Abstract
Background
Metabolic syndrome (MetS) defines a well‐known cluster of metabolic disturbances associated with an increased risk of cardiovascular disease and diabetes. The aim of this study was to examine the distribution of soluble lectin‐like oxidized low‐density lipoprotein (LDL) receptor‐1 (sLOX‐1) levels in patients with MetS, possible association of sLOX‐1 with oxidized LDL (oxLDL), endothelial nitric oxide synthase (eNOS), nitric oxide (NOx), endothelin‐1 (ET‐1), paraoxonase 1 (PON1), and arylesterase (ARE) activities, and these parameters compared with healthy controls.
Methods
A total of 55 patients (37 women, 18 men) with MetS and 29 healthy controls (19 women, 10 men) with a body mass index (BMI) less than 25 kg/m2 were enrolled in the study.
Results
sLOX‐1, oxLDL, and ET‐1 levels were significantly higher in patients with MetS than in control subjects (P = 0.023 P < 0.001, and P < 0.001, respectively). MetS patients have significantly lower eNOS and NOx levels, and PON1 and ARE activities than control subjects (P = 0.017, P < 0.004, P < 0.001, and P = 0.010, respectively). A positive correlation was observed between the sLOX‐1 levels and the oxLDL, ET‐1, BMI, glucose levels. ET‐1 levels also exhibited significant negative correlation with ARE activity.
Conclusion
sLOX‐1 levels are associated with cardiovascular risk factors, such as increased oxLDL, obesity, and diabetes, in patients with MetS. An increased concentration of sLOX‐1 could be an early predictor of endothelial damage in MetS. In addition, it appears that oxLDL, ET‐1, eNOS, NOx, PON1, and ARE activities may accurately reflect the levels of endothelial dysfunction in MetS patients.
Keywords: metabolic syndrome, soluble lectin‐like oxidized LDL receptor 1, oxidized LDL, endothelial nitric oxide synthase, nitric oxide, endothelin‐1
INTRODUCTION
The metabolic syndrome (MetS) refers to the clustering of cardiovascular risk factors that include diabetes, obesity, dyslipidemia, and hypertension 1. Numerous metabolic abnormalities found in the MetS, including hyperglycemia, excessive fatty acids, and insulin resistance (IR), cause an endothelial cell dysfunction by affecting nitric oxide (NO) synthesis or degradation 2.
Endothelial dysfunction and oxidized low‐density lipoprotein (oxLDL) is believed to play a key role in the initiation of the atherosclerotic process. In 1998, the lectin‐like oxidized low‐density lipoprotein receptor 1 (LOX‐1) was identified as the receptor for oxLDL on endothelial cells. Lectin‐like oxidized LOX‐1 is a type II membrane protein that belongs to the C‐type lectin family of molecules that can act as a cell‐surface endocytosis receptor for atherogenic oxLDL 3. Measurement of soluble lectin‐like oxidized LDL receptor 1 (sLOX‐1) in vivo may provide a novel diagnostic tool for the evaluation and prediction of atherosclerosis and vascular disease 4. sLOX‐1 is identified endothelial receptor for oxLDL that plays a centric role in oxLDL‐induced endothelial dysfunction 5. Level of circulating sLOX‐1 may reflect the expression of LOX‐1, and is increasingly viewed as a biomarker for coronary artery disease (CAD). LOX‐1 plays a pivotal role in the cross‐talk between MetS and CAD 6.
Low levels of HDL‐cholesterol (HDLC; where HDL is high‐density lipoprotein), which is one of the most important antioxidant defense system in plasma, are typical in MetS. The antioxidant properties of HDL are at last partly attributable to paraoxonase 1 (PON1). PON1 and arylesterase (ARE) are lipophylic antioxidant enzymes. Studies showed that subjects with MetS have lower PON1 activity 7. NO is known to play a role in the pathogenesis of obesity, MetS, hypertension, and IR 8, 9. Endothelin‐1 (ET‐1) is a potent vasoconstrictor peptide and elevated level of ET‐1 is a well‐known marker of endothelial dysfunction and generates IR in visceral adipose tissue 10.
NO also plays a major role as a regulator during the onset of immunological and inflammatory reactions. Polymorphism in endothelial nitric oxide synthase (eNOS) gene is associated with MetS in humans 8, 9.
In this study, we investigated the sLOX‐1 levels in patients with MetS, possible association of sLOX‐1 with the oxLDL, PON1, ARE, eNOS, NOx, ET‐1 levels, to determine their relationships with demographic and clinical parameters, and we compared these parameters with healthy controls. So, we aimed to see if sLOX‐1 level could be an earlier predictor of endothelial dysfunction along with the aforementioned biomarkers.
MATERIALS AND METHODS
Subjects
A total of 55 patients with the MetS based on the Adult Treatment Panel III (ATP III) criteria 11 were included in the current study. All patients had been examined by the same physician at the Internal Medicine Outpatient Clinic of our hospital. MetS was diagnosed as the presence of at least three of the following parameters according to ATP III criteria: abdominal obesity (waist circumference >102 cm for males and >88 cm for females), hypertension (SBP > 130 mmHg and/or DBP > 85 mmHg) or history of antihypertensive usage, hypertriglyceridemia (≥150 mg/dl) or presence of treatment for this disorder, low HDL‐C (<40 mg/dl in males and <50 mg/dl in females), and high fasting plasma glucose (≥100 mg/dl) or presence of diagnosis of type 2 diabetes. Smokers and patients who were taking drugs known to affect oxidant and antioxidant status were excluded.
The control group consisted of 29 healthy subjects who were selected from the hospital staff and did not have a personal or family history of diabetes or dyslipidemia and had normal thyroid, hepatic, and renal function. The 2‐hr glucose tolerance test was used to rule out impaired glucose regulation in healthy controls. Smokers and healthy subjects who were taking drugs that are known to affect carbohydrate and lipid metabolism or oxidant and antioxidant status were also excluded from the control group. All patients and controls were of Turkish descent. The height and weight of each subject were measured without shoes but with clothes on using a calibrated stadiometer and a balance beam scale. The body mass index (BMI) of each subject was calculated using the following formula: weight (kg)/height (m)2. Waist circumference was measured twice to the nearest 0.1 cm with a flexible tape measure at the level of the minimum circumference, which was usually at the level of the navel.
Ethics
All participants were informed about the survey and freely signed and dated the consent form. The protocol was approved by the Ethics Committee of Cerrahpasa Medical Faculty and was conducted in accordance with the Declaration of Helsinki.
Laboratory Analysis
Sample collection and preparation
Drugs were administered at least 24 hr prior to blood collection. Clinical parameters, including routine biochemical parameters, were measured using standard protocols. Blood samples were collected in ethylenediaminetetraacetic acid (EDTA)‐containing tubes and anticoagulant‐free tubes after an overnight fast. After immediate centrifugation (3,000 × g) for 10 min at 4°C, plasma and serum were separated in Eppendorf tubes and frozen immediately at −80°C until analysis.
The homeostasis model assessment (HOMA) was used to detect the degree of IR by measuring the levels of basal (fasting) glucose and insulin. HOMA‐IR was calculated using the following formula: HOMA‐IR = (fasting glucose [mg/dl] × fasting insulin [μU/ml])/05.
Measurements of the serum soluble LOX‐1 concentrations
Serum sLOX‐1 levels were measured by a commercially available enzyme‐linked immunosorbent assay kit (USCN, Life Science, Inc. E91859Hu, Export Processing Zone, Economic and Tecnological Development Zone, Wuhan 430056, P. R. China). The coefficients of intra‐ and interassay variation were 5.3% (n = 10) and 7.5% (n = 10), respectively.
Measurement of plasma oxLDL concentrations
Plasma MDA‐oxLDL concentrations were measured by enzyme‐linked immunoassay using commercially available kit (Biomedica, Germany, Lot No. BI‐20022). The coefficients of intra‐ and interassay variations were 4.8% (n = 10) and 5.6% (n = 10), respectively.
Measurement of plasma eNOS activity
Plasma eNOS activity was measured by enzyme‐linked immunoassay using commercially available kit (USCN, Life Science, Inc.). The coefficients of intra‐ and interassay variations were 5.7% (n = 10) and 6.9% (n = 10), respectively.
Measurement of plasma ET‐1 concentrations
Plasma ET‐1 concentrations were measured by enzyme immunoassay using commercially available kit (Enzo Life Sciences catalog no. ADI‐900‐020A; Lorrach, Germany) in our laboratory. The coefficients of intra‐ and interassay variations were 5.9% (n = 10) and 6.9% (n = 10), respectively.
Measurement of serum NOx concentrations
Serum NOx concentrations were determined by measuring serum NO2/NO3 levels using nitrate/nitrite colorimetric assay kit (Enzo Life Sciences catalog no, ADI‐917‐020). The coefficients of intra‐ and interassay variations were 4.1% (n = 15) and 4.9% (n = 15), respectively.
Measurement of serum PON1 ARE Activity
ARE activity was also measured spectrophotometrically using phenylacetate (Sigma Co., London, UK) as the substrate. The assay mixture contained 100 μl of 10 mmol/l substrate solution, 5 μl serum, and 1 mmol/l CaCl2 (Sigma, USA) in 50 mmol/l Tris buffer (Fluka Chemie, Switzerland), pH = 8.0. Production of phenol was determined spectrophotometrically after 2 min at 270 nm. The assay mixture was prepared daily before use. PON1 ARE activity was monitored in triplicate and the results are presented as micromole per minute per milliliter 12. Mean intra‐ and interassay coefficients of variation were up to 5.2 % (n = 15) and 7.6 % (n = 15), respectively.
Measurement of serum PON1 paraoxonase Activity
PON1 activity was assayed using synthetic paraoxon (diethyl‐p‐nitrophenyl phosphate) as substrate. PON1 activity was determined by measuring the initial rate of substrate hydrolysis to p‐nitrophenol, whose absorbance was monitored at 412 nm in the assay mixture containing 2.0 mM paraoxon, 2.0 mM CaCl2, and 20 μl of plasma in 100 mM Tris‐HCI buffer (pH = 8.0). The blank sample containing incubation mixture without plasma was run simultaneously to correct for spontaneous substrate breakdown. The enzyme activity was calculated from E412 of p‐nitrophenol (18.290 per M/cm) and was expressed as U/ml; 1 U of enzyme hydrolyzes 1 nmol of paraoxon per minute 13. Mean intra‐ and interassay coefficients of variation for this analysis were 8.2% (n = 15) and 9.5% (n = 15), respectively.
The other biochemical parameters were measured by routine methods with commercial kits.
Statistical Analysis
Statistical analyses were performed using SPSS 20.0 for Windows. The results are expressed as the mean ± standard deviation. Mann–Whitney U tests were used to compare the mean values between the groups. Spearman's rho test was used to determine the correlations with MetS risk factors. Pearson's correlation was used for numerical data. To assess the diagnostic accuracy, we performed receiver operating characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was then estimated. The endothelial dysfunction variable, does the model also include age, gender, BMI, cutoff values were also determined according to ROC analysis. Multivariate logistic regression model was performed to determine the effect of independent risk factors for MetS. P < 0.05 values were considered to be statistically significant.
RESULTS
The general characteristics of the studied groups are shown in Table 1. No significant differences were observed between the patient and control groups with regards to age, total protein, albumin, LDL‐C, and HDL‐C levels. However, the number of female patients within the MetS group was higher significantly (P <0.001) than in the control group. As expected, a comparison of the diagnostic criteria between the two groups revealed significant differences in hypertension, hyperlipidemia, hyperglycemia, obesity, family history for CAD, systolic and diastolic blood pressure, waist circumference, and hip circumference. Patients in the MetS group had significantly higher levels of uric acid (P <0.001), total cholesterol (P <0.001), triglyceride (P <0.001), fibrinogen (P <0.001), glucose (P <0.001), HbA1C (P <0.001), insulin (P <0.001), and HOMA‐IR (P <0.001) than those in the control group.
Table 1.
Demographic and Biochemical Values of MetS Patients and Controls
| Control group, n = 29 | Metabolic syndrome, n = 55 | P | |
|---|---|---|---|
| Age | 45.24 ± 7.52 | 50.73 ± 6.41 | NS |
| Sex (F/M) | 10/19 | 37/18 | <0.001/<0.001 |
| Waist circumference (cm) | 81.21 ± 9.12 | 103.55 ± 7.24 | <0.001 |
| Hip circumference (cm) | 99.59 ± 9.36 | 116.16 ± 7.96 | <0.001 |
| BMI (kg/m2) | 24.11 ± 2.71 | 34.07 ± 3.67 | <0.001 |
| Systolic blood pressure (mmHg) | 112.41 ± 6.36 | 135.18 ± 7.26 | <0.001 |
| Diastolic blood pressure (mmHg) | 74.14 ± 5.68 | 83.45 ± 6.23 | <0.001 |
| Total protein (g/dl) | 7.37 ± 0.35 | 7.42 ± 0.31 | NS |
| Albumin (g/dl) | 4.61 ± 0.25 | 4.45 ± 0.24 | NS |
| Uric acid (mg/dl) | 4.10 ± 1.07 | 5.31 ± 1.14 | <0.001 |
| Total cholesterol (mg/dl) | 162.03 ± 23.25 | 198.56 ± 41.31 | <0.001 |
| HDL‐C (mg/dl) | 46.45 ± 6.23 | 47.62 ± 9.64 | NS |
| LDL‐C (mg/dl) | 97.48 ± 23.22 | 110.98 ± 37.49 | NS |
| Triglyceride (mg/dl) | 98.10 ± 28.89 | 196.18 ± 86.86 | <0.001 |
| Fibrinogen (mg/dl) | 277.34 ± 47.65 | 338.60 ± 69.03 | <0.001 |
| Glucose (mg/dl) | 93.03 ± 9.78 | 154.24 ± 47.93 | <0.001 |
| C‐peptide (ng/ml) | 1.65 ± 0.59 | 2.18 ± 1.03 | NS |
| HbA1C (%) | 5.62 ± 0.36 | 8.00 ± 1.83 | <0.001 |
| Insulin (μU/ml) | 9.84 ± 4.45 | 37.82 ± 44.72 | <0.001 |
| HOMA‐IR | 2.25 ± 1.00 | 15.02 ± 23.10 | <0.001 |
BMI, body mass index; LDL, low‐density lipoprotein; HDL, high density lipoprotein; NS, nonsignificant.
The endothelial dysfunction parameters in the two groups are shown in Table 2. sLOX‐1 levels were significantly higher in patients with MetS compared with healthy control (P <0.023). oxLDL levels were significantly higher in patients with MetS compared with healthy control (P <0.001). PON1 and ARE activities were found to be decreased (P <0.001, P <0.010, respectively) in serum of patients with MetS compared to controls. eNOS activities and NOx levels were significantly lower in the MetS group compared with healthy control (P <0.017, P <0.004, respectively). ET‐1 levels were significantly higher in patients with MetS compared with healthy control (P <0.001).
Table 2.
Endothelial Damage Parameters of MetS Patients and Controls
| Control (n = 29) | Metabolic syndrome (n = 55) | P | |
|---|---|---|---|
| sLOX –1 (ng/ml) | 0.68 ± 0.46 | 1.08 ± 0.86 | 0.023 |
| oxLDL (μU/l) | 0.89 ± 0.21 | 1.90 ± 1.14 | <0.001 |
| eNOS (pg/ml) | 933.54 ± 138.09 | 852.60 ± 147.69 | 0.017 |
| NOx (μmol/l) | 83.22 ± 35.93 | 64.96 ± 20.71 | 0.004 |
| ET‐1 (pg/ml) | 1.72 ± 0.98 | 5.74 ± 3.41 | <0.001 |
| PON1 (U/l) | 91.43 ± 21.19 | 58.26 ± 11.27 | <0.001 |
| ARE (kU/l) | 67.56 ± 35.26 | 52.55 ± 17.32 | 0.010 |
sLOX‐1, soluble lectin‐like oxidized LDL receptor 1; oxLDL, oxidized LDL; eNOS, endothelial nitric oxide synthase; NOx, nitric oxide; ET‐1, endothelin‐1; PON1, paraoxonase; ARE, arylesterase.
sLOX‐1 was positively correlated with oxLDL (r = 0.552, P < 0.001), ET‐1 (r = 0.640, P <0.001), and BMI (r = 0.321, P = 0.017) in MetS patients (Fig. 1), but no correlations were observed between laboratory endothelial dysfunction parameters and any of the components of MetS. Positive correlations were observed between eNOS activity and the NOx and insulin levels (r = 0.826, P < 0.001; and r = 0.303, P = 0.025, respectively). ET‐1 levels were also significantly negatively correlated with ARE (r = −0.297, P = 0.028). PON1 was positively correlated with HDL (r = 0.267, P <0.049), total protein (r = 0.286, P = 0.034) in MetS patients.
Figure 1.
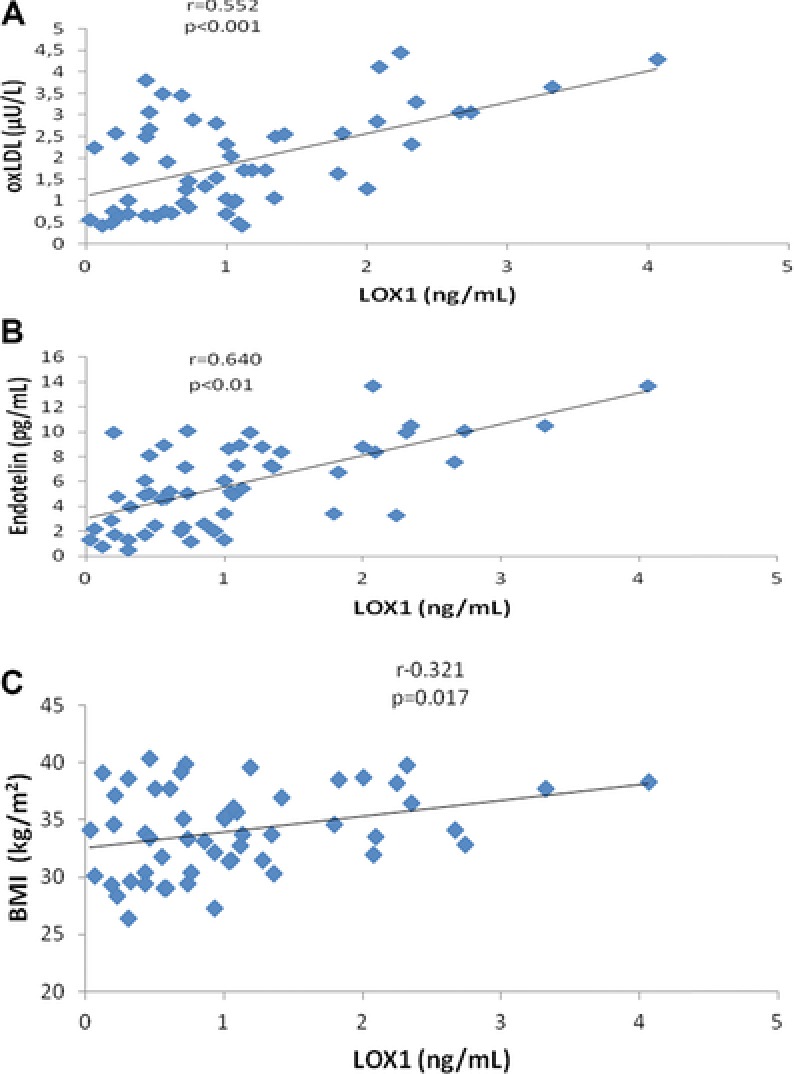
The relationship between oxLDL (A), ET‐1 (B), and BMI (C) with sLOX‐1 in MetS patients.
A comparison of the ROC curves for sensitivity, specificity, AUC, cutoff, and asymptotic significance of BMI, sLOX‐1, eNOS, NOx, ET‐1, and PON1 in all subjects are shown in Table 3 and Figure 2.
Table 3.
Sensitivity, Specificity, AUC, Cutoff, and Asymptotic Significance of LOX‐1, eNOS, NOx, ET‐1, and PON1 Levels in All Subjects
| Sensitivity (%) | Specifity (%) | AUC | Cutoff | Asymptotic significance | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 98.2 | 10.3 | 0.981 | 27 | <0.001 |
| sLOX −1 (pg/ml) | 76.4 | 41.4 | 0.640 | 0.45 | <0.05 |
| oxLDL (μU/l) | 61.8 | 96.6 | 0.745 | 1.3 | <0.001 |
| eNOS (pg/ml) | 58.6 | 70.9 | 0.336 | 900 | <0.05 |
| NOx (μmol/l) | 79.3 | 41.8 | 0.355 | 56 | <0.05 |
| ET‐1 (pg/ml) | 74.5 | 89.7 | 0.860 | 2.5 | <0.001 |
| PON1 (U/l) | 93.1 | 94.5 | 0.073 | 75 | <0.001 |
Figure 2.
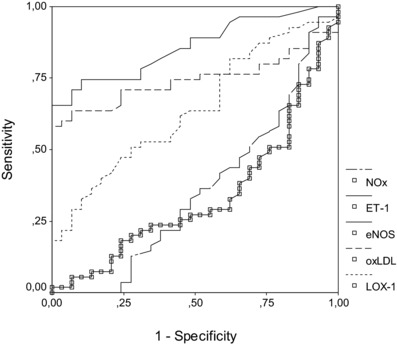
ROC curves for NOx, ET‐1, eNOS, oxLDL, and sLOX‐1 values.
The endothelial dysfunction variables include age, gender, sLOX‐1, eNOS, NOx, ET‐1, and PON1. Multivariate logistic regression analysis revealed that PON1 and oxLDL were the variable that had a significant effect on MetS (P <0.001 OR = 154.308; 95% CI: 21.095–1128.760; P = 0.033 OR = 19.22; 95% CI, 1.267–291.554, respectively).
DISCUSSION
sLOX‐1 is a recently discovered specific oxLDL receptor that is expressed in many kinds of cells to mediate multiple pathological injuries 14. OxLDL also plays an important role in the development of atherosclerotic processes and endothelial dysfunction 15, 16. MetS refers to the clustering of cardiovascular risk factors that include diabetes, obesity, dyslipidemia, and hypertension. As expected, dyslipidemia, particularly increased plasma level of oxLDL, plays the major role in the upregulation of sLOX‐1 17. The major finding of the present study was sLOX‐1 levels were positively correlated with oxLDL, ET‐1, and BMI. We found that sLOX‐1, oxLDL, and ET‐1 levels were significantly higher in patients with MetS when compared with healthy controls.
Li et al. 6 demonstrated that LOX‐1 plays a pivotal role in the cross‐talk between MetS and CAD, and high LOX‐1 level could perhaps be used to predict the occurrence of future cardiovascular events in patients with MetS. sLOX‐1 levels are significantly increased in metabolic disorders, including obesity 18 and diabetes mellitus 19, and are positively correlated with reduction in body weight 20. All this results are consistent with our study. In vitro and animal studies showing that LOX‐1 expression is increased by the hyperglycemic milieu 21, 22, 23. Tan et al. 19 reported that serum log(sLOX‐1) correlated only with serum AGEs in the diabetic patients and no correlation was seen with age, BMI, systolic or diastolic BP, fasting glucose, plasma lipids, or log(CRP). Then observed an increase in sLOX‐1 with extremely high glucose levels in vitro. LOX‐1 expression may be increased by glucose both in macrophages and in endothelial cells 21, 22, but not blood stream. No correlations were observed between sLOX‐1 and glucose levels in our study. According to ROC analysis, LOX‐1 showed only a borderline level of significance (AUC = 0.640, P <0.05). We believe that this assay may be statistically meaningful, but it may not have any clinical significance in MetS.
Reactive oxygen species (ROS) is usually considered to be a toxic by‐product of cell metabolism. It interacts with lipids, proteins, or DNA, leading to histological changes and cellular malfunction; furthermore, this reaction varies in different cells 14. The decrease of total PON1, ARE, eNOS activities, and NOx levels may indicate that there is a tendency to atherothrombotic process in patients with MetS. Our previous study shows that the increased advanced protein oxidation products (AOPPs) levels and higher pro‐oxidant‐antioxidant balance (PAB) values are likely to be a result of oxidative stress 23. The current study shows a substantial impairment of PON1 and ARE activities that have lipophilic antioxidant characteristics in patients with MetS. PON1 was also positively correlated with HDL and total protein in MetS patients. Some previous studies observed a progressive decrease of PON1 activity by increasing the number of diseases associated with MetS 7, 24, 25, 26. Our results were similar with other researches. PON1 protects LDL and HDL against oxidative modification and oxidation of LDL is recognized as an early stage in the development of atherosclerosis 27. According to the ROC curves, PON1 activity were both highly sensitive (93.1%) and specific (94.5%). However, we could not find any data regarding the association of PON1 activities with the MetS.
Recent studies have suggested that endothelial NO‐mediated vasorelaxation is impaired in MetS 28. Although the results are controversial, high serum NOx level seems as an independent predictor of incident MetS and it may be a potential biomarker for assessing cardiometabolic disturbances 29, 30, 31, 32 The major finding of the present study was sLOX‐1 levels were positively correlated with oxLDL, ET‐1, and BMI. We found that both eNOS and NOx are lower in MetS patients. Decreased eNOS and NOx levels significantly contribute to endothelial dysfunction observed in MetS. NOx can be considered a double‐edged sword, so that while both very‐low and very‐high concentrations could be pathologic, its low concentration is normal. LOX‐1 may also indirectly inhibit eNOS pathway via activation of arginase II that competitively inhibits eNOS through the utilization of their common substrate, l‐arginine 33. Positive correlations were also observed between eNOS activity and the NOx and insulin levels in our study. Inhibition of eNOS may decrease the production of NOx. Consequently, NO deficiency upregulates LOX‐1 expression, leading to even more LOX‐1‐mediated NO deficiency. It is unclear whether the trigger of this cycle is the decrease of NO or the activation of LOX‐1 33.
ET‐1 levels are a well‐known marker of vascular endothelial dysfunction and contribute to pathological manifestations. ET‐1 upregulation and/or NO release imbalance by endothelial cells represents one of many putative factors linking IR and the development of atherosclerotic vascular disease. Our study demonstrates that ET‐1 levels were significantly higher in patients with MetS compared with healthy control and ET‐1 provides useful information for stratifying cardiovascular status with respect to endothelial dysfunction in MetS. Insulin stimulates both NO activity and ET‐1. But positive correlations were also observed between insulin levels and eNOS activity, except ET‐1 in our study. Verma et al. 34 demonstrated that insulin‐mediated vasorelaxation is only well patented when antagonizing ET‐1 receptors. Insulin exhibits a dual and opposite action on blood vessels with NO‐mediated vasodilation and not ET‐1‐mediated vasoconstriction. The binding of platelets to LOX‐1 enhances the release of ET‐1 and suppresses the release of NO from endothelial cells. This suggests that platelet‐endothelium interaction via LOX‐1 might induce endothelial changes in a similar way to oxLDL 35. sLOX‐1 was also positively correlated with ET‐1 and BMI in MetS patients. Induction of sLOX‐1 mediates endothelial dysfunction through a number of pathways, including induction of ET‐1 33.
In summary, sLOX‐1 levels are associated with cardiovascular risk factors, such as increased oxLDL, obesity, and diabetes in patients with MetS. An increased concentration of sLOX‐1 could be an early predictor of endothelial damage in MetS. sLOX‐1 binding to oxLDL may decrease NOx release via attenuated eNOS activity. Increased sLOX‐1 may cause damage in many kinds of cells to mediate multiple pathological injuries in MetS, thus anti‐LOX‐1 treatment may reverse this damage. We hope that sLOX1, as an easy, cheap, and noninvasive biomarker of endothelial injury, should be more widely used in the future in patients with MetS.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest related to the publication of this manuscript.
ABBREVIATIONS
- ARE
arylesterase
- AUC
area under the curve
- BMI
body mass index
- CAD
coronary artery disease
- eNOS
endothelial nitric oxide synthase
- ET‐1
endothelin‐1
- HDL‐C
high‐density lipoprotein cholesterol
- HOMA
homeostasis model assessment
- IR
insulin resistance
- MetS
metabolic syndrome
- NOx
nitric oxide
- oxLDL
oxidized LDL
- PON1
paraoxonase 1
- ROC
receiver operating characteristic
- sLOX‐1
soluble lectin‐like oxidized LDL receptor 1
Grant sponsor: The Research Fund of Istanbul University; Grant number: 21330.
All authors contributed equally to the research and writing of this article.
REFERENCES
- 1. Duvnjak L, Duvnjak M. The metabolic syndrome—an ongoing story. J Physiol Pharmacol 2009;60:19–24. [PubMed] [Google Scholar]
- 2. Cozma A, Orăşan O, Sâmpelean D, et al. Endothelial dysfunction in metabolic syndrome. Rom J Intern Med 2009;47:133–140. [PubMed] [Google Scholar]
- 3. Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low‐density lipoprotein. Nature 1997;386:73–77. [DOI] [PubMed] [Google Scholar]
- 4. Schmitz‐Huebner U, Knop J. Evidence for an endothelial dysfunction in association with Behcet's disease. Thromb Res 1984;3:277–285. [DOI] [PubMed] [Google Scholar]
- 5. Von Feldt JM. Premature atherosclerotic cardiovascular disease and systemic lupus erythematosus from bedside to bench. Bull NYU Hosp Jt Dis 2008;66:184–187. [PubMed] [Google Scholar]
- 6. Li B, Zhang LH, Yang XG, Liu XT, Ren YG. Serum sLOX‐1 levels are associated with the presence and severity of angiographic coronary artery disease in patients with metabolic syndrome. Clin Invest Med 2010;33:E398–E404. [DOI] [PubMed] [Google Scholar]
- 7. Martinelli N, Micaglio R, Consoli L, et al. Low levels of serum paraoxonase activities are characteristic of metabolic syndrome and may influence the metabolic‐syndrome‐related risk of coronary artery disease. Exp Diabetes Res 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodis J, Gaier N, Farghali H. Nitric oxide in patients with obesity and metabolic syndrome. Cas Lek Cesk 2009;148:34–38. [PubMed] [Google Scholar]
- 9. Carlström M, Larsen FJ, Nyström T, et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase‐deficient mice. Proc Natl Acad Sci USA 2010;107:17716–17720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendizábal Y, Llorens S, Nava E. Hypertension in metabolic syndrome: Vascular pathophysiology. Int J Hypertens 2013;2013:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific Statement. Circulation 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 12. Haagen L, Brock A. A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4‐nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 1992;30:391–395. [DOI] [PubMed] [Google Scholar]
- 13. Cakatay U, Kayali R, Uzun H. Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin Exp Med 2008;8:51–57. [DOI] [PubMed] [Google Scholar]
- 14. Wang R, Ding G, Liang W, Chen C, Yang H. Role of LOX‐1 and ROS in oxidized low‐density lipoprotein induced epithelial‐mesenchymal transition of NRK52E. Lipids Health Dis 2010;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kume N, Kita T. Roles of lectin‐like oxidized LDL receptor‐1 and its soluble forms in atherogenesis. Curr Opin Lipidol 2001;12:419–423. [DOI] [PubMed] [Google Scholar]
- 16. Morawietz H. LOX‐1 receptor as a novel target in endothelial dysfunction and atherosclerosis. Dtsch Med Wochenschr 2010;135:308–312. [DOI] [PubMed] [Google Scholar]
- 17. Chen H, Li D, Sawamura T, Inoue K, Mehta JL. Upregulation of LOX‐1 expression in aorta of hypercholesterolemic rabbits: modulation by losartan. Biochem Biophys Res Commun 2000;276:1100–1104. [DOI] [PubMed] [Google Scholar]
- 18. Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. Elevated soluble lectin‐like oxidized LDL receptor‐1 (sLOX‐1) levels in obese postmenopausal women. Obesity (Silver Spring) 2008;16:1454–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan KC, Shiu SW, Wong Y, Leng L, Bucala R. Soluble lectin‐like oxidized low density lipoprotein receptor‐1 in type 2 diabetes mellitus. J Lipid Res 2008;49:1438–1444. [DOI] [PubMed] [Google Scholar]
- 20. Nomata Y, Kume N, Sasai H, et al. Weight reduction can decrease circulating soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 levels in overweight middle‐aged men. Metabolism 2009;58:1209–1214. [DOI] [PubMed] [Google Scholar]
- 21. Li L, Sawamura T, Renier G. Glucose enhances endothelial LOX‐1 expression: Role for LOX‐1 in glucose‐induced human monocyte adhesion to endothelium. Diabetes 2003;52:1843–1850. [DOI] [PubMed] [Google Scholar]
- 22. Li L, Sawamura T, Renier G. Glucose enhances human macrophage LOX‐1 expression: Role for LOX‐1 in glucose‐induced macrophage foam cell formation. Circ Res 2004;94:892–901. [DOI] [PubMed] [Google Scholar]
- 23. Korkmaz GG, Altınoglu E, Civelek S, et al. The association of oxidative stress markers with conventional risk factors in the metabolic syndrome. Metabolism 2013;62:828–835. [DOI] [PubMed] [Google Scholar]
- 24. Tabak O, Gelişgen R, Cicekçi H, et al. Circulating levels of adiponectin, orexin‐A, ghrelin and the antioxidant paraoxonase‐1 in metabolic syndrome. Minerva Med 2012;103:323–329. [PubMed] [Google Scholar]
- 25. Meaney E, Sierra‐Vargas P, Meaney A, et al. Does metformin increase paraoxonase activity in patients with the metabolic syndrome? Additional data from the MEFISTO study. Clin Transl Sci 2012;5:265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Usta M, Turan E, Aral H, Inal BB, Gurel MS, Guvenen G. Serum paraoxonase‐1 activities and oxidative status in patients with plaque‐type psoriasis with/without metabolic syndrome. J Clin Lab Anal 2011;25:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Juretić D, Motejlkova A, Kunović B, et al. Paraoxonase/arylesterase in serum of patients with type II diabetes mellitus. Acta Pharm 2006;56:59–68. [PubMed] [Google Scholar]
- 28. Koh KK, Quon MJ, Han SH, Chung WJ, Kim JA, Shin EK. Vascular and metabolic effects of candesartan: Insights from therapeutic interventions. J Hypertens Suppl 2006;24:S31–S38. [DOI] [PubMed] [Google Scholar]
- 29. Ghasemi A, Zahediasl S, Azizi F. High serum nitric oxide metabolites and incident metabolic syndrome. Scand J Clin Lab Invest 2012;72:523–530. [DOI] [PubMed] [Google Scholar]
- 30. Zahedi Asl S, Ghasemi A, Azizi F. Serum nitric oxide metabolites in subjects with metabolic syndrome. Clin Biochem 2008;41:1342–1347. [DOI] [PubMed] [Google Scholar]
- 31. Kondo T, Ueyama J, Imai R, Suzuki K, Ito Y. Association of abdominal circumference with serum nitric oxide concentration in healthy population. Environ Health Prev Med 2006;11:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simão AN, Lozovoy MA, Simão TN, Dichi JB, Matsuo T, Dichi I. Nitric oxide enhancement and blood pressure decrease in patients with metabolic syndrome using soy protein or fish oil. Arq Bras Endocrinol Metabol 2010;54:540–545. [DOI] [PubMed] [Google Scholar]
- 33. Taye A, El‐Sheikh AA. Lectin‐like oxidized low‐density lipoprotein receptor 1 pathways. Eur J Clin Invest 2013;43:740–745. [DOI] [PubMed] [Google Scholar]
- 34. Verma S, Yao L, Stewart DJ, Dumont AS, Anderson TJ, McNeill JH. Endothelin antagonism uncovers insulin‐mediated vasorelaxation in vitro and in vivo. Hypertension 2001;37:328–333. [DOI] [PubMed] [Google Scholar]
- 35. Sakurai K, Cominacini L, Garbin U, et al. Induction of endothelin‐1 production in endothelial cells via co‐operative action between CD40 and lectin‐like oxidized LDL receptor (LOX‐1). J Cardiovasc Pharmacol 2004;44:S173–S180. [DOI] [PubMed] [Google Scholar]


