Abstract
Background
Netrin‐1 is a diagnostic biomarker that has been identified recently for chronic renal failure (CRF) in animal experiments. Netrin‐1 levels in urine have been shown to have increased significantly at the acute kidney damage. Human studies on the relation between the CRF and plasma netrin‐1 levels have not been found in the literature. This study aimed to investigate whether plasma netrin‐1 levels increased in the early stages of the CRF in diabetic patients.
Methods
Plasma samples from healthy volunteers and diabetic patients with and without microalbuminuria were collected after receiving consent. Netrin‐1 in plasma was quantified by enzyme‐linked immunosorbent assay and the data were analyzed to determine whether plasma netrin‐1 correlates significantly with disease progression.
Result
Plasma netrin‐1 level in microalbuminuric diabetic patients was significantly higher than in normoalbuminuric diabetic patients and the control group. However, no significant difference between normoalbuminuric patients and control group was determined. Plasma netrin‐1 level was significantly associated with albuminuria and estimated glomerular filtration rate, independently of age and sex.
Conclusion
This study supports that plasma netrin‐1 level increases significantly when glomerular damage occurs in diabetic nephropathy.
Keywords: netrin‐1, albuminuria, diabetic nephropathy
Introduction
Of the 7.125 billion people in the world 382 million are diabetic; Diabetes prevalence in adult Turkish society has reached 14.85% 1. Diabetic nephropathy is a major cause worldwide in end‐stage renal disease (ESRD) 2. For example, in the Unites States 45.2% of the new diagnosed ESRD cases are caused by diabetic nephropathy 3, 4. The early diagnosis of diabetic nephropathy can be advantageous to prevent the development of Diabetic Kidney Disease 5. When the commonly used microalbuminuria is determined, glomerular damage has already developed. Although diabetic nephropathy is classified in glomerular diseases, latest studies have reported that tubulointestinal damage may occur before glomerular damage 3, 5, 6, 7. In recent studies netrin‐1 has been indicated as a novel biomarker in acute renal failure 8, 9. Netrins are important precursor protein for the neural and vascular development 10. It was determined that the first reported netrin located in the Caenorhabditis elegans with nematode worm 11. There are multiple netrin genes in vertebrates. Five kinds of netrin have been identified for mammals (1, 3, 4, G1, and G2). All netrins are the members of the superfamily of laminin. Netrin‐1 is a laminin‐like protein of 50–75 kD weight 12. Netrin‐1 is a laminin‐related secreted proteins highly induced after acute and chronic kidney injury and excreted in urine in both mice and humans 13, 14.
Recent studies have pointed out that urinary netrin‐1 level might be an early biomarker for diabetic nephropathy 4, 8, 9. In these studies, generally, urine netrin‐1 levels were examined. 4, 8, 9, 12, 13. We have not encountered any studies on plasma netrin‐1 levels and diabetic nephropathy in humans in our wide range literature screening. The relationship between diabetic nephropathy development and plasma netrin‐1 levels is unknown. This study was designed to evaluate the relation between blood netrin‐1 levels and albuminuria in individuals over 18‐years‐old with and without diabetes.
Materials and Methods
Patient Recruitment and Sample Collection
This study is a type of case‐control study. Sixty patients with diabetes and 56 nondiabetic people admitted to family medicine clinic for different reasons were included in the study. This study was conducted at Diabetes Education Clinic of Family Medicine of Selcuk University and Periodic Outpatient Clinic between October 30, 2014 and August 06, 2015. It was planned to carry out with the Decision No. 2014/15 and dated September 09, 2014 by Selcuk University Faculty of Medicine Ethics Commission. Informed consent forms compatible with Helsinki Declaration of World Medical Association were received from each participant prior to the study.
Individuals in both groups with and without diabetes were equated at age, gender, and sociocultural levels. The ones with a history of Cerebrovascular accident, neurological disease, cigarette smokers, with the infection, pregnant women, the ones with diagnosed liver failure, with previously diagnosed malignancy, lactation, patients using anticonvulsant drugs, nephrotoxic drug users, with kidney diseases except diabetic nephropathy, and unwilling patients to participate the study were excluded from the study.
The blood samples of the individuals were taken from antecubital area in front of the elbows after cleaning with 70% alcohol swab and put into in Etilendiamin Tetraasetik Asit (EDTA) tubes. After centrifugation at 2800 rpm and at 4°C for 20 min, plasma samples were placed into the eppendorf tubes and were stored at −80°C until runtime.
Netrin‐1 Quantification in Plasma
Netrin measurements in plasma samples were performed with Biotek ELX 800 device using Human Netrin‐1 (NTN1) enzyme‐linked immunosorbent assay (ELISA) kit (MyBioSource, USA). The lowest concentration the method could determine was 1 pg/ml, and precision values were under 15% within and between the runs.
Measurements
The patients' blood samples were taken after fasting for 8–12 h. Blood pressure was assessed with a single measurement. Spot urinary albumin concentration was determined by nephelometry (Architect C16000 Abbott). Serum and spot urine creatinine were measured with an enzymatic creatinine assay (Architect C16000 Abbott). Glycated hemoglobin (HbA1c) was measured with high performance liquid chromatography method. Glucose and cholesterol levels were measured with standard laboratory testing.
Definitions
We defined history of cardiovascular disease as occurrence of myocardial infarction, stroke, cardiovascular surgery, or endovascular treatment for coronary carotid or peripheral (legs, abdominal, or aortic) artery disease. Hypertension was defined as systolic blood pressure >140 mmHg and diastolic blood pressure >90 mmHg or use of blood pressure‐lowering medication. Normoalbuminuri was defined as an albumin to creatinine ratio (ACR) <30 mg/g day, microalbuminuria as ACR 30–299 mg/g day 15. Serum creatinine values were used to calculate glomerular filtration rate (GFR) using the abbreviated Modification of Diet in Renal Disease formula 14.
Statistical Analysis
Data were assessed for normality and appropriate transformations were used when necessary. Median (min, max) values are reported for continuous nonnormal data, mean ± SD is reported otherwise. Chi‐square tests were used to assess the relationship between control and albuminuria groups with categorical variables. Student's t‐test was used to compare the measurements of a particular variable of two separate groups and for normal scattered groups, the Mann–Whitney U test was used for abnormally distributed groups. Tamhane's T2 test together with the one‐way ANalysis Of VAriance (ANOVA) was used to compare multiple groups. Pearson correlation analysis was used to determine the relationship between numerical variables for normally distributed groups, and Spearman test was used for abnormally distributed groups. Correlation coefficient (r); between 0.000 and 0.249 was considered as weak relation; middle from 0.250 to 0.499; strong from 0.500 to 0.749; and very strong relation between 0.750 and 1.000. Significance was determined at α = 0.05. In the analysis of all the data, SPSS (Statistical Package for Social Sciences) for Windows 16.0 statistical software was used.
Results
Individuals enrolled in the study were divided into two as 60 diabetic and 56 nondiabetic patients. The average age of the 116 individuals who participated in our study was 54.06 ± 9.63 (min: 32, max: 73, median: 54), body mass index (BMI) average was 30.11 ± 4.85 kg/ m² (min: 19.63, max: 44.44, median: 29.62).
Diabetic patients were divided among themselves as 30 normoalbumiunric diabetic patients, and 30 micromoalbumiunric diabetic patients. The individuals with and without diabetes in both groups were equal in terms of age, gender, and cultural levels. Details are shown in Tables 1 and 2.
Table 1.
Similar Held Features and Sociodemographic Characteristics of Nondiabetic and Diabetic Patients
| Nondiabetic patients (n = 56) | Diabetic patients (n = 60) | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| n | % | n | % | X2/t | P | |
| Age (years) | 54.10 ± 8.96 | 54.01 ± 10.30 | 0.050 | 0.960 | ||
| BMI (kg/m2) | 29.64 ± 4.97 | 30.56 ± 4.72 | −1.023 | 0.309 | ||
| Sex | ||||||
| Female | 28 | 50.0 | 28 | 46.7 | 0.129 | 0.720 |
| Male | 28 | 50.0 | 32 | 53.3 | ||
| Job | ||||||
| Housewife | 25 | 44.6 | 27 | 45.0 | 4.324 | 0.364 |
| Employee | 9 | 16.1 | 6 | 10.0 | ||
| Officer | 10 | 17.9 | 6 | 10.0 | ||
| Private sector | 5 | 8.9 | 11 | 18.3 | ||
| Retired | 7 | 12.5 | 10 | 16.7 | ||
| Educational status | ||||||
| Primary education | 30 | 53.6 | 41 | 68.4 | 2.659 | 0.265 |
| High school | 15 | 26.8 | 11 | 18.3 | ||
| University | 11 | 19.6 | 8 | 13.3 | ||
| Marital status | ||||||
| Married | 52 | 92.9 | 54 | 90.0 | 0.300 | 0.584 |
| Not married | 4 | 7.1 | 6 | 10 | ||
| Total | 56 | 100.0 | 60 | 100.0 | ||
Table 2.
Comparison of Blood Parameters of Patients With Nondiabetes and Diabetes
| Nondiabetic patients (n = 56) | Diabetic patients (n = 60) | |||
|---|---|---|---|---|
| Mean ± SD/median | Mean ± SD/median | t/z | P | |
| Netrin‐1 | 4.105 | 4.655 | −2.536 | 0.011 a |
| FPG | 99 | 144.500 | −7.952 | <0.0001 a |
| LDL | 148.63 ± 58.19 | 123.86 ± 37.05 | 2.753 | 0.007 b |
| HDL | 41.500 | 39 | −2.583 | 0.010 a |
| TG | 136.5 | 180.5 | −1.757 | 0.79a |
| TK | 215 | 206 | −1.658 | 0.097 a |
| GFR | 100.95 ± 13.42 | 92.10 ± 21.02 | 2.718 | 0.008 b |
| Creatinine | 0.760 | 0.810 | −2.292 | 0.022 a |
| Spot urine microalbumin/creatinine | 8 | 29 | −3.731 | <0.0001 a |
| HbA1C | — | 8.10 ± 1.67 | — | — |
| Duration of DM | — | 7.30 ± 6.81 | — | — |
aMann–Whitney U test.
bStudent's t‐test.
DM, diabetes mellitus; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; TG, triglycerides; TK, total cholesterol.
Plasma netrin‐1 median was statistically significantly higher (P = 0.011) when comparing the group with diabetes to the group without diabetes. There was no statistically significance (P = 0.882) between the group without diabetes and normoalbumiunric diabetic patients. The plasma netrin‐1 level in micromoalbumiunric diabetic patients is statistically significantly higher (P = 0.004 and P = 0.023) than in the group without diabetes and normoalbumiunric diabetic patients (Fig. 1).
Figure 1.
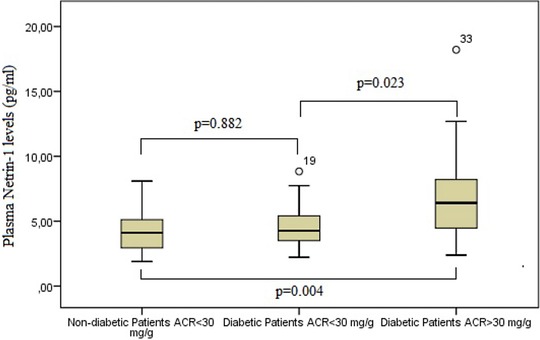
Comparison of netrin‐1 between group without diabetes, diabetic group with microalbumin/creatinine ratio <30 in the spot urine, and diabetic group with microalbumin/creatinine ratio >30.
When GFR of case and control groups were compared, the mean GFR of the case group (n = 60) was 92.10 ± 21.02 ml/min, while the mean GFR of the control group (n = 56) was 100.95 ± 13.42 ml/min. In terms of GFR, mean GFR of the group with diabetes was significantly lower (P = 0.008) than that of the group without diabetes. Creatinine median of the case group (n = 60) was 0.81 mg/dl, creatinine median of the control group (n = 56) was 0.76 mg/dl, and serum creatinine median of the group with diabetes was statistically significantly higher (P = 0.022) than the nondiabetic group. Microalbumin/creatinine median of the case group (n = 60) in spot urine was 8 mg/dl and microalbumin/creatinine median of the control group (n = 56) in spot urine was 29 mg/dl, and the microalbumin creatinine medians in the spot urine were statistically significantly different (P = 0.000). The mean HbA1c of the case group (n = 60) was 8.10% ± 1.67% (Table 2).
There was positive statistically significant correlation between netrin‐1 level and the duration of diabetes and HbA1c. There was slightly strong correlation and negative statistically significant difference between netrin‐1 level and GFR. While there was slightly strong correlation between netrin‐1 level and the duration of diabetes and GFR, there was mediocre strong correlation between HbA1c (Table 3). The correlation of Netrin‐1 was evaluated with ten parameters (Table 3).
Table 3.
Age, BMI, Duration of DM, FPG, LDL, HDL, Creatinine, GFR, and HbA1c Correlation With Netrin‐1
| Age | BMI | Duration of DM | FPG | LDL | HDL | Creatinine | GFR | HbA1c | Microalbumin/creatinine | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Netrin‐1 | R | 0.055 | −0.014 | 0.199 | 0.157 | 0.051 | −0.031 | 0.157 | −0.230 | 0.251 | 0.437 |
| P | 0.555a | 0.881a | 0.033 a | 0.093b | 0.589a | 0.743b | 0.091b | 0.013 a | 0.007 a | P < 0.0001 b |
aPearson test.
bSpearman test.
DM, diabetes mellitus; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Discussion
This study was designed to see the relationship between diabetic nephropathy development and plasma netrin‐1levels. We compared plasma levels of netrin‐1 in three groups as albumiunric diabetic patients, normoalbumiunric diabetic patients, and nondiabetic patients. Plasma levels of netrin‐1 with diabetic nephropathy group were significantly higher than the other groups.
Netrin‐1 is recently discovered biomarker that has been shown to increase in the urine at the acute kidney damage 9, 13. Netrin‐1 levels have been shown to increase in the urine in the variety of acute kidney injuries caused by different reasons in both studies on animal models and human models 4, 8, 9, 12, 13. After it has been determined that netrin‐1 level has increased in animals with diabetic nephropathy in chronic renal diseases 15, the level of netrin‐1 has also been shown to increase significantly in the early stages in the urine in humans with diabetic nephropathy 4. The fact that Jayakumar et al. have reported that netrin‐1 level has increased significantly in the urine without microalbuminuria in patients with diabetic nephropathy demonstrates that urine may be a biomarker for early diagnosis urinary netrin‐1 level, and they have also pointed out that tubular damage develops before any glomerular damage in diabetic nephropathy classified in glomerular renal failure. Our study is the first to compare plasma netrin‐1 levels with albuminuria in patients with diabetic nephropathy. Our results have shown that when microalbuminuria begins in diabetic patients, plasma netrin‐1 levels elevates significantly. However, plasma netrin‐1 level is not a helpful biomarker to show the tubular damage that might occur in diabetic patients without microalbuminuria. Animal experiments have indicated that the source of netrin‐1 released in the urine at acute and chronic renal failure is the proximal tubule epithelial cells 13. We think that the source of rising plasma netrin‐1 level in patients with diabetic nephropathy may be diabetes‐damaged glomerular endothelial. We may say that rising netrin‐1 levels in urine indicates tubular damage and rising plasma netrin‐1 levels shows glomerular damage in diabetic patients. According to these results, if netrin‐1 is used for the early diagnosis of diabetic nephropathy as a marker, it seems that urine is useful than plasma. Elevated plasma levels of netrin‐1 are important because it indicates that no longer start in glomerular injury. It comes to mind that rising plasma netrin‐1 levels may influence the other diabetic complications.
Recent studies have shown that netrin‐1 has a protective feature for diabetic nephropathy, and some studies have established relationship between netrin and other complications of diabetes 16, 17, 18, 19, 20. Experiments on diabetic mice have shown that oversecretion of netrin‐1 increases the nerve conduction velocity and promotes angiogenesis 16. Diabetic nephropathy has been attributed to increased netrin‐1 level in vitreous fluid in a recent study 17, 18. Van Gils et al. 19 have shown that netrin‐1 has a role in the growth of atherosclerosis plaque by preventing the migration of lipid‐laden macrophage cells and by increasing the migration of smooth muscle cells into the intima. Netrin‐1 is also regarded as a new atherogenic protein 21, 22, 23. In light of these studies, high level netrin‐1 in the general circulation could encourage atherosclerosis in patients with diabetic nephropathy.
Our study has some limitations. First, our clinical data were obtained from only one center. A prospective study with larger groups of patients should be performed to show the relations of plasma netrin‐1 levels with diabetic nephropathy better. Furthermore, only plasma netrin‐1 level has been investigated, but at what stages netrin‐1 level elevates in the urine and blood can be displayed better in a study comparing plasma netrin‐1 levels and urine netrin‐1 levels in the same patients.
In conclusion, this is the first study showing that raised netrin‐1 levels can be found in diabetic nephropathy patients. Further randomized, prospective studies with larger samples are required to support our findings.
Conflict of Interest
The authors have no relationship with any industry.
Acknowledgments
This study was supported by Selcuk University, Scientific Research Projects (Project Number: 14102047).
References
- 1. Prevalence of Diabetes in the World, 2013 . Available from: http://www.idf.org/diabetesatlas. Accessed on June 11, 2015.
- 2. Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nxephropathy in Type 1 (insulin‐dependent) diabetes: An epidemiological study. Diabetologia 1983;25:496–501. [DOI] [PubMed] [Google Scholar]
- 3. Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structuralfunctional relationships in diabetic nephropathy. J Clin Invest 1984;74:1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jayakumar C, Ferdau LN, Stephan JLB, et al. Netrin‐1, a urinary proximal tubular injury marker, is elevated early in the time course of human diabetes. J Nephrol 2014;27:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amsellem S, Gburek J, Hamard G, et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 2010;21:1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gekle M. Renal proximal tubular albumin reabsorption: Daily prevention of albuminuria. News Physiol Sci 1998;13:5–11. [DOI] [PubMed] [Google Scholar]
- 7. Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 2009;20:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reeves WB, Kwon O, Ramesh G. Netrin‐1 and kidney injury. II. Netrin‐1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol 2008;294:731–738. [DOI] [PubMed] [Google Scholar]
- 9. Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin‐1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 2010;5:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freitas C, Larrivee B, Eichmann A. Netrins and UNC5 receptors in angiogenesis. Angiogenesis 2008;11:23–29. [DOI] [PubMed] [Google Scholar]
- 11. Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC‐6, a lamininrelated protein, guides cell and pioneer axon migrations in C. elegans. Neuron 1992;9:873–881. [DOI] [PubMed] [Google Scholar]
- 12. Basnakian AG. Netrin‐1: A potential universal biomarker for acute kidney injury. Am J Physiol Renal Physiol 2008;294:729–730. [DOI] [PubMed] [Google Scholar]
- 13. Reeves WB, Kwon O, Ramesh G. Netrin‐1 and kidney injury. II. Netrin‐1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol 2008;294:731–738. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–2100. [DOI] [PubMed] [Google Scholar]
- 15. White JJ, Mohamed R, Jayakumar C, Ramesh G. Tubular injury marker netrin‐1 is elevated early in experimental diabetes. J Nephrol 2013;26:1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson BD, Ii M, Park KW, et al. Netrins promote developmental and therapeutic angiogenesis. Science 2006;313:640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Xia X, Xiong S, Le Y, Xu H. Intravitreous high expression level of netrin‐1 in patients with proliferative diabetic retinopathy. Yan Ke Xue Bao 2011;26:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tian XF, Xia XB, Xiong SQ, Jiang J, Liu D, Liu JL. Netrin‐1 overexpression in oxygen induced retinopathy correlates with breakdown of the blood‐retina barrier and retinal neovascularization. Ophthalmologica 2011;226:37–44. [DOI] [PubMed] [Google Scholar]
- 19. Van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin‐1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunolt 2012;13:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tak E, Ridyard D, Badulak A, et al. Protective role for netrin‐1 during diabetic nephropathy. J Mol Med 2013;91:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bongo JB, Peng DQ. The neuroimmune guidance cue netrin‐1: A new therapeutic target in cardiovascular disease. J Cardiol 2014;63:95–98. [DOI] [PubMed] [Google Scholar]
- 22. Ramkhelawon B, Yang Y, van Gils JM, et al. Hypoxia induces netrin‐1 and Unc5b in atherosclerotic plaques: Mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol 2013;33:1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore KJ, Fisher EA. Macrophages, atherosclerosis and the potential of netrin‐1 as a novel target for future therapeutic intervention. Future Cardiol 2012;8:349–352. [DOI] [PMC free article] [PubMed] [Google Scholar]


