Abstract
Background
This study is aimed at investigating the frequency of different functional IL‐22+CD4+ T cells in Chinese patients with type 2 diabetes mellitus (T2DM).
Methods
The frequency of circulating IFN‐γ+IL‐17‐IL‐22‐CD4+ (Th1), IFN‐γ‐IL‐17A+IL‐22‐CD4+ (Th17), and IFN‐γ‐IL‐17A‐IL‐22+CD4+ (Th22), and other subsets of IL‐22+CD4+ T cells in 31 patients with new onset T2DM and 16 healthy controls was characterized by flow cytometry. The levels of serum IL‐22, IL‐17, IFN‐γ, insulin C‐peptide, hemoglobin A1c (HbA1c), fasting plasma glucose, and insulin were examined.
Results
The frequency of Th1, Th17, Th22, IFN‐γ+IL‐17−IL‐22+, and IFN‐γ−IL‐17+IL‐22+ CD4+ T cells and the concentrations of IL‐22, but not IL‐17 and IFNγ, in the patients were significantly higher than controls. The percentages of Th22 cells were correlated positively with the frequency of IFN‐γ−IL‐17+IL‐22+CD4+ T cells, the values of body mass index (BMI) and homeostatic model assessment insulin resistance (HOMA‐IR), and the levels of serum IL‐22 in those patients.
Conclusion
Our data suggest that IL‐22+CD4+ T cells may contribute to the early process of T2DM.
Keywords: type 2 diabetes mellitus, Th22, Th17
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a significant health problem worldwide. T2DM is characterized by chronic low grade inflammation and islet β‐cell dysfunction, leading to insulin resistance in the skeletal muscle, liver, and adipose tissues 1. Currently, the etiology and pathogenesis of T2DM have not been fully understood. Previous studies have suggested that genetic, host‐related, and environmental factors contribute to the development and progression of T2DM 2, 3, 4, 5. However, recent studies have indicated that chronic low‐grade inflammation is a critical factor of the development and progression of T2DM because increased levels of acute‐phase proteins, cytokines, and mediators linked to endothelial activation are detected in patients with T2DM 6, 7, 8. Furthermore, proinflammatory Th1 cells are involved in the pathogenesis of T2DM, while anti‐inflammatory Th2 and regulatory T cells negatively regulate inflammation and macrophage activation, improving insulin sensitivity in rodents 9, 10, 11. However, there is little information about the role of other proinflammatory T cells in the development of insulin resistance and T2DM.
Interleukin‐22 (IL‐22) can be produced by CD4+ T cells, natural killer (NK) cells, NKT cells, and activated dendritic cells 12, 13. IL‐22 can protect against tissue damage and regulate inflammation and autoimmunity 5, 6, 12. Previous studies have shown that IL‐22 can induce anti‐inflammatory follistatin and IL‐11 expression, and also proinflammatory IL‐6 and chemokine expression 13, 14, 15. Indeed, elevated levels of plasma IL‐22 are detected in patients with autoimmune diseases, such as psoriasis, rheumatoid arthritis (RA), and Crohn's disease (CD) 16. A recent study has shown that significantly higher levels of IL‐22 are produced by stimulated peripheral blood mononuclear cells (PBMCs) from diabetes patients with active retinopathy 17, suggesting that Th22 may participate in the pathogenesis of diabetes‐related retinopathy. However, what the frequency of circulating Th22 cells in patients with new onset T2DM is and whether Th22 and IL‐22 contribute to the early process of T2DM have not been clarified. Furthermore, some functional CD4+ T cells can secrete IL‐22 and interferon‐γ(IFNγ) or IL‐17 12. It is unclear how frequently IL‐22+IFNγ+ or IL‐22+IL‐17+ CD4+ T cells occur in patients with new onset T2DM and what the role of those different functional IL‐22+CD4+ T cells play in the pathogenesis of T2DM.
Th17 cells are important players and participate in the process of inflammation 18. Th17 cells secrete IL‐17A, IL‐17F, and IL‐21, and are positively regulated by IL‐6, IL‐23, and IL‐1β 19, 20, 21, 22. Previous studies have shown that Th17 cells contribute to the pathogenesis of T2DM and adipose tissue inflammation 23, 24, 25. However, whether and how Th17 cells contribute to the early process of T2DM in Chinese patients have not been clarified. In the current study, we examined the frequency of different functional IL‐22+CD4+ T cells and explored their potential role in the early process of T2DM in Chinese patients.
MATERIALS AND METHODS
Patients and Subjects
A total of 31 patients with newly diagnosed T2DM were recruited in the inpatient service of the First Hospital of Jilin University in China from September 2011 to March 2012. Individual patients with T2DM were diagnosed, according to the diagnosis standards for T2DM (fasting plasma glucose [FPG] >120 mg/dl and hemoglobin A1c [HbA1c] >7%) of the American Diabetes Association (ADA) 2011 12. The inclusion criteria contained: (1) both male and female patients between 20 and 70 years of age; (2) diagnosis of T2DM within 1 month and without diabetic ketoacidosis (DKA); (3) body mass index (BMI) ≥25 kg/m2; (4) plasma insulin c‐peptide (Pc‐peptide) at 60 min post‐Sustacal > 0.4 mmol/l, Pc‐peptide (mmol/l) at 120 min post‐Sustacal > 0.8 mmol/l; (5) having no formal treatment for diabetes; and (6) having no indications of other chronic diseases. Furthermore, individuals were excluded if they had chronic respiratory disease, smoking habit, clinic history of a neuromuscular disease, narcolepsy, stroke, transient ischemic attack, chronic heart failure, craniofacial abnormality, and alcoholic abuse or use of sedative drugs, current pregnancy, other endocrinological diseases, and recent infection. In addition, 16 gender‐ and age‐matched healthy volunteers were recruited from the Physical Examination Center of our hospital and they had no a history of any chronic disease. Written informed consent was obtained from individual participants, and the experimental protocol was approved by the Institute Review Board of the First Hospital of Jilin University.
Clinical Measurements
Individuals were subjected to physical examination, and their BMI and waist circumference (WC) were measured. Individual subjects were fasted overnight, and their venous blood samples were obtained. The concentrations of FGP, serum insulin c‐peptide, and HbA1c were measured by the glucose oxidase method using a HITACHI 7600–020 Automatic Analyzer (Hitachi High‐Technologies, Tokyo, Japan), radioimmunology (SR 300; STRATEC, Birkenfeld, Germany), and by immunoturbidimetry, respectively. Furthermore, their diabetic status, fasting lipids, hematological and biochemical parameters were examined. In addition, individual subjects were subjected to a standard oral glucose tolerance test for the concentrations of c‐peptide at 30, 60, 120, and 180 min post‐Sustacal stimulation. The degrees of insulin resistance in individuals were assessed by the homeostatic model assessment (HOMA; insulin resistance index = [FPG (mmol/l) × insulin (mU/ml)]/22.5). The levels of insulin sensitivity in individuals were estimated by calculating the quantitative insulin‐sensitivity check index (QUICKI; QUICKI = 1/[log(I(0)) + log(G(0))], I(0) means fasting insulin, G(0) means fasting glucose levels).
Blood Samples and PBMCs Preparation
Venous blood samples (10 ml) from each subject were collected in heparinized tubes, and PBMCs were prepared by density‐gradient centrifugation using Ficoll‐Paque Plus (Amersham Biosciences, Little Chalfont, UK).
Flow Cytometry Analysis of Intracellular Staining
For the detection of IFN‐γ+, IL‐17+, and IL‐22+ CD4+ T cells, PBMCs (106 cells/well) were stimulated in duplicate with 50 ng/ml of phorbol myristate acetate (PMA, Sigma‐Aldrich, St. Louis, MO) and 1.0 μg/ml of ionomycin (Sigma‐Aldrich) in complete Roswell Park Memorial Institute 1640 (RPMI 1640) medium (Invitrogen, Carlsbad, CA) for 2 h at 37°C in 5% CO2 and exposed to Brefeldin A (GolgiPlug, Becton Dickinson, San Diego, CA) for an additional 4 h. Subsequently, the nonadherent cells were harvested, washed with cold Phosphate Buffered Saline (PBS), and stained with PerCP‐anti‐CD4 (Becton Dickinson). After being fixed with 4% paraformaldehyde (30 min/RT), the cells were permeabilized with 0.5% saponin in 10% Fetal Bovine Serum (FBS) in PBS (30 min/reaction time) and stained with FITC (fluorescein isothiocyanate)‐anti‐IFN‐γ, Alexa‐Flour‐anti‐IL‐17 (Becton Dickinson), and PE (phycoerythrin)‐anti‐IL‐22 (R&D Systems, Minneapolis, MN), followed by flow cytometry analysis using the FlowJo software (v7.6.2; TreeStar, Ashland, OR). Th22, Th17, Th1, and Th1/Th17 cells were defined as CD4+IFN‐γ−IL17A−IL‐22+, CD4+IFN‐γ−IL17A+IL‐22−, CD4+IFN‐γ+, and CD4+IFN‐γ+IL17+ T cells, respectively.
Measurement of Serum IL‐17, IL‐22, and IFN‐γ Concentrations by Enzyme‐Linked Immunosorbent Serologic Assay (ELISA)
The concentrations of serum IL‐17, IL‐22, and IFN‐γ in individual patients and HC were determined by ELISA using human IL‐17, IL‐22, and IFN‐γ ELISA kits, according to the manufacturer's instruction (Roche Diagnostics, USA). Briefly, individual sera at 1:4 dilutions were subjected to ELISA analysis, and the concentrations of serum IL‐17, IL‐22, and IFN‐γ in individual samples were calculated according to the standard curve established using the recombinant IL‐17, IL‐22, and IFN‐γ provided. The detection limit of IL‐17, IL‐22, or IFN‐γ ELISA kit was 4.8, 4.8, and 8.0 pg/ml, respectively.
Statistical Analysis
All data are expressed as individual values, median, and range of each group of subjects. Multiple comparisons between two groups were analyzed by the Kruskal‐Wallis H nonparametric test. Correlations between variables were evaluated by the Spearman rank correlation test using SPSS 19.0 for Windows (SPSS, Inc., Chicago, IL). A two‐sided P‐value of <0.05 was considered statistically significant.
RESULTS
A Higher Frequency of Th22, Th17, and Th1 Cells and Elevated Levels of Serum IL‐22 in Patients With New Onset T2DM
Proinflammation has been associated with the development and progression of T2DM. To determine the role of different types of proinflammatory CD4+ T cells in the pathogenesis of T2DM, a total of 31 patients with new onset T2DM and 16 healthy controls were recruited. Their demographic and clinical characteristics are shown in Table 1. There was no significant difference in the distribution of age and gender and in the number of white blood cell and lymphocytes between the patient and control groups. As expected, the values of HbA1c, BMI, and WC in the patient group were significantly higher than that in the controls. Similarly, the concentrations of FPG in the patients were significantly higher than that in the controls and the levels of FPI and c‐peptide were significantly lower than that in the controls. Following glucose challenge, the concentrations of serum c‐peptide at 60 and 120 min post‐Sustacal were significantly elevated. As a result, the values of HOMA‐IR in the patients were significantly higher than that in the controls, while the values of QUICKI in the patients were lower than that in the controls. These data indicated that those patients had impaired glucose tolerance and a high level of insulin resistance.
Table 1.
The Demographic and Characteristics of Subjects
| T2DM (n = 31) | Controls (n = 16) | |
|---|---|---|
| Age (years) | 43 (18–62) | 45 (20–58) |
| F/M (n) | 18/13 | 10/6 |
| HbA1c (%) | 10.6 (8.3–14.7)* | 5.25 (4.5–6.1) |
| BMI (kg/m2) | 29.54 (25.3–35.29)* | 20 (17.35–23.29) |
| WC (cm) | 95 (86–107)* | 75 (69–85) |
| P‐glucose (mmol/l) pre‐Sustacal | 12 (7.8–36.58)* | 5.3 (4.2–6.3) |
| Pc‐peptide (mmol/l) pre‐Sustacal | 1.15 (0.39–3.83)* | 3.85 (3.49–4.02) |
| Pc‐peptide (mmol/l) post‐Sustacal (60 min) | 2.34 (0.57–5.91) | N/A |
| Pc‐peptide (mmol/l) post‐Sustacal (120 min) | 3.32 (1.6–8.91) | N/A |
| Insulin (mU/ml) | 5.01 (0.38–20.83)* | 7.32 (5.13–24.56) |
| HOMA‐IR | 4.7 (0.27–13.83)* | 1.38 (1.01–2.69) |
| QUICKI | 0.325 (0.305–0.58)* | 0.585 (0.464–0.859) |
| White blood cells (×109/l) | 6.23 (5.81–7.60) | 6.19 (5.63–7.36) |
| Lymphocytes (×109/l) | 2.21 (1.10–2.83) | 2.18 (1.12–2.79) |
| Th1 cells (%) | 5.92(0.73–54.4) | 1.485(0–6.86) |
| Th17 cells (%) | 0.85(0.02–3.63) | 0.07 (0–2.72) |
| Th22 cells (%) | 0.76(0–5.34) | 0.025 (0–0.81) |
| IFN‐γ‐IL‐17+IL‐22+ CD4+ (%) | 0.07(0–0.72) | 0 (0–0.13) |
| IFN‐γ+IL‐22+ CD4+ (%) | 25.13 (1.8–60.58) | 0.82(0.06–22.5) |
Data shown are median (range) of each group of subjects. The “%” represents a percentage of this type of CD4+ T cells in total CD4+ T cells.
BMI, body mass index; WC, waist circumference; HbA1c, hemoglobin A1C; HOMA‐IR, homeostatic model assessment insulin resistance; QUICKI, quantitative insulin sensitivity check index.
*P < 0.05 versus the controls.
Next, we isolated PBMCs from individual subjects and stimulated them with PMA and ionomycin in the presence BFA. Subsequently, the cells were stained with fluorescent antibody against CD4 and intraplasmically stained with anti‐IFN‐γ, anti‐IL‐17, and anti‐IL‐22, respectively, followed by flow cytometry analysis. We found that the frequency of peripheral blood IFN‐γ+IL‐17−IL‐22−CD4+ (Th1), IFN‐γ−IL‐17+IL‐22−CD4+ (Th17), and IFN‐γ−IL‐17−IL‐22+CD4+ (Th22) cells in the patients was significantly higher than that in the controls (Fig. 1). Therefore, a higher frequency of proinflammatory Th1, Th17, and Th22 cells existed in patients with new onset of T2DM.
Figure 1.
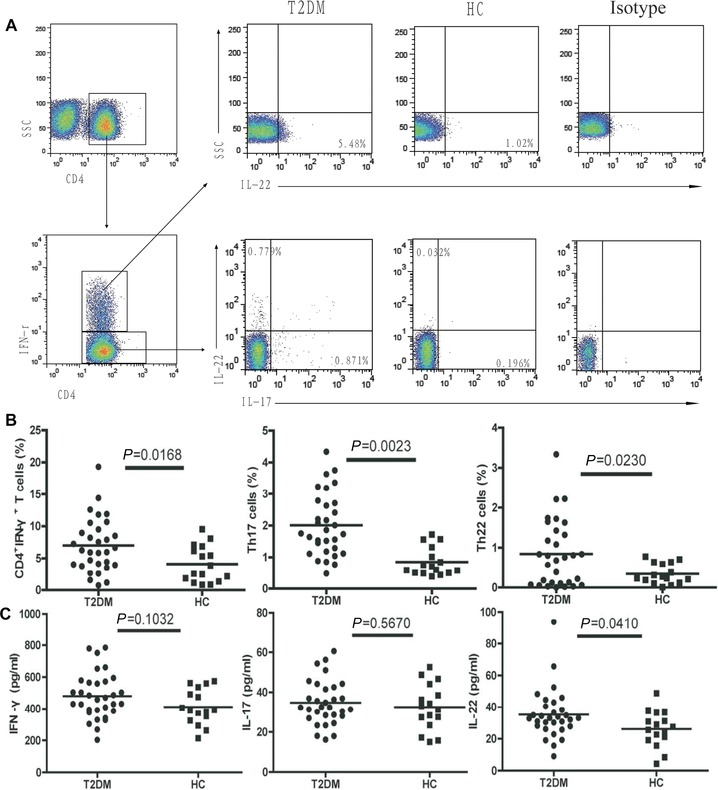
The frequencies of peripheral blood IFN‐γ+CD4+, IFN‐γ−IL‐17+IL‐22−CD4+ (Th17), and IFN‐γ−IL‐17−IL‐22+CD4+ (Th22) cells and ELISA analysis of the concentrations of serum IL‐22, IL‐17, and IFN‐γ. At least 20,000 events were analyzed. Data shown are representative charts from 31 patients and 16 controls or expressed as individual values of the percentage of each type of cells from three separate experiments.
We further examined the concentrations of serum IL‐22, IL‐17, and IFN‐γ in individual subjects by ELISA. We found that the concentrations of serum IL‐22 in the patients were significantly higher than that in the controls (P = 0.041, Fig. 1). However, there was no significant difference in the levels of serum IL‐17 and IFN‐γ between the T2DM patients and healthy controls (P = 0.1032; P = 0.5670, Fig. 1).
Given that some subsets of CD4+ T cells secrete both IL‐22 and IFNγ or IL‐17, we further characterized the percentages of IFN‐γ+IL‐17−IL‐22+CD4+ and IFN‐γ−IL‐17+IL‐22+CD4+ T cells in both the patients and controls by flow cytometry analysis. We found that IFN‐γ+IL‐17−IL‐22+CD4+ and IFN‐γ−IL‐17+IL‐22+CD4+ T cells in the T2DM patients were significantly higher than that in the controls (Fig. 2).
Figure 2.
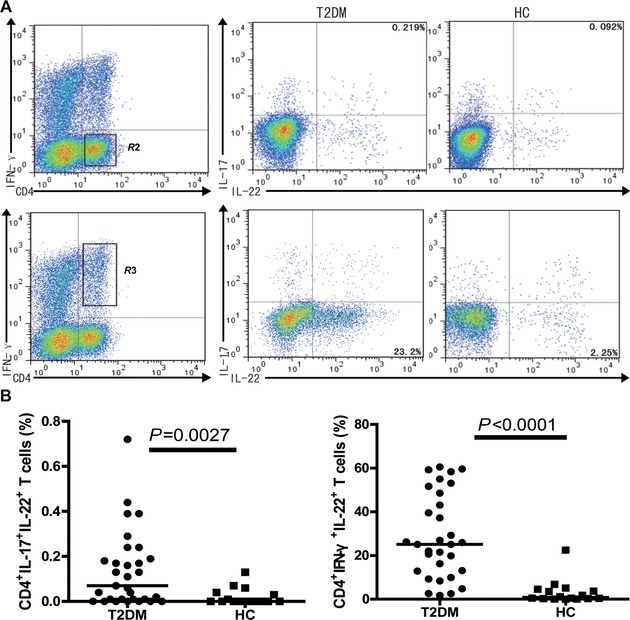
The frequencies of peripheral blood IL‐22+CD4+ T cells in subjects, respectively, for the frequency of IFN‐γ+IL‐22+CD4+ and IFN‐γ−IL‐17+IL‐22+CD4+ in the total CD4+ T cells. At least 20,000 events were analyzed. Data shown are representative charts from patients and controls or expressed as individual values of the percentage of each type of cells from three separate experiments.
The Percentages of Th22 Cells Are Correlated Positively With the Frequency of IFN‐γ−IL‐17+IL‐22+CD4+ T Cells and Levels of Serum IL‐22 in Patients With T2DM
We further investigated the relationships between different subsets of IL‐22+CD4+ T cells in patients with new onset T2DM. We found that the percentages of Th22 cells were correlated positively with the frequency of IFN‐γ−IL‐17+IL‐22+CD4+ T cells in patients with T2DM (R = 0.779, P < 0.0001, Fig. 3). However, there was no significant correlation between the frequency of Th22 and IFN‐γ+IL‐17−IL‐22+CD4+ T and between the frequency of IFN‐γ+IL‐17−IL‐22+CD4+ and IFN‐γ−IL‐17+IL‐22+CD4+ T cells in patients with new onset T2DM (data not shown).
Figure 3.
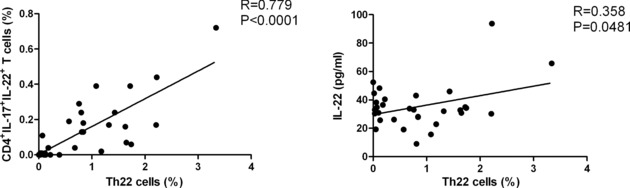
The frequency of Th22 cells is significantly correlated with the frequency of IL17+IL‐22+CD4+ T cells and levels of serum IL‐22 in patients with new onset T2DM. Data shown are individual mean values of those subjects from three separate experiments.
Furthermore, we found that the concentrations of serum IL‐22 were also correlated positively with the frequency of Th22 cells in the T2DM patients (R = 0.358, P = 0.0431; Fig. 3). However, the concentrations of serum IL‐22 were not correlated positively with the frequency of IFNγ+IL‐17−IL‐22+CD4+ and IFNγ−IL‐17+IL‐22+CD4+ T cells in this population of patients (data not shown). In addition, the levels of serum IFN‐γ or IL‐17 were not correlated significantly with the frequency of IFNγ+IL‐17−IL‐22+CD4+ or IFNγ−IL‐17+IL‐22+CD4+ T cells in those patients (data not shown).
The Percentages of Th22 and IFNγ−IL‐17+IL‐22+CD4+ T Cells Are Correlated Positively With the Values of BMI and HOMA‐IR in T2DM
Finally, we analyzed the potential association between the frequency of IL‐22+CD4+ T cells and the values of BMI and HOMA‐IR in T2DM patients. We found that the percentages of Th22 and IFNγ−IL‐17+IL‐22+CD4+ T cells, but not IFNγ+IL‐17−IL‐22+CD4+ T cells were correlated significantly with the values of BMI in T2DM patients (R = 0.386, P = 0.0318; R = 0.485, P = 0.0056; Fig. 4). Similarly, the percentages of Th22 cells were correlated positively with the values of HOMA‐IR in this population of patients (R = 0.395 P = 0.0279). However, the percentages of Th1 or Th17 were not correlated significantly with the values of BMI and HOMA‐IR in those patients, and the concentrations of serum IL‐22, IL‐17, or IFN‐γ were not significantly associated with the values of BMI in those patients (data not shown).
Figure 4.
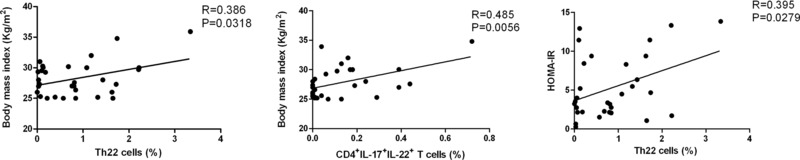
The correlation analysis. The potential correlation between the frequency of IL‐22+CD4+ T cells Th22 cells and the values of diabetes‐related measures in patients was analyzed. Data shown are the mean values of both measures from individual patients. The frequency of Th22 and IL‐17+IL‐22+CD4+ T cells was correlated with the values of BMI in those patients, and the percentages of Th22 cells were positively correlated.
DISCUSSION
Chronic low grade of inflammation and insulin resistance are critical for the development of T2DM 26. During the pathogenesis of T2DM, low‐grade inflammation and oxidative stress can be toxic to the pancreatic β‐cells, leading to insufficient insulin production, and exacerbating insulin resistance. Recent studies have shown that many inflammatory immune cells and their cytokines participate in the pathogenesis of T2DM, and they include Th1, Th17, CD8+ T cells, B cells, and mast cells 6, 27, 28, 29. Th22 cells produce IL‐22, but neither IFN‐γ nor IL‐17 have been considered as active players in the pathogenesis of inflammatory diseases 16, 30, 31, 32. A recent study has shown higher frequency of circulating Th22 and higher levels of plasma IL‐22 in T2DM patients, which are correlated positively with the values of HOMA‐IR in T2DM patients 33. These findings suggest that Th22 responses may participate in the pathogenesis of T2DM. However, little is known about the frequency of circulating Th22 cells in patients with new onset T2DM. In this study, we characterized the frequency of different functional IL‐22+CD4+ T cells in patients with new onset of T2DM and healthy controls by flow cytometry analysis following in vitro stimulation and intraplasmic staining. We found that the frequency of peripheral blood IFN‐γ+CD4+, Th17, and Th22 cells in T2DM patients was significantly higher than that in the controls. Similarly, the percentages of IFN‐γ+IL‐22+CD4+ and IL‐17+IL‐22+CD4+ T cells were significantly higher than that in the controls. Our data are consistent with previous findings that indicate the importance of Th1 and Th17 cells in the pathogenesis of T2DM 34. Our findings extended previous findings 34 and indicated that IL‐22+CD4+ T cells not only contributed to the pathogenesis of diabetes‐related retinopathy and other autoimmune diseases 17, 30, but also participated in the early process of T2DM. To the best of our knowledge, our findings are the first report on the higher frequency of different functional IL‐22+CD4+ T cells in patients with new onset diabetes.
We found that the concentrations of serum IL‐22 in patients with new onset T2DM were significantly higher than that in the controls and were correlated positively with the percentages of peripheral blood Th22 cells in those patients. Notably, a previous study has shown that the frequency of Th22 cells is relatively higher than those of other types of IL‐22+CD4+ T cells in total IL‐22+CD4+ T cells 35. Accordingly, our data suggest that Th22 cells may be the major players to produce IL‐22 in those patients, although many other types of cells also produce IL‐22. Accordingly, the concentrations of serum IL‐22 may be used as a measure to evaluate the frequency of peripheral blood Th2 cells, at least in patients with new onset T2DM.
A previous study has shown that Th17 cells participate in the pathogenesis of T2DM 34. Given that some activated CD4+ T cells secrete both IL‐17 and IL‐22, we examined the frequency of IFN‐γ−IL‐17+IL‐22+, IFN‐γ−IL‐17+IL‐22− (Th17), and IFN‐γ−IL‐17−IL‐22+ (Th22) CD4+ T cells and the potential relationship among these different functional CD4+ T cells in patients with new onset T2DM. Notably, IL‐6 and IL‐23 are crucial regulators of Th17 development and can augment IL‐22 expression in T cells 36, 37, 38. However, the Th17 cell functional development and IL‐22 expression by T cells are differentially regulated by TGFβ1. It is possible that initially activated CD4+ T cells differentiate in an isotropic manner and form various types of functional cells, including IFN‐γ−IL‐17+IL‐22+ CD4+ T cells. In an environment with low levels of TGFβ1 or high levels of inducers for aryl hydrocarbon receptor expression, those mixed types of IFN‐γ−IL‐17+IL‐22+ CD4+ T cells further differentiate into Th22 cells. Therefore, IFN‐γ−IL‐17+IL‐22+ CD4+ T cells are differentiating cells and preferably differentiate into Th22 in the pathogenic process of T2DM. We are interested in further investigating the molecular mechanisms by which inflammatory signaling regulates Th22 development in an inflammatory environment.
Obesity is a risk factor of the development of metabolic syndrome related T2DM, hypertension, and nonalcoholic fat liver diseases as well as other macro‐ and microvascular diseases 39, 40. Insulin resistance is a critical hallmark of the pathogenesis in obesity‐related T2DM and other macro‐ and microvascular diseases 4. Interestingly, we found that the frequency of Th22 cells and IL‐22+IL‐17+CD4+ T cells was positively correlated with the values of BMI and HOMA‐IR. Given that chronic inflammation contributes to the development of insulin resistance and related metabolic disorders 4, 9, 41, the positive correlation between the frequency of Th22 and IL‐22+IL‐17+CD4+ T cells and the degrees of obesity or insulin resistance strongly suggests that those cells are pathogenic factors, participating in the early stage of the pathogenic process of T2DM. Conceivably, Th22 and IL‐22+IL‐17+CD4+ T cells as well as IL‐22 may be targets for the design of new therapies for the intervention of T2DM. We recognize that our studies had limitations, including a small sample size, one time point, and the lack of a functional study of these different subsets of T cells in those patients. Therefore, further studies in a bigger population are needed to validate these findings and to determine the role of IL‐22+CD4+ T cells in the pathogenesis of T2DM.
In conclusion, our data indicated that IL‐22+CD4+ T cells may contribute to chronic inflammation and the early pathogenic process of T2DM in Chinese patients. Our findings suggest that IL‐22+CD4+ T cells and IL‐22 may be the therapeutic targets for the intervention of T2DM.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript. This work was supported by grants from the National Natural Science Foundation of China (nos. 30972610 and 81273240), Jilin Province Science and Technology Agency (no. 20110716), the Health Department Research Project in Jilin Province (2009Z054), and Norman Bethune Program of Jilin University (2012206).
Grant sponsor: National Natural Science Foundation of China; Grant numbers: 30972610 and 81273240; Grant sponsor: Jilin Province Science and Technology Agency; Grant number: 20110716; Grant sponsor: Health Department Research Project; Grant number: 2009Z054; Grant sponsor: Norman Bethune Program of Jilin University; Grant number: 2012206.
REFERENCES
- 1. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005;365:1333–1346. [DOI] [PubMed] [Google Scholar]
- 2. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787. [DOI] [PubMed] [Google Scholar]
- 3. Alberti KG. Treating type 2 diabetes—today's targets, tomorrow's goals. Diabetes Obes Metab 2001;3(Suppl 1):S3–S10. [PubMed] [Google Scholar]
- 4. Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7. [DOI] [PubMed] [Google Scholar]
- 5. Dandona P, Aljada A, Chaudhuri A, Bandyopadhyay A. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis‐related complications in type 2 diabetes. J Clin Endocrinol Metab 2003;88:2422–2429. [DOI] [PubMed] [Google Scholar]
- 6. Kolb H, Mandrup‐Poulsen T. An immune origin of type 2 diabetes? Diabetologia 2005;48:1038–1050. [DOI] [PubMed] [Google Scholar]
- 7. Ford ES. The metabolic syndrome and C‐reactive protein, fibrinogen, and leukocyte count: Findings from the Third National Health and Nutrition Examination Survey. Atherosclerosis 2003;168:351–358. [DOI] [PubMed] [Google Scholar]
- 8. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–823. [DOI] [PubMed] [Google Scholar]
- 9. Winer S, Chan Y, Paltser G, et al. Normalization of obesity‐associated insulin resistance through immunotherapy. Nat Med 2009;15:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cintra DE, Pauli JR, Araujo EP, et al. Interleukin‐10 is a protective factor against diet‐induced insulin resistance in liver. J Hepatol 2008;48:628–637. [DOI] [PubMed] [Google Scholar]
- 12. Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin‐22: A cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev 2010;21:365–379. [DOI] [PubMed] [Google Scholar]
- 13. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL‐22 increases the innate immunity of tissues. Immunity 2004;21:241–254. [DOI] [PubMed] [Google Scholar]
- 14. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL‐22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005;174:3695–3702. [DOI] [PubMed] [Google Scholar]
- 15. Andoh A, Zhang Z, Inatomi O, et al. Interleukin‐22, a member of the IL‐10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005;129:969–984. [DOI] [PubMed] [Google Scholar]
- 16. Zhang N, Pan HF, Ye DQ. Th22 in inflammatory and autoimmune disease: Prospects for therapeutic intervention. Mol Cell Biochem 2011;353:41–46. [DOI] [PubMed] [Google Scholar]
- 17. Chen H, Wen F, Zhang X, Su SB. Expression of T‐helper‐associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Mol Vis 2012;18:219–226. [PMC free article] [PubMed] [Google Scholar]
- 18. Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Rohowsky‐Kochan C. Regulation of IL‐17 in human CCR6+ effector memory T cells. J Immunol 2008;180:7948–7957. [DOI] [PubMed] [Google Scholar]
- 20. Acosta‐Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor‐beta are essential for the differentiation of interleukin 17‐producing human T helper cells. Nat Immunol 2007;8:942–949. [DOI] [PubMed] [Google Scholar]
- 21. Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ. Distinct regulation of interleukin‐17 in human T helper lymphocytes. Arthritis Rheum 2007;56:2936–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17‐producing helper T cells. Nat Immunol 2007;8:950–957. [DOI] [PubMed] [Google Scholar]
- 23. Yang H, Youm YH, Vandanmagsar B, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: Implications for systemic inflammation and insulin resistance. J Immunol 2010;185:1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller AM, Asquith DL, Hueber AJ, et al. Interleukin‐33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res 2010;107:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kintscher U, Hartge M, Hess K, et al. T‐lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity‐mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008;28:1304–1310. [DOI] [PubMed] [Google Scholar]
- 26. Accili D. Mechanisms of β cell failure in the pathogenesis of Type 2 diabetes. Drug Dev Res 2008;69:111–115. [Google Scholar]
- 27. Abbasi F, Amiri P, Sayahpour FA, et al. TGF‐beta and IL‐23 gene expression in unstimulated PBMCs of patients with diabetes. Endocrine 2012;41:430–434. [DOI] [PubMed] [Google Scholar]
- 28. Stentz FB, Kitabchi AE. Transcriptome and proteome expressions involved in insulin resistance in muscle and activated T‐lymphocytes of patients with type 2 diabetes. Genomics Proteomics Bioinformatics 2007;5:216–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type‐2 innate immunity in vivo. Nature 2002;420:825–829. [DOI] [PubMed] [Google Scholar]
- 30. Zhang L, Li JM, Liu XG, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. J Clin Immunol 2011;31:606–614. [DOI] [PubMed] [Google Scholar]
- 31. Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009;119:3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010;130:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao R, Tang D, Yi S, Li W, Wu C. Elvated peripheral frequencies of Th22 cells: Novel potent participant in obesity and type 2 diabetes. PLoS One 2014;9(1):e85770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng C, Shi X, Zhang B, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: Relationship with metabolic factors and complications. J Mol Med (Berl) 2012;90:175–186. [DOI] [PubMed] [Google Scholar]
- 35. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin‐homing memory T cells. Nat Immunol 2009;10:857–863. [DOI] [PubMed] [Google Scholar]
- 36. Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor‐β induces development of the TH17 lineage. Nature 2006;441:231–234. [DOI] [PubMed] [Google Scholar]
- 37. Zheng Y, Danilenko DM, Valdez P, et al. Interleukin‐22, a T(H)17 cytokine, mediates IL‐23‐induced dermal inflammation and acanthosis. Nature 2007;445:648–651. [DOI] [PubMed] [Google Scholar]
- 38. Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor‐beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)‐17 responses. Nat Immunol 2008;9:650–657. [DOI] [PubMed] [Google Scholar]
- 39. Bouskela E, Kraemer de Aguiar LG, Nivoit P, Bahia LR, Villela NR, Bottino DA. Vascular dysfunction in metabolic disorders: Evaluation of some therapeutic interventions. Bull Acad Natl Med 2007;191:475–492; discussion 492–473. [PubMed] [Google Scholar]
- 40. Gupta D, Krueger CB, Lastra G. Over‐nutrition, obesity and insulin resistance in the development of beta‐cell dysfunction. Curr Diabetes Rev 2012;8:76–83. [DOI] [PubMed] [Google Scholar]
- 41. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–246. [DOI] [PubMed] [Google Scholar]


