Abstract
Background
Monitoring urinary albumin is a useful method in clinical practice for the management of diabetic nephropathy, chronic kidney disease, and hypertension. Currently there are neither standardized methods nor reference material for the determination of urinary albumin; for this reason it is useful to compare different assays used in clinical laboratory.
Objectives
The aim of this study is to verify analytical performance of an immunoturbidimetric assay on Roche Cobas 8000 platform and to compare urinary albumin results with those obtained by immunonephelometry on Siemens Dade Behring BN II Nephelometer.
Results
The method comparison showed a good linear relationship, confirmed by Passing–Bablok and Bland–Altman plots. The turbidimetric assay meets the requirements of accuracy and precision for the practice of medical diagnostics and clinical use.
Conclusions
The present study can contribute to the methods standardization and harmonization of urinary albumin assay.
Keywords: urinary albumin, method evaluation
Introduction
Diabetic nephropathy is the most common cause of end‐stage renal disease. An increased urinary albumin excretion represents a characteristic sign of diabetic kidney damage and is important predictor of future cardiovascular disease and hypertension. Regular screening for albuminuria provides early detection and timely intervention. In overt diabetic nephropathy, quantification of albuminuria helps monitoring disease progression 1. International guidelines suggest the measurement of albumin in the urine annually both in patients with type 1 diabetes with disease duration >5 years, and in all patients with type 2 diabetes 2. For the diagnosis of diabetic nephropathy, given the high biological variability of albuminuria, the determination of this parameter must be positive in at least two of three samples, collected at an interval of 3–6 months. The decision levels for diabetic nephropathy have long been defined and internationally accepted 2. Moreover, the measurement of albumin in the urine is important even to assess renal involvement in other diseases such as rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, and amyloidosis. Microalbuminuria was defined as an albumin excretion in the range of 30–300 mg/day. It is useful to furnish an accurate and precise measure of urinary albumin, despite the fact that standardization of this determination has not yet been reached. Data harmonization and specific measurement procedures are essential to allow the clinical use of the fixed decision points for urinary albumin excretion recommended by clinical practice guidelines for managing chronic kidney disease. The recent report from the National Kidney Disease Education Program (NKDEP) Laboratory Working Group and the International Federation of Clinical Chemistry (IFCC) Work Group for Standardization of Albumin in Urine identified information needed to develop recommendations for standardization 3. Traditionally, urinary albumin is measured using antibody‐based methods, including radioimmunoassay (RIA), enzyme‐linked immunosorbent assay (ELISA), nephelometry, turbidimetry, High Performance Liquid Chromatography (HPLC), and Liquid Chromatography Mass Spectrometry (LC‐MS) 4, 5. For the urinary albumin, an accurate definition is not easily achieved because of the multiple molecular forms (fragments, partial degradation, glycated forms, etc.) of protein found in urine. A few years ago this problem appeared particularly important as it had been suggested that “modified” albumin is not recognized by antisera of immunochemical assays and therefore the common laboratory methods would provide marked underestimation of the concentration of the protein 6. In fact, other studies have been unable to demonstrate that polyclonal antisera are also able to recognize the modified forms of the protein, and therefore the problem has been solved 7. The aim of this study is to verify analytical performance of an immunoturbidimetric assay on Cobas 8000 (Roche Diagnostics, Mannheim, Germany) platform and compare urinary albumin results, in the general population, with those obtained with the conventional immunonephelometry on Dade Behring BN II Nephelometer (Siemens, Munich, Germany).
Material and Methods
Sample Collection
Aliquots of patients’ 24‐hr urine samples (n = 100) were collected from residual samples submitted from routine medical care and stored in plastic tubes at −80°C. The maximum interval from collection to measurement was 5 days. Samples were resumed to room temperature before testing and the comparison test was completed in six nonconsecutive days, covering 2 weeks in total.
Instruments and Assay Methods
In this experiment, we compared two methods for the albumin measurement in urine. The first method (or “reference method”) is immunonephelometric, implemented on Siemens Dade Behring BN II Nephelometer (using N Antiserum to Human Albumin, Siemens) and is currently in use at our laboratory. The second method (from now on referred to as “test method”) is immunoturbidimetric, implemented on Roche Cobas 8000 platform (using Tina‐quant Albumin Gen2, Roche Diagnostics). In the first assay, protein contained in samples forms immune complexes in an immunochemical reaction with specific antibodies; these complexes scatter a beam of light passed through the sample. The intensity of the scattered light is proportional to the concentration of the relevant protein in the sample. In the second method, albumin is detected using antibody directed to human albumin in automated immunoprecipitation analysis that detects turbidity and increased light scatter. Urine samples were centrifuged at 650 × g for 10 min before analysis. The manufacturer claimed the detection limit to be 2 mg/l and the limit of quantification to be 3 mg/l.
Methodological Evaluation
Each analytical session included calibration (two calibrations during 2 weeks), the execution of third part internal quality control (IQC), and the measurement of the urinary albumin on both instruments. The two methods were performed according to the instructions of each manufacturer.
Linearity
Urine sample with high albumin concentration (approximately 717 mg/l) was serially diluted at fixed ratios (1:1; 1:2; 1:4; 1:8; 1:16; 1:32; 1:64) with sterile water to cover the most clinically significant range of concentrations. Serial dilutions were analyzed in duplicate and the theoretical values were calculated from the measured values of the undiluted specimens (NCCLS EP‐6A). Linearity was assessed with calculation of linear regression analysis and Spearman's correlation coefficient.
Recovery
A human urine reference preparation calibrator for automated systems (C.f.a.s.), PUC (Cat. no. 03121305122, reference material CRM2 470), was reconstituted with distilled water and then added in 1:1 ratio to urinary sample with low concentration (19.3 mg/l) and high concentration (147.8 mg/l), carefully mixed and assayed in duplicate on Cobas 8000. The recovery rate was determined according to EP‐15A3 guideline.
Imprecision Studies
For the repeatability of the new method, the rapid protocol scheme 3 × 5 (triple × 5 days) was performed to verify the statement of the manufacturer (CLSI EP‐15). The intra‐assay imprecision was performed on two different control levels (UrichemGol Level I, lot. no. 667 UC and UrichemGol Level II, lot. no. 672 UC, BioDev, Parabiago (Milano) Italia). These materials were reconstituted according to manufacturer's instructions, split and frozen at −20°C until measurement.
Inter‐assay imprecision was evaluated with commercial normal and pathological control, on daily basis. The study was assessed, during 30 days, using different reagent lots and calibrations. The between‐run imprecision was compared with the current analytical quality specifications derived from biological variability 8.
Comparison Study
The comparison study was performed on 100 urine samples displaying a broad range of albuminuria (0.5–206 mg/l). Results of albuminuria on Behring Nephelometer II analyzer were compared with those obtained from the same samples on Cobas 8000. The nonparametric regression of Passing–Bablok and the Bland–Altman difference plots approaches were used to evaluate the comparability and the agreement between microalbuminuria results obtained by nephelometry and turbidimetry. Results are given as a mean percentage bias and 95% confidence intervals.
Statistics
The statistical evaluation was performed with Analyse‐it for Microsoft Excel (Analyse‐it Software Ltd., Leeds, UK). Comparative analysis was performed between the two assays studied by correlation and regression analysis, as well as by difference plots (Bland–Altman plot) combined with bias calculations and 95% confidence intervals.
Results
Linearity showed a strong correlation (r = 1.00). The average percentage recovery was optimal for both calibrator‐spiked (98%) and water‐spiked (96%) samples. The intra‐assay imprecision showed a CV % of 0.87 at low control level (29.9 mg/l) and 0.61% at high control level (174.2 mg/l). The between‐run CV% was 2.7 at level concentration of 33 mg/l and 2.6 at 185 mg/l of concentration, reaching optimum goals for imprecision (Table 1).
Table 1.
Intra‐Assay and Inter‐Assay Imprecision Compared With the Current Analytical Quality Specifications for the CV% Derived From Biological Variability
| Intra‐assay | Inter‐assay | Recovery (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | CV% | Mean | SD | CV% | Observed imprecision performance | Linearity | Calibrator + sample | Water + sample | |
| Low | 30.0 | 0.26 | 0.87 | 33 | 0.89 | 2.7 | Optimum | 1.00 | 98 | 96 |
| High | 174 | 1.06 | 0.61 | 185 | 4.81 | 2.6 | Optimum | |||
The Spearman's correlation coefficient and the percentage of recovery are also shown.
The bold values shown the coefficient of variation is reported as a percentage.
The correlation and regression studies evaluated for method comparison showed a good linear relationship between nephelometry and turbidimetry (R 2 = 0.995). These results were confirmed by Passing–Bablok as shown in Figure 1, with an intercept of −1.873 and a slope of 1.091. Moreover, Figure 2 displays the Bland–Altman plot. The solid line represents the bias between assays (0.98), the dashed lines represent 95% limits of agreement (−6.24 to 8.20), and P‐value (0.02) exhibits statistically significant mean difference.
Figure 1.
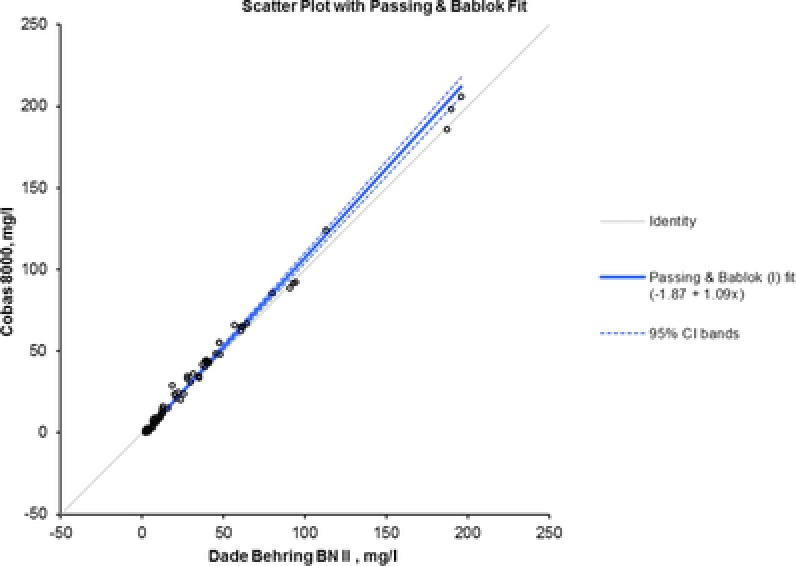
Passing–Bablok regression analysis. It also shows the value of slope (1.09) and intercept (−1.87).
Figure 2.
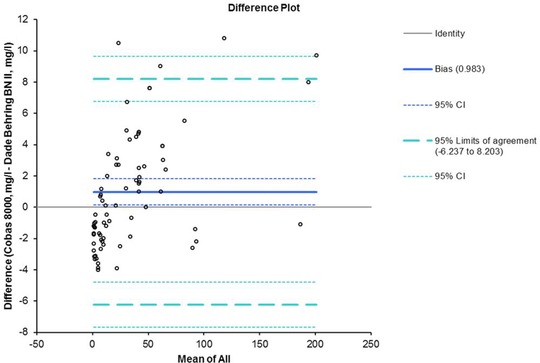
Bland–Altman plot. The solid line represents the bias between assays (0.98), the dashed lines represent 95% limits of agreement (−6.24 to 8.20).
Discussion
Monitoring urinary albumin is the first marker of renal impairment and its determination is a useful method in clinical practice for the management of diabetic nephropathy, chronic kidney disease, and hypertension. Currently there are neither standardized methods nor reference material for the determination of urinary albumin; for this reason, it is useful to compare different assays used in clinical laboratory. The methodology chosen to quantify urinary albumin is important when assessing the presence of nephropathy in diabetic patients because inaccurate measurement could lead to incorrect therapeutic choices 9. The group IFCC/NKDEP has not provided advice about the quality of analytical measurement of albumin in the urine. For immunochemical methods performed in the laboratory, the CV should be <15% and the detection limit should be approximately 2 mg/l 10. Some other indications, based on observations in clinical studies, indeed suggest a more stringent goal for imprecision, which should be within 13% for albumin. Obviously, it is essential that the laboratories adhere to the procedures of good practice, running the International Quality Control (IQC) for each assay and participating regularly in European Quality Assurance (EQA) programs. On the basis of these indications, it was interesting to evaluate the turbidimetric method to determine if these requirements had been met in full. Our results show the full quality of the tested analytical method. The statistical analysis displays a strong linear correlation between two methods. Also, limited bias shown by Bland–Altman plot confirms a good agreement. In addition, the plot shows an absence of systematic error, since the points corresponding to the difference between the two methods accumulate randomly around the zero line. Moreover, our results show that the turbidimetric method is a good alternative to the nephelometric assay because it meets the requirements of accuracy and precision for the practice of medical diagnostics and clinical use. Since 2002, the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines state that the Pr/Cr ratio in an untimed urine specimen should replace protein excretion in a 24‐hr collection as the preferred method for detecting and monitoring proteinuria 11. In this context, it is thus of fundamental importance to dose albumin and creatinine on the same instrument without resorting to nephelometry. The practical advantages of immunoturbidimetric assay include random access analysis instead of batch testing, relatively rapid turnaround time (TAT), high volume testing capability, cost reduction through consolidation of testing on a single platform, elimination of stand‐alone specialized analyzers, and time and effort required to maintain them 12. Collectively, these characteristics of immunoturbidimetric assay have resulted in more reliable tests and reduced laboratory variances to help clinicians. The present study can contribute to the methods standardization and harmonization of urinary albumin assay.
Conflict of Interest
None.
References
- 1. Kistler AD. Albuminuria in the diabetic patient: Practical management. Praxis 2013;102:1229–1235. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes—2010. Diabetes Care 2010;33(S1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller WG, Narva A, Bruns DE, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 2009;55:24–38. [DOI] [PubMed] [Google Scholar]
- 4. Shaikh A, Seegmiller JC, Borland TM, et al. Comparison between immunoturbidimetry, size‐exclusion chromatography, and LC‐MS to quantify urinary albumin. Clin Chem 2008;54:1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinkman JW, Bakker SJ, Gansevoort RT, et al. Which method for quantifying urinary albumin excretion gives what outcome? A comparison of immunonephelometry with HPLC. Kidney Int 2004;92:S69–S75 [DOI] [PubMed] [Google Scholar]
- 6. Osika TM, Comper WD. Characterisation of immunochemically nonreactive urinary albumin. Clin Chem 2004;50:2286–2291. [DOI] [PubMed] [Google Scholar]
- 7. Sviridov D, Drake SK, Hortin GL. Reactivity of urinary albumin (microalbumin) assays with fragmented or modified albumin. Clin Chem 2008;54:61–68. [DOI] [PubMed] [Google Scholar]
- 8. Ricos C, Alvarez V, Cava F, et al. Current databases on biologic variation: Pros, cons and progress. Scand J Clin Lab Invest 1999;59:491–500. (Final version 2014 database.) [DOI] [PubMed] [Google Scholar]
- 9. Liu R, Li G, Xiao‐Fan Cui, et al. Methodological evaluation and comparison of five urinary albumin measurements. J Clin Lab Anal 2011;25(5):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin Biochem Rev 2011;32(2):97–102. [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 2003;139(2):137–147. [DOI] [PubMed] [Google Scholar]
- 12. Mali B, Armbruster D, Serediak E, Ottenbreit T. Comparison of immunoturbidimetric and immunonephelometric assays for specific proteins. Clin Biochem 2009;42:1568–1571. [DOI] [PubMed] [Google Scholar]


