Abstract
Background
Psoriasis is a chronic immune‐mediated inflammatory skin disease associated with increase of some pro‐inflammatory mediators. We wanted to investigate whether there is a relationship between psoriasis and visfatin, fetuin‐A and pentraxin 3 (PTX3)—pro‐inflammatory mediators implicated in the development of insulin resistance (IR), metabolic syndrome, and atherosclerosis.
Methods
Visfatin, fetuin‐A, and PTX3 concentrations were measured in 45 patients with plaque‐type psoriasis and 45 healthy controls using enzyme‐linked immunosorbent assay (ELISA).
Results
Serum levels of visfatin, fetuin‐A, and PTX3 in patients with psoriasis were found to be higher than in healthy controls (P = 0.002, P = 0.009, P < 0.001, respectively). Psoriasis area and severity index (PASI) score correlated significantly with visfatin and fetuin‐A levels (P = 0.011, P = 0.040, respectively). There was a significant positive correlation between visfatin and fetuin‐A (P < 0.001). PTX3 levels were correlated positively with homeostasis model assessment (HOMA‐IR), insulin, triglyceride (TG), and very low density lipoprotein cholesterol (VLDL; P = 0.009, P = 0.007, P = 0.023, P = 0.024, respectively).
Conclusions
Increased serum visfatin, fetuin‐A, and PTX3 levels, and the presence of positive correlation between visfatin, fetuin‐A, and PASI score, probably reflect the inflammatory state and IR seen in psoriasis.
Keywords: visfatin, fetuin‐A, pentraxin 3, psoriasis, inflammation, insulin resistance
INTRODUCTION
Psoriasis is a common inflammatory skin disease affecting approximately 2% of population 1. In psoriasis, increased prevalence of metabolic syndrome, insulin resistance (IR), and atherosclerosis have been reported 2, 3, 4, 5, 6, 7. Although the pathologic link between psoriasis and its comorbidities has been well established, it is poorly understood. The ultimate pathological process leading to both skin determinants and comorbidities is chronic inflammation 8. This concept is named as psoriatic march 9. It describes the development of atherosclerosis as a consequence of systemic inflammation on the basis of psoriasis that results in IR followed by atherosclerosis. Both keratinocytes and inflammatory immune cells including neutrophils, monocytes, macrophages, hepatocytes, epithelial, and endothelial cells are rich sources of cytokines, chemokines, and mediators with various pro‐inflammatory properties in patients with psoriasis. Visfatin, fetuin‐A, and pentraxin 3 (PTX3) are included with the impressive list of these biologically active compounds.
Visfatin is a 52 kDa pro‐inflammatory adipokine secreted mainly in adipose tissue. Visfatin synthesis is stimulated by various factors such as tumor necrosis factor α (TNFα), interleukin (IL)‐1, and IL‐6. It has several pro‐inflammatory and immune‐modulating properties, as it promotes T‐cell activation by inducing costimulatory molecules such as CD80, CD40, and intercellular adhesion molecule 1 (ICAM‐1) 10. It has been reported that visfatin influences insulin receptors by binding to a different place than insulin, thus leading to IR 11, 12. Increased visfatin levels in psoriatic patients 13, 14 correlated with psoriasis area and severity index (PASI) score 14 have been previously reported.
Fetuin‐A is a 60 kDa protein synthesized in hepatocytes. Fetuin‐A binds to insulin receptors in adipose and muscular tissue and inhibits insulin receptor tyrosine kinase activity as well as insulin receptor autophosphorilation in vivo and in vitro. Fetuin‐A has been suggested to potentially cause IR and/or metabolic syndrome 15. Till date, there is only one study in the literature describing decreased fetuin‐A levels in patients with psoriasis 16.
PTX3 is an acute phase protein belonging to the pentraxin superfamily. PTX3 is produced in response to primary inflammatory stimuli such as IL‐1 and TNFα 17. Although the role of PTX3 in the pathogenesis of atherosclerosis and heart failure has been identified, studies still report conflicting data on the association of PTX3 with IR among many different health conditions 18, 19. Chu et al. suggested that PTX3 plays an important role in the regulation of IR 20. Little information is available in the literature about PTX3 in psoriasis. The data on the relationship between psoriasis and PTX3 are controversial. While one study described elevated levels in psoriatic patients correlated with PASI score 21, another reported unchanged PTX3 levels in psoriatic arthritis 22.
Therefore, the aim of this study was to investigate the levels of visfatin, fetuin‐A, and PTX3 in plaque‐type psoriatic patients, their correlation to disease severity, and the relationship with other clinical and biochemical characteristics of psoriasis.
MATERIALS AND METHODS
Forty‐five patients with plaque‐type psoriasis, not previously diagnosed with psoriatic arthritis, were included in this study. Diagnosis of psoriasis was based on clinical findings. Depending on the age at diseases onset, patients were classified as suffering from either early‐age (<40 years) or late‐onset (≥40 years) psoriasis. For definition of the severity of the cutaneous manifestations of psoriasis, we used the PASI that assesses erythema, infiltration, desquamation, and the percentage of the involved body surface. The PASI total score ranges from 0 to 72. Higher scores indicate greater psoriasis severity. All patients were clinically assessed by the same dermatologist (GO) to establish disease severity using the PASI with the same standards. The control group consisted of 45 individuals matched for age and sex. None of the controls had personal or family history of any dermatologic disease on examination. Exclusion criteria for patients and controls were the existence of any comorbid cardiac, autoimmune, infectious, musculoskeletal, or malignant disease and a recent history of operation or trauma. Patients were excluded if they were receiving concurrent systemic or topical antipsoriasis therapy (Table 1). The study was approved by the Institutional Review Board. Informed consent was obtained from each subject.
Table 1.
Clinical and Antropometric Characteristics of Healthy Controls and Patients With Psoriasis
| Control | Psoriasis | Significance | |
|---|---|---|---|
| Age (years) | |||
| Mean (range) | 36.3 (12–56) | 33.3 (17–51) | NS |
| Disease onset | |||
| <40 years, n (%) | – | 25 (55.6) | – |
| >40 years, n (%) | – | 20 (44.4) | – |
| Sex | |||
| Male, n (%) | 29 (64.4) | 31 (68.9) | NS |
| Female, n (%) | 16 (35.6) | 14 (31.1) | NS |
| Familial history, n (%) | – | 11 (24.4) | – |
| Smoking, n (%) | 8 (17.8) | 7 (15.6) | NS |
| BMI (kg/m2) (mean, range) | 26.9 (18–37) | 26.1 (19–38) | NS |
| Systolic BP (mmHg) (mean ± SD) | 111.2 ± 11.5 | 114.3 ± 13.3 | NS |
| Diastolic BP (mmHg) (mean ± SD) | 75.7 ± 7.5 | 72.7 ± 9.2 | NS |
| PASI score (mean, range) | – | 18.5 (2.0–35.7) | – |
BMI, body mass index; BP, blood pressure; PASI, psoriasis area and severity index.
Venous blood samples were taken in the morning in plain tubes following an overnight (12 h) fast. The blood collected in the tubes was centrifuged at 2,000 × g for 10 min after clot formation to obtain the serum samples. Routine biochemical parameters were measured on the same day. Remaining serum samples were stored at −80ºC until the day on which they were analyzed for the concentrations of study markers. Serum glucose, total cholesterol (TC), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), and very low density lipoprotein cholesterol (VLDL‐C), insulin, and C‐reactive protein (CRP) were determined using commercially available assay kits with autoanalyzer (Architect CI 8200; Abbott Diagnostics, IL). Low‐density lipoprotein cholesterol (LDL‐C) was calculated by Frigewal's formula: LDL‐C = TC (mg/dl) – [(TG/5 (mg/dl) + HDL‐C (mg/dl)]. HOMA‐IR was computed by the following formula: HOMA‐IR = [Fasting insulin (μU/ml) × Fasting glucose (mg/dl)]/405.
Visfatin, fetuin‐A, and PTX3 levels were assayed using a commercially available enzyme‐linked immunosorbent assay (ELISA) kits (Shanghai Yehua Biological Technology Co., Shanghai, China). The sensitivity of above‐mentioned ELISA kits was as follows: visfatin—0.25 ng/ml, fetuin‐A—4.96 mg/l, and PTX3—0.05 ng/ml. The intra‐assay and interassay coefficients of variation for the three parameters were <10% and <12%, respectively. All statistical analyses were performed with IBM SPSS statistics for Windows (version 21; SPSS Inc., Chicago, IL). Mann–Whitney U‐test and Spearman correlation test were used for the evaluation of clinical and biochemical parameters. Univariate analysis of variance (General Linear Model (GLM), univariate) was used for constructing the model to explain the variations in visfatin, fetuin‐A, HOMA‐IR, and insulin. Age, gender, BMI, and smoking status were included as covariates.
RESULTS
Clinical characteristics, lipid profile, and parameters of IR of controls and patients with psoriasis are shown in Tables 1 and 2. There were no significant differences between groups in terms of age, gender, BMI, systolic‐diastolic blood pressure values, and lipid profile parameters. Mean insulin levels and HOMA‐IR values were significantly increased in patients according to controls (P = 0.001). In addition, serum visfatin, fetuin‐A, and PTX3 levels in patients with psoriasis were increased (P = 0.002, P = 0.009, P < 0.001, respectively) according to healthy controls (Table 2).
Table 2.
Lipid Profile, Insulin Resistance Parameters and Serum Visfatin, Fetuin‐A, and Pentraxin 3 in Healthy Controls and Patients With Psoriasis (Mean, Range)
| Control | Psoriasis | P‐value | |
|---|---|---|---|
| TC (mg/dl) | 155.11 (86–329) | 165.88 (51–245) | NS |
| TG (mg/dl) | 97.27 (28–402) | 119.18 (12–389) | NS |
| HDL‐C (mg/dl) | 37.20 (15–60) | 42.90 (20–76) | NS |
| LDC‐C (mg/dl) | 98.46 (16–204) | 98.93 (22–162) | NS |
| VLDL‐C (mg/dl) | 19.48 (6–80) | 23.81 (2–78) | NS |
| Glucose (mg/dl) | 87.16 (60–166) | 92.45 (50–144) | NS |
| Insulin (μIU/ml) | 15.61 (4.30–48.8) | 29.54 (1.8–77.9) | 0.001 |
| HOMA‐IR | 3.23 (0.69–11.85) | 6.98 (0.28–27.70) | 0.001 |
| CRP (mg/l) | 3.01 (0.20–11.40) | 2.17 (0.40–7.30) | NS |
| Visfatin (ng/ml) | 10.62 (5.09–39.78) | 18.71 (4.76–54.79) | 0.002 |
| Fetuin‐A (mg/l) | 215.3 (101.2–710.6) | 354.6 (100–1,299) | 0.009 |
| Pentraxin 3 (ng/ml) | 7.79 (4.22–15.94) | 11.21 (5.68–15.94) | < 0.001 |
Mann–Whitney U‐test. CRP, C‐reactive protein; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment; LDL‐C, low‐density lipoprotein cholesterol; PASI, psoriasis area and severity index; TC, total cholesterol; TG, triglyceride; VLDL‐C, very low density lipoprotein cholesterol.
Further, to check whether the factors such as age, gender, BMI, and smoking status influence the visfatin, fetuin‐A, pentraxin, HOMA‐IR, and insulin, we performed analysis of covariance. When stratified by age, gender, BMI, and smoking status, visfatin, fetuin‐A, HOMA‐IR, and insulin were still significantly increased in patients with psoriasis in comparison with controls (Table 3).
Table 3.
General Lineal Model for Visfatin, Fetuin‐A, Pentraxin 3, HOMA‐IR, and Insulin Significance Taking Age, Gender, BMI, and Smoking Status As Covariates
| General lineal model | F‐Value | P‐value |
|---|---|---|
| Age | 0.001 | 0.980 |
| Gender | 0.876 | 0.353 |
| BMI | 0.105 | 0.747 |
| Smoking | 0.396 | 0.531 |
| Visfatin | 13.412 | 0.000 |
| Age | 0.038 | 0.845 |
| Gender | 0.569 | 0.453 |
| BMI | 0.097 | 0.757 |
| Smoking | 0.835 | 0.364 |
| Fetuin‐A | 7.830 | 0.007 |
| Age | 2.294 | 0.134 |
| Gender | 0.051 | 0.823 |
| BMI | 0.775 | 0.382 |
| Smoking | 0.001 | 0.982 |
| Pentraxin 3 | 29.746 | 0.000 |
| Age | 3.889 | 0.053 |
| Gender | 3.219 | 0.077 |
| BMI | 4.411 | 0.060 |
| Smoking | 0.629 | 0.431 |
| HOMA‐IR | 19.483 | 0.000 |
| Age | 2.53 | 0.116 |
| Gender | 1.038 | 0.312 |
| BMI | 3.192 | 0.079 |
| Smoking | 0.003 | 0.956 |
| Insulin | 16.802 | 0.000 |
BMI, body mass index; HOMA‐IR, homeostasis model assessment.
Correlation analysis revealed that PASI score correlated significantly with visfatin and fetuin‐A in study group (Figure 1, Table 4). There was a significant correlation between visfatin and fetuin‐A as well (Table 4). PTX3 levels were correlated with HOMA‐IR, insulin, TG, and VLDL (Table 5). CRP was significantly correlated with BMI, glucose, TC, TG, and VLDL in patients with psoriasis (Table 6). As we expected, there were strong correlations among parameters of IR and lipid profile (data not shown).
Figure 1.
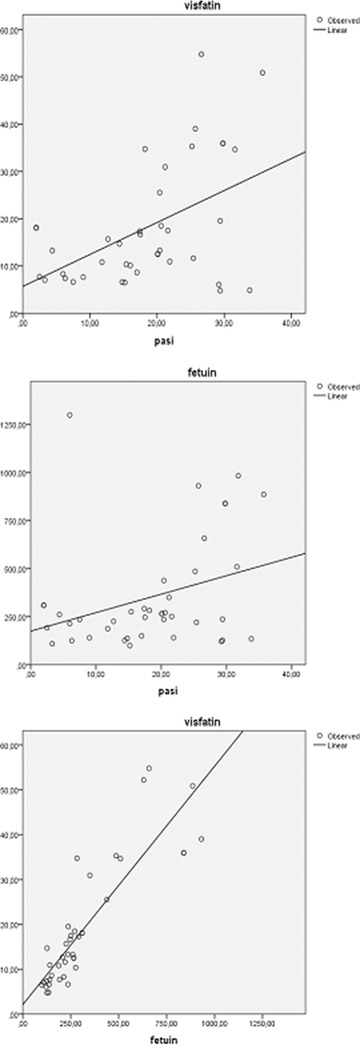
Correlations between PASI score, visfatin, and fetuin‐A.
Table 4.
Correlations Between PASI Score, Visfatin, and Fetuin‐A
| Visfatin, r (P‐value) | Fetuin‐A, r (P‐value) | |
|---|---|---|
| PASI | 0.396 (0.011) | 0.322 (0.040) |
| Fetuin‐A | 0.895 (<0.001) | – |
PASI, psoriasis area and severity index.
Table 5.
Correlations Between Pentraxin 3 and HOMA‐IR, Insulin, TG, and VLDL in Patients With Psoriasis
| Pentraxin 3, r (P‐value) | |
|---|---|
| HOMA‐IR | 0.404 (0.009) |
| Insulin | 0.413 (0.007) |
| TG | 0.341 (0.023) |
| VLDL | 0.339 (0.024) |
HOMA‐IR, homeostasis model assessment; TG, triglyceride; VLDL‐C, very low density lipoprotein cholesterol.
Table 6.
Correlations Between CRP and BMI, Glucose, TC, TG, and VLDL in Patients With Psoriasis
| CRP, r (P‐value) | |
|---|---|
| BMI | 0.383 (0.019) |
| Glucose | 0.366 (0.017) |
| TC | 0.314 (0.043) |
| TG | 0.444 (0.003) |
| VLDL | 0.448 (0.004) |
CRP, C‐reactive protein; TC, total cholesterol; TG, triglyceride; VLDL‐C, very low density lipoprotein cholesterol.
DISCUSSION
The results of present study demonstrated that (1) insulin and HOMA‐IR values were elevated in patients with psoriasis when compared to healthy subjects; (2) visfatin, fetuin‐A, and PTX3 levels were increased in patients according to controls; (3) visfatin and fetuin‐A were significantly correlated with PASI score; (4) PTX3 levels were significantly correlated with HOMA‐IR, insulin, TG, and VLDL; (5) there were significant correlations between CRP with BMI, glucose, TC, TG, and VLDL.
IR, hyperinsulinemia, and/or metabolic syndrome are widely acknowledged to be common biochemical features of psoriasis 2, 3, 4, 5, 6. High prevalence of IR and diabetes mellitus in psoriatic patients has been reported 7, 23. Romano et al. 24 found the prevalence of psoriasis is 9% in diabetic patients, which is higher than normal populatıon. The prevalence of psoriasis is higher than normal population in diabetics. Moreover, increased insulin levels and HOMA‐IR values 5, 6 correlated with PASI score 2, 3 has been shown. In the present study, we found elevated insulin levels and HOMA‐IR in patients with psoriasis. These results are in agreement with other observations.
Visfatin is a pro‐inflammatory adipokine that increased both psoriasis 8, 9 and other chronic inflammatory diseases such as rheumatoid arthritis (RA), inflammatory bowel disease, and IR as well 13, 14. Visfatin binds to the insulin receptor at a site different from insulin, thus leading to IR 11, 12. It is observed from the results that in psoriatic patients, visfatin was significantly higher than controls independent of age, gender, BMI, and smoking status. Moreover, we demonstrated that visfatin levels correlated positively with PASI score, proposing visfatin as marker of disease severity. PASI score was significantly correlated with resistin as well—another pro‐inflammatory adipokine known to be related with IR 2.
The role of visfatin in psoriasis might include modulation of the inflammatory or immune response, as it induces chemotaxis and increases the production of IL‐1, TNFα, IL‐6, and costimulatory molecules by CD14+ monocytes. On the other hand, visfatin may be upregulated as response to pro‐inflammatory cytokines during inflammation 10. An in vivo study showed an increased expression of visfatin gene 19, and in vitro data showed that visfatin enhanced TNFα‐induced α‐chemokine (CXC) ligand 8 and 10 as well as β‐chemokine (CC) ligand 20 secretion and mRNA expression in keratinocytes 25. Elevated serum visfatin levels may be related to its increased production in lesional skin because cells involved in psoriasis synthesize this adipokine. The other sources of visfatin may be neutrophils, monocytes, macrophages, epithelial, and endothelial cells as well 26. Also, as visfatin production is increased in atherosclerotic plaques 27, adipokine may provide a link between psoriasis, endothelial dysfunction, and cardiovascular morbidity. Indeed, visfatin was reported to be independently associated with soluble vascular cell adhesion molecule 1 (sVCAM‐1)—a marker of endothelial dysfunction 28.
Fetuin‐A is a hepatokine secreted from the liver. In vitro, fetuin‐A binds the insulin receptor tyrosin kinase in peripheral tissues, thus inhibiting the insulin‐induced intracellular cascade and leading to peripheral IR 29. Consistent with above‐mentioned function, fetuin‐A knockout mice are insulin sensitive 30, whereas wild‐type mice under exogenous fetuin‐A treatment develop IR 31. In previous studies in humans, higher fetuin‐A levels were associated with IR 32, 33. Since psoriasis shares many biochemical features of IR and metabolic syndrome, the elevation of fetuin‐A in psoriatic patients is not a surprise. Moreover, our results showed that fetuin‐A levels were significantly correlated with PASI score, suggesting that fetuin‐A together with visfatin is a marker for psoriasis severity. The fetuin‐A results in our study is in contradiction with Gerdes et al. 16. In our study, the reason for different fetuin‐A levels in patients with psoriasis and Gerdes’ group may be due to the differences in demographic characteristics. Psoriatic patients in our study were younger than the patients in Gerdes et al. 16. It has been shown previously that younger age was strongly associated with higher fetuin‐A levels in patients with diabetes mellitus 34.
In our study, PTX3 was found to be higher in psoriatic patients than in healthy controls. Increased PTX3 levels correlated with disease severity has been reported previously, and this correlation was interpreted as an evidence of cellular activation of monocytes/macrophages during inflammatory process in psoriasis 21.
In conclusion, psoriatic march describes the development of atherosclerosis as a consequence of systemic inflammation on the basis of psoriasis that leads to IR followed by atherosclerosis. Increased visfatin, fetuin‐A, and PTX3; and the presence of correlation between visfatin, fetuin‐A, and PASI score probably reflects the inflammatory state and IR seen in psoriasis. IR in psoriatic patients probably precedes the alterations of CRP and lipid parameters—the main indices of atherosclerosis and cardiovascular morbidity. Presence of positive correlations between CRP and BMI, glucose, TC, TG, and VLDL in patients with psoriasis supports this explanation.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supplementary Material
REFERENCES
- 1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361(5):496–509. [DOI] [PubMed] [Google Scholar]
- 2. Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol 2007;157(6):1249–1251. [DOI] [PubMed] [Google Scholar]
- 3. Tobin AM, Hackett CB, Rogers S, et al. Body mass index, waist circumference and HOMA‐IR correlate with PASI in psoriasis patients receiving phototherapy. Br J Dermatol 2014;171(2):436–438. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: A systematic review and meta‐analysis of observational studies. J Am Acad Dermatol 2013;68(4):654–662 [DOI] [PubMed] [Google Scholar]
- 5. Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre‐atherosclerotic disease? Increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol 2010;49(6):642–646. [DOI] [PubMed] [Google Scholar]
- 6. Ucak S, Ekmekci TR, Basat O, et al. Comparison of various insulin sensitivity indices in psoriatic patients and their relationship with type of psoriasis. J Eur Acad Dermatol Venereol 2006;20(5):517–522. [DOI] [PubMed] [Google Scholar]
- 7. Khunger N, Gupta D, Ramesh V. Is psoriasis a new cutaneous marker for metabolic syndrome? A study in Indian patients. Indian J Dermatol 2013;58(4):313–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnston A, Arnadottir S, Gudjonsson JE, et al. Obesity in psoriasis: Leptin and resistin as mediators of cutaneous inflammation. Br J Dermatol 2008;159(2):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The “psoriatic march”: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol 2011;20(4):303–307. [DOI] [PubMed] [Google Scholar]
- 10. Stofkova A. Resistin and visfatin: Regulators of insulin sensitivity, inflammation and immunity. Endoc Regul 2010;44:25–36. [DOI] [PubMed] [Google Scholar]
- 11. Samal B, Sun Y, Stearns G, et al. Cloning and characterization of cDNA encoding a novel human pre‐B‐cell colony‐enhancing factor. Mol Cell Biol 1994;14:1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukuhara A, Matsuda M, Nishizawa M et al. Visfatin : A protein secreted by visceral fat that mimics the effects of insulin. Science 2005;307:426–430. [DOI] [PubMed] [Google Scholar]
- 13. Gerdes S, Osadtschy S, Rostami‐Yazdi M, et al. Leptin, adiponectin, visfatin and retinol‐binding protein‐4‐mediators of comorbidities in patients with psoriasis? Exp Dermatol 2012;21(1):43–47. [DOI] [PubMed] [Google Scholar]
- 14. Ismail SA, Mohamed SA. Serum levels of visfatin and omentin‐1 in patients with psoriasis and their relation to disease severity. Br J Dermatol 2012;167(2):436–439. [DOI] [PubMed] [Google Scholar]
- 15. Mori K, Emoto M, Araki T, et al. Effects of pioglitazone on serum fetuin‐A levels in patients with type diabetes mellitus. Metabolism 2008; 57:1248–1252. [DOI] [PubMed] [Google Scholar]
- 16. Gerdes S, Osadtschy S, Buhles N, et al. Cardiovascular biomarkers in patients with psoriasis. Exp Dermatol 2014;23(5):322–325. [DOI] [PubMed] [Google Scholar]
- 17. Bottazzi B, Doni A, Garlanda C, et al. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu Rev Immunol 2010;28:157–158. [DOI] [PubMed] [Google Scholar]
- 18. Zanetti M, Bosutti A, FerreiraC, et al. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: Evidence for association with atherogenic lipid profile. Clin Exp Med 2009;9(3):243–248. [DOI] [PubMed] [Google Scholar]
- 19. Koczan D, Guthke R, Thiesen HJ, et al. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur J Dermatol 2005;15(4): 251–257. [PubMed] [Google Scholar]
- 20. Chu SH, Park JH, Lee MK, et al. The association between pentraxin 3 and insulin resistance in obese children at baseline and after physical activity intervention. Clin Chim Acta 2012;413(19–20):1430–1437. [DOI] [PubMed] [Google Scholar]
- 21. Bevelacqua V, Libra M, Mazzarino MC, et al. Long pentraxin 3: A marker of inflammation in untreated psoriatic patients. Int J Mol Med 2006;18(3):415–423. [PubMed] [Google Scholar]
- 22. Ramonda R, Modesti V, Ortolan A, et al. Serological markers in psoriatic arthritis: Promising tools. Exp Biol Med 2013;238(12):1431–1436. [DOI] [PubMed] [Google Scholar]
- 23. Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol 1995;32(6):982–986. [DOI] [PubMed] [Google Scholar]
- 24. Romano G, Moretti G, Di Benedetto A et al. Skin lesions in diabetes mellitus: Prevelance and clinical correlations. Diabetes Res Clin Pract 1998;39:101–106. [DOI] [PubMed] [Google Scholar]
- 25. Kanda N, Hau CS, Tada Y, et al. Visfatine enhances CXCL8, CXCL10 and CCL20 production in human keratinocytes. Endocrinology 2011;152(8):3155–3164. [DOI] [PubMed] [Google Scholar]
- 26. Luk T, Malam Z, Marshall JC. Pre‐B cell colony enhancing factor (PBEF)/visfatin: A novel mediator of innate immunity. J Leukoc Biol 2008;83:804–816. [DOI] [PubMed] [Google Scholar]
- 27. Dahl TB, Yndestad A, Skjelland M, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possible role in inflammation and plaque destabilization. Circulation 2007;115:972–980. [DOI] [PubMed] [Google Scholar]
- 28. Axelsson J, Witasp A, Carrero JJ, et al. Circulating levels of visfatin/pre‐B‐cell colony‐enhancing factor 1 in relation to genotype, GFR, body composition, and survival in patients with CKD. Am J Kidney Dis 2007;49(2):237–244. [DOI] [PubMed] [Google Scholar]
- 29. Ix JH, Biggs ML, Mukamal KJ, et al. Association of fetuin‐a with incident diabetes mellitus in community‐living older adults: The cardiovascular health study. Circulation 2012;125(19):2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathews ST, Singh GP, Ranalletta M, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene Diabetes 2002;51(8):2450–2458. [DOI] [PubMed] [Google Scholar]
- 31. Hennige AM, Staiger H, Wicke C, et al. Fetuin‐A induces cytokine expression and suppresses adiponectin production. PLoS One 2008;3(3):e1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erdmann J, Salmhofer H, Knauß A, et al. Relationship of fetuin‐A levels to weight‐dependent insulin resistance and type 2 diabetes mellitus. Regul Pept 2012;178(1–3):6–10. [DOI] [PubMed] [Google Scholar]
- 33. Weikert C, Stefan N, Schulze MB, et al. Plasma fetuin‐a levels and the risk of myocardial infarction and ischemic stroke. Circulation 2008;118(24):2555–2562. [DOI] [PubMed] [Google Scholar]
- 34. Ix JH, Wassel CL, Kanaya AM, et al. Fetuin‐A and incident diabetes mellitus in older persons. JAMA 2008;300(2):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supplementary Material


