Abstract
Background
High C‐reactive protein (CRP) and mean platelet volume (MPV) levels are associated with poor prognosis in patients with ST‐segment elevation myocardial infarction (STEMI). The aim of this study was to evaluate the relationship between CRP level or MPV and infarct transmurality in patients with STEMI.
Methods
We retrospectively reviewed CRP level, MPV, and infarct transmurality in 112 STEMI patients who were assessed with contrast‐enhanced cardiac magnetic resonance imaging.
Results
When the cut‐off peak CRP level and MPV were set at 2.35 mg/dl and 7.3 fl using receiver operating characteristic curves analysis, the sensitivity was 67.3/69.2% and specificity was 76.7/76.7% for differentiating between the groups with and those without transmural involvement. Peak CRP level, MPV, peak creatine kinase‐MB (CK‐MB) level, and peak high‐sensitivity cardiac troponin T (hs‐cTnT) level had comparable predictive values for transmural involvement (area under the curve, 0.749, 0.761, 0.680, and 0.696, respectively). High peak CRP level and MPV were independent predictors of transmural involvement after adjusting for the peak CK‐MB level, peak hs‐cTnT level, baseline thrombolysis in myocardial infarction flow grade, and left ventricular ejection fraction (odds ratio: 5.16/5.42, 95% confidence interval: 1.84–14.50/2.03–14.47, P = 0.002/0.001, respectively) in the logistic regression analysis.
Conclusion
The results of this study show that peak CRP level and MPV are predictive markers for transmural involvement. Their predictive power for transmural involvement is independent of and comparable to that of peak CK‐MB and hs‐cTnT levels.
Keywords: C‐reactive protein, mean platelet volume, myocardial infarction, magnetic resonance imaging
Introduction
Mean platelet volume (MPV), the most commonly used measure of platelet size, is an indicator of platelet reactivity 1, 2, 3, 4, 5. Recently, a meta‐analysis reported that higher MPV is related to coronary artery disease 6. Moreover, MPV is a predictor of cardiovascular risk, quantified on automated hemograms, and is routinely measured prior to coronary revascularization 2, 5, 7, 8. High preprocedural MPV is associated with increased mortality in patients with ST‐segment elevation myocardial infarction (STEMI) 9.
In acute myocardial infarction (AMI), ischemia‐reperfusion damage and mechanical stress cause acute inflammation 10. Excessive systemic inflammatory activity may hamper tissue repair, enhance apoptosis at the margin of the infarcted tissue, and increase the risk of short‐ or long‐term mortality 11, 12, 13, 14. C‐reactive protein (CRP), a systemic indicator of inflammation, is the established acute‐phase protein that attaches to ligands exposed in injured tissue and stimulates the complement system 15, 16, 17. During AMI, circulating CRP levels rise after the onset of ischemia 18, 19, 20. Peak CRP concentration measured 24–72 hr following symptom onset is considered a significant prognostic indicator of 1‐year outcomes 21, 22.
Contrast‐enhanced cardiac magnetic resonance (CE‐CMR) imaging is gaining approval and being increasingly used for application in quantifying the at‐risk or chronically injured myocardium following STEMI, and can identify and display infarct size and transmurality, myocardial area at risk (AAR), myocardial viability, microvascular obstruction, and myocardial hemorrhage 23, 24, 25, 26, 27, 28, 29. CE‐CMR imaging parameters appear to be related to abnormal electrocardiograms in anterior AMI patients 30.
The predictive values of CRP and MPV for infarct transmurality using CE‐CMR imaging in patients with STEMI have not been studied. The aim of this study was to determine the relationship between peak CRP level/MPV and infarct transmurality using CE‐CMR imaging in STEMI patients.
Materials and Methods
Subjects
A total of 374 consecutive patients with STEMI who underwent measurement of CRP level and MPV between November 2010 and July 2014 were enrolled in this study. Patients were included if they were older than 18 years and had undergone primary percutaneous coronary intervention (PCI) within 12 hr after symptom onset. Patients who did not give consent to undergo CE‐CMR imaging or who had contraindications for CE‐CMR imaging were excluded; 112 patients were finally included. The present study protocol was approved by the Chosun University Hospital Research Ethics Committee (approval CHOSUN 2014‐12‐001).
Definition of STEMI
STEMI was defined as an ST‐segment elevation of at least 1 mm in two or more standard leads, at least 2 mm in two or more neighboring precordial leads, or presumed new‐onset left bundle branch block.
Percutaneous Coronary Intervention
All patients received dual oral antiplatelet treatment (300 mg aspirin, 600 mg clopidogrel) prior to intervention, and maintenance doses of aspirin (100–200 mg daily) and clopidogrel (75 mg daily) thereafter. Coronary angiography and stent implantation were performed by standard interventional techniques. Glycoprotein IIb/IIIa receptor antagonists were administered intravenously as required.
Blood Collection and High‐Sensitivity CRP (hsCRP) Level and MPV Measurement
Venous blood samples were collected in K2‐ethylenediaminetetraacetic acid (EDTA) and serum separator blood‐drawing tubes (Becton Dickinson, Franklin Lakes, NJ) before and 24–72 hr after PCI, and on the day of discharge. hsCRP levels and MPV were analyzed within 2 hr after sample collection using a BN II System (Siemens Healthcare Diagnostics GmbH, Eschborn, Germany) and an ADVIA 2120 Hematology System (Siemens Healthcare Diagnostics GmbH), respectively.
CE‐CMR Imaging Protocol and Analysis
A specified depiction of the CE‐CMR procedure and techniques used for imaging has been stated elsewhere 31 and is outlined below. A complete CE‐CMR study was carried out to assess myocardial infarction and cardiac function. Examinations were performed with a 1.5‐T MR scanner (Avanto, Siemens Medical Solutions, Erlangen, Germany) and a 3.0‐T MR scanner (Magnetom Skyra, Siemens Medical Solutions, Erlangen, Germany) with dedicated cardiac surface coils.
T2‐ and T1‐weighted images were obtained as a stack of contiguous 8‐mm thick images in the cardiac short‐axis view. Cine images were acquired by a fast gradient echo sequence (steady state free precession) in the short‐axis, 2‐chamber, and 4‐chamber views. The slice thickness was set at 8 mm without gaps, and short‐axis images of the left ventricle (LV) were obtained from the apex to the base to contain the whole LV volume. Stress perfusion imaging was performed following scouting and cine imaging. Adenosine (140 μg·kg−1·min−1) was infused for 6 min. Subsequently, a dosage of 0.2 mmol/kg gadolinium‐diethylene triamine pentaacetic acid (Magnevist, Bayer Schering Pharma, Berlin, Germany) was infused intravenously at a speed of 3 ml/s followed by a 20 ml saline flush for 4 min under adenosine infusion. Delayed hyperenhancement and the extent of microvascular obstruction (MVO) were evaluated 5 and 15 min after contrast administration in 10–12 contiguous 8‐mm thick slices with no gap. The field of view and image matrix were 224 × 340 mm (230 × 350 mm in 3T MR) and 256 × 146 (256 × 156 in 3T MR), respectively.
The entire cardiac MR image parameters were determined at our MRI core laboratory. LV end‐diastolic volume (LVEDV), LV end‐systolic volume (LVESV), and ejection fraction (EF) were calculated. Myocardial mass was analyzed by multiplying the myocardial volume by the myocardial density (1.05 g/ml). LV mass was indexed to the body surface area. Infarct volume of the LV was measured based on delayed enhancement. The volume and the extent of MVO, defined as a late hypoenhanced region within the infracted myocardium on the delayed enhancement image, were calculated in the same manner as the infarct volume. The myocardial AAR was defined as myocardium with a signal intensity larger than 2 standard deviations (SDs) above the mean signal intensity of a distant normal myocardium and expressed as a percentage of LV myocardial volume. Myocardial salvage index was calculated as follows: myocardial salvage index = (AAR − infarct size) × 100/AAR. We analyzed infarct transmurality for all segments by dividing the maximal hyperenhanced thickness by the full myocardial thickness in each segment. The transmural extent of infarction was expressed as >75% of infarct transmurality.
Statistical Analysis
All values are expressed as means ± SDs/medians (interquartile ranges [IQRs]) or numbers (percentages). The Mann–Whitney U‐test for continuous variables and chi‐square (statistic) analysis for noncontinuous variables were used to compare baseline characteristics between groups.
Receiver operating characteristic (ROC) curve analysis was performed to determine the sensitivity and specificity with 95% confidence intervals (CIs) of the cutoff hsCRP level and MPV detecting the transmural extent of infarction. In addition, multivariable logistic regression analysis of factors associated with the transmural extent of infarction was performed using a forward conditional stepwise model. Baseline clinical factors with P values < 0.1 on univariate analysis were entered into the model. Independent variables included hsCRP ≥ 2.35 mg/dl, MPV ≥ 7.3 fl, peak CK‐MB level, peak high‐sensitivity cardiac troponin T (hs‐cTnT) level, LVEF, and baseline thrombolysis in myocardial infarction (TIMI) flow grade. Relationships between hsCRP level or MPV and other clinical variables were evaluated using Pearson's correlation analysis. P values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS, version 15 for Windows (SPSS, Inc., Chicago, IL) and MedCalc 14.10.2 (MedCalc Software bvba, Acacialaan, Ostend, Belgium).
Results
Baseline Characteristics of the Cohort
Mean patient age was 59.0 years, and 85.7% patients were men. Other clinical, angiographic, and CE‐CMR imaging characteristics of the overall cohort are shown in Table 1. Mean baseline and peak hsCRP levels for the overall study population were 1.60 ± 2.25 and 3.32 ± 4.43 mg/dl (median, 0.54 and 1.35 mg/dl; IQR, 0.20–2.20 and 0.30–5.07 mg/dl; normal range, 0.0–0.1 mg/dl), respectively. Mean MPV for the overall study population was 7.58 ± 1.16 fl (median, 7.30 fl; IQR, 6.80–8.00; normal range, 7.20–11.1 fl).
Table 1.
Baseline Characteristics, Angiographic Data, and CE‐CMR Imaging Data on the Basis of Peak hsCRP Level
| Characteristic | Total (n = 112) | Peak hsCRP <2.35 mg/dl (n = 62) | Peak hsCRP ≥2.35 mg/dl (n = 50) | P‐value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 59.0 ± 10.4 | 59.4 ± 10.5 | 58.6 ± 10.3 | 0.697 |
| Male sex (%) | 85.7 | 80.6 | 92.0 | 0.088 |
| Hypertension (%) | 41.1 | 46.8 | 34.0 | 0.172 |
| Diabetes mellitus (%) | 17.9 | 16.1 | 20.0 | 0.595 |
| Dyslipidemia (%) | 11.6 | 12.9 | 10.0 | 0.633 |
| Smoking (%) | 72.3 | 67.7 | 78.0 | 0.228 |
| Prior PCI (%) | 5.4 | 4.8 | 6.0 | 0.786 |
| Killip class ≥2 (%) | 51.8 | 45.2 | 60.0 | 0.118 |
| Anterior infarction (%) | 42.9 | 40.3 | 46.0 | 0.546 |
| SBP at admission (mmHg) | 126.1 ± 24.3 | 127.6 ± 23.2 | 124.2 ± 25.7 | 0.455 |
| Initial heart rate(beat/min) | 73.5 ± 17.1 | 70.6 ± 15.6 | 77.2 ± 16.2 | 0.042 |
| Door to balloon time (min) | 79.5 ± 21.3 | 82.1 ± 24.5 | 76.1 ± 16.2 | 0.150 |
| Symptom to balloon time (min) | 264.9 ± 166.7 | 261.5 ± 162.3 | 269.1 ± 173.7 | 0.811 |
| Peak CK‐MB (ng/dl) | 222.1 ± 123.5 | 190.5 ± 115.7 | 261.3 ± 122.6 | 0.002 |
| Peak hs‐cTnT (ng/ml) | 6.39 ± 3.78 | 5.08 ± 3.41 | 8.02 ± 3.60 | <0.001 |
| Creatinine (mg/dl) | 1.00 ± 0.19 | 1.00 ± 0.18 | 1.00 ± 0.20 | 0.907 |
| MPV (fl) | 7.58 ± 1.16 | 7.42 ± 1.00 | 7.78 ± 1.32 | 0.102 |
| Baseline hsCRP (mg/dl) | 1.60 ± 2.25 | 0.48 ± 0.51 | 3.00 ± 2.76 | <0.001 |
| Peak hsCRP (mg/dl) | 3.32 ± 4.43 | 0.54 ± 0.52 | 6.78 ± 4.69 | <0.001 |
| Angiographic data | ||||
| Culprit artery | ||||
| LAD (%) | 42.9 | 40.3 | 46.0 | 0.546 |
| LCx (%) | 14.3 | 14.5 | 14.0 | 0.938 |
| RCA (%) | 42.9 | 45.2 | 40.0 | 0.583 |
| Multivessel disease (%) | 56.3 | 59.7 | 52.0 | 0.416 |
| Baseline TIMI flow grade 0–1 (%) | 79.5 | 69.4 | 92.0 | 0.003 |
| Final TIMI flow grade 3 (%) | 92.0 | 96.8 | 86.0 | 0.037 |
| Angiographic no‐reflow (%) | 2.7 | 0.0 | 6.0 | 0.051 |
| Thrombus aspiration (%) | 23.2 | 21.0 | 26.0 | 0.531 |
| Bare‐metal stents (%) | 24.1 | 21.0 | 28.0 | 0.387 |
| Stent diameter at culprit artery, mm | 3.13 ± 0.59 | 3.11 ± 0.57 | 3.15 ± 0.63 | 0.781 |
| Stent length at culprit artery, mm | 31.5 ± 18.0 | 30.6 ± 18.8 | 32.5 ± 17.2 | 0.599 |
| Glycoprotein IIb/IIIa inhibitor (%) | 57.1 | 50.0 | 66.0 | 0.089 |
| CE‐CMR imaging data | ||||
| LVEDV (ml) | 140.1 ± 32.9 | 132.8 ± 31.1 | 149.1 ± 33.1 | 0.009 |
| LVESV (ml) | 70.4 ± 28.8 | 63.0 ± 24.9 | 79.7 ± 30.9 | 0.002 |
| LV mass index (g/m2) | 89.1 ± 16.2 | 85.9 ± 14.0 | 93.5 ± 17.8 | 0.012 |
| LV EF (%) | 49.8 ± 9.8 | 52.9 ± 9.5 | 46.0 ± 8.9 | <0.001 |
| Infarct size, % of LV | 6.88 ± 5.5 | 5.30 ± 4.5 | 8.83 ± 5.9 | 0.001 |
| AAR, % of LV | 17.4 ± 11.1 | 16.2 ± 11.3 | 18.9 ± 10.9 | 0.201 |
| Myocardial salvage index (%) | 0.58 ± 0.26 | 0.63 ± 0.27 | 0.53 ± 0.24 | 0.048 |
| Hemorrhagic infarction (%) | 0.57 ± 1.27 | 0.44 ± 0.93 | 0.74 ± 1.59 | 0.220 |
| MVO area, % of LV | 0.24 ± 0.55 | 0.16 ± 0.45 | 0.34 ± 0.64 | 0.096 |
| Number of segments with >75% of infarct transmurality | 1.45 ± 1.73 | 0.94 ± 1.41 | 2.10 ± 1.88 | <0.001 |
| Maximal infarct transmurality (%) | 66.0 ± 29.0 | 55.8 ± 27.9 | 78.6 ± 25.3 | <0.001 |
| Frequency of transmural extent of infarction (%) | 46.4 | 27.4 | 70.0 | <0.001 |
a“Smoking” means active smokers as well as ex‐smokers who stopped smoking less than 1 year before enrollment.
hsCRP, high‐sensitivity CRP; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; CK, creatine kinase; hs‐cTnT, high‐sensitivity cardiac troponin T; MPV, mean platelet volume; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction; CE‐CMR, contrast‐enhanced cardiac magnetic resonance; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; MVO, microvascular obstruction.
Cut‐Off hsCRP Level and MPV for Predicting Transmural Extent of Infarction
Z‐statistics for comparisons of individual ROC curves between baseline hsCRP and peak hsCRP levels were significantly different (Fig. 1A). The ROC curve analysis indicated a cut‐off value of 2.35 mg/dl for peak hsCRP level, with 67.3% sensitivity (95% CI: 52.9–79.7) and 76.7% specificity (95% CI: 64.0–86.6; area under the ROC curve [AUC] = 0.749, P < 0.001), for the detection of transmural extent of infarction (Fig. 1B). ROC analysis indicated a cut‐off value of 7.3 fl for MPV with 69.2% sensitivity (95% CI: 54.9–81.3) and 76.7% specificity (95% CI: 64.0–86.6; AUC = 0.761, P < 0.001) for the detection of transmural extent of infarction (Fig. 1B).
Figure 1.
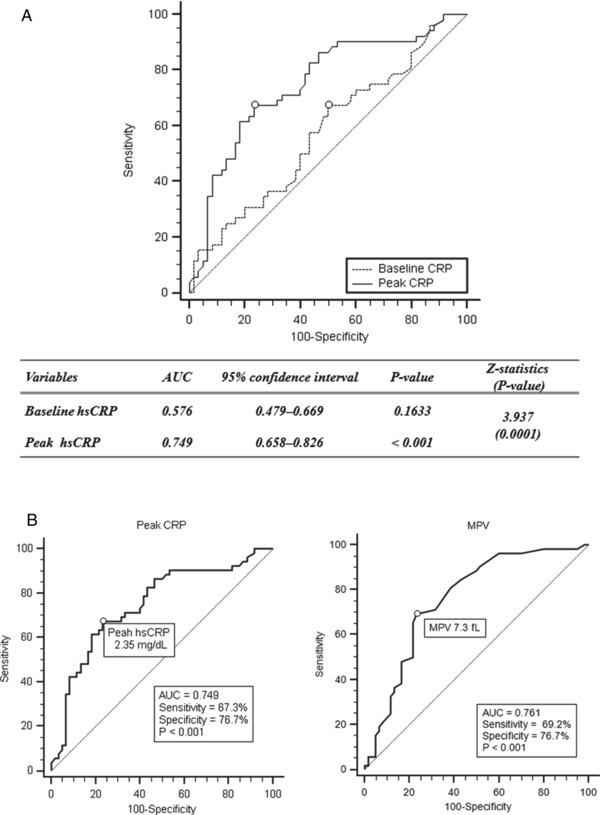
Receiver operating characteristics curves of baseline and peak high‐sensitivity C‐reactive protein (CRP) levels and mean platelet volume (MPV) for predicting transmural infarction. Comparison between baseline CRP and peak CRP levels (A); peak CRP level and MPV, respectively (B).
The mean peak hsCRP and MPV value was higher in patients with transmural extent of infarction (1.86 ± 2.98 vs. 5.01 ± 5.19 mg/dl, P < 0.001; 7.22 ± 1.07 vs. 7.99 ± 1.14, P < 0.001; Fig. 2) than in those without.
Figure 2.
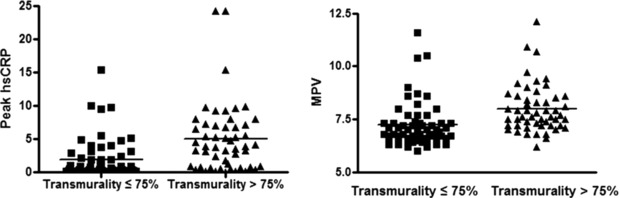
Peak high‐sensitivity CRP level and mean protein volume according to transmural infarction categories.
Clinical, Angiographic, and CE‐CMR Imaging Data Based on Cut‐Off Peak hsCRP Level
Patients were stratified into two groups according to the cut‐off peak hsCRP values: peak hsCRP < 2.35 mg/dl (62 patients) and peak hsCRP ≥ 2.35 mg/dl (50 patients). The clinical, angiographic, and CE‐CMR imaging data of these two groups are shown in Table 1. Although we noted that the proportion of men was higher in the high hsCRP level group than in the low hsCRP level group, these trends were not statistically significant. Initial heart rate and peak CK‐MB and hs‐cTnT levels in patients with high hsCRP levels were higher than those in patients with low hsCRP levels. Otherwise, there were no differences in clinical and demographic characteristics between the two groups. Patients with a high peak hsCRP level tended to develop angiographic no‐reflow phenomenon and use glycoprotein IIb/IIIa inhibitors. A high rate of baseline TIMI flow grade 0–1 and low rate of final TIMI flow grade 3 were associated with high peak hsCRP level. There were no differences in other angiographic or procedural characteristics between the two groups.
CE‐CMR imaging was performed at a median of 41 days after the index event (IQR, 31–52 days). No significant differences were found in the interval from procedure to CE‐CMR imaging between the high peak hsCRP level group (47.6 ± 37.9 days) and the low peak hsCRP level group (53.6 ± 57.2 days, P = 0.529). Figure 3 demonstrates representative coronary angiography before reperfusion and CE‐CMR images of reperfused STEMI. CE‐CMR imaging findings are presented in Table 1. LVEDV, LVESV, LV mass index, infarct size, number of segments with >75% of infarct transmurality, and maximal infarct transmurality were higher in the high peak hsCRP level group than in the low peak hsCRP level group on CE‐CMR imaging. In addition, LVEF and myocardial salvage index were lower and transmural extent of infarction was detected more frequently in the high peak hsCRP level group than in the low peak hsCRP level group. There was a trend of increased MVO area in the high peak hsCRP level group compared with that in the low peak hsCRP level group.
Figure 3.
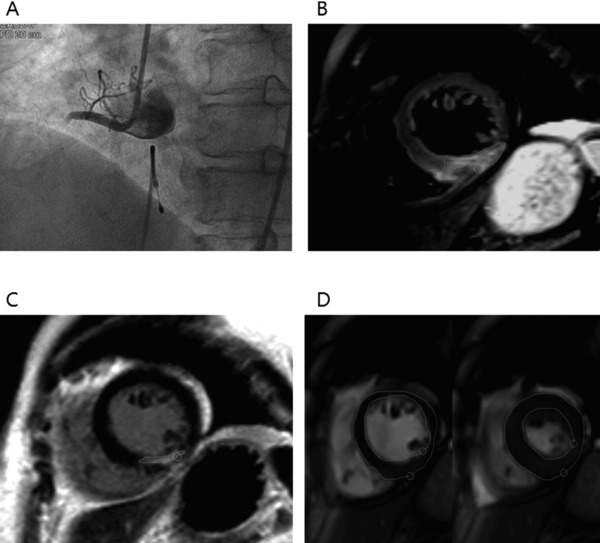
An ST‐elevation myocardial infarction patient with a culprit lesion in the right coronary artery. Coronary angiogram showing total occlusion of the proximal right coronary artery (A). T2‐weighted short‐axis image showing edema in the inferior wall and inferoseptum of the left ventricle (B) and corresponding delayed enhancement (C). Myocardial mass and other functional parameters derived from stacked images measured by endocardial and epicardial contours at diastole (right image in D) and systole (left image in D).
Clinical, Angiographic, and CE‐CMR Imaging Data Based on Cut‐Off MPV
Patients were stratified into two groups according to the cut‐off MPV value: MPV < 7.3 fl (56 patients) and MPV ≥ 7.3 fl (56 patients). The clinical and demographic characteristics of these two groups are shown in Table 2. Although we noted that the prevalence of history of PCI was higher and door‐to‐balloon time was lower in patients with high MPV than in those with low MPV, these trends were not statistically significant. Peak CK‐MB and hs‐cTnT levels were higher in patients with high MPV than in those with low MPV.
Table 2.
Baseline Characteristics, Angiographic Data, and CE‐CMR Imaging Data on the Basis of MPV
| Characteristic | Total (n = 112) | MPV < 7.3 fl (n = 56) | MPV ≥ 7.3 fl (n = 56) | P‐value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 59.0 ± 10.4 | 59.7 ± 10.6 | 58.3 ± 10.2 | 0.498 |
| Male gender (%) | 85.7 | 82.1 | 89.3 | 0.280 |
| Hypertension (%) | 41.1 | 41.1 | 41.1 | 1.000 |
| Diabetes mellitus (%) | 17.9 | 16.1 | 19.6 | 0.622 |
| Dyslipidemia (%) | 11.6 | 12.5 | 10.7 | 0.768 |
| Smoking (%) | 72.3 | 67.9 | 76.8 | 0.291 |
| Prior PCI (%) | 5.4 | 1.8 | 8.9 | 0.093 |
| Killip class ≥2 (%) | 51.8 | 53.6 | 50.0 | 0.705 |
| Anterior infarction (%) | 42.9 | 46.4 | 39.3 | 0.445 |
| SBP at admission (mmHg) | 126.1 ± 24.3 | 128.6 ± 20.7 | 123.5 ± 27.4 | 0.266 |
| Initial heart rate(beat/min) | 73.5 ± 17.1 | 72.3 ± 16.5 | 74.8 ± 17.6 | 0.451 |
| Door to balloon time (min) | 79.5 ± 21.3 | 83.4 ± 26.3 | 75.6 ± 13.9 | 0.051 |
| Symptom to balloon time (min) | 264.9 ± 166.7 | 278.7 ± 172.3 | 251.0 ± 161.4 | 0.381 |
| Peak CK‐MB (ng/dl) | 222.1 ± 123.5 | 193.7 ± 123.5 | 250.6 ± 117.7 | 0.014 |
| Peak hs‐cTnT(ng/ml) | 6.39 ± 3.78 | 5.33 ± 3.34 | 7.45 ± 3.93 | 0.003 |
| Creatinine (mg/dl) | 1.00 ± 0.19 | 1.02 ± 0.18 | 0.97 ± 0.19 | 0.181 |
| MPV (fl) | 7.58 ± 1.16 | 6.77 ± 0.32 | 8.39 ± 1.13 | <0.001 |
| Baseline hsCRP (mg/dl) | 1.60 ± 2.25 | 1.70 ± 2.35 | 1.50 ± 2.17 | 0.647 |
| Peak hsCRP (mg/dl) | 3.32 ± 4.43 | 3.03 ± 4.46 | 3.61 ± 4.41 | 0.488 |
| Angiographic data | ||||
| Culprit artery | ||||
| LAD (%) | 42.9 | 46.4 | 39.3 | 0.445 |
| LCx (%) | 14.3 | 12.5 | 16.1 | 0.589 |
| RCA (%) | 42.9 | 41.1 | 44.6 | 0.703 |
| Multivessel disease (%) | 56.3 | 48.2 | 64.3 | 0.086 |
| Baseline TIMI flow grade 0–1 (%) | 79.5 | 73.2 | 85.7 | 0.102 |
| Final TIMI flow grade 3 (%) | 92.0 | 91.1 | 92.9 | 0.728 |
| Angiographic no‐reflow (%) | 2.7 | 1.8 | 3.6 | 0.558 |
| Thrombus aspiration (%) | 23.2 | 21.4 | 50.0 | 0.654 |
| Bare‐metal stents (%) | 24.1 | 16.1 | 32.1 | 0.047 |
| Stent diameter at culprit artery (mm) | 3.13 ± 0.59 | 3.09 ± 0.55 | 3.17 ± 0.64 | 0.488 |
| Stent length at culprit artery (mm) | 31.5 ± 18.0 | 31.4 ± 17.4 | 31.5 ± 18.9 | 0.988 |
| Glycoprotein IIb/IIIa inhibitor (%) | 57.1 | 46.4 | 67.9 | 0.022 |
| CE‐CMR imaging data | ||||
| LVEDV (ml) | 140.1 ± 32.9 | 136.1 ± 29.6 | 144.1 ± 35.7 | 0.202 |
| LVESV (ml) | 70.4 ± 28.8 | 63.8 ± 18.6 | 77.0 ± 35.2 | 0.015 |
| LV mass (g) | 89.1 ± 16.2 | 86.9±14.2 | 91.6 ± 17.8 | 0.124 |
| LV EF (%) | 49.8 ± 9.8 | 53.0 ± 8.1 | 46.7 ± 10.4 | 0.001 |
| Infarct size, % of LV | 6.88 ± 5.5 | 5.54±4.7 | 8.22 ± 5.9 | 0.009 |
| AAR, % of LV | 17.4 ± 11.1 | 18.2 ± 12.1 | 16.6 ± 10.1 | 0.470 |
| Myocardial salvage index (%) | 0.58 ± 0.26 | 0.66 ± 0.25 | 0.51 ± 0.25 | 0.003 |
| Hemorrhagic infarction (%) | 0.57 ± 1.27 | 0.51 ± 1.38 | 0.64 ± 1.16 | 0.607 |
| MVO area, % of LV | 0.24 ± 0.55 | 0.15 ± 0.40 | 0.34 ± 0.65 | 0.078 |
| Number of segments with >75% of infarct transmurality | 1.45 ± 1.73 | 0.71 ± 1.26 | 2.20 ± 1.82 | <0.001 |
| Maximal infarct transmurality (%) | 66.0 ± 29.0 | 55.9 ± 29.6 | 76.0 ± 24.7 | <0.001 |
| Frequency of transmural extent of infarction (%) | 46.4 | 26.8 | 66.1 | <0.001 |
a“Smoking” means active smokers as well as ex‐smokers who stopped smoking less than 1 year before enrollment.
hsCRP, high sensitivity CRP; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; CK, creatine kinase; hs‐cTnT, high‐sensitivity cardiac troponin T; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction; CE‐CMR, contrast‐enhanced cardiac magnetic resonance; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; MVO, microvascular obstruction.
Patients in the high MPV group had longer intervals from the procedure to CE‐CMR imaging than those in the low MPV group (58.7 ± 62.7 vs. 43.1 ± 29.3 days, P = 0.095). CE‐CMR imaging findings are presented in Table 2. LVESV, infarct size, number of segments with >75% of infarct transmurality, and maximal infarct transmurality were higher in the high MPV group than in the low MPV group on CE‐CMR imaging. In addition, LVEF and myocardial salvage index were lower, and transmural extent of infarction was detected more frequently in the high peak hsCRP level group than in the low peak hsCRP level group. Patients in the high peak hsCRP group had larger MVO area than those in the low peak hsCRP group, and no significant differences were observed in LVEDV, LV mass index, AAR, and hemorrhagic infarction incidence between the two groups.
Comparison of Predictive Values of Peak Cardiac Enzyme Levels, Peak hsCRP Level, and MPV for Transmural Extent of Infarction
ROC curve analysis revealed a cut‐off value of 156 ng/dl for CK‐MB, with 88.5% sensitivity (95% CI: 76.6–95.6) and 45% specificity (95% CI: 32.1–58.4; AUC = 0.680, P = 0.0004), and 4.42 ng/dl for hs‐cTnT, with 88.5% sensitivity (95% CI: 76.6–95.6) and 51.7% specificity (95% CI: 38.4–67.8; AUC = 0.696, P = 0.0001), for transmural extent of infarction detection (Fig. 4). Z‐statistics for comparing individual ROC curves between peak hsCRP level, MPV, and peak cardiac enzyme levels were not significantly different (Fig. 4).
Figure 4.
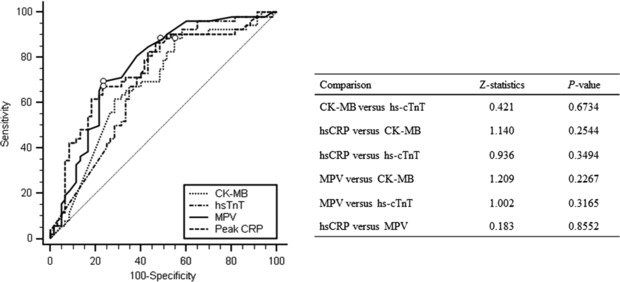
Comparison of receiver operating characteristic curves for peak high‐sensitivity CRP level, MPV, creatine kinase‐MB, and high‐sensitivity cardiac troponin T level for predicting transmural infarction.
Independent Predictors of Transmural Extent of Infarction
Univariate analysis showed that peak hsCRP level (≥2.35 mg/dl), MPV (≥7.3 fl), peak CK‐MB level (≥156 ng/dl), peak hs‐cTnT level (≥4.42 ng/dl), low baseline TIMI flow grade (0 to 1), and low LVEF (<55%) were significantly associated with transmural extent of infarction. Multivariate logistic regression analysis included the significant univariate variables. From this model, the variables found to be independent risk factors of transmural extent of infarction were high peak hsCRP level (≥2.35 mg/dl) and high MPV (≥7.3 fl; Table 3).
Table 3.
Univariate and Multivariate Logistic Regression Analyses Determining the Significant and Independent Predictors for Transmural Extent of Infarction, Respectively
| Factor | Univariate OR (95% CI), P‐value | Multivariate OR (95% CI), P‐value |
|---|---|---|
| Peak hsCRP (≥2.35 mg/dl) | 6.18 (2.71–14.17), <0.001 | 5.16 (1.84–14.50), 0.002 |
| MPV (≥7.3 fl) | 5.32 (2.37–11.96), <0.001 | 5.42 (2.03–14.47), 0.001 |
| Peak CK‐MB (≥156 ng/dl) | 5.86 (2.17–15.81), <0.001 | 2.90 (0.68–12.37), 0.151 |
| Peak hs‐cTnT (≥4.42 ng/dl) | 7.67 (2.85–20.63), <0.001 | 1.57 (0.37–6.73), 0.542 |
| Baseline low TIMI flow grade (0–1) | 4.03 (1.38–11.80), 0.011 | 1.10 (0.25–4.78), 0.899 |
| Low LVEF (<55%) | 6.71 (2.49–18.06), <0.001 | 1.97 (0.56–6.96), 0.294 |
Discussion
To our knowledge, this is the first study to evaluate the relationship between peak hsCRP level/MPV and infarct transmurality. The main finding was that the high peak hsCRP level and MPV were independent predictors of transmural extent of infarction in patients with STEMI. After adjusting for peak CK‐MB level, peak hs‐cTnT level, baseline TIMI flow grade, and LVEF, the odds ratio was significant in the high peak hsCRP level and MPV groups. Furthermore, the predictive power of peak hsCRP level and MPV for transmural involvement was comparable to that of peak CK‐MB and hs‐cTnT levels.
MI extent is the main determinant of mortality risk and probability of heart failure 32, 33. CRP level is elevated in patients with acute coronary syndrome, and is recognized as a significant risk factor for coronary artery disease 22, 34. Moreover, admission CRP level is a significant independent predictor of short‐term (30 days) mortality 35. During AMI, peak CRP concentration measured at 24–72 hr following symptom onset is now recognized as a prognostic indicator of 1‐year outcomes 21, 22. Evidence of the role of CRP in predicting the extent of myocardial tissue damage in AMI has been found in infarction models with endogenous and exogenous augmented CRP levels 36, 37. Recently, some authors have reported that selective CRP apheresis significantly reduces CRP levels and the volume of the infarction zone in a pig model for AMI 38, 39.
Platelets are a major cause of atherosclerotic lesions, plaque destabilization, and atherothrombosis 40, 41, 42, 43. MPV is an easily evaluable parameter for determining platelet reactivity, and is suggested as a risk factor for overall vascular mortality, such as MI 44. Numerous investigations have demonstrated poor prognoses after acute coronary syndrome in patients with high MPV and an association between MPV and sluggish coronary stream and microvascular injury 45, 46, 47.
CE‐CMR imaging is a sensitive and reliable technique for identifying the morphological and functional complications of AMI, and has considerable potential as a method of risk stratification and direct management after AMI. CE‐CMR imaging can discriminate viable myocardium from infarcted myocardium and can establish transmurality precisely after MI. Transmurality of infarction is vital to predicting the progress of LV remodeling, alterations of LV function, and clinical prognoses 27, 48, 49, 50, 51. The majority of these studies using delayed enhanced imaging showed an inverse association between the transmural degree of delayed enhancement (>75%) and improvement of LV regional contractile function and minimal or no improvement in dysfunctional segments 52.
Recently, the relationship between MPV and infarct size (percentage with respect to the total myocardial mass) in STEMI patients was reported 53. There are no reports of a significant association between peak hsCRP level or MPV and infarct transmurality in STEMI patients who underwent primary PCI. To our knowledge, this is the first study to report the significant predictive values of peak hsCRP and MPV as biomarkers for transmural extent of infarction in patients with STEMI.
This study was limited by the relatively small sample size. In addition, it was not a prospective study. Consequently, the outcomes and conclusions are subject to the limitations inherent to this type of investigation.
We cannot exclude the possibility of a selection bias in the present study because of the original inclusion criteria used (STEMI patients in whom hsCRP level, MPV, and infarct transmurality were measured using CE‐CMR imaging).
In conclusion, high peak hsCRP level and MPV were found to be independent predictors of transmural extent of infarction in STEMI patients. This predictive power for transmural involvement was independent of peak CK‐MB level, peak hs‐cTnT level, baseline TIMI flow grade, and LVEF. Moreover, it was comparable to that of peak CK‐MB and hs‐cTnT levels.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014028083 and 2014064216). None of the authors has any conflict of interest to declare.
Grant sponsor: Ministry of Education, Science and Technology; Grant numbers: 2014028083 and 2014064216.
References
- 1. Ha SI, Choi DH, Ki YJ, et al. Stroke prediction using mean platelet volume in patients with atrial fibrillation. Platelets 2011;22(6):408–414. [DOI] [PubMed] [Google Scholar]
- 2. Choi SW, Choi DH, Kim HW, et al. Clinical outcome prediction from mean platelet volume in patients undergoing percutaneous coronary intervention in Korean cohort: Implications of more simple and useful test than platelet function testing. Platelets 2014;25(5):322–327. [DOI] [PubMed] [Google Scholar]
- 3. Han JY, Choi DH, Choi SW, et al. Stroke or coronary artery disease prediction from mean platelet volume in patients with type 2 diabetes mellitus. Platelets 2013;24(5):401–406. [DOI] [PubMed] [Google Scholar]
- 4. Hong SP, Choi DH, Kim HW, et al. Stroke prevention in patients with non‐valvular atrial fibrillation: New insight in selection of rhythm or rate control therapy and impact of mean platelet volume. Curr Pharm Design 2013;19(32):5824–5829. [DOI] [PubMed] [Google Scholar]
- 5. Ki YJ, Park S, Ha SI, Choi DH, Song H. Usefulness of mean platelet volume as a biomarker for long‐term clinical outcomes after percutaneous coronary intervention in Korean cohort: A comparable and additive predictive value to high‐sensitivity cardiac troponin T and N‐terminal pro‐B type natriuretic peptide. Platelets 2014;25(6):427–432. [DOI] [PubMed] [Google Scholar]
- 6. Sansanayudh N, Anothaisintawee T, Muntham D, McEvoy M, Attia J, Thakkinstian A. Mean platelet volume and coronary artery disease: A systematic review and meta‐analysis. Int J Cardiol 2014;175(3):433–440. [DOI] [PubMed] [Google Scholar]
- 7. Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta‐analysis. J Thromb Haemost 2010;8(1):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah B, Oberweis B, Tummala L, et al. Mean platelet volume and long‐term mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol 2013;111(2):185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long‐term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am College Cardiol 2005;46(2):284–290. [DOI] [PubMed] [Google Scholar]
- 10. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002;53(1):31–47. [DOI] [PubMed] [Google Scholar]
- 11. Pietila KO, Harmoinen AP, Jokiniitty J, Pasternack AI. Serum C‐reactive protein concentration in acute myocardial infarction and its relationship to mortality during 24 months of follow‐up in patients under thrombolytic treatment. Eur Heart J 1996;17(9):1345–1349. [DOI] [PubMed] [Google Scholar]
- 12. Elmas E, Popp T, Lang S, et al. Sudden death: Do cytokines and prothrombotic peptides contribute to the occurrence of ventricular fibrillation during acute myocardial infarction? Int J Cardiol 2010;145(1):118–119. [DOI] [PubMed] [Google Scholar]
- 13. Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 2004;94(12):1543–1553. [DOI] [PubMed] [Google Scholar]
- 14. Saraste A, Pulkki K, Kallajoki M, et al. Apoptosis in human acute myocardial infarction. Circulation 1997;95(2):320–323. [DOI] [PubMed] [Google Scholar]
- 15. Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C‐reactive protein and coronary heart disease: A critical review. J Int Med 2008;264(4):295–314. [DOI] [PubMed] [Google Scholar]
- 16. Volanakis JE. Human C‐reactive protein: Expression, structure, and function. Mol Immunol 2001;38(2‐3):189–197. [DOI] [PubMed] [Google Scholar]
- 17. Pepys MB, Hirschfield GM. C‐reactive protein: A critical update. J Clin Invest 2003;111(12):1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bursi F, Weston SA, Killian JM, et al. C‐reactive protein and heart failure after myocardial infarction in the community. Am J Med 2007;120(7):616–622. [DOI] [PubMed] [Google Scholar]
- 19. Kavsak PA, MacRae AR, Newman AM, et al. Elevated C‐reactive protein in acute coronary syndrome presentation is an independent predictor of long‐term mortality and heart failure. Clin Biochem 2007;40(5‐6):326–329. [DOI] [PubMed] [Google Scholar]
- 20. Suleiman M, Khatib R, Agmon Y, et al. Early inflammation and risk of long‐term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C‐reactive protein. J Am Coll Cardiol 2006;47(5):962–968. [DOI] [PubMed] [Google Scholar]
- 21. Dimitrijevic O, Stojcevski BD, Ignjatovic S, Singh NM. Serial measurements of C‐reactive protein after acute myocardial infarction in predicting one‐year outcome. Int Heart J 2006;47(6):833–842. [DOI] [PubMed] [Google Scholar]
- 22. Berton G, Cordiano R, Palmieri R, et al. C‐reactive protein in acute myocardial infarction: Association with heart failure. Am Heart J 2003;145(6):1094‐1101. [DOI] [PubMed] [Google Scholar]
- 23. Wu KC. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson 2012;14(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ugander M, Bagi PS, Oki AJ, et al. Myocardial edema as detected by pre‐contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imag 2012;5(6):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim RJ, Shah DJ. Fundamental concepts in myocardial viability assessment revisited: When knowing how much is “alive” is not enough. Heart 2004;90(2):137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klem I, Kim RJ. Assessment of microvascular injury after acute myocardial infarction: Importance of the area at risk. Nat Clin Pract Cardiovasc Med 2008;5(12):756–757. [DOI] [PubMed] [Google Scholar]
- 27. Choi KM, Kim RJ, Gubernikoff G, et al. Transmural extent of acute myocardial infarction predicts long‐term improvement in contractile function. Circulation 2001;104(10):1101–1107. [DOI] [PubMed] [Google Scholar]
- 28. Friedrich MG, Abdel‐Aty H, Taylor A, et al. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51(16):1581–1587. [DOI] [PubMed] [Google Scholar]
- 29. Wright J, Adriaenssens T, Dymarkowski S, Desmet W, Bogaert J. Quantification of myocardial area at risk with T2‐weighted CMR: Comparison with contrast‐enhanced CMR and coronary angiography. JACC Cardiovasc Imag 2009;2(7):825–831. [DOI] [PubMed] [Google Scholar]
- 30. Yang HS, Lee CW, Hong MK, et al. Terminal QRS complex distortion on the admission electrocardiogram in anterior acute myocardial infarction and association with residual flow and infarct size after primary angioplasty. Korean J Intern Med 2005;20(1):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim DH, Choi DH, Kim HW, et al. Prediction of infarct severity from triiodothyronine levels in patients with ST‐elevation myocardial infarction. Korean J Intern Med 2014;29(4):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res 2005;66(1):22‐32. [DOI] [PubMed] [Google Scholar]
- 33. Takemura G, Nakagawa M, Kanamori H, Minatoguchi S, Fujiwara H. Benefits of reperfusion beyond infarct size limitation. Cardiovasc Res 2009;83(2):269–276. [DOI] [PubMed] [Google Scholar]
- 34. Brunetti ND, Correale M, Pellegrino PL, Cuculo A, Biase MD. Acute phase proteins in patients with acute coronary syndrome: Correlations with diagnosis, clinical features, and angiographic findings. Eur J Intern Med 2007;18(2):109–117. [DOI] [PubMed] [Google Scholar]
- 35. Vrsalovic M, Pintaric H, Babic Z, et al. Impact of admission anemia, C‐reactive protein and mean platelet volume on short term mortality in patients with acute ST‐elevation myocardial infarction treated with primary angioplasty. Clin Biochem 2012;45(16–17):1506–1509. [DOI] [PubMed] [Google Scholar]
- 36. Barrett TD, Hennan JK, Marks RM, Lucchesi BR. C‐reactive‐protein‐associated increase in myocardial infarct size after ischemia/reperfusion. J Pharmacol Exp Ther 2002;303(3):1007–1013. [DOI] [PubMed] [Google Scholar]
- 37. Griselli M, Herbert J, Hutchinson WL, et al. C‐reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med 1999;190(12):1733‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheriff A, Schindler R, Vogt B, et al. Selective apheresis of C‐reactive protein: A new therapeutic option in myocardial infarction? J Clin Apher 2015;30(1):15–21. [DOI] [PubMed] [Google Scholar]
- 39. Slagman AC, Bock C, Abdel‐Aty H, et al. Specific removal of C‐reactive protein by apheresis in a porcine cardiac infarction model. Blood Purif 2011;31(1‐3):9–17. [DOI] [PubMed] [Google Scholar]
- 40. Davi G, Patrono C. Platelet activation and atherothrombosis. N Eng J Med 2007;357(24):2482–2494. [DOI] [PubMed] [Google Scholar]
- 41. Pratico D, Tillmann C, Zhang ZB, Li H, FitzGerald GA. Acceleration of atherogenesis by COX‐1‐dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc Natl Acad Sci USA 2001;98(6):3358–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002;8(11):1227–1234. [DOI] [PubMed] [Google Scholar]
- 43. Davi G, Gresele P, Violi F, et al. Diabetes mellitus, hypercholesterolemia, and hypertension but not vascular disease per se are associated with persistent platelet activation in vivo. Evidence derived from the study of peripheral arterial disease. Circulation 1997;96(1):69–75. [DOI] [PubMed] [Google Scholar]
- 44. Slavka G, Perkmann T, Haslacher H, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol 2011;31(5):1215–1218. [DOI] [PubMed] [Google Scholar]
- 45. Sezer M, Okcular I, Goren T, et al. Association of haematological indices with the degree of microvascular injury in patients with acute anterior wall myocardial infarction treated with primary percutaneous coronary intervention. Heart 2007;93(3):313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sen N, Basar N, Maden O, et al. Increased mean platelet volume in patients with slow coronary flow. Platelets 2009;20(1):23–28. [DOI] [PubMed] [Google Scholar]
- 47. Celik T, Yuksel UC, Bugan B, et al. Increased platelet activation in patients with slow coronary flow. J Thromb Thrombolysis 2010;29(3):310–315. [DOI] [PubMed] [Google Scholar]
- 48. Tarantini G, Razzolini R, Cacciavillani L, et al. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol 2006;98(8):1033–1040. [DOI] [PubMed] [Google Scholar]
- 49. Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast‐enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation 2002;106(9):1083–1089. [DOI] [PubMed] [Google Scholar]
- 50. Baks T, van Geuns RJ, Biagini E, et al. Recovery of left ventricular function after primary angioplasty for acute myocardial infarction. Eur Heart J 2005;26(11):1070–1077. [DOI] [PubMed] [Google Scholar]
- 51. Hombach V, Grebe O, Merkle N, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J 2005;26(6):549–557. [DOI] [PubMed] [Google Scholar]
- 52. Kim RJ, Wu E, Rafael A, et al. The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343(20):1445–1453. [DOI] [PubMed] [Google Scholar]
- 53. Fabregat‐Andres O, Cubillos A, Ferrando‐Beltran M, et al. Mean platelet volume is associated with infarct size and microvascular obstruction estimated by cardiac magnetic resonance in ST segment elevation myocardial infarction. Blood Coagul Fibrinolysis. 2013;24(4):424–427. [DOI] [PubMed] [Google Scholar]


