Abstract
Background
The most commonly used method of polyethylene glycol (PEG) precipitation for macroprolactinemia (MP) screening has some significant drawbacks. The aim of this study was to establish a new method using PEG for precipitation of macroprolactin (macroPRL) to detect genuine hyperprolactinemia (genuine HP).
Methods
The optimal PEG concentration for precipitation and the effect of PEG on the precipitation of PRL were analyzed to establish and optimize our PEG precipitation method. The PRL recovery rate and genuine HP detection rate were compared between our method and MP screening method.
Results
About 25% PEG6000 was determined to be the optimal PEG concentration for precipitation. Along with an increase in protein concentration in the PRL calibration solution, the PRL recovery rate after precipitation decreased gradually. The PRL recovery rate increased when the precipitation was carried out with diluted PRL calibration solution; the recovery rate reached greater than 90% after a 5‐fold dilution of the calibration solution. The genuine HP detection rate and PRL recovery rate using our diluted serum PEG precipitation method were significantly higher than those obtained with the MP screening method. Our method successfully detected 31 cases of genuine HP, which was significantly higher than the detection rate obtained using the MP screening method (25 cases; P < 0.001).
Conclusion
Precipitation using 5‐fold diluted serum with 25% PEG6000 can effectively reduce the macroPRL concentration, increasing the PRL recovery rate and detection rate of genuine HP after precipitation, which is an effective and convenient method for the detection of genuine HP.
Keywords: hyperprolactinemia, macroprolactin, macroprolactinemia, polyethylene glycol, prolactin
Introduction
There are three molecular forms of prolactin (PRL) presented in circulating human blood, which are monoPRL with a molecular weight around 23 kD (also called free PRL), bigPRL with a molecular weight of 40–60 kD, and macroPRL with a molecular weight greater than 100kD 1. MacroPRL is a complex formed by PRL and its antibody 2, 3. Although macroPRL is believed to be biologically inactive due to its large molecular weight and steric constrains 4, 5, it impacts the immunological detection of PRL 6. Macroprolactinemia (MP) associated with the accumulation of macroPRL is generally false hyperprolactinemia (HP) 7, which can lead to improper examination and drug treatment 8, 9. PEG precipitation is the most commonly used method for MP screening in clinical laboratories, which uses the PRL recovery rate (following serum PEG precipitation) as an indicator to estimate the content of macroPRL in the serum as a means to confirm the diagnosis of MP, providing corresponding reports based on measured results 10. This method has some significant drawbacks, including an unclear determination of MP and a low concentration of active PRL (micoPRL + bigPRL) 11, 12. To improve the precipitation efficacy of smaller active PRLs, this study explored a new method using PEG6000 to effectively precipitate macroPRL and consequently increase the detection rate of genuine HP.
Materials and methods
Materials
Sample source
All 72 patients with elevated serum PRL levels (>25.0 ng/ml) 13 were admitted into our hospital, with an age range 11–55 years and a median age of 28 years.
Instruments and reagents for detection
An I2000sr chemical luminescence analyzer and the supporting PRL detection reagent box (lot: 35917UI00) were obtained from Abbott (Longford, Ireland). A LX20 biochemical analyzer and the supporting total protein detection reagent box (lot: 4962) were obtained from Beckman (Brea, CA). PEG6000 was obtained from Sigma (St. Louis, MO), which was prepared as a 15%, 20%, 25%, and 30% PEG working solution in 0.9% sodium chloride (NaCl). PRL calibration solution was obtained from Roche (Basel, Switzerland) (lot: 175007‐01, total protein: 60 g/l, containing specific concentrations of PRL). Protein calibration solution CAL 1 was a special protein calibration solution obtained from Beckman (lot: M305372, total protein: 105 g/l, containing an extremely low concentration of PRL).
Methods
Establishment of gold standard for MP
The PRLs in the 32 samples were separated using Sephacryl sephacryls‐100 hr column chromatography (GE Healthcare, Pittsburgh, PA) combined with an Akata explore protein purification instrument (GE Healthcare), and the eluents were collected using a Frac‐900 collector (1.2 ml/tube). The PRL content in the eluents was detected by an i2000sr analyzer. MacroPRL accounting for 50% or more of the total PRL content was used as the gold standard for MP determination 14.
Establishment of MP screening standard by PEG precipitation
One eluent sample with an obvious macroPRL composition and one with macroPRL + bigPRL were randomly selected. A 200 μl eluent collected from each sample tube was precipitated with different concentrations of PEG, according to the method previously described 15. The PRL content of the supernatant was then measured by the i2000sr to determine the most suitable concentration of PEG for later experiments. The same 32 serum samples were subjected to PEG precipitation. The PRL recovery rate (<50%) after PEG precipitation was used as the standard for MP screening.
Effects of increased protein concentration on PEG precipitation of PRL
We randomly selected one portion of PRL calibration solution, and each one of eluents containing monoPRL, bigPRL, and macroPRL, respectively. A different ratio of 0.9% NaCl and calibration protein solution CAL 1 was added to these solutions, which were subjected to PEG precipitation. The PRL levels in the supernatant were measured by i2000sr.
Selection of the optimal dilution factor
One portion of PRL calibration solution and two portions of calibration protein solution were mixed to prepare a mixture with a protein content of 90.0 g/l, which was then diluted with 0.9% NaCl by a factor of 1, 3, 5, 10, or 15. The PRL contents in the supernatant of these solutions after PEG precipitation were then measured using an i2000sr. Once the PRL recovery rate was calculated, the appropriate dilution factor was selected for clinical serum samples.
Comparison of serum dilution method and MP screening method
For the diluted serum method, serum samples were subjected to PEG precipitation after dilution, with concentrations of active PRL calculated as 2 × PRL levels in the supernatant × the corresponding dilution factor. Another 40 cases of serum samples with elevated PRL levels were subjected to the diluted serum method and the MP screening method simultaneously.
Statistical Analysis
The serum PRL concentrations followed a non‐normal distribution, which are presented as median and quartile [M (QR)]. Comparisons of MP detection rates was conducted with a chi square (χ2) test, and the PRL concentrations detected by different methods were analyzed using rank sum test (Wilcoxon test) for paired data. SPSS 16.0 statistical software (SPSS Inc., Chicago, IL) was used, with P < 0.05 considered statistically significant.
Results
As demonstrated in Figure 1a, 15% PEG failed to precipitate macroPRL. The precipitation rate of macroPRL by 20%, 25%, and 30% PEG6000 was 42.0%, 77.5%, and 78.4%, respectively. There was no significant difference between the precipitation rates of macroPRL using 25% or 30% PEG6000 (P = 0.374). As shown in Figure 1b, both 25% and 30% PEG6000 failed to effectively precipitate monoPRL or bigPRL. Therefore, 25% PEG6000 was selected as the most suitable concentration for PEG precipitation.
Figure 1.
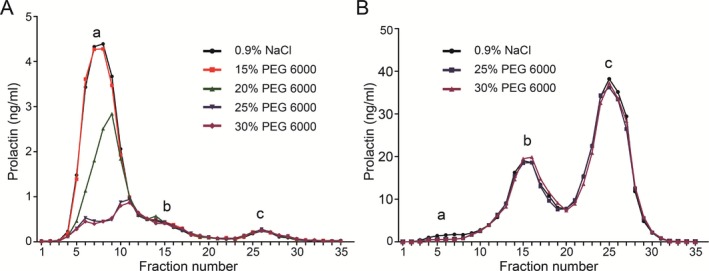
Precipitation of PRL in the eluents using different concentrations of PEG6000.
Figure 2 was plotted using the percentage of macroPRL within the total PRL after gel chromatography (>50% as the gold standard for MP determination) and the PRL recovery rate after 25% PEG6000 precipitation. As shown in Figure 2, the MP screening method and gel chromatography method showed a high coincidence rate (90.6%).
Figure 2.
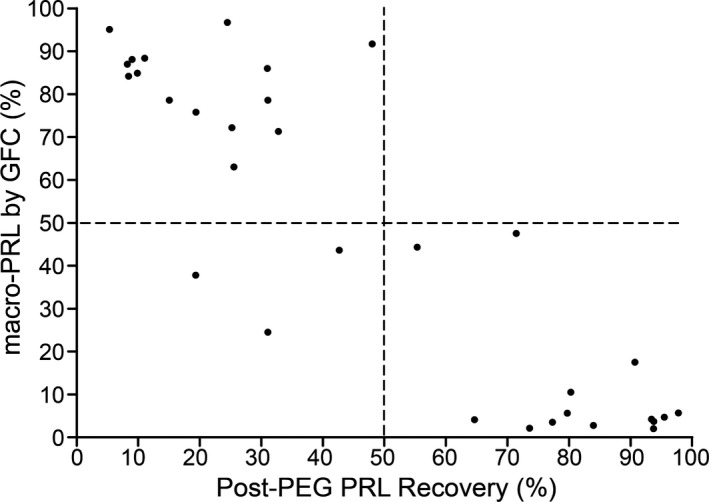
Establishment of the MP screening standard by PEG precipitation.
With the increase in total protein concentration in the PRL calibration solution, the PRL content in the supernatant after PEG precipitation decreased gradually (Fig. 3a). At the same time, with the increase in protein added to the eluents containing monoPRL, bigPRL, and macroPRL, the contents of each PRL decreased after PEG precipitation (Fig. 3b).
Figure 3.
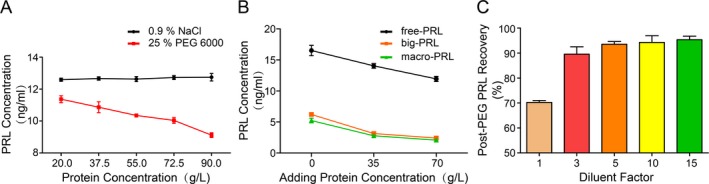
Effects of protein concentration on PEG‐based precipitation of PRL.
PEG precipitation was conducted after diluting the PRL calibration solution with different proportions of 0.9% NaCl. The observed PRL recovery rate gradually increased with increasing dilution factor. When the calibration solution was diluted greater than 5‐fold with a corresponding serum protein concentration below 18.0 g/l, the PRL recovery rate reached 93.5% (5‐fold), 94.3% (10‐fold), and 95.3% (15‐fold), respectively (Fig. 3c). Therefore, a 5‐fold dilution was chosen as the optimal dilution factor.
Among the 40 patients with elevated serum PRL levels, the MP screening method diagnosed MP in 15 cases and genuine HP in 25 cases. When the serum dilution method was used, the PRL recovery rate of genuine HP and MP patients was 101.3 (94.7–105.8)% and 43 (32.6–53.4)%, respectively. These values were significantly higher than those obtained by the MP screening method. Compared with the MP screening method, the serum dilution method significantly increased the reported PRL levels of MP patients. However, there were no effects on the reported PRL levels in genuine HP patients (P > 0.05). These results are listed in Table 1.
Table 1.
Comparison of the Effects of the Serum Dilution Method and the MP Screening Method on the Recovery Rate and Reported Concentration of PRL
| MP group | Genuine HP group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Before precipitation PRL (ng/ml) | After precipitation PRL (ng/ml) | Recovery rate of PRL (%) | Reported concentration of PRL (ng/ml) | n | Before precipitation PRL (ng/ml) | After precipitation PRL (ng/ml) | Recovery rate of PRL (%) | Reported concentration of PRL (ng/ml) | |
| Serum dilution method | 15 | 64.0(46.1˜81.1) | 26.0(21.4˜31.8) | 43.0(32.6˜53.4) | 26.0(21.4˜31.8) | 25 | 33.0(30.4˜39.7) | 33.6(30.6˜40.4) | 101.3(94.7˜105.8) | 33.6(30.6˜40.4) |
| MP screening method | 15 | 64.0(46.1˜81.1) | 11.7(10.7˜15.9) | 18.9(13.7˜34.2) | 11.7(10.7˜15.9) | 25 | 33.0(30.4˜39.7) | 28.1(24.7˜34.6) | 83.4(78.1˜91.3) | 33.0(30.4˜39.7) |
| z | −3.408 | −3.408 | −3.408 | −4.372 | −4.372 | −0.377 | ||||
| P | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.706 | ||||
Among the 40 patients with elevated PRL levels, the serum dilution method detected 31 cases of genuine HP, which was significantly higher than the detection rate for the MP screening method (25 cases, P < 0.01). There were a total of 22 patients with symptoms related to genuine HP (17 cases of menstrual disorders, 2 cases of infertility, 2 cases of menopause, and 1 case of intracranial space occupying lesion). Our serum dilution method detected 18 cases of genuine HP with a sensitivity of 81.8%, which was significantly higher than that of the MP screening method (59.1%; P < 0.05).
Discussion
As early as 1994, Hattori et al. developed a radioimmunoassay to detect PRL autoantibodies 16, based on the principle that immune complexes could be precipitated by PEG. However, no detailed reports have been published regarding PEG precipitation of PRLs. Using different concentrations of PEG6000 to precipitate PRLs, we found that 25% PEG6000 can precipitate most macroPRL, with a precipitation rate of ~77.5%. Meanwhile, this PEG concentration did not effectively precipitate monoPRL or bigPRL. Furthermore, after testing, we found that the total protein content in the eluents was very low (less than 2.0 g/l), indicating that, in solutions with low protein concentration, 25% PEG6000 may selectively precipitate PRL based on molecular weight. When we added additional protein to the eluent solution containing monoPRL, bigPRL, and macroPRL, the PRL contents detected in the supernatant decreased with increasing amounts of added protein (Fig. 3b). Consistent results were obtained when different amounts of protein were added to the PRL calibration solution (containing monoPRL, calibrated with W.H.O third international standard 84/500). When the concentration of total protein increased from 55.0 to 90.0 g/l, the recovery rate of monoPRL decreased from 81.9 to 71.3% (Fig. 3a), with nearly 20–30% of monoPRL being non‐specifically co‐precipitated. Therefore, PEG precipitation of PRL from serum samples with a rich protein content is likely the result of both the molecular weight selectivity and non‐specific co‐precipitation.
In addition to the effect of total serum protein on non‐specific precipitation, the ratio of globulin in serum protein is also one of the most important factors that influence co‐precipitation 17. In addition, the molecular weight of macroPRL varies 18. Due to all of these factors, the amount of active PRL after PEG precipitation cannot be calculated simply as the PRL content in the supernatant multiplied by the dilution factor. With the MP screening method, a high recovery rate of PRL after precipitation suggests that monoPRL is the most abundant PRL in the sample, whereas a low recovery rate suggests that macroPRL is the most abundant PRL; a recovery rate of <40% for MP and >60% for genuine HP are therefore suitable selection criteria 10. However, we believe that there are some significant drawbacks with this method. First, the gel chromatography method is very time consuming, expensive, and the “gray zone” between the two recovery rates of 40–60% is difficult to be confirmed in clinical laboratory where a single cut‐off value is usually used as the screening standard 19, 20, 21, 22. Therefore, a screening standard of a recovery rate <50% after precipitation was selected in this study, similar to the study by Fahie‐Wilson et al. 23, 24. Among the 32 samples used for the determination of screening standard, three cases (9.4%) demonstrated a recovery rate in this “gray zone” (42.7%, 48.0%, and 55.3%), with one genuine HP sample (macroPRL composition accounted for 43.6%) misclassified as MP (recovery rate was 42.7%). Secondly, because of the effect of co‐precipitation, the predetermined content of monoPRL significantly decreased after PEG precipitation, whereas our experimental data (Fig. 3a, b) were consistent with related literature reports 12, 17. Although it has been suggested that this influence may be eliminated by building another PRL reference interval after precipitation 25, 26, such an approach would still bring undesired complexity into the interpretation of PRL laboratory reports.
After realizing that co‐precipitation occurred in high protein solution but not low protein solution, we believe it is feasible to effectively precipitate macroPRL meanwhile retain active PRL by lowering the serum protein content (to a midpoint) for PEG precipitation. In this study, we showed that PEG precipitation (after dilution of the high protein PRL calibration solution) significantly increased the PRL recovery rate (as shown in Fig. 3c), proving that this approach is both feasible and effective. At the same time, a 5‐fold dilution was determined to be the optimal dilution factor in this study. As shown in Figure 2, the MP screening method had a high coincidence rate with the gold standard method of gel chromatography for macroPRL determination (90.6%). It is believed that post‐diluted serum PEG method would have a higher coincidence with gel chromatography method than that of MP screening method. When comparing this method with the MP screening method, the serum dilution method not only eliminated the process of MP determination but also improved the recovery rate of PRL after precipitation, which had no effect on the reported PRL levels in genuine HP cases, but significantly increased the reported PRL concentration in patients with MP, thereby increasing the detection rate of and sensitivity for genuine HP.
Conclusion
Precipitation of PRL using 25% PEG6000 can filter PRLs based on molecular weight selectivity, while avoiding non‐specific co‐precipitation. A 25% PEG solution in 5‐fold diluted serum can effectively remove macroPRL, improving the detection rate of and sensitivity for genuine HP.
Author contributions
Conceived and designed the experiments: Yongjian Chen and Guangzhong Song. Performed the experiments: Huan Wang, Wei Yang, Weidong Jin, Wenge Yu, and Wei Wang. Analyzed the data: Kailin Zhang.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81301406). The authors are grateful to the subjects who participated in this study. The authors would like to thank Duoease Scientific Center for excellent language editing service and suggestions for figure revision.
References
- 1. Fahie‐Wilson MN, John R, Ellis AR. Macroprolactin; high molecular mass forms of circulating prolactin. Ann Clin Biochem 2005;42:175–192. [DOI] [PubMed] [Google Scholar]
- 2. Hattori N, Inagaki C. Anti‐prolactin (PRL) autoantibodies cause asymptomatic hyperprolactinemia: Bioassay and clearance studies of PRL‐immunoglobulin G complex. J Clin Endocrinol Metab 1997;82:3107–3110. [DOI] [PubMed] [Google Scholar]
- 3. Hattori N, Ishihara T, Saiki Y, et al. Macroprolactinaemia in patients with hyperprolactinaemia: Composition of macroprolactin and stability during long‐term followup. Clin Endocrinol 2010;73:792–797. [DOI] [PubMed] [Google Scholar]
- 4. Teilum K, Hoch JC, Goffin V, et al. Solution structure of human prolactin. J Mol Biol 2005;351:810–823. [DOI] [PubMed] [Google Scholar]
- 5. Hattori N, Nakayama Y, Kitagawa K, et al. Anti‐prolactin (PRL) autoantibody‐binding sites (epitopes) on PRL molecule in macroprolactinemia. J Endocrinol 2006;190:287–293. [DOI] [PubMed] [Google Scholar]
- 6. Smith TP, Suliman AM, Fahie‐Wilson MN, et al. Gross variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. J Clin Endocrinol Metab 2002;87:5410–5415. [DOI] [PubMed] [Google Scholar]
- 7. Hattori N, Nakayama Y, Kitagawa K, et al. Development of anti‐PRL (prolactin) autoantibodies by homologous PRL in rats: A model for macroprolactinemia. Endocrinology 2007;148:2465–2470. [DOI] [PubMed] [Google Scholar]
- 8. Suliman AM, Smith TP, Gibney J, et al. Frequent misdiagnosis and mismanagement of hyperprolactinemic patients before the introduction of macroprolactin screening: Application of a new strict laboratory definition of macroprolactinemia. Clin Chem 2003;49:1504–1509. [DOI] [PubMed] [Google Scholar]
- 9. Vanbesien J, Schiettecatte J, Anckaert E, et al. Circulating anti‐prolactin auto‐antibodies must be considered in the differential diagnosis of hyperprolactinaemia in adolescents. Eur J Pediatr 2002;161:373–376. [DOI] [PubMed] [Google Scholar]
- 10. Hattori N, Shimatsu A. Macroprolactinemia: Diagnostic, clinical, and pathogenic significance. Clin Dev Immunol 2012;2012:167132–167138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McKenna TJ. Should macroprolactin be measured in all hyperprolactinaemic sera? Clin Endocrinol (Oxf) 2009;71:466–469. [DOI] [PubMed] [Google Scholar]
- 12. Kavanagh L, McKenna TJ, Fahie‐Wilson MN, et al. Specificity and clinical utility of methods for the detection of macroprolactin. Clin Chem 2006;52:1366–1372. [DOI] [PubMed] [Google Scholar]
- 13. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:273–288. [DOI] [PubMed] [Google Scholar]
- 14. McCudden CR, Sharpless JL, Grenache DG. Comparison of multiple methods for identification of hyperprolactinemia in the presence of macroprolactin. Clin Chim Acta 2010;411:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibney J, Smith TP, McKenna TJ. The impact on clinical practice of routine screening for macroprolactin. J Clin Endocrinol Metab 2005;90:3927–3932. [DOI] [PubMed] [Google Scholar]
- 16. Hattori N, Ikekubo K, Ishihara T, et al. Correlation of the antibody titers with serum prolactin levels and their clinical course in patients with anti‐prolactin autoantibody. Eur J Endocrinol 1994;130:438–445. [DOI] [PubMed] [Google Scholar]
- 17. Ram S, Harris B, Fernando JJ, et al. False‐positive polyethylene glycol precipitation tests for macroprolactin due to increased serum globulins. Ann Clin Biochem 2008;45(Pt 3):256–259. [DOI] [PubMed] [Google Scholar]
- 18. Diver MJ, Ewins DL, Worth RC, et al. An unusual form of big, big (macro) prolactin in a pregnant patient. Clin Chem 2001;47:346–348. [PubMed] [Google Scholar]
- 19. Sari F, Sari R, Ozdem S, et al. Serum prolactin and macroprolactin levels in diabetic nephropathy. Clin Nephrol 2012;78:33–39. [DOI] [PubMed] [Google Scholar]
- 20. Strachan MW, Teoh WL, Don‐Wauchope AC, et al. Clinical and radiological features of patients with macroprolactinaemia. Clin Endocrinol (Oxf) 2003;59:339–346. [DOI] [PubMed] [Google Scholar]
- 21. Donadio F, Barbieri A, Angioni R, et al. Patients with macroprolactinaemia: Clinical and radiological features. Eur J Clin Invest 2007;37:552–557. [DOI] [PubMed] [Google Scholar]
- 22. Jamaluddin FA, Sthaneshwar P, Hussein Z, et al. Importance of screening for macroprolactin in all hyperprolactinaemic sera. Malays J Pathol 2013;35:59–63. [PubMed] [Google Scholar]
- 23. Fahie‐Wilson MN. Polyethylene glycol precipitation as a screening method for macroprolactinemia. Clin Chem 1999;45:436–437. [PubMed] [Google Scholar]
- 24. De Schepper J, Schiettecatte J, Velkeniers B, et al. Clinical and biological characterization of macroprolactinemia with and without prolactin‐IgG complexes. Eur J Endocrinol 2003;149:201–207. [DOI] [PubMed] [Google Scholar]
- 25. Beltran L, Fahie‐Wilson MN, McKenna TJ, et al. Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: Evaluation and validation on common immunoassay platforms. Clin Chem 2008;54:1673–1681. [DOI] [PubMed] [Google Scholar]
- 26. Beda‐Maluga K, Pisarek H, Komorowski J, et al. Evaluation of hyperprolactinaemia with the use of the intervals for prolactin after macroforms separation. J Physiol Pharmacol 2014;65:359–364. [PubMed] [Google Scholar]


