Abstract
Background
In this study, the effects of viral infections on platelet (PLT) count have been reported. This study examined the correlation between PLT count and respiratory virus (RV) infections.
Methods
Patients who visited Dankook University Hospital between December 2006 and February 2014 with symptoms of suspected RV infection were recruited. Multiplex reverse transcriptase polymerase chain reactions identified the causative virus(es). PLT counts were analyzed with respect to virus, age and sex of the patient, and length of hospital stay.
Results
Of the 8,147 patients, 62.8% were RV‐positive, and 18.6% of RV‐positive patients had abnormal PLT counts. There were no differences in the rates of abnormal PLT counts between single‐infection and virus co‐infection cases. In RV infection patients, the incidence of abnormal PLT count increased with age and varied depending on the RV infection type. Patients with abnormal PLT count stayed in hospital longer than those with normal PLT count.
Conclusions
The incidence of thrombocytopenia was higher among the elderly; in younger patients, thrombocytosis was more prevalent than thrombocytopenia. Respiratory tract infections caused by different viruses resulted in varied PLT count changes. Further systematic research of PLT count changes related to viral infections is required.
Keywords: thrombocytosis, thrombocytopenia, influenza virus, respiratory sincytial virus, multiplex PCR, age group, length of hospital stay
INTRODUCTION
The critical role of blood platelets (PLTs) in hemostasis and thrombosis is clearly recognized, but their function in host defense against infection has received much less attention 1. The relationship between PLT count and various viral infections has been reported previously. Infections with varicella zoster virus 2, Epstein–Barr virus 3, and human immunodeficiency type 1 virus 4 have been shown to cause a reduction in PLT count, whereas infection with respiratory syncytial virus 5 has been shown to result in increased PLT count. Abnormal changes in PLT count may affect clinical progression and may be related to a patient's progression to a severe condition.
A number of studies have examined the relationship between PLT count and virus infections. In Japan, the occurrence of immune thrombocytopenia following influenza virus infection 6 has been examined. Similarly, the relationship between avian influenza virus infection and PLT count has been examined in China 7. However, few studies have assessed the relationship between commonly observed clinical respiratory virus (RV) infections, other than influenza, and PLT count. Thus, we examined the relationship between RV infection and PLT count in patients admitted to Dankook University Hospital. To the best of our knowledge, this is the first study to assess the relationship between RV infection and abnormal PLT count in the Korean population.
MATERIALS AND METHODS
Subjects
A total of 8,147 patients who were treated at Dankook University Hospital for acute respiratory symptoms between December 2006 and February 2014 were included in the study. Patients were admitted via the emergency room with symptoms of acute respiratory illness or via outpatient services. General blood tests and tests for examining the presence of RV were performed on all patients.
Sample Collection and Extraction of Viral Nucleic Acid
Within 24 h of admission, nasopharyngeal fluids were collected by inserting a mucus extractor connected to a sterile 8‐French catheter (SEWOON MEDICAL CO. LTD, Ipjang, Cheonan, Korea) 5–7 cm into the nostril and suctioning with a pressure of 60–80 mmHg. Samples were stored at 4°C until nucleic acid extraction was performed. The extracted nucleic acids were stored at −70°C until the tests were performed.
RV Detection
Ribonucleic acid (RNA) was isolated from nasopharyngeal fluid and reconstructed as complementary deoxyribonucleic acid (cDNA). The cDNA was then amplified using the Seeplex RV detection kit‐1 (Seegene, Seoul, Korea) to test for 1 DNA virus, human adenovirus, and 13 RNA viruses that cause respiratory infection: respiratory syncytial virus A and B; influenza virus A and B; parainfluenza virus types 1, 2, and 3; human rhinovirus A and B; coronavirus 229E, NL63, and OC43; and human metapneumovirus. PCR was performed using the PTC 200 PCR system (MJ Research, Watertown, MA) and a program of 40 cycles of 30 s at 94°C, 90 s at 60°C, and 90 s at 72°C followed by one cycle of 10 min at 72°C. The amplified PCR products were analyzed following 30 min of electrophoresis at 100–150 V in 2% agarose gels stained with ethidium bromide.
Definition of PLT Abnormality
Thrombocytopenia was defined as a PLT count <150,000/mm3; thrombocytosis was defined as a PLT count >450,000/mm3 8. Both thrombocytopenia and thrombocytosis were considered to be abnormal PLT counts.
Statistical Analysis
Patient characteristics at the time of admission, including age, sex, and initial blood test results, were analyzed retrospectively. Regression analysis and Welch's t‐test were performed. A P value <0.05 was considered to be statistically significant.
RESULTS
Subject Analysis
Of the 8,147 cases included in this study, 5,114 (62.8%; 2,998 males, 2,116 females) patients tested positive for RV (Table 1). The average age of RV‐positive patients was 6.7 years, and the median age was 1.4 years. The 0‐ to 9‐year age group had the highest number of patients who had RV infections (4,618 cases), and the positive test rate was also highest in this age group (71.9%). Rhinovirus was the most frequently detected virus (1,308 cases; 21.0%), followed by respiratory syncytial virus A (1,034 cases; 16.6%), adenovirus (915 cases; 14.7%), and respiratory syncytial virus B (775 cases; 12.4%).
Table 1.
Analysis of Cases, Positive Rate, Infection Types, and Pathogenic Viruses
| Average | Median | Positive | ||
|---|---|---|---|---|
| Number | age | age | rate (%) | |
| Enrolled patients | 8,147 | 13.0 | 1.9 | 100.0 |
| RV infection positive | 5,114 | 6.7 | 1.4 | 62.8 |
| Male | 2,998 | 6.8 | 1.8 | 36.8 |
| Female | 2,116 | 6.5 | 1.5 | 26.0 |
| Single infection | 4,101 | 7.4 | 1.3 | 50.4 |
| Double infection | 916 | 3.9 | 1.4 | 11.2 |
| More than triple | 97 | 4.1 | 1.6 | 1.2 |
| Causative virus | 6,228 | – | – | 100.0 |
| INF A | 518 | 16.7 | 4.4 | 8.3 |
| INF B | 148 | 16.2 | 5.0 | 2.4 |
| RSV A | 1,034 | 9.6 | 0.5 | 16.6 |
| RSV B | 775 | 4.5 | 0.7 | 12.4 |
| Meta Pn | 413 | 2.8 | 1.7 | 6.6 |
| PIV 1 | 248 | 5.3 | 1.4 | 4.0 |
| PIV 2 | 78 | 8.1 | 1.8 | 1.3 |
| PIV 3 | 422 | 3.7 | 1.1 | 6.8 |
| Rhino | 1,308 | 5.0 | 1.4 | 21.0 |
| Cov 229 | 170 | 5.8 | 1.5 | 2.7 |
| Cov OC43 | 199 | 4.4 | 1.3 | 3.2 |
| ADV | 915 | 8.6 | 2.2 | 14.7 |
RV, respiratory virus; INF, influenza virus; RSV, respiratory syncytial virus; Meta Pn, human metapneumovirus; PIV, parainfluenza virus; Rhino, human rhinovirus; Cov, coronavirus; ADV, adenovirus.
Analysis of Viral Infection and PLT Count
Virus–virus co‐infection and PLT count
In the RV‐positive cases, the rate of abnormal PLT count was 18.6% (953 of 5,114 cases). In the single RV infection cases, the rate of abnormal PLT count was 19.2% (787 of 4,101 cases), and in virus co‐infection cases (double and triple RV infection), the rate of abnormal PLT count was 16.4% (166 of 988 cases). Specifically, abnormal PLT counts were observed in 16.3% (149 of 916 cases) of double‐infection cases and 17.5% (17 of 97 cases) of triple‐infection cases (Table 1).
Age and PLT count
Of the RV‐positive patients, those in the 40‐ to 50‐year age group had the highest rate of abnormal PLT count (Fig. 1). The rate of thrombocytopenia was highest in the 40‐ to 50‐year age group, whereas the rate of thrombocytosis was highest in the 0‐ to 1‐year age group (26.1%). Regression analysis showed that the number of patients with decreased PLT count increased with the increasing age (P < 0.001, Fig. 1).
Figure 1.
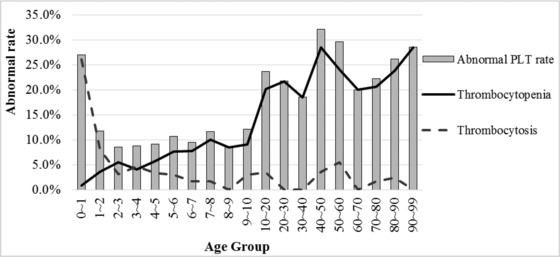
Abnormal PLT rate in each age group. The solid line indicates the rate of thrombocytopenia and broken line indicates the rate of thrombocytosis.
PLT count abnormality secondary to viral infection
The rate of abnormal PLT count was highest in patients following infection with influenza virus B (24.3%) and lowest following infection with influenza type A (11.4%; Fig. 2).
Figure 2.
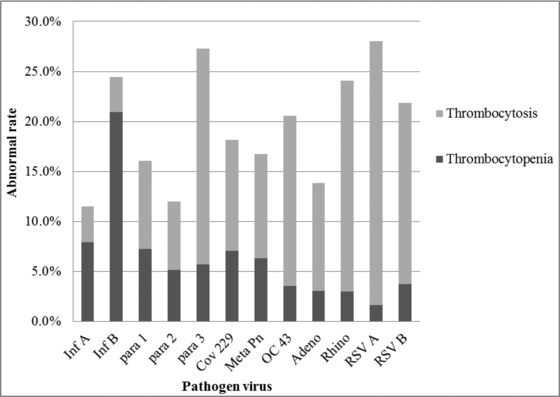
Abnormal PLT rate by causative virus. The rate of thrombocytopenia is indicated by the dark gray shading and the rate of thrombocytosis is indicated by the light gray shading. Inf, influenza virus; para, parainfluenza virus; Cov, coronavirus; Meta Pn, human metapneumovirus; OC 43, coronavirus OC43; Adeno, adenovirus; Rhino, human rhinovirus; RSV, respiratory syncytial virus.
Length of hospital stay and PLT count
The length of hospital stay for patients with an abnormal PLT count was significantly longer than for patients with a normal PLT count (P < 0.001, Welch's t‐test; Table 2).
Table 2.
The Difference in Hospital Stay Length Between Patients With a Normal PLT Count and Those With an Abnormal PLT Count
| Average length of hospital stay | Variance | Number | P value | |
|---|---|---|---|---|
| Normal PLT count | 6.9 | 156.7 | 6,403 | <0.001 |
| Abnormal PLT count | 13.6 | 550.4 | 592 |
PLT, platelet.
DISCUSSION
The recent advances in methods used in the diagnosis of viral infections, such as the development of multiplex reverse transcriptase PCR, have allowed the simultaneous examination for the presence of multiple RVs and simplified identification of causative viruses. Therefore, it is now possible to examine the clinical differences of specific viruses 9. Viral infections can cause a number of hematological abnormalities that commonly include abnormalities in blood cell counts, including anemia, neutropenia, and thrombocytopenia. It is thought that autoimmune responses and suppression of bone marrow progenitor cells are the causative factors of such abnormalities 1, 10. Although several mechanisms have been proposed to explain the effects of viruses on PLT count, the factors that determine whether thrombocytopenia develops in response to a viral infection are not well understood.
Of the 8,147 patients included in this study, 62.8% tested positive for RV, with a male‐to‐female ratio of 1.42:1. The distribution of age and sex of the patients who tested positive for RV was similar to what has been observed previously 5, 8, 11, 12; however, the overall rate of patients who tested positive for viruses differed from the rate of 77.4% 5 and 34.1% 12 reported previously.
A total of 18.6% of patients who tested positive for RV infection presented with PLT count abnormality. Thrombocytopenia was observed in 4.9% of patients, whereas thrombocytosis was observed in 13.7% of patients. In contrast, 6.1% 13 and 36% 7 of thrombocytopenia incidents have been reported previously. It is likely that these observed differences are as a result of the different patient population or the different viral infections, as PLT count abnormality is thought to manifest differently depending on the causative virus.
It has been reported previously that thrombocytopenia is associated with Epstein–Barr virus, varicella zoster virus, and human immunodeficiency type 1 virus infections 14. Thrombocytosis has been reported after infection with bocavirus 15. In our study, the proportion of patients with thrombocytopenia or thrombocytosis after viral infection varied depending on the virus. Patients with influenza virus A and B or parainfluenza virus 1 infections tend to have lower PLT count than patients who did not have RV infection. Specifically, in patients with influenza virus type B infection, 20.9% of patients had thrombocytopenia and 3.5% had thrombocytosis. In contrast, patients with human metapneumovirus, parainfluenza virus 2, coronavirus 229, or adenovirus infections did not show significant changes in PLT count. Furthermore, patients with parainfluenza virus 3, rhinovirus, coronavirus OC43, respiratory syncytial virus A, or respiratory syncytial virus B infections showed a tendency for increased PLT count. In patients with respiratory syncytial virus A infections, 1.6% of the patients had thrombocytopenia and 26.4% had thrombocytosis. While we found that in this study thrombocytosis was more prevalent than thrombocytopenia, further systematic research on PLT count changes associated with viral infections is required.
When PLT count abnormalities were analyzed according to age group, the rate of thrombocytopenia increased as age increased, whereas there was no significant correlation between thrombocytosis and age. It is thought that thrombocytopenia is prevalent in younger patients. It has been reported previously that childhood idiopathic thrombocytopenia occurs most frequently in young children and it occurs following viral respiratory tract infection 16. However, thrombocytosis was more prevalent in young patients in this study. This observed difference is likely as a result of the causative viruses. The younger patients are more likely to be infected with viruses that cause thrombocytosis, such as rhinovirus and respiratory syncytial virus A, than other viruses 17, whereas viruses that primarily cause thrombocytopenia, such as influenza viruses A and B, more often infect older patients.
The most common mechanism of virus‐induced decreases in PLT count is bone marrow suppression; however, it has been reported that viral infection may cause PLT destruction by immune mechanisms, segregation of PLT in the spleen, impairment of thrombopoietin production, and PLT destruction via direct interaction with viruses 1. In most cases, thrombocytopenia caused by viral infection is transient without serious bleeding and resolved spontaneously within a few weeks without treatment 9, 18.
While there have been a number of studies that have investigated specific age groups, such as young children or the elderly 5, 11, 13, 14, 19, there are limited studies that have examined the correlation between abnormal PLT count and RV infections in patients of all age groups.
CONCLUSION
This study examined the relationship between RV infection and PLT count abnormalities in patients of all age groups in Korea and showed that respiratory syncytial virus, influenza virus type A, and rhinovirus infections are associated with high rates of PLT count abnormalities. In contrast parainfluenza virus, adenovirus, human metapneumovirus, human coronavirus, and human bocavirus infections did not significantly affect PLT count. For further examination of the association between PLT count abnormalities and RV infection, additional studies with larger sample sizes are required.
Grant sponsor: The present research was conducted by the research fund of Dankook University in 2014.
Correction added on 15th March, after first online publication: Co‐author Ga‐Yeon kim is added in this paper.
REFERENCES
- 1. Flaujac C1, Boukour S, Cramer‐Bordé E. Platelets and viruses: An ambivalent relationship. Cell Mol Life Sci 2010;67:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Espinoza C, Kuhn C. Viral infection of megakaryocytes in varicella with purpura. Am J Clin Pathol 1974;61:203–208. [DOI] [PubMed] [Google Scholar]
- 3. Michele L, Pipp, Norman D , Means, John W , et al. Acute Epstein‐Barr Virus Infection Complicated by Severe Thrombocytopenia. 1997;25:1237–1239. [DOI] [PubMed] [Google Scholar]
- 4. Kouri YH, Borkowsky W, Nardi M, Karpatkin S, Basch RS. Human megakaryocytes have a CD4 molecule capable of binding human immunodeficiency virus‐1. Blood 1993;81:2664–2670. [PubMed] [Google Scholar]
- 5. Bilavsky E, Yarden‐Bilavsky H, Shouval DS, et al. Respiratory syncytial virus‐positive bronchiolitis in hospitalized infants is associated with thrombocytosis. Isr Med Assoc J 2010;12:39–41. [PubMed] [Google Scholar]
- 6. Shizuma T. Immune thrombocytopenia following influenza virus infection and influenza vaccine administration. Virol Mycol 2014;S2:003. doi:10.4172/2161‐0517.S2‐003 [Google Scholar]
- 7. Chen W, Hongjie Y, Peter HW, et al. Comparison of patients hospitalized with influenza A H7N9, H5N1, and 2009 pandemic H1N1. Clin Infect Dis 2014;58:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park YK, Park SH, Kim NH, Park EH, Hwang SJ, Jin SH. Isolated respiratory virus and clinical features analysis of acute respiratory illness in Busan. Annual Report of Busan Metropolitan City Institute of Health & Environment, Vol. 20; 2011. p 16–26. [Google Scholar]
- 9. Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis 2006;43:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peter E. Leukopenia Kliegman RM, Stanton BF, Schor NF, Behrman RE, St. Geme JW., III (eds.). Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier Saunders; 2011. p 746–752. [Google Scholar]
- 11. Calvo C, García‐García ML, Blanco C, et al. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J ClinVirol 2008;42:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roh EJ, Chang YP, Kim JK, Rheem IS, Park KS, Chung EH. Clinical significance of codetection of the causative agents for acute respiratory tract infection in hospitalized children. Korean J Pediatr 2009;52:661–666. [Google Scholar]
- 13. Park IH, Lee SH, You ST, Choi DY. Hematologic complication of respiratory virus infection. Korean J Pediatr Infect Dis 2013;20:178–185. [Google Scholar]
- 14. Rand ML, Wright JF. Virus‐associated idiopathic thrombocytopenic purpura. Transfus Sci 1998;19:253–259. [DOI] [PubMed] [Google Scholar]
- 15. Körner RW, Söderlund‐Venermo M, van Koningsbruggen‐Rietschel S, Kaiser R, Malecki M, Schildgen O. Severe human bocavirus infection, Germany. Emerg Infect Dis 2011;17:2303–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–186. [DOI] [PubMed] [Google Scholar]
- 17. Consolini DM. Thrombocytopenia in infants and children. Pediatric Rev 2011;32:135–151 [DOI] [PubMed] [Google Scholar]
- 18. Labarque V, Van Geet C. Clinical practice: Immune thrombocytopenia in paediatrics. Eur J Pediatr 2014;173:163–172. [DOI] [PubMed] [Google Scholar]
- 19. Dame C, Sutor AH. Primary and secondary thrombocytosis in childhood. Br J Haematol 2005;129:165–177. [DOI] [PubMed] [Google Scholar]


