Abstract
Objective
Diagnostic significance of interleukin 6 (IL‐6) for lung cancer patients with radiation pneumonitis (RP) was examined within various studies, but yielded conflicting results. Thus, this meta‐analysis was performed to demonstrate correlations between serum IL‐6 levels and RP in lung cancer patients.
Method
Electronic databases updated to March 2014 were searched to find relevant studies. Relevant literatures were searched under the PubMed, Embase, Web of Science, Cochrane Library, CISCOM, CINAHL, Google Scholar, CBM and CNKI databases. STATA statistical software (Version 12.0, Stata Corporation, and College Station, TX) Standardized mean difference (SMD), and its corresponding 95% confidence intervals (CIs) were used for this meta‐analysis. In addition, nine cohort studies met the inclusion criteria and involved a total of 137 RP patients and 295 non‐RP patients.
Results
The results of combined SMD suggested that serum IL‐6 levels in RP patients was significantly higher than in non‐RP patients before radiotherapy. While, there was a significant difference in serum IL‐6 levels of RP patients between before and after radiotherapy, we observed no difference in serum IL‐6 levels between RP patients and non‐RP patients after radiotherapy. Ethnicity‐stratified analyses indicated that increased serum IL‐6 levels were related to the risk of RP in lung cancer patients among Caucasians, but not detected among Asians (all P > 0.05).
Conclusion
The main finding of our meta‐analysis reveals that increased serum IL‐6 levels may contribute to the incidence of RP in lung cancer patients, especially among Caucasians.
Keywords: interleukin‐6, radiotherapy, radiation pneumonitis, lung cancer, meta‐analysis
INTRODUCTION
Lung cancer, regarded as the most frequently diagnosed cancer worldwide, was estimated to be the leading cause of cancer mortality in developed countries 1. Approximately, 30∼40% of patients with lung cancer were treated with radiotherapy, which is usually associated with toxicities that possibly limit the treatment dose, and may increase the risk of developing pneumonitis and fibrosis 2. Radiation pneumonitis (RP), which usually occurs within 1.5∼3 months after radiotherapy, was a serious complication and also one of the most common dose‐limiting toxicities for lung cancer patients treated with radiotherapy 3, 4. Generally, RP was characterized by inflammatory immune cells and infiltrated the alveolar side from the vascular side, with approximately 10% to 20% of patients experiencing moderate or severe RP, and the effects led by RP on the treatment of lung cancer have been increasingly recognized 5, 6. Multiple risk factors, such as age, location of tumor, gender, smoking status, and lung dosimetric factors, have been identified for the development of RP 7. Inaccurate assessment of the severity and inappropriate treatment for RP may lead to a potential risk of mortality. Therefore, selection of an auxiliary serum marker could be essential for aiding radiation oncologists to set individual constraints on dosimetric parameters and accurately assess the severity of RP for the lung 3. In clinical practice, the traditional predictors, such as serum amyloid A and plasma cytokines, proved inefficient and inaccurate, hence, replacing an identifying biologic marker may be urgently needed. Also, interleukin‐6 (IL‐ 6) that involves inflammatory processes in the lung has been reported as a risk factor correlated with development of RP 6, 8.
IL‐6, whose family members include IL‐11, IL‐27, IL‐31, leukemia inhibitory factor, was a cytokine consisting of 184 amino acids produced by a variety of host and tumor cells in response to trauma, infection, or immunological challenges 9, 10. Furthermore, IL‐6 has a critical role on the immune system for host defense and tumorigenic process and is often regarded as one of the best pleiotropic pro‐inflammatory cytokines with a wide range of biological activities 11. For instance, IL‐6 holds effects on the regulation of cell growth, cell proliferation, metabolism, the acute‐phase reaction, and hematopoiesis 12. According to previous studies, increased serum IL‐6 levels were associated with individual morbidity and disease activity and were found in many cancers such as breast cancer, gastric cancer, colorectal cancer, lung cancer, prostate cancer, and melanoma 10, 13. Under normal physiological conditions, serum levels of IL‐6 may be quite low or may not be detected; however, it has been reported that IL‐6 can act as an autocrine growth factor in cancers as it is produced in a large amount stimulated by physiological factors such as diet, exercise, and stress 13. Coincidently, it has been presented that overproduction of IL‐6 has been described in the acute radiation‐induced process and it may be associated with the risk of RP in patients with lung cancer 14, 15. It is speculated that if the serum levels of IL‐6 in patients are significantly higher after radiotherapy, they are more likely to develop RP, while on the contrary, if the serum levels are lower, they are less likely to develop RP 8. In this regard, IL‐6 concentration could be considered as a very important predictor for the assessment of RP 8. A number of studies have suggested that IL‐6 could be used as a powerful predictive tool for the possible development of RP or could be used in association with the dosimetric parameters for a more accurate estimation of the occurrence of severe RP. 8, 15, 16. Therefore, the present meta‐analysis was conducted to explore further potential correlations between serum IL‐6 levels and RP in lung cancer patients.
MATERIALS AND METHODS
Search Strategy
We conducted a systematic literature search to identify relevant studies published before March 2014 using PubMed, Embase, Web of Science, Cochrane Library, CISCOM, CINAHL, Google Scholar, CBM, and CNKI databases. Disease‐specific search terms (“radiation pneumonitis” or “radiation pneumonitis” or “radiation pneumonia” or “radiation fibrosis”) were combined with inflammatory marker specific search terms (“interleukin‐6” or “interleukin‐6” or “plasmacytoma growth factor” or “B‐cell differentiation factor‐2” or “B‐cell stimulatory factor 2” or “BSF‐2” or “hepatocyte‐stimulating Factor” or “hybridoma growth factor” or “IFN‐beta 2” or “IL‐6” or “IL 6” or “MGI‐2” or “myeloid differentiation‐inducing protein” or “B cell stimulatory factor‐2” or “B‐cell differentiation factor”) in all of our searches with no language restriction. The electronic searches were supplemented by manually scanning the reference lists from retrieved articles to identify additional studies that may have been missed during the initial search. We also contacted the primary authors for additional data and/or clarification of data, when necessary, to ensure that all relevant articles were represented in the meta‐analysis.
Selection Criteria
The primary outcome of this systematic review was to compare serum IL‐6 levels between study participants with and without RP. To be enrolled in the analysis, these studies must be in accordance with the following criteria: (i) cohort studies focus on the correlations between serum IL‐6 levels and RP in lung cancer patients; (2) the minimum number of cases in included studies should be greater than 30; (3) all lung cancer patients diagnosed with RP should be confirmed by X‐ray or other laboratory examinations; (4) published data about serum IL‐6 levels must be sufficiency; (5) data only from those participants who had a history of smoking were included, while data from lifetime nonsmokers were censored from the main analyses. Studies were excluded if they did not meet all of these inclusion criteria. If more than one study by the same author, using the same case series, was published, both the study with the largest sample size and the most recent publication were included. Any questions or discrepancies regarding these data were resolved through iteration and consensus.
Data Extraction
From each eligible article, two investigators abstracted the following information independently using a standardized form: surname of first author, year of publication, source of publication, country of origin, ethnicity, language of publication, study type, study design, total number of subjects or samples, source of subjects or samples, pathological subtype, baseline characteristics of study participants including age, gender. We also evaluated the laboratory methods used to detect the levels of serum IL‐6. In cases of conflicting evaluations, disagreements on inconsistent data from the eligible studies were resolved through discussion and careful reexamination of the full text by the investigators.
With the use of the Newcastle–Ottawa Scale (NOS) criteria, two investigators separately assessed methodological quality 17. The NOS criteria comprised three aspects: (i) subject selection: 0∼4; (ii) comparability of subject: 0∼2; (iii) clinical outcome: 0∼3. NOS scores ranged from 0 to 9; and a score with good quality should be ≥7.
Statistical Analysis
The primary outcome of serum IL‐6 levels and RP risk was performed by standardized mean difference (SMD) and 95% confidence intervals (CIs). A fixed‐ or random‐effects model was used wherever it was appropriate. The significance of the pooled estimate was made using the Z‐test. Statistical heterogeneity among studies was evaluated by using the Cochran's Q‐statistic, which is considered significant at P < 0.05 18. The I2 test was also used to quantify the heterogeneity (ranges from 0% to 100%) 19. Random‐effects model (DerSimonian Laird method) was used when a significant Q‐test with P < 0.05 or I 2 > 50%. When there was no statistical heterogeneity, we used a fixed‐effects model (Mantel–Haenszel method). In order to explore potential sources of heterogeneity, subgroup analyses were performed based on ethnicity, and radiotherapy method. Sensitivity analysis was performed to evaluate whether the results could have been affected significantly by repeating the meta‐analysis but excluding one study at a time. The symmetry of the funnel plot was further evaluated by Egger's linear regression test 20. All tests were two‐sided and a P‐value of <0.05 was considered statistically significant. All analyses were calculated using the STATA version 12.0 (Stata Corp, College Station, TX).
RESULTS
Baseline Characteristics of Included Studies
A summary of the search strategy is shown in Figure 1. The original search yielded a total of 196 citations related to the searched keywords. Of these articles, 88 were excluded after reviewing their titles and key words; then, abstract and full text were reviewed, and another 99 papers were excluded. Finally, nine studies met our inclusion criteria for this meta‐analysis 8, 21, 22, 23, 24, 25, 26, 27, 28. These publications ranged from 2005 to 2012. The distribution of the number of topic‐related literatures in the electronic database during the last decade is shown in Figure 2. A total number of 137 RP patients and 295 non‐RP patients were involved in this meta‐analysis. Among the nine studies, four were conducted in Caucasians and five in Asians. Peripheral blood samples were used for detection of serum IL‐6 levels. Enzyme‐linked immunosorbent assay (ELISA), Luminex multi‐analyte profiling (xMAP) assay, and Avidin–Biotin Complex (ABC) method were utilized. The characteristics and methodological quality of the included studies are summarized in Table 1.
Figure 1.
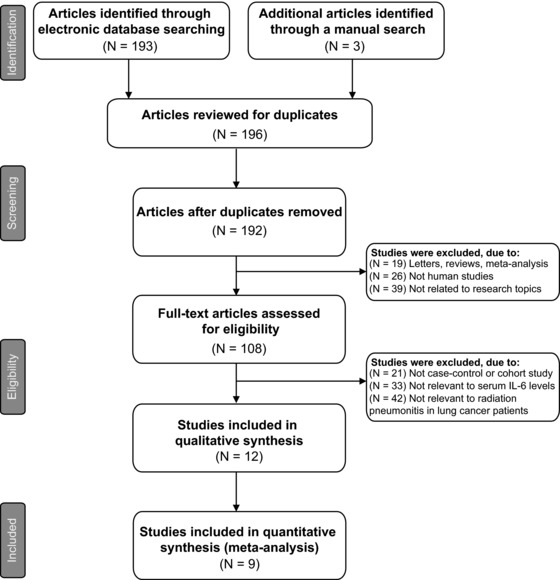
Flow chart of literature search and study selection. Nine clinical cohort studies were included in this meta‐analysis.
Figure 2.
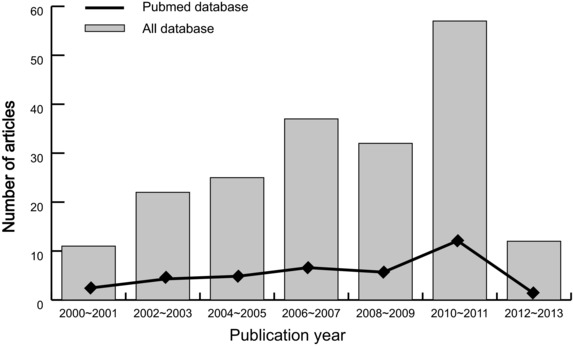
Distribution of topic‐related literature in electronic databases over the last decade.
Table 1.
Characteristics and Methodological Quality of the Included Studies
| Case number | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | Year | Country | Ethnicity | RP | non‐RP | Gender (M/F) | Age (years) | Detection method | Radiotherapy | Dose (Gy) | NOS score |
| Yao YH 23 | 2012 | China | Asians | 11 | 38 | 36/13 | 56 (46∼66) | ELISA | IMRT | 60∼70 | 6 |
| He MW 21 | 2012 | China | Asians | 17 | 41 | 35/23 | 56.7 ± 13.5 | ELISA | 3D‐CRT | 40∼66 | 7 |
| Wang D 28 | 2012 | China | Asians | 5 | 21 | 48/9 | 68 (34∼71) | ELISA | IMRT | 59.6∼68.0 | 6 |
| Stenmark MH 8 | 2012 | USA | Caucasians | 10 | 48 | 51/7 | 69 (60∼76) | xMAP assay | 3D‐CRT | 66.9 (64.2 ∼70.0) | 7 |
| Wang G 22 | 2008 | China | Asians | 16 | 21 | 19/18 | 61 (33∼92) | ELISA | 3D‐CRT | 55.6 (46∼66) | 6 |
| Rube CE 27 | 2008 | Germany | Caucasians | 21 | 31 | 41/11 | 65.1 (36∼85) | ABC | IMRT | 66 | 7 |
| Zhao LJ 24 | 2006 | China | Asians | 13 | 29 | 38/4 | 57 (40∼81) | ELISA | 3D‐CRT | 60 (46∼70) | 6 |
| Hart JP 26 | 2005 | USA | Caucasians | 22 | 33 | – | – | ELISA | 3D‐CRT | – | 5 |
| Arpin D 25 | 2005 | France | Caucasians | 22 | 33 | 88/12 | 60 (27∼77) | ELISA | 3D‐CRT | 66 (46∼72) | 6 |
M, male; F, female; IMRT, intensity‐modulated radiation therapy; 3D‐CRT, three‐dimensional conformal radiotherapy.
Change of serum IL‐6 levels
The results of meta‐analysis on the associations between serum IL‐6 levels and RP in lung cancer patients are shown in Figure 3. Considering significant heterogeneity among studies, the random‐effects model was used. The pooled estimate indicated that serum IL‐6 levels in RP patients were significantly higher than in non‐RP patients before radiotherapy (SMD = 0.60, 95% CI = 0.31∼0.90, P < 0.001). Besides, there was significant difference in serum IL‐6 levels of RP patients between before and after radiotherapy (SMD = 0.90, 95% CI = 0.54∼1.25, P < 0.001). However, we observed no difference in serum IL‐6 levels between RP patients and non‐RP patients after radiotherapy (SMD = 0.02, 95% CI = −0.19∼0.24, P = 0.837).
Figure 3.
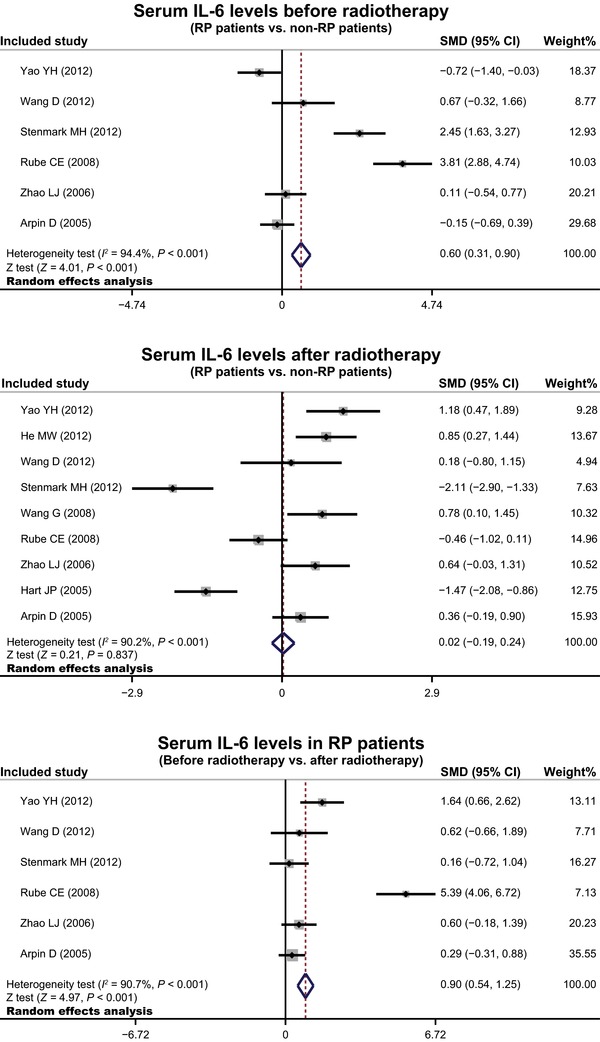
Forest plots for the differences in serum IL‐6 level between lung cancer patients with and without RP before and after radiotherapy.
In order to explore potential sources of heterogeneity, we also performed subgroup analysis based on ethnicity. Ethnicity‐stratified analyses indicated that increased serum IL‐6 levels were related to the risk of RP in lung cancer patients among Caucasians (SMD = 1.24, 95% CI = 0.84∼1.65, P < 0.001), but not detected among Asians (all P > 0.05, Fig. 4).
Figure 4.
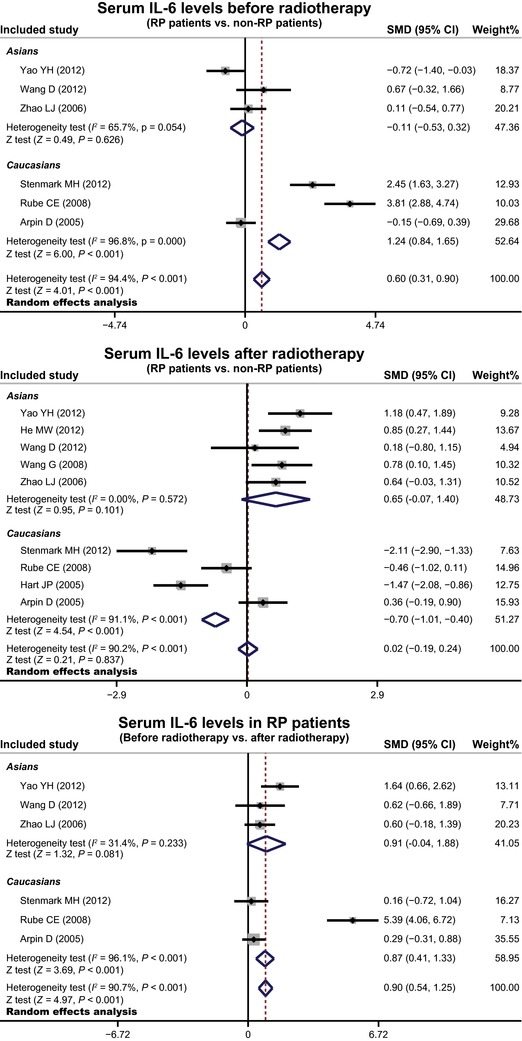
Subgroup analysis by ethnicity for the differences in serum IL‐6 level between lung cancer patients with and without RP before and after radiotherapy.
Evaluation of Heterogeneity and Publication Bias
Sensitivity analysis was performed to assess the influence of each individual study on the pooled SMD by omitting each. The analysis results suggested that no individual studies influenced the pooled SMDs (Fig. 5). Funnel plots and Egger's linear regression test were used to assess potential publication bias of included studies. The shapes of the funnel plots did not reveal any evidence of obvious asymmetry (Fig. 6). Egger's test also did not show strong statistical evidence of publication bias (all P > 0.05).
Figure 5.
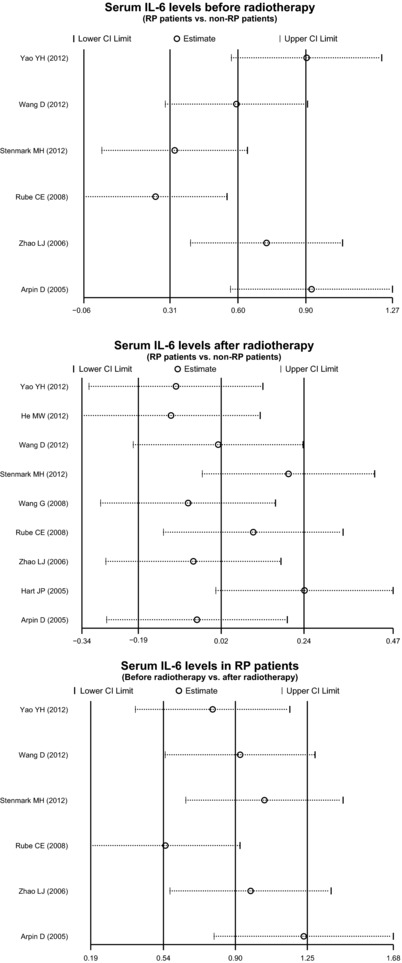
Sensitivity analysis of the summary odds ratio coefficients for the differences in serum IL‐6 level between lung cancer patients with and without RP before and after radiotherapy.
Figure 6.
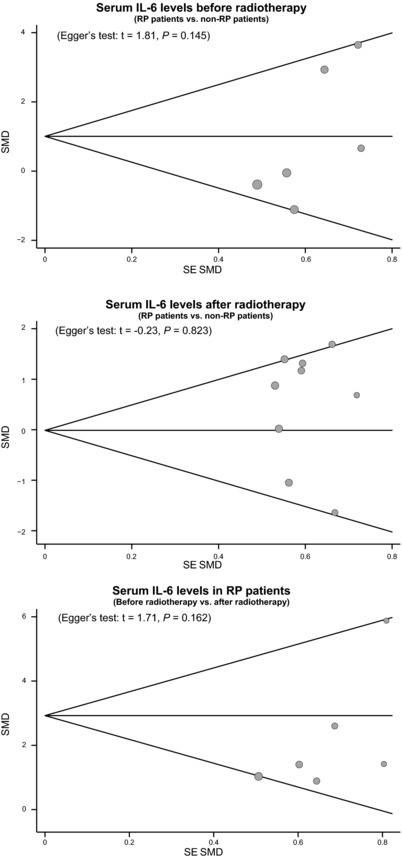
Funnel plot of publication biases for the differences in serum IL‐6 level between lung cancer patients with and without RP before and after radiotherapy.
DISCUSSION
The present meta‐analysis was performed to study the relationships between serum IL‐6 levels and RP in lung cancer patients treated with radiotherapy. The major result of our meta‐analysis revealed that the serum levels of IL‐6 were significantly higher in RP patients than those in non‐RP patients before radiotherapy, suggesting that elevated serum IL‐6 level may serve as a potential predictor in the incidence of RP. In recent years, some researches in radiation pulmonary injury have supported that the cytokine factors, such as TGF‐beta1 and IL‐6, may be involved in the pathogenesis of RP, although the specific mechanism is still unclear 29, 30. It is widely accepted that IL‐6 is a cytokine involved in inflammation and infection responses and acts as a pro‐inflammatory cytokine 31. As a multifunctional cytokine, human IL‐6 is secreted by T‐lymphocytes, macrophages, type II pneumocytes, and fibroblasts, and regulates the immune reactions, inflammation, and the acute phase protein response 32. Furthermore, the cytokines, including IL‐6, and some growth factors are pleiotropic and have a wide range of activities, which can expressed after injury and are nonspecific for the radiation injury 33. Thus, the measurement of serum IL‐6 level changes in blood circulation may eventually play an important role in predicting the risk of radiation‐induced complications and estimating the inflammatory state of lung tissues 25, 28. Some previous clinical reports have indicated that the plasma concentration of IL‐6 of patients have been changed during the radiation therapy, suggesting that the variations of IL‐6 plasma concentration could identify patients at risk of RP 29, 34. In addition, our study also found that the serum level of IL‐6 was significantly higher in posttreatment periods than that of pretreatment periods in two groups. This research finding may indicate that the inflammatory process of radiation‐induced lung injury is correlated with an increased production of IL‐6 within the irradiation field, which is launched into the blood circulation leading to elevated plasma levels 27. Some previous studies have also indicated that IL‐6 serum level increased more strikingly for patients who experienced RP than those who did not develop this complication after treated with the radiation therapy and chemotherapy 35, 36.
We also carefully performed stratified analysis on the basis of ethnicity to investigate the potential possibility of obvious heterogeneity, which may influence our results negatively. Our results showed that increased level of IL‐6 reveals a significant associated risk of RP in lung cancer patients among Caucasians, whereas no such observations were detected among Asians, suggesting that ethnicity differences may be the potential heterogeneity resource of this outcome. A possible explanation for the ethnicity differences could be that the divergence in environments, genetic backgrounds, and risk factors relate to the lifestyles among these populations. Furthermore, we also found that the serum level of IL‐6 was significantly higher in posttreatment periods than that in pretreatment periods in two Asian and Caucasian groups.
Although our meta‐analysis was a practical way to generate a more powerful estimate of true effect‐size with less random error than individual studies, it did come with some limitations. First, most of the included publications were retrospective studies, where the level of evidence provided by retrospective articles was lower than that of randomized controlled trails. Second, it is important to note that due to the restriction of the small number of primary studies analyzed in each group, the power to detect potentially important differences is limited, though no significant heterogeneity among the primary studies was detected. We especially enrolled eligible English studies that relate to the occurrence of potential biases because of excluding parts of qualified studies based on language criteria. A third limit in our meta‐analysis is that we did not take into account unpublished articles and abstracts, since a myriad of needed information cannot be successfully obtained. It must be pointed out that, when the sample size of the studies or the number of eligible studies is small, power of detecting publication bias is reduced. Therefore, the results from this meta‐analysis must be interpreted with caution. Despite the above limitations, this is the first example of meta‐analysis on the association of serum IL‐6 levels with the incidence of RP. More importantly, our meta‐analysis uses a statistical approach to combine the results from multiple studies. In order to achieve strong objectivity, all the research methods were carried out on strict inclusion and exclusion criteria. Besides, inconsistency of results was rigorously quantified and analyzed in our meta‐analysis, which will lead to a more accurate conclusion.
In conclusion, our meta‐analysis demonstrated that increased serum IL‐6 levels may contribute to the incidence of RP in lung cancer patients, especially among Caucasians. Serum IL‐6 level could be a candidate and useful diagnostic biomarker for RP in lung cancer patients. Nevertheless, due to several limitations addressed previously, to strengthen our results, well‐designed prospective studies should help to explore the relations between elevated serum IL‐6 levels and the incidence of RP, combined with a better standardized assessment of diagnostic markers.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
We thank the reviewers for their helpful comments on this paper.
REFERENCES
- 1. Wangari‐Talbot J, Hopper‐Borge E. Drug resistance mechanisms in non‐small cell lung carcinoma. J Can Res Updates 2013;2:265–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le Pechoux C, Laplanche A, Faivre‐Finn C, et al. Clinical neurological outcome and quality of life among patients with limited small‐cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99–01, EORTC 22003–08004, RTOG 0212 and IFCT 99–01). Ann Oncol 2011;22:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsuo Y, Shibuya K, Nakamura M, et al. Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012;83:e545–e549. [DOI] [PubMed] [Google Scholar]
- 4. Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis after hypofractionated radiotherapy: Evaluation of the LQ(L) model and different dose parameters. Int J Radiat Oncol Biol Phys 2010;77:1596–1603. [DOI] [PubMed] [Google Scholar]
- 5. Tajvidi M, Sirous M, Sirous R, et al. Partial frequency of radiation pneumonitis and its association with the energy and treatment technique in patients with breast cancer, Isfahan, Iran. J Res Med Sci 2013;18:413–416. [PMC free article] [PubMed] [Google Scholar]
- 6. Wang YS, Chang HJ, Chang YC, et al. Serum amyloid a as a predictive marker for radiation pneumonitis in lung cancer patients. Int J Radiat Oncol Biol Phys 2013;85:791–797. [DOI] [PubMed] [Google Scholar]
- 7. Kong FM, Ao X, Wang L, et al. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Cancer Control 2008;15:140–150. [DOI] [PubMed] [Google Scholar]
- 8. Stenmark MH, Cai XW, Shedden K, et al. Combining physical and biologic parameters to predict radiation‐induced lung toxicity in patients with non‐small‐cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:e217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spooren A, Kolmus K, Laureys G, et al. Interleukin‐6, a mental cytokine. Brain Res Rev 2011;67:157–183. [DOI] [PubMed] [Google Scholar]
- 10. Taniguchi K, Karin M. IL‐6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014;26:54–74. [DOI] [PubMed] [Google Scholar]
- 11. Lee JJ, Kim HJ, Yang CS, et al. A high‐affinity protein binder that blocks the IL‐6/STAT3 signaling pathway effectively suppresses non‐small cell lung cancer. Mol Ther 2014;22(7):1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raj DS. Role of interleukin‐6 in the anemia of chronic disease. Semin Arthritis Rheum 2009;38:382–388. [DOI] [PubMed] [Google Scholar]
- 13. Ara T, Declerck YA. Interleukin‐6 in bone metastasis and cancer progression. Eur J Cancer 2010;46:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pine SR, Mechanic LE, Enewold L, et al. Increased levels of circulating interleukin 6, interleukin 8, C‐reactive protein, and risk of lung cancer. J Natl Cancer Inst 2011;103:1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazeron R, Etienne‐Mastroianni B, Perol D, et al. Predictive factors of late radiation fibrosis: A prospective study in non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2010;77:38–43. [DOI] [PubMed] [Google Scholar]
- 16. Grosso MJ, Frangiamore SJ, Saleh A, et al. Poor utility of serum interleukin‐6 levels to predict indolent periprosthetic shoulder infections. J Shoulder Elbow Surg 2014;23(9):1277–1281. [DOI] [PubMed] [Google Scholar]
- 17. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 18. Jackson D, White IR, Riley RD. Quantifying the impact of between‐study heterogeneity in multivariate meta‐analyses. Stat Med 2012;31:3805–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta‐analysis. JAMA 2006;295:676–680. [DOI] [PubMed] [Google Scholar]
- 20. Zintzaras E, Ioannidis JP. HEGESMA: Genome search meta‐analysis and heterogeneity testing. Bioinformatics 2005;21:3672–3673. [DOI] [PubMed] [Google Scholar]
- 21. He MW, Zhang NB. Serum imflammatory factors in radiation pneumonitis patients induced by three‐diemensional conformal radiotherapy and chemotherapy of lung cancer. Chin J Nosocomiol 2012;22:240–242. [Google Scholar]
- 22. Wang G, Lv CJ, Chen SS, et al. Study of correlation between TGF‐β1,TNF‐α and IL‐6 levels in plasma and radiation pneumonitis in patients with non‐small‐cell lung cancer. J Binzhou Med Univ 2011;34:81–84. [Google Scholar]
- 23. Yao YH, Zhang LZ, Wu Y, et al. The correlation between plasma TGF‐beta 1 and IL‐6 levels and radiation pneumonitis in locally advanced non‐small cell lung cancer patients with concurrent chemoradiotherapy. Guangdong Med J 2012;33:604–607. [Google Scholar]
- 24. Zhao LJ, Wang LH, Wang XZ, et al. Study the value of TGF‐β1,IL‐6 and ACE levels in plasma to predict radiation pneumonitis. Chin J Radiation Oncol 2006:217–221. [Google Scholar]
- 25. Arpin D, Perol D, Blay JY, et al. Early variations of circulating interleukin‐6 and interleukin‐10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. J Clin Oncol 2005;23:8748–8756. [DOI] [PubMed] [Google Scholar]
- 26. Hart JP, Broadwater G, Rabbani Z, et al. Cytokine profiling for prediction of symptomatic radiation‐induced lung injury. Int J Radiat Oncol Biol Phys 2005;63:1448–1454. [DOI] [PubMed] [Google Scholar]
- 27. Rube CE, Palm J, Erren M, et al. Cytokine plasma levels: Reliable predictors for radiation pneumonitis? PLoS One 2008;3:e2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang D, Zhu J, Sun J, et al. Functional and biologic metrics for predicting radiation pneumonitis in locally advanced non‐small cell lung cancer patients treated with chemoradiotherapy. Clin Transl Oncol 2012;14:943–952. [DOI] [PubMed] [Google Scholar]
- 29. Okunieff P, Chen Y, Maguire DJ, et al. Molecular markers of radiation‐related normal tissue toxicity. Cancer Metastasis Rev 2008;27:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JY, Kim YS, Kim YK, et al. The TGF‐beta1 dynamics during radiation therapy and its correlation to symptomatic radiation pneumonitis in lung cancer patients. Radiat Oncol 2009;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scheller J, Chalaris A, Schmidt‐Arras D, et al. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta 2011;1813:878–888. [DOI] [PubMed] [Google Scholar]
- 32. Nishimoto N. Interleukin‐6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Ther 2010;87:483–487. [DOI] [PubMed] [Google Scholar]
- 33. Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281–1293. [DOI] [PubMed] [Google Scholar]
- 34. Provatopoulou X, Athanasiou E, Gounaris A. Predictive markers of radiation pneumonitis. Anticancer Res 2008;28:2421–2432. [PubMed] [Google Scholar]
- 35. Graves PR, Siddiqui F, Anscher MS, et al. Radiation pulmonary toxicity: From mechanisms to management. Semin Radiat Oncol 2010;20:201–207. [DOI] [PubMed] [Google Scholar]
- 36. Zhang XJ, Sun JG, Sun J, et al. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol 2012;138:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]


