ABSTRACT
Highlights
Quality-of-life measures improve as early as 3 months after bariatric surgery.
There is wide variability in reporting that renders direct comparisons difficult.
The available comparisons between RYGB and sleeve gastrectomy could not detect any difference.
Improvement in PRO measures might be related to weight loss.
Larger and better designed studies are required to achieve discrimination in PRO measures.
Background:
Obesity is associated with reduced quality-of-life (QoL), which generally improves after bariatric surgery. The differential effect of each type of surgery (gastric sleeve [SG] and gastric bypass [RYGB]) on QoL is not yet fully understood.
Objectives:
To understand which of these surgeries offers greatest improvement in QoL and patient satisfaction.
Methods:
Systematic literature search on Pubmed in July 2014. Relevant articles were selected in a step-wise approach. The 2482 titles were scanned for relevance and 191 were selected for abstract reviewing; and 88 papers were selected for full text analysis.
Results:
Only 5 papers compared the 2 techniques and only 17 more had retrievable data either on SG or RYGB. The reports were very heterogeneous, preventing a direct comparison of patient reported outcomes (PRO) among studies.
Improved results have been reported as early has 3 months and SF-36 scores were improved in all domains in medium to long-term. The question remains whether the improvement in QoL is related to the weight loss and which factors are associated with improved patients' perceptions.
Conclusions:
There is wide heterogeneity in the reporting of PRO measures after bariatric surgery, but data is consistent with a significant improvement after both surgeries.
Larger and better-designed studies are required to understand if there are significant differences in the quality of life after SG or RYGB.
Keywords: Bariatric surgery, Metabolic surgery, Obesity surgery, Roux-en-Y gastric bypass, Sleeve gastrectomy, Quality-of-life
Introduction
The worldwide epidemic of obesity, fueled by a sedentary lifestyle and an energetically dense diet, has contributed to an unprecedented event in the history of mankind: for the first time, the number of obese people is greater than the number of undernourished.1 Obesity is a growing public health concern in developed countries, and it is estimated that there are over than 1.5 billion obese people worldwide.2 This represents about 5% of all the health related expenditures in the USA3 and leads to a significant decrease in life expectancy.4
Bariatric surgery is the most effective available treatment and the one that allows more significant and durable weight loss.5 It leads to reversal of co-morbidities and components of the metabolic syndrome,6,7,8,9,10 as well as a reduction in all-cause mortality6,7,8,9,10,11,12,13,14 by as much as 89%.15 Its cost-effectiveness has been widely reported, and we conducted a cost-utility study based on a Markov model that concluded that RYGB is a cost-saving strategy.16
However, up to 18% of patients fail to achieve a body mass index (BMI)<35kg/m2, and unsuccessful weight loss has been reported in 10–30% of patients who have undergone bariatric surgery.17
Roux-en-Y gastric bypass (RYGB) was one of the first approved surgical options for morbid obesity. In 1991 the National Institutes of Health (NIH) determined that RYGB was indicated for patients with a BMI greater than 35 with obesity related comorbidities and for patients with BMI greater than 40 without comorbidities.18
Since its inception in 1994, the totally laparoscopic RYGB, though technically demanding, has become the procedure of choice for morbid obesity.18 The laparoscopic technique is associated with a decrease in complications and an improvement in quality-of-life.18 There are several variations to the technique, but all include the creation of a small gastric reservoir, a gastro-jejunal anastomosis and a biliary limb and Roux-limb of varying lengths.
Sleeve gastrectomy (SG), originally performed as the restrictive component of the duodenal switch procedure,19 has recently been recognized as a valid option for the surgical treatment of morbid obesity.20 In the past 10-years it has evolved from an investigational stand-alone procedure to ˜%5 of all bariatric surgeries.21 It usually is performed through a laparoscopic approach and involves the creation of a narrow tubular stomach due to a vertical gastrectomy based along the greater curvature.22 It has shown an intermediate result between gastric banding and RYGB in terms of weight loss, and an improvement in co-morbidities similar to that seen after RYGB.21,23,24
Development of gastroesophageal reflux disease (GERD) is one of the most frequent complications of SG with up to 26% of patients experiencing new symptoms after surgery25 and up to 30% requiring reoperation due to GERD or weight increase caused by dilatation of the gastric tube.26,27
The evaluation of outcomes in bariatric surgery is of utmost importance, although it is not easy to do. There are several different risks at stake (including death) and different outcomes to be achieved: health related quality of life (QoL), weight loss and resolution of associated diseases. Several studies evaluating QoL measures have shown conflicting results.28,29,30,31
Also, due to the technical nature of the procedures, small variations in the technique (such as pouch size, anastomoses diameter or limb lengths) or different techniques (RYGB, gastric band, SG, and others) being used by different surgeons and different centers makes it more difficult to analyze the results and compare data. Patient follow-up and reporting are usually sub-optimal and the parameters used to measure improvement or resolution of comorbidities have not been standardized.32 Many of the published studies are retrospective cohorts or case series rather than prospective randomized trials, and may not be truly representative of differences in QoL between different procedures. So, despite the more than 350,000 surgeries performed worldwide each year, we are still lacking consensus on which is the best surgery for each patient.33
Obesity is associated with reduced QoL and in general, QoL improves after bariatric surgery.34 The differential effect of each surgery on QoL is not yet fully understood, but it seems that RYGB is associated with better patient-centered outcome measures and greatest improvement in QoL.26,32,35
Since SG numbers are increasing and the clinical results seem to be comparable to RYGB, at least in the short term, it is important to understand which of these surgeries offers greater improvement in QoL and patient satisfaction.
Materials and methods
We performed a systematic review of the literature, with the primary objective being to compare the quality of life of morbidly obese patients after RYGB and SG. A secondary focus was related to comparing weight loss and measures of alimentary satisfaction.
Literature search: We performed a systematic literature search on Pubmed in July 2014, with the following terms: [“patient satisfaction” or “quality of life” or “patient reported outcomes”] and [“gastric sleeve” or “gastric bypass” or “bariatric surgery” or “metabolic surgery”]. Since the MeSH thesaurus is not up to date, and several relevant papers were published in the latter year, we opted to use general language search. The search was restricted to humans and there was no date restriction nor any other restrictions to types of studies, participants or interventions. Only articles in English, Spanish, French or Portuguese were selected.
Relevant articles were selected in a step-wise approach. The search retrieved 2482 titles that were scanned for relevance and 191 of these were selected for abstract reviewing. The abstracts were reviewed and 88 papers were selected for full text analysis (Fig. 1). Papers selection and reviewing was performed unblinded by the first author, based on relevance and the presence of retrievable (numeric) data in the full-text of the paper. Only papers that evaluated QoL after each intervention using validated QoL questionnaires were selected. Papers that mentioned “quality of life” but did not use any measure were excluded, as well as papers evaluating QoL in other types of surgery. Data was extracted from the papers in tables (Tables 1 and 2). Only 22 papers had retrievable data and were used in the analysis. One of the papers used the Food Tolerance Questionnaire, 3 used the M-A-II, 3 used the BAROS, 3 used the GIQLI and 13 used the SF-36.
Fig. 1.
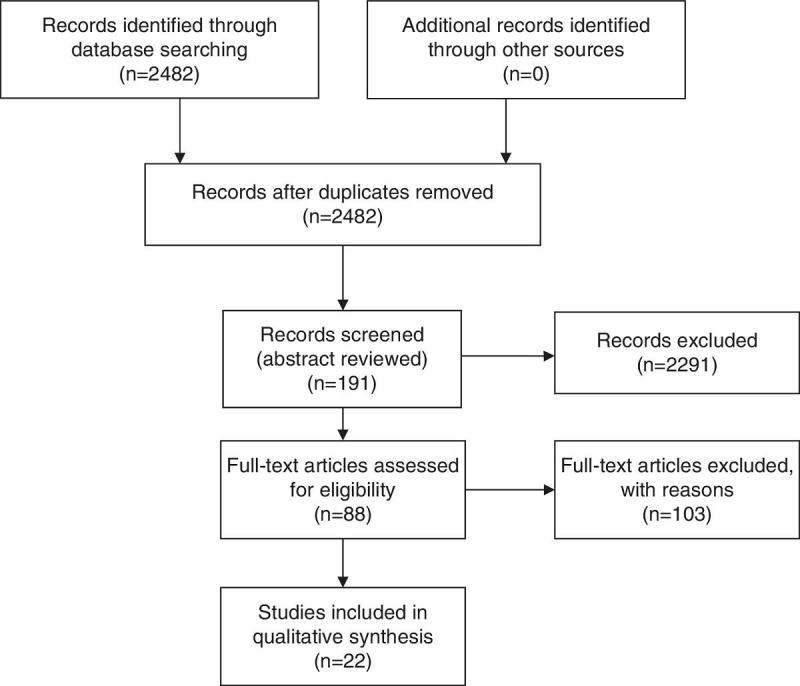
Prisma flow diagram. From the 88 papers selected for full text revision, only 22 had retrievable objective data, which were used in the summary tables. Only 5 studies had a direct comparison between both techniques, 2 of which were randomized controlled trials.
Table 1.
Studies with direct comparison between SG and RYGB outcomes.
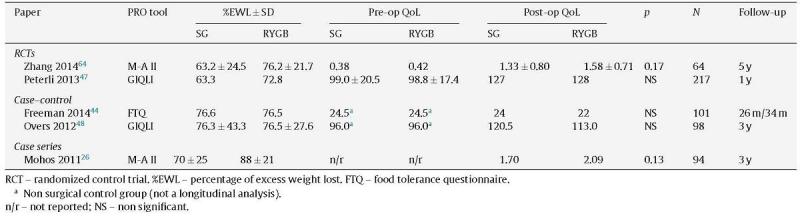
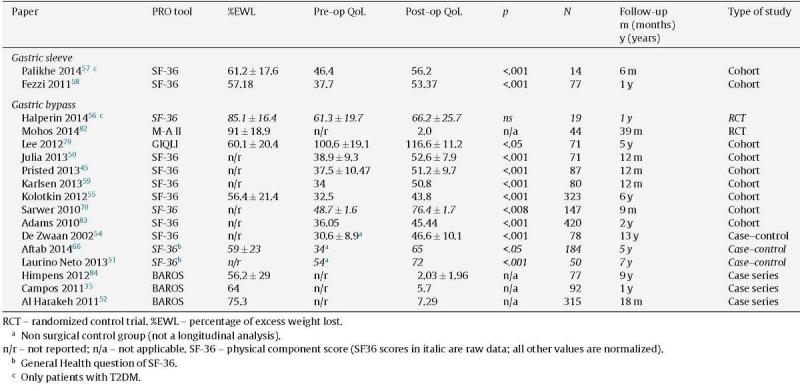
Table 2.
Summary of studies reporting PRO either in SG or RYGB.
Outcomes used
Many validated generic questionnaires can be used to assess QoL. The most frequently used questionnaire is the Short Form-36 (SF-36).36 However, these instruments are often times exhaustive, might require interviewers and are not validated for follow-up of bariatric patients since they are not disease specific.37 The condition specific Moorehead-Ardelt Quality of Life Questionnaire II38 (MA-II, alone or integrated in the BAROS) is a simple, 1-page, valid, reliable, appropriate and reproducible instrument to assess patient's QoL before and after bariatric surgery. Since it uses pictures to represent different health states, it bridges linguistic, cultural and educational barriers.37 However, both BAROS and MA-II are copyrighted, which might preclude their widespread use.
The Gastrointestinal Quality of Life Index (GIQLI) is a questionnaire capable of providing information on generic and specific aspects of digestive symptoms and QoL. For this reason, GIQLI has been extensively used in different pathologies and to evaluate medical treatments or surgical procedures performed on the digestive tract.
BAROS
The Bariatric Analysis and Reporting Outcome System was proposed in 1998.39 It included analysis of weight loss, improvement in co-morbidities and changes in QoL using the specifically designed Moorehead-Ardelt Quality of Life Questionnaire. Each of these 3 domains was granted up to 3 points and re-operations and complications deduct points. Thus, a scale from 0-9 points could be constructed. The outcomes were translated as ≤1 point – failure2,3; fair4,5; good6,7; very good8,9; excellent. This system has been one of the most widely used QoL instruments for bariatric surgery and allows direct comparison of results between centers or between different surgical techniques. It later incorporated the Moorehead-Ardelt Quality of Life Questionnaire II38 and recently the updated BAROS II has been published,37 which includes new criteria for diabetes and clarifies the concept of improvement.
Given its simplicity, BAROS is associated with better patient compliance than the longer, more generic SF-36.40
However, many reports only present the aggregate results of the BAROS score and only in a single post-operative assessment. This greatly reduces understanding of the underlying behaviors related to success or failure of the procedure.
M-A Quality of Life Questionnaire II
M-A Quality of Life Questionnaire II has been validated as a tool to assess quality of life. The questionnaire is composed of six items to measure the patient's quality of life in the areas of general self-esteem, physical activity, social contact, work satisfaction, sexuality and eating behavior. Points are added or deducted based on the patients' subjective perception. Each item is answered in a Likert-type 10 point scale, with variations from −0.5 to +0.5. It is simple, user friendly and can be completed within 1min. The total score is the sum of six dimensions with a range of −3.0 to +3.0.38
The MA-II was validated by a study38 on RYGB patients, 90 women and 20 men, with a mean BMI of 50kg/m2. Cronbach's α coefficient of. 84 demonstrated internal consistency and the instrument's reliability. The scores correlated significantly (p<01) with 7 of the 8 scales of the SF-36 Health Survey. A literature review has concluded that from 11 questionnaires measuring obesity the MA-II seems to be the most promising.41
SF36
SF-36 is a generic health status questionnaire, developed to evaluate self-reported physical and mental health across a wide range of medical conditions.40 It is an instrument studied and used in bariatric surgery, and is endorsed by the International Bariatric Surgery Registry.
Obesity has been correlated with lower scores, especially in the physical component of SF-36. Several trials of bariatric surgery have concluded that after surgery, there is an improvement in the SF-36 scores, and thus, this is a sensitive instrument to capture this construct.40
In spite of lower pre-operative values associated with obesity, bariatric surgery patients with long-term follow-up seem to have SF-36 scores similar to the normal US40 and Spanish population.42 Overall, SF-36 has been the patient reported outcome (PRO) measure most often reported.36
GIQLI
Compared to other questionnaires, the GIQLI provides information on the quality of life of the patient in relation to both generic aspects (physical status, social relations, emotional status) and specific aspects of the upper and lower digestive tract. Also, it is a self-contained, easy-to-understand and easy-to-complete questionnaire that does not require much time to enter the data. It can assess the changes in quality of life of an obese patient who has undergone bariatric surgery (due to the changes caused in the intestinal tract by this type of surgery), and has been validated in this group of patients.43
Several other questionnaires have been developed. However, since they have been scarcely used and there is no direct comparison for SG or RYGB, we opted not to include them in this analysis. The only exception was the report from Freeman et al.,44 since it was one of the few studies with direct comparison between both techniques.
Results
Although the expression “quality of life” is intuitively understood by most people, there is not yet a comprehensive definition.45 Quality of life is a construct of physical, psychological and social domains of health that can be measured by a series of questionnaires.46 These measures can be influenced by a person's experiences, beliefs, expectations and perceptions. Generic instruments measure a wide array of health states, conditions and diseases, allowing for comparisons between disease states. Disease-specific instruments focus on the domains most relevant to the disease under study and might be more sensitive to detect changes due to interventions.46
Of the 88 papers selected for reviewing, only 5 papers compared RYGB and SG (Table 1), and only 17 more had retrievable data and could be thoroughly analyzed (Table 2). The reports were very heterogeneous, preventing a direct comparison of PRO between studies. Not only were the populations different, but also there were a wide variety of instruments used. Even if the same instruments were used, there was no uniform reporting of the results. Several studies only showed aggregate results, others did not show results at all (only stating that QoL was improved) and some presented graphs with no significant legends. Furthermore, some studies with complete patient outcomes reporting did not have information about which type of surgery had been done and how. The great majority of studies analyzed outcomes at one time-point alone, thus limiting the interpretation of the measure of change.
A recently published RCT47 concluded that the 1 year QoL was significantly improved after both SG and RYGB (p<.001 from baseline) and was even higher than in healthy individuals (GIQLI 127 & 128 vs 121; p<.01). At the end of the first post-operative year there were no significant differences between groups (SG vs RYGB) either in terms of weight loss, resolution of co-morbidities or GIQLI scores. For the patients who had reached the 3rd follow-up year there was a small and not statistically significant difference in weight loss, favoring patients with RYGB (% excess BMI lost 63.3% vs 72.8%; p=NS).
Mohos et al.,26 in a BMI matched analysis, concluded that there is a significant improvement in weight loss and QoL after both types of surgery, but the results seem better after RYGB (although the follow-up period is greater for SG and there is no baseline analysis of the QoL). Again, both groups of patients achieved aggregate SF-36 scores similar to the normal population.
It was also reported that decreased food tolerance was associated with a reduced GIQLI, thus impacting the overall QoL.48 A direct comparison between SG and RYGB (although with shorter follow-up for SG) shows a non-significant difference in the GIQLI score in favor of SG (120.5 vs 113.0).48
A 2013 meta-analysis conducted by Yang et al.49 comparing the clinical outcomes between SG and RYGB could analyze only 8 papers, 6 of which were RCT's. According to this meta-analysis, RYGB has higher weight loss than SG, but no information of patient reported outcomes is given.
Improvement in the physical domains of QoL have been reported as early as 3 months after RYGB.50
A study in Brazil concluded that after RYGB, the short-term effects (1 year) were stronger than those achieved at 7-years follow-up, both in terms of QoL and weight loss, with an increase in BMI of 6kg/m2 between these time-periods.51
A large cohort of RYGB patients analyzed by Al Harakeh52 reported significant improvement in BAROS scores at almost all time-points (3 weeks to 5 years). The results were better at 18 months and were slightly more positive in women and in patients with lower baseline BMI. Since baseline values were not presented, these patients may have different baseline values.
A small study from New Zealand concluded that 6 months after both SG and RYGB, SF-36 component summary scores were comparable to the average population.53 Pristed et al.45 reported that QoL 12 months after surgery in a cohort of 87 RYGB patients was not different from the national norm. A long-term study by de Zwaan et al.54 with 13 years of follow-up concludes the same: compared to the pre-operative group, patients who had RYGB present values close to the reference of the population. Kolotkin et al.55 report an improvement of 1.17SD in the physical component score of the SF-36 at 6 years after RYGB. This effect was greatest at the 2nd post-operative year and slightly decreased at 6 years.
A recent report by Halperin et al.56 in patients with type 2 diabetes concluded that even though cardiometabolic risk factors and weight improvement were better after gastric bypass than optimized lifestyle intervention, the 12 months SF-36 scores were not different between the groups. However, the more disease-specific IWQOL revealed a significant improvement in obesity related QoL (81.7–46.5; p<0.001) after gastric bypass.
After SG, QoL is improved in most patients and scales used. In a group of patients primarily treated for obesity associated T2DM, SF-36 was improved in every domain57 at 6 months after surgery. One year after SG, non-selected patients reported significant improvements in all the SF-36 measures.58,59,60
Long-term follow up of the SOS study concluded that 10 years after bariatric surgery, large groups of patients started to regain weight. However, a long-term weight loss of ˜10% was enough for positive long-term effects on QoL.5
Discussion
Empirical data has suggested that subjects with morbid obesity might experience worse QoL than normal-weight subjects, including symptoms of psychological discomfort since many areas of their everyday life are adversely affected (including inter-personal relations).61 However, the major impact of obesity on QoL has been reported in the physical dimensions.42 Obese individuals were more likely to report poor health, increased unhealthy days caused by physical or mental problems and increased total unhealthy days per month.40 Although obesity has been associated with a decreased QoL,40 several studies report that there is no linear relation between higher BMI's and lower QoL.62
Patients with morbid obesity who seek bariatric surgery seem to have lower QoL scores,63 and bariatric surgery improves QoL.40 The clinical effectiveness regarding weight loss and resolution of co-morbidities in the long-term seems to favor RYGB.49,64
In spite of its importance, weight loss is not the only measure of success of bariatric surgery. Quality of life parameters and patient satisfaction are increasingly recognized as important measures. The first report of patient satisfaction after bariatric surgery dates back to 1983 and here, Hall65 concludes that satisfaction was associated with improved health, better self-image and greater social activity. Also, satisfaction increased with weight loss and decreased with unrealistic expectations.
Improved results have been reported as early as 3 months50 and SF-36 scores were improved in all domains in medium to long-term follow up of RYGB patients.42,55,66,67,68
Unfortunately, most studies that evaluate QoL only present the global satisfaction level of the patients, and there are few studies comparing baseline with post-operative scores. Furthermore, a 2013 systematic review of PRO in bariatric surgery identified 68 different PRO measures, which prevents direct comparisons.36
Some authors5 have concluded that a modest weight loss was associated with significant effect on quality of life. In spite of greater weight loss after RYGB, one study concluded that the QoL after gastric band or RYGB was the same.69
Long-term (5 years) studies with high follow-up rates,66 have concluded that both weight loss and QoL improvement are sustained. Most studies, however, report short-term evaluations or have low follow-up rates.
Larger effect sizes have been reported in the physical dimensions of the QoL questionnaires.59 This might be explained by the reduction of body pain, increased stamina and physical activity, and improvements in shortness of breath and foot problems.59
Some reports conclude that improvement in QoL is related to weight loss and improvement of the co-morbidities.57,59,70,71,72 In a population based cohort study, Batsis72 concluded that the self-reported QoL after surgery could be predicted by a profound weight loss and by the absence of co-morbidities. Improved patient satisfaction (which is a different construct from QoL) has also been reported in association with increasing weight loss.73
The relation between QoL and weight loss is difficult to interpret, since it appears not to be linear42,74 and other reports concluded that improvement in QoL occurred early on and was not correlated with weight loss.50,58 Kolotkin et al.55 concluded that the weight loss accounted for only 28.5% of the variance in the physical component score of the SF-36. D'Hondt,75 reported that 6 years after SG the physical components of the SF-36 were higher in the patients that had >50% of EWL. The recent randomized control trial comparing RYGB with intensive lifestyle intervention56 in type 2 diabetes patients concluded that in both groups, the weight-specific quality-of-life improvements (as measured by IWQOL) were proportional to weight lost.
On the other hand, QoL measures might be correlated not with weight loss, but with fulfillment of patients' expectations.45 Even for patients that remained morbidly obese, RYGB has been reported to have a positive effect on QoL.66,68 According to Testa and Simonson, two individuals with the same health status may report different health related QoL because of differences in their expectations and ability to cope with expectations.46
Specifically in bariatric surgery patients, the expectations are often times high and unrealistic, which might impact on the QoL reported.45 The improvement in the emotional QoL domains might be related to an improved perception of self, related to weight loss.59
Klingemann74 reports that it is not the amount of weight lost that is related to the QoL, but rather the achieved BMI and Sarwer70 suggests that body image and QoL were directly correlated.
Al Harakeh52 concluded that BAROS scores were higher in women and in patients with lower BMIs, which raises the question of comparison between different studies that have different distributions of participants. Reports from Sarwer et al.70 and Leiva et al.76 also concluded that QoL improvement was greater in higher educated patients.
Furthermore, Fezzi58 concluded that surgical complications were not associated with the improvement of the physical domain QoL measures, but a smaller percentage of patients achieved significant improvements in the psychological domains. Patients with psychiatric disorders and depression are not able to achieve QoL comparable to the population norm,77,78 which confirms that the emotional and psychological domains also influence the overall results.
The GIQLI score is most sensitive for measuring gastrointestinal symptoms. Although the mean scores improved after RYGB,79 some patients developed new symptoms, mainly related to vomiting, slow eating and abdominal discomfort.
Food tolerance and diet quality might also be related to increased perceptions of QoL. The definition of good food tolerance has been proposed to be the ability to consume a variety of foods without difficulty and with minimal regurgitation/vomiting,80 and seem to be different according to the type of surgery and the time elapsed.44,80,81 SG and RYGB had results comparable to the baseline population in terms of overall food tolerance and increased satisfaction with eating.44 Increase food quality was also associated with “healthier” food choices, which might help to explain its association with increased weight loss.
So, the question remains whether the improvement in QoL is related to the weight loss and which factors are associated with increased patients' perceptions. Also, it is not clear if there are specific aspects in the QoL that are related to each type of surgery.
Conclusions
There is wide heterogeneity in the reporting of PRO measures after bariatric surgery, but the data are consistent with a significant improvement after both types of surgeries. The improvement starts within the first few months after the surgery and lasts up to 10 years. Obese patients present lower values of QoL, but generally 1 year after the surgery are indistinguishable from the general population. The few studies that compared QoL between RYGB and SG could not detect any significant difference.
Larger and better-designed studies are required in order to understand if there are significant differences in the quality of life after SG or RYGB. The development of computer assisted dynamic tools might help achieving greater discrimination in the evaluation of obesity related QoL.
Funding
There were no external sources of funding for this manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Available online 24 January 2017
References
- 1.Tanumihardjo SA, Anderson C, Kaufer-Horwitz M, Bode L, Emenaker NJ, Haqq AM, et al. Poverty, obesity, and malnutrition: an international perspective recognizing the paradox. J Am Diet Assoc. 2007;107:1966-1972. [DOI] [PubMed] [Google Scholar]
- 2.Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg. 2003;13:329-330. [DOI] [PubMed] [Google Scholar]
- 3.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102-107. discussion 107–8. [DOI] [PubMed] [Google Scholar]
- 4.Drenick EJ, Bale GS, Seltzer F, Johnson DG. Excessive mortality and causes of death in morbidly obese men. JAMA. 1980;243:443-445. [PubMed] [Google Scholar]
- 5.Karlsson J, Taft C, Ryden A, Sjöström L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (2005). 2007;31:1248-1261. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg D, Duffy A-J, Bell R-L. Update on obesity surgery. World J Gastroenterol. 2006;12:3196-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings DE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608-2615. [DOI] [PubMed] [Google Scholar]
- 8.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793-2796. [DOI] [PubMed] [Google Scholar]
- 9.Mun EC, Blackburn GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology. 2001;120:669-681. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [DOI] [PubMed] [Google Scholar]
- 11.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753-761. [DOI] [PubMed] [Google Scholar]
- 12.Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484-487. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RJ, Johnson BL, Blackhurst DW, et al. Bariatric surgery is associated with a reduced risk of mortality in morbidly obese patients with a history of major cardiovascular events. Am Surg. 2012;78:685-692. [PubMed] [Google Scholar]
- 14.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65. [DOI] [PubMed] [Google Scholar]
- 15.Christou N, Maclean L. Effect of bariatric surgery on long-term mortality. Adv Surg. 2005;39:165-179. [DOI] [PubMed] [Google Scholar]
- 16.Faria G, Preto JR, Costa-Maia J. Gastric bypass is a cost-saving procedure: results from a comprehensive markov model. Obes Surg. 2013;23:460-466. [DOI] [PubMed] [Google Scholar]
- 17.Ortega E, Morinigo R, Flores L, Moize V, Rios M, Lacy AM, et al. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. 2012;26:1744-1750. [DOI] [PubMed] [Google Scholar]
- 18.Powell MS, Fernandez AZ. Surgical treatment for morbid obesity: the laparoscopic Roux-en-Y gastric bypass. Surg Clin North Am. 2011;91:1203-24-viii. [DOI] [PubMed] [Google Scholar]
- 19.Brethauer SA, Chand B, Schauer PR. Risks and benefits of bariatric surgery: current evidence. Cleve Clin J Med. 2006;73:993-1007. [DOI] [PubMed] [Google Scholar]
- 20.Mechanick JI, Youdim A, Jones DB, Timothy-Garvey W, Hurley DL, McMahon M, et al. Clinical Practice Guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013;9:159-191. [DOI] [PubMed] [Google Scholar]
- 21.Brethauer SA. Sleeve gastrectomy. Surg Clin North Am. 2011;91:1265-79-ix. [DOI] [PubMed] [Google Scholar]
- 22.Gumbs AA, Gagner M, Dakin G, Pomp A. Sleeve gastrectomy for morbid obesity. Obes Surg. 2007;17:962-969. [DOI] [PubMed] [Google Scholar]
- 23.Lee W-J, Chen C-Y, Chong K, Lee Y-C, Chen S-C, Lee S-D. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:683-690. [DOI] [PubMed] [Google Scholar]
- 24.Vidal J, Ibarzabal A, Romero F, Delgado S, Momblán D, Flores L, et al. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. vol. 18. 2008. 1077-1082. [DOI] [PubMed] [Google Scholar]
- 25.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319-324. [DOI] [PubMed] [Google Scholar]
- 26.Mohos E, Schmaldienst E, Prager M. Quality of life parameters, weight change and improvement of co-morbidities after laparoscopic Roux Y gastric bypass and laparoscopic gastric sleeve resection – comparative study. Obes Surg. 2011;21:288-294. [DOI] [PubMed] [Google Scholar]
- 27.Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450-1456. [DOI] [PubMed] [Google Scholar]
- 28.Burgmer R, Legenbauer T, Muller A, de Zwaan M, Fischer C, Herpertz S. Psychological outcome 4 years after restrictive bariatric surgery. Obes Surg 2014. [DOI] [PubMed] [Google Scholar]
- 29.van Hout GCM, Verschure SKM, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15:552-560. [DOI] [PubMed] [Google Scholar]
- 30.Bocchieri LE, Meana M, Fisher BL. Perceived psychosocial outcomes of gastric bypass surgery: a qualitative study. Obes Surg. 2002;12:781-788. [DOI] [PubMed] [Google Scholar]
- 31.Chang C-Y, Huang C-K, Chang Y-Y, Tai C-M, Lin J-T, Wang J-D. Prospective study of health-related quality of life after Roux-en-Y bypass surgery for morbid obesity. Br J Surg. 2010;97:1541-1546. [DOI] [PubMed] [Google Scholar]
- 32.Dumon KR, Murayama KM. Bariatric surgery outcomes. Surg Clin North Am. 2011;91:1313-38-x. [DOI] [PubMed] [Google Scholar]
- 33.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605-1611. [DOI] [PubMed] [Google Scholar]
- 34.Herpertz SS, Kielmann RR, Wolf AMA, Langkafel MM, Senf WW, Hebebrand JJ. Does obesity surgery improve psychosocial functioning? A systematic review. Int J Obes (2005). 2003;27:1300-1314. [DOI] [PubMed] [Google Scholar]
- 35.Campos GM, Rabl C, Roll GR, et al. Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass vs banding: results of a 2-cohort pair-matched study. Arch Surg (Chicago, IL: 1960). 2011;146:149-155. [DOI] [PubMed] [Google Scholar]
- 36.Coulman KD, Abdelrahman T, Owen-Smith A, Andrews RC, Welbourn R, Blazeby JM. Patient-reported outcomes in bariatric surgery: a systematic review of standards of reporting. Obes Rev 2013. [DOI] [PubMed] [Google Scholar]
- 37.Oria HE, Moorehead MK. Updated bariatric analysis and reporting outcome system (BAROS). Surg Obes Relat Dis. 2009;5:60-66. [DOI] [PubMed] [Google Scholar]
- 38.Moorehead MK, Ardelt-Gattinger E, Lechner H, Oria HE. The validation of the Moorehead-Ardelt Quality of Life Questionnaire II. Obes Surg. 2003;13:684-692. [DOI] [PubMed] [Google Scholar]
- 39.Oria HE, Moorehead MK. Bariatric analysis and reporting outcome system (BAROS). Obes Surg. 1998;8:487-499. [DOI] [PubMed] [Google Scholar]
- 40.Ballantyne GH. Measuring outcomes following bariatric surgery: weight loss parameters, improvement in co-morbid conditions, change in quality of life and patient satisfaction. Obes Surg. 2003;13:954-964. [DOI] [PubMed] [Google Scholar]
- 41.Duval K, Marceau P, Perusse L, Lacasse Y. An overview of obesity-specific quality of life questionnaires. Obes Rev. 2006;7:347-360. [DOI] [PubMed] [Google Scholar]
- 42.Mar J, Karlsson J, Arrospide A, Mar B, Martinez de Aragon G, Martinez-Blazquez C. Two-year changes in generic and obesity-specific quality of life after gastric bypass. Eat Weight Disord. 2013;18:305-310. [DOI] [PubMed] [Google Scholar]
- 43.Poves Prim I, Macias GJ, Cabrera Fraga M, Situ L, Ballesta Lopez C. Quality of life in morbid obesity. Revista española de enfermedades digestivas: organo oficial de la Sociedad Española de Patología Digestiva. 2005;97:187-195. [DOI] [PubMed] [Google Scholar]
- 44.Freeman RA, Overs SE, Zarshenas N, Walton KL, Jorgensen JO. Food tolerance and diet quality following adjustable gastric banding, sleeve gastrectomy and Roux-en-Y gastric bypass. Obes Res Clin Pract. 2014;8:e115-e200. [DOI] [PubMed] [Google Scholar]
- 45.Pristed SG, Omar HK, Kroustrup JP. Association between fulfilment of expectations and health-related quality of life after gastric bypass. Appl Res Qual Life. 2013;8:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med. 1996;334:835-840. [DOI] [PubMed] [Google Scholar]
- 47.Peterli R, Borbély Y, Kern B, Gass M, Peters T, Thurnheer M, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258:690-694. discussion 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overs SE, Freeman RA, Zarshenas N, Walton KL, Jorgensen JO. Food tolerance and gastrointestinal quality of life following three bariatric procedures: adjustable gastric banding, Roux-en-Y gastric bypass, and sleeve gastrectomy. Obes Surg. 2012;22:536-543. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, Yang G, Wang W, Chen G, Yang H. A meta-analysis: to compare the clinical results between gastric bypass and sleeve gastrectomy for the obese patients. Obes Surg. 2013;23:1001-1010. [DOI] [PubMed] [Google Scholar]
- 50.Julia C, Ciangura C, Capuron L, Bouillot JL, Basdevant A, Poitou C, et al. Quality of life after Roux-en-Y gastric bypass and changes in body mass index and obesity-related comorbidities. Diabetes Metab. 2013;39:148-154. [DOI] [PubMed] [Google Scholar]
- 51.Laurino Neto RM, Herbella FAM. Changes in quality of life after short and long term follow-up of Roux-en-Y gastric bypass for morbid obesity. Arq Gastroenterol. 2013;50:186-190. [DOI] [PubMed] [Google Scholar]
- 52.Harakeh Al AB, Larson CJ, Mathiason MA, Kallies KJ, Kothari SN. BAROS results in 700 patients after laparoscopic Roux-en-Y gastric bypass with subset analysis of age, gender, and initial body mass index. Surg Obes Relat Dis. 2011;7:94-98. [DOI] [PubMed] [Google Scholar]
- 53.McLeod B, Beban G, Sanderson J, McKillop A, Jull A. Bariatric surgery makes dramatic difference to health-related quality of life. N Z Med J. 2012;125:46-52. [PubMed] [Google Scholar]
- 54.de Zwaan M, Lancaster KL, Mitchel JE, Howell LM, Monson N, Roerig JL, et al. Health-related quality of life in morbidly obese patients: effect of gastric bypass surgery. Obes Surg. 2002;12:773-780. [DOI] [PubMed] [Google Scholar]
- 55.Kolotkin RL, Davidson LE, Crosby RD, Hunt SC, Adams TD. Six-year changes in health-related quality of life in gastric bypass patients versus obese comparison groups. Surg Obes Relat Dis. 2012;8:625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halperin F, Ding SA, Simonson DC, Panosian J, Goebel-Fabbri A, Wewalka M, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes. JAMA Surg. 2014;149:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palikhe G, Gupta R, Behera BN, Sachdeva N, Gangadhar P, Bhansali A. Efficacy of laparoscopic sleeve gastrectomy and intensive medical management in obese patients with type 2 diabetes mellitus. Obes Surg. 2014;24:529-535. [DOI] [PubMed] [Google Scholar]
- 58.Fezzi M, Kolotkin RL, Nedelcu M, Jaussent A, Schaub R, Cahuvet MA, et al. Improvement in quality of life after laparoscopic sleeve gastrectomy. Obes Surg. 2011;21:1161-1167. [DOI] [PubMed] [Google Scholar]
- 59.Karlsen TI, Lund RS, Roislien J, Tonstad S, Natvig G, Sandbu R, et al. Health related quality of life after gastric bypass or intensive lifestyle intervention: a controlled clinical study. Health Qual Life Outcomes. 2013;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dymek MP, Le Grange D, Neven K, Alverdy J. Quality of life after gastric bypass surgery: a cross-sectional study. Obes Res. 2002;10:1135-1142. [DOI] [PubMed] [Google Scholar]
- 61.Callegari A, Michelini I, Sguazzin C, Catona A, Klersy C. Efficacy of the SF-36 questionnaire in identifying obese patients with psychological discomfort. Obes Surg. 2005;15:254-260. [DOI] [PubMed] [Google Scholar]
- 62.Sendi P, Brunotte R, Potoczna N, Branson R, Horber FF. Health-related quality of life in patients with class II and class III obesity. Obes Surg. 2005;15:1070-1076. [DOI] [PubMed] [Google Scholar]
- 63.Kolotkin RL, Crosby RD, Pendleton R, Strong M, Gress RE, Adams T. Health-related quality of life in patients seeking gastric bypass surgery vs non-treatment-seeking controls. Obes Surg. 2003;13:371-377. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Zhao H, Cao Z, Sun X, Zhang C, Cai W, et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in china: a 5-year outcome. Obes Surg 2014. [DOI] [PubMed] [Google Scholar]
- 65.Hall JC, Horne K, O'Brien PE, Watts JM. Patient well-being after gastric bypass surgery for morbid obesity. Aust N Z J Surg. 1983;53:321-324. [DOI] [PubMed] [Google Scholar]
- 66.Aftab H, Risstad H, Sφvik TT, Bernklev T, Hewitt S, Kristinsson JA, et al. Five-year outcome after gastric bypass for morbid obesity in a Norwegian cohort. Surg Obes Relat Dis. 2014;10:71-78. [DOI] [PubMed] [Google Scholar]
- 67.Suter M, Donadini A, Romy S, Demartines N, Giusti V. Laparoscopic Roux-en-Y gastric bypass: significant long-term weight loss, improvement of obesity-related comorbidities and quality of life. Ann Surg. 2011;254:267-273. [DOI] [PubMed] [Google Scholar]
- 68.Suter M, Calmes J-M, Paroz A, Romy S, Giusti V. Results of Roux-en-Y gastric bypass in morbidly obese vs super obese patients: similar body weight loss, correction of comorbidities, and improvement of quality of life. Arch Surg (Chicago, IL: 1960). 2009;144:312-318. discussion 318. [DOI] [PubMed] [Google Scholar]
- 69.Müller MK, Wenger C, Schiesser M, Clavien P-A, Weber M. Quality of life after bariatric surgery – a comparative study of laparoscopic banding vs. bypass. Obes Surg. 2008;18:1551-1557. [DOI] [PubMed] [Google Scholar]
- 70.Sarwer DB, Wadden TA, Moore RH, Eisenberg MH, Raper SE, Williams NN. Changes in quality of life and body image after gastric bypass surgery. Surg Obes Relat Dis. 2010;6:608-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robert M, Denis A, Badol-Van Straaten P, Jaisson-Hot I, Gouillat C. Prospective longitudinal assessment of change in health-related quality of life after adjustable gastric banding. Obes Surg. 2013;23:1564-1570. [DOI] [PubMed] [Google Scholar]
- 72.Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, Lopez-Jimenez F. Effect of bariatric surgery on the metabolic syndrome: a population-based, long-term controlled study. Mayo Clin Proc. 2008;83:897-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edholm D, Svensson F, Näslund I, Karlsson FA, Rask E, Sundbom M. Long-term results 11 years after primary gastric bypass in 384 patients. Surg Obes Relat Dis. 2013;9:708-713. [DOI] [PubMed] [Google Scholar]
- 74.Klingemann J, Pataky Z, Iliescu I, Golay A. Relationship between quality of life and weight loss 1 year after gastric bypass. Dig Surg. 2009;26:430-433. [DOI] [PubMed] [Google Scholar]
- 75.D'Hondt M, Sergeant G, Deylgat B, Devriendt D, Van Rooy F, Vansteenkiste F. Prophylactic cholecystectomy, a mandatory step in morbidly obese patients undergoing laparoscopic Roux-en-Y gastric bypass? J Gastrointest Surg. 2011;15:1532-1536. [DOI] [PubMed] [Google Scholar]
- 76.Leiva MJ, Fuentealba C, Boggiano C, Gattas V, Barrera G, Leiva L, et al. Quality of life of patients subjected to gastric bypass more than one year ago: influence of socioeconomic status. Rev Med Chil. 2009;137:625-633. [PubMed] [Google Scholar]
- 77.Lier HO, Biringer E, Hove O, Stubhaug B, Tangen T. Quality of life among patients undergoing bariatric surgery: associations with mental health – a 1 year follow-up study of bariatric surgery patients. Health Qual Life Outcomes. 2011;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanchez-Santos R, Del Barrio MJ, Gonzalez C, Madico C, Terrado I, Gordillo ML, et al. Long-term health-related quality of life following gastric bypass: influence of depression. Obes Surg. 2006;16:580-585. [DOI] [PubMed] [Google Scholar]
- 79.Lee W-J, Ser K-H, Lee Y-C, Tsou J-J, Chen S-C, Chen J-C. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg. 2012;22:1827-1834. [DOI] [PubMed] [Google Scholar]
- 80.Suter M, Calmes J-M, Paroz A, Giusti V. A new questionnaire for quick assessment of food tolerance after bariatric surgery. Obes Surg. 2007;17:2-8. [DOI] [PubMed] [Google Scholar]
- 81.Schweiger C, Weiss R, Keidar A. Effect of different bariatric operations on food tolerance and quality of eating. Obes Surg. 2010;20:1393-1399. [DOI] [PubMed] [Google Scholar]
- 82.Mohos E, Jano Z, Richter D, Schmaldienst E, Sandor G, Mohos P, et al. Quality of life, weight loss and improvement of co-morbidities after primary and revisional laparoscopic Roux Y gastric bypass procedure-comparative match pair study. Obes Surg 2014. [DOI] [PubMed] [Google Scholar]
- 83.Adams TD, Robert C, Strong MB, Kolotkin R, Walker J, Litwin SE, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring). 2010;18:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Himpens J, Verbrugghe A, Cadière GB, Everaerts W, Greve J-W. Long-term results of laparoscopic Roux-en-Y Gastric bypass: evaluation after 9 years. Obes Surg. 2012;22:1586-1593. [DOI] [PubMed] [Google Scholar]


