistically significant risk factors for vitamin D deficiency in anemic patients.
Table 3.
Odds Ratios for Vitamin D Deficiency Using Multiple Logistic Regression Analysis
| Independent variables | Odds ratio (95% CI) | P‐value |
|---|---|---|
| Age (≥60 years) | 1.061 (0.528–2.133) | 0.867 |
| Gender (female) | 5.769 (2.824–11.785) | <0.001 |
| Hb (<7 g/dl) | 1.514 (0.790–2.900) | 0.211 |
| Ferritin (<30 ng/ml) | 0.659 (0.234–1.851) | 0.428 |
| (≥100 ng/ml) | 1.020 (0.391–2.662) | 0.968 |
| Transferrin saturation (<16 %) | 1.862 (0.866–4.006) | 0.112 |
| eGFR (<60 ml/min/1.73m2) | 2.389 (0.936–6.099) | 0.069 |
| CRP (≥1.0 mg/dl) | 2.751 (1.161–6.519) | 0.021 |
DISCUSSION
In this study, Korean anemic patients had a higher prevalence of vitamin D deficiency and significantly lower 25(OH)D levels compared with controls. In the KNHANES IV study 17, the prevalence of vitamin D inadequacy was slightly less than 70% in both healthy male and female patients who were 40–49 years of age. They also reported mean 25(OH)D levels of 21.4 ng/ml in males and 17.4 ng/ml in females in this same age group. Mean vitamin D levels were found to be highest in patients who were 60–69 years of age, at 23.8 ng/ml in males and 20.0 ng/ml in females. In this study, however, the prevalence of vitamin D inadequacy was 86.1% in anemic patients with a mean vitamin D level of 11.69 ng/ml at 40–49 years of age. Anemic patients at age 60–69 years had significantly lower 25(OH)D levels of 14.41 ng/ml.
Vitamin D deficiency was found to be associated with an increased risk of anemia independent of age, gender, and ethnicity. In a previous population‐based study, the odds ratio for anemia was increased by approximately 60% in the presence of vitamin D deficiency 20. Anemia may also be an influencing factor for vitamin D deficiency. Anemia can be caused by a number of factors such as nutritional deficiencies including iron, vitamin B12, and folate, chronic inflammation, and CKD. It has been suggested that anemia may predispose patients to vitamin D deficiency because of inadequate sun exposure as a result of decreased outdoor activity 11.
Although endogenous synthetic vitamin D from exposure of sunlight may be the primary contributor to the maintenance of serum vitamin D levels, the role of dietary intake cannot be disregarded. Young females have several risk factors for vitamin D deficiency including poor nutritional status and limited sun exposure due to decreased outdoor activity and excessive use of sun block. IDA is the most common nutritional deficiency worldwide, and is frequently observed in women of reproductive age. Half of the IDA patients enrolled in this study were females younger than 50 years of age. Therefore, IDA patients in this study likely exhibited a high prevalence of vitamin D deficiency. The correlation between IDA and vitamin D deficiency has also been reported in Korean children 15. While there are differences in these studies including study population characteristics, causes of IDA, and risk factors for vitamin D deficiency, all suggest that vitamin D deficiency should be considered in IDA patients regardless of age.
An increased risk of vitamin D deficiency in patients with AI has also been reported. AI, which has historically been known as anemia of chronic disease, is associated with infection, rheumatologic disorders, malignancy, and other chronic diseases. Perlstein et al. previously reported that vitamin D deficiency was strongly associated with AI, particularly in elderly persons ≥60 years of age 6. Additionally, Shin et al. reported that pre‐ and postmenopausal Korean women with low levels of 25(OH)D are at higher risk of IDA and AI 21.
This study found no statistically significant differences in the prevalence or distribution of vitamin D deficiency among anemia subtypes. Potential reasons for this include differences in characteristics of the study population, prevalence of anemia, criteria for anemia subtypes, distribution of anemia subtypes and sample size. Above all, ethnicity of the enrolled patients was likely the primary cause of this difference. Skin color is an adaptive trait that has evolved in part to regulate 25(OH)D 22. Non‐Hispanic blacks were previously shown to have a sevenfold greater risk of having AI, and their vitamin D levels were also lower than those seen in white patients 6. Since only Koreans were enrolled in this study, the association of vitamin D deficiency with AI was not as strong as that reported by Perlstein et al. The basic physical condition of enrolled anemic patients might be different. Compared to the national health survey based studies by Perlstein et al. and Shin et al., all anemic patients in this study had health problems. In addition, relatively higher prevalence of AI and small sample size are also considered as other reasons of different result. A larger dataset from multi‐institutional studies is needed to draw any definitive conclusions.
Vitamin D may also have a potential role in the regulation of hepcidin synthesis and inflammatory pathways. Vitamin D increases anti‐inflammatory cytokines 23 and suppresses pro‐inflammatory cytokines such as IL‐6, thereby inducing hepcidin synthesis 24. Decreased levels of vitamin D may contribute to increases in hepcidin expression, resulting in an increased risk for AI 25. Thus, analysis of hepcidin levels in anemic patients may offer additional information about the correlation between vitamin D metabolism and erythropoiesis. In this study, we instead assessed the correlation between high CRP levels and anemia. Although CRP was not associated with urinary hepcidin levels 26, it is the most commonly used acute‐phase reactant for measuring the degree of inflammation 27. A CRP level >1.0 ng/ml in anemic patients was found to be a risk factor for vitamin D deficiency after controlling for other confounding factors such as age, gender, eGFR, and transferrin saturation.
Kendrick et al. previously reported that lower vitamin D and higher CRP levels were independently associated with lower Hb in patients with kidney disease not requiring dialysis 10. Kidney disease is also an inflammatory process, and CRP levels may reflect the degree of inflammation. The median serum 25(OH)D level was the lowest in patients with ACKD, although the distribution of serum 25(OH)D level was not statistically significant in this study.
ACKD is a multifactorial disease, and anemia is a common complication of CKD due to low erythropoietin (EPO) levels, chronic inflammation, iron deficiency, and reduced RBC lifespan. In CKD, vitamin D deficiency may cause immune activation and cytokine production, thereby inducing impaired erythropoiesis in the bone marrow microenvironment. Consequences of this inflammatory cascade are EPO resistance and anemia 28. Hepcidin may contribute to impaired erythropoiesis in ACKD as well 28, 29. As a result, these combined underlying factors may contribute to more severe vitamin D deficiency in ACKD patients.
Vitamin D deficiency has been observed even in the early stages of CKD 30. Both serum 25(OH)D and 1,25(OH)2D levels are decreased in CKD patients, and vitamin D deficiency is a known risk factor for mortality in this population. Moderate renal dysfunction (eGFR <45 ml/min/1.73 m2) is an important predictor of vitamin D deficiency, as serum 25(OH)D levels begin to decrease at an eGFR of 60 ml/min/1.73 m2 independent of other risk factors 31. The adjusted odds ratio of eGFR <60 ml/min/1.73 m2 for vitamin D deficiency was 2.389; however, it was not statistically significant in this study.
Although there are several limitations to this study including small sample size, seasonal variation, and unanalyzed hepcidin levels, we clearly demonstrate that vitamin D deficiency was much more prevalent in anemic patients regardless of age, gender, or anemia subtype. Therefore, serum vitamin D measurement should be considered in the management of anemia, particularly in females and patients with high CRP level.
Grant sponsor: Konyang University Myunggok Research Fund 2011.
REFERENCES
- 1. Katrin M, Klemm MJK. Biochemical Markers of Bone Metabolism. Henry's Clinical Diagnosis and Management by Laboratory Methods, twenty‐second edition, Philadelphia, PA: Elsevier Saunders; 2011. p. 193–209. [Google Scholar]
- 2. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80:1689s–1696s. [DOI] [PubMed] [Google Scholar]
- 4. Penna G, Roncari A, Amuchastegui S, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3 +regulatory T cells by 1,25‐dihydroxyvitamin D3. Blood 2005;106:3490–3497. [DOI] [PubMed] [Google Scholar]
- 5. Tian J, Liu Y, Williams LA, de Zeeuw D. Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant 2007;22:321–328. [DOI] [PubMed] [Google Scholar]
- 6. Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25‐hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: Association with anemia of inflammation. Blood 2011;117:2800–2806. [DOI] [PubMed] [Google Scholar]
- 7. Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med 1989;320:980–991. [DOI] [PubMed] [Google Scholar]
- 8. Norman AW. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006;147:5542–5548. [DOI] [PubMed] [Google Scholar]
- 9. Blazsek I, Farabos C, Quittet P, et al. Bone marrow stromal cell defects and 1 alpha,25‐dihydroxyvitamin D3 deficiency underlying human myeloid leukemias. Cancer Detect Prev 1996;20:31–42. [PubMed] [Google Scholar]
- 10. Kendrick J, Targher G, Smits G, Chonchol M. 25‐Hydroxyvitamin D deficiency and inflammation and their association with hemoglobin levels in chronic kidney disease. Am J Nephrol 2009;30:64–72. [DOI] [PubMed] [Google Scholar]
- 11. Sim JJ, Lac PT, Liu IL, et al. Vitamin D deficiency and anemia: A cross‐sectional study. Ann Hematol 2010;89:447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albitar S, Genin R, Fen‐Chong M, Serveaux MO, Schohn D, Chuet C. High‐dose alfacalcidol improves anaemia in patients on haemodialysis. Nephrol Dial Transplant 1997;12:514–518. [DOI] [PubMed] [Google Scholar]
- 13. Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract 2007;105:c132–c138. [DOI] [PubMed] [Google Scholar]
- 14. Zittermann A, Jungvogel A, Prokop S, et al. Vitamin D dieficiency is an independent predictor of anemia in end‐stage heart failure. Clin Res Cardiol 2011;100:781–788. [DOI] [PubMed] [Google Scholar]
- 15. Yoon JW, Kim SW, Yoo EG, Kim MK. Prevalence and risk factors for vitamin D deficiency in children with iron deficiency anemia. Korean J Pediatr 2012;55:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zittermann A, Kuhn J, Drieier J, et al. Association of 25‐hydroxyvitamin D with anemia risk in patients scheduled for cardiac surgery. Int J Lab Hematol 2014;36:29–36. [DOI] [PubMed] [Google Scholar]
- 17. Choi HS, Oh HJ, Choi H, et al. Vitamin D insufficiency in Korea—A greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 2011;96:643–651. [DOI] [PubMed] [Google Scholar]
- 18. Kim SK, Kang HS, Kim CS, Kim YT. The prevalence of anemia and iron depletion in the population aged 10 years or older. Korean J Hematol 2011;46:196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross AC, Taylor CL, Yaktine AL, del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: US National Academies Press; 2011. [PubMed] [Google Scholar]
- 20. Vanasse GJ, Berliner N. Anemia in elderly patients: An emerging problem for the 21st century. Hematology Am Soc Hematol Educ Program 2010;2010:271–275. [DOI] [PubMed] [Google Scholar]
- 21. Shin JY, Shim JY. Low vitamin D levels increase anemia risk in Korean women. Clin Chim Acta 2013;421:177–180. [DOI] [PubMed] [Google Scholar]
- 22. Durazo‐Arvizu RA, Aloia JF, Dugas LR, et al. 25‐Hydroxyvitamin D levels in African American and Nigerian women. Am J Hum Biol 2013;25:560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double‐blind, randomized, placebo‐controlled trial. Am J Clin Nutr 2006;83:754–759. [DOI] [PubMed] [Google Scholar]
- 24. Andrews NC. Anemia of inflammation: The cytokine‐hepcidin link. J Clin Invest 2004;113:1251–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carvalho C, Isakova T, Collerone G, et al. Hepcidin and disordered mineral metabolism in chronic kidney disease. Clin Nephrol 2011;76:90–98. [DOI] [PubMed] [Google Scholar]
- 26. Ferrucci L, Semba RD, Guralnik JM, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood 2010;115:3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yilmaz MI, Carrero JJ, Axelsson J, Lindholm B, Stenvinkel P. Low‐grade inflammation in chronic kidney disease patients before the start of renal replacement therapy: Sources and consequences. Clin Nephrol 2007;68:1–9. [DOI] [PubMed] [Google Scholar]
- 28. Icardi A, Paoletti E, de Nicola L, Mazzaferro S, Russo R, Cozzolino M. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: The potential role of inflammation. Nephrol Dial Transplant 2013;28:1672–1679. [DOI] [PubMed] [Google Scholar]
- 29. Tsuchiya K, Nitta K. Hepcidin is a potential regulator of iron status in chronic kidney disease. Ther Apher Dial 2013;17:1–8. [DOI] [PubMed] [Google Scholar]
- 30. Zehnder D, Landray MJ, Wheeler DC, et al. Cross‐sectional analysis of abnormalities of mineral homeostasis, vitamin D and parathyroid hormone in a cohort of pre‐dialysis patients. The chronic renal impairment in Birmingham (CRIB) study. Nephron Clin Pract 2007;107:c109–c116. [DOI] [PubMed] [Google Scholar]
- 31. Oh YJ, Kim M, Lee H, et al. A threshold value of estimated glomerular filtration rate that predicts changes in serum 25‐hydroxyvitamin D levels: 4th Korean National Health and Nutritional Examination Survey 2008. Nephrol Dial Transplant 2012;27:2396–2403. [DOI] [PubMed] [Google Scholar]


