Abstract
Background
A novel ischemia marker named ischemia modified albumin was previously considered as an early marker of myocardial ischemia, however due to recent reports, its contribution was demonstrated in different pathologies such as oxidative stress, diabetes, stroke and cancer. We aimed to investigate the relation between oxidative stress and thyroid dysfunctions determining IMA levels since IMA is closely related with increased oxidative stress.
Methods
A total of 88 individuals were participated in this study: 34 cases in hypothyroid, 27 cases in hyperthyroid and 27 cases in euthyroid group. Ischemia‐modified albumin levels were measured by albumin cobalt binding test and thyroid hormone levels were determined with electrochemiluminescent method.
Results
Ischemia modified albumin levels were significantly decreased in hypothyroid group compared to hyperthyroid and euthyroid groups (p < 0.001). In hyperthyroid individuals ischemia modified albumin levels were higher compared to euthyroid ones (p < 0.001). Ischemia modified albumin was negatively correlated with TSH levels (r = −0.473, p < 0.001), and positively correlated with FT4 and FT3 levels (r = 0.496, p < 0.001 and r = 0.275, p = 0.010, respectively).
Conclusion
We suggest that albumin adjusted IMA levels are significantly lower in hypothyroid group than hyperthyroid and euthyroid groups.
Keywords: ischemia‐modified albumin, thyroid hormone levels, hypothyroid, hyperthyroid, euthyroid
INTRODUCTION
Thyroid dysfunctions, namely hypothyroidism and hyperthyroidism, are the most common endocrine abnormalities diagnosed either in subclinical or clinical form. According to the NHANES III study, the prevalence of hypothyroidism was 4.6% (0.3% clinical and 4.3% subclinical) and of hyperthyroidism 1.3% (0.5% clinical and 0.7% subclinical), in population aged at least 12, showing an age and sex dependence 1.
Hyperthyroidism is associated with an increased metabolic rate due to increments in the rate of oxygen consumption in target tissues. Acceleration of aerobic metabolism by thyroid hormones enhances the generation of oxidative stress. Oxidative stress is regarded as a pathogenic factor in hyperthyroidism. Clinical and experimental studies reported increased levels of free oxygen radicals and a decreased antioxidant status in thyrotoxicosis 2.
Thyroid hormones are associated with the oxidant and antioxidant status of the organism. Depression of metabolism due to hypothyroidism has been reported to decrease oxidant production and thus protects tissues against oxidant damage 3.
In late 1990s, Bar‐Or et al. described a novel, rapid, and simple marker named ischemia‐modified albumin (IMA) for detection of myocardial ischemia in patients with acute symptoms such as chest pain 4. IMA was suggested as a rapid and sensitive marker for myocardial ischemia and would reduce unnecessary tests and health care costs. The colorimetric test performed on serum is based on decreased binding of exogenous cobalt to albumin. In intact human albumin, N‐terminal region with the amino acid sequence N‐Asp‐Ala‐His‐Lys has been shown to be a strong binding site for transition metals such as Co, Cu, and Ni. It has been suggested that of these amino acids, histidine at position 3 in human albumin is essential for copper binding 5. The test has recently been licensed by the US Food and Drug Administration for diagnostic use in suspected myocardial ischemia. It is important to emphasize that the decreased ability of albumin to bind cobalt occurs in hypoxia, acidosis, sodium, and calcium pump malfunctions, and tissue damage caused by free radicals 6. This issue was supported by the reports suggesting that IMA is strongly related with oxidative stress rather than being a myocardial ischemia marker 7, 8, 9, 10, 11, 12.
In this study, we aimed to investigate the relation between oxidative stress and thyroid dysfunctions determining IMA levels since IMA is closely related with increased oxidative stress.
MATERIALS AND METHODS
Study Groups
A total of 88 individuals were contributed in this study. The participants were grouped as hypothyroid (n = 34), hyperthyroid (n = 27), and euthyroid (n = 27) due to baseline thyroid function tests. All the subjects were aged between 16 and 81 years, newly diagnosed and untreated for hypothyroidism or hyperthyroidism. The patients with diabetes mellitus, hypertension, coronary artery disease, ischemic cerebrovascular disease and also smokers, alcohol users, and cases who were under regular medications for chronic diseases were excluded. Study protocol was approved by local ethics committee of Selcuk University Medical Faculty. Informed consents were obtained from all participants.
Biochemical Analysis
Fasting venous blood samples were obtained at 09:00 A.M. Serum portions were separated within 30 min after centrifugation at 800g for 10 min. Thyroid hormone levels were quantified with electrochemiluminescence method on cobas 6000 analyzer (Roche Diagnostics, Indianapolis, USA) using original reagents. The remaining serum samples were stored in Eppendorf tubes at −20°C for 1 month.
IMA levels were determined using rapid colorimetric method described by Bar‐Or et al. 13. A total of 200 μl of serum was placed into glass tubes, and 50 μl of 1 g/l CoCl2 was added. After being gently shaken, the solution was incubated at room temperature for 10 min to ensure sufficient cobalt albumin binding. Then 50 μl of dithiothreitol (DTT; 1.5 g⁄l) was added as a colorizing agent and the reaction was quenched 2 min later by adding 1.0 ml 0.9% NaCl. A sample blank was prepared for each sample without DTT. Absorbance values were measured at 470 nm using a spectrophotometer (UV1601, Shimadzu, Sydney, Australia). The color of the DTT‐containing specimens was compared with that of the sample blank tubes and the results were reported as absorbance units (ABSUs). Within‐run imprecisions of low (mean IMA 0.420 ABSU) and high (mean IMA 0.867 ABSU) serum pools (n = 20, in each) were 5.27 and 7.63%, respectively. IMA levels of all samples were measured in the same run, but to minimize systematic bias were run in a random order of cases and controls within the same batch (eight samples per batch. Interassay CV values for the same sera pools were 7.73 and 9.34%, respectively. Albumin concentrations were measured with BCG method on BS800 analyzer (Mindray, Shenzhen, China) and adjusted IMA levels were calculated as described by Lippi et al. The formula for this adjustment is as follows: [(individual serum albumin concentration/median albumin concentration of the group) × IMA value] 14. All chemicals were analytical grade and obtained from Sigma‐Aldrich (Munich, Germany).
STATISTICAL EVALUATION
SPSS 13 for Windows was used for statistical evaluation of the data. Shapiro–Wilk test was used to test the distribution pattern of the numerical data. ANOVA test with post hoc Bonferroni test was used for comparison of parametric numeric data (IMA, albumin and adjusted IMA levels). Kruskal–Wallis and Mann–Whitney U‐test were used for comparison of nonparametric data (thyroid hormone levels). Correlations between IMA and thyroid hormone levels were tested with Spearman's correlation analysis. All data are expressed as mean ± standard deviation and p < 0.05 was assumed as statistically significant.
RESULTS
IMA levels of hypothyroid group were significantly lower than hyperthyroid and euthyroid groups (p < 0.001 and p < 0.001, respectively). Also IMA levels of hyperthyroid group were higher compared to the euthyroid group (p < 0.001). Albumin levels were similar in the groups. Albumin‐adjusted IMA levels of hypothyroid group were also significantly lower than hyperthyroid and euthyroid groups (p < 0.001 and p < 0.001, respectively; Table 1).
Table 1.
Demographic Characteristics and Results of Biochemical Analysis
| Parameter | Hyperthyroid | Hypothyroid | Euthyroid |
|---|---|---|---|
| n | 27 | 34 | 27 |
| Gender (F/M) | 23/4 | 32/2 | 24/3 |
| Age (year) | 45 (26–80) | 37 (16–81) | 38 (16–66) |
| TSH (μU/ml) | 0.02 ± 0.03 | 8.7 ± 5.18 | 2.14 ± 1.17 |
| FT3 (ng/ml) | 5.25 ± 3.37 | 2.95 ± 0.39 | 3.06 ± 0.46 |
| FT4 (μg/dl) | 2.22 ± 1.09 | 1.08 ± 0.2 | 1.55 ± 0.53 |
| Albumin (g/dl) | 4.62 ± 0.44 | 4.89 ± 0.5 | 4.83 ± 0.33 |
| IMA (ABSU) | 0.490 ± 0.13 | 0.344 ± 0.08a, b | 0.453 ± 0.07 |
| Albumin‐adjusted IMA (ABSU) | 0.474 ± 0.09 | 0.351 ± 0.09c, d | 0.449 ± 0.06 |
p < 0.001 IMA levels of hypothyroid group vs. hyperthyroid group.
p < 0.001 IMA levels of hypothyroid group vs. euthyroid group.
p < 0.001 albumin‐adjusted IMA levels of hypothyroid group vs. hyperthyroid group.
p < 0.001 albumin‐adjusted IMA levels of hypothyroid group vs. euthyroid group.
There was a negative correlation between albumin‐adjusted IMA and TSH levels (r = −0.456, p < 0.001) and a positive correlation between albumin‐adjusted IMA and FT3, FT4 levels, (r = 0.328, p = 0.002 and r = 0.466, p < 0.001, respectively; Figs. 1, 2, and 3).
Figure 1.
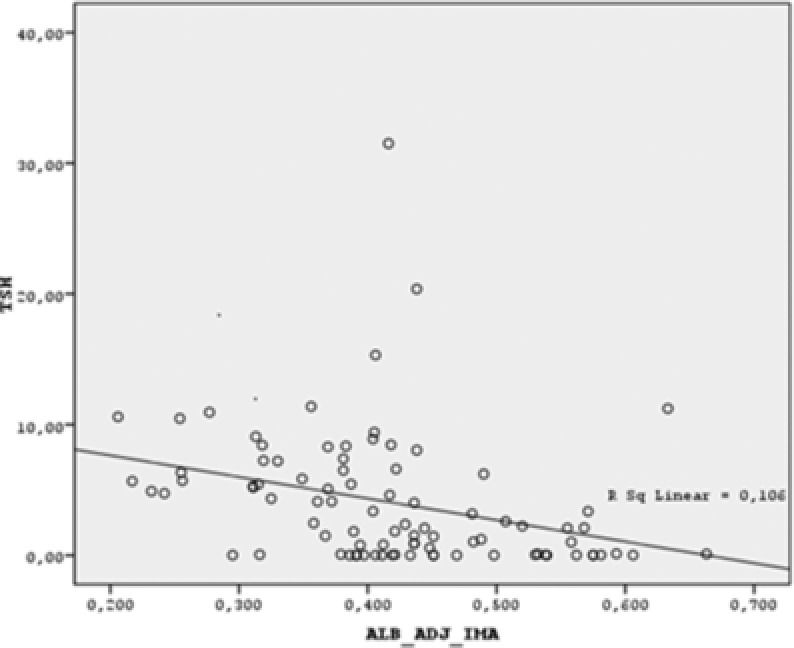
Scatter dot graph of TSH vs. albumin‐adjusted IMA. (Albumin‐adjusted IMA is negatively correlated with TSH. r = −0.456, p < 0.001)
Figure 2.
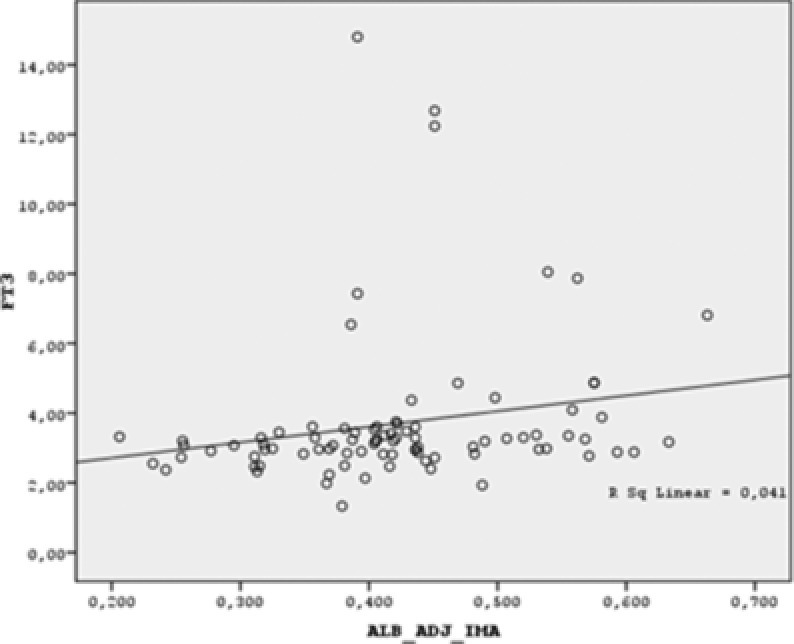
Scatter dot graph of FT3 vs. albumin‐adjusted IMA. (Albumin adjusted IMA is positively correlated with FT3. r = 0.328, p = 0.002)
Figure 3.
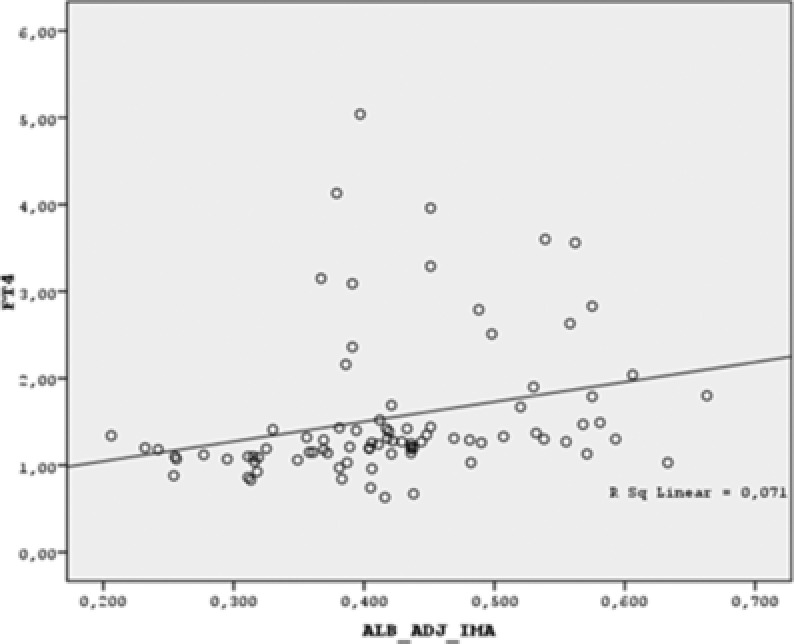
Scatter dot graph of FT4 vs. albumin‐adjusted IMA. (Albumin adjusted IMA is positively correlated with FT4. r = 0.466, p < 0.001)
DISCUSSION
Thyroid dysfunctions bring about pathological changes in different organs of the body. The findings obtained from in vivo and in vitro studies show that thyroid hormones have a strong impact on oxidative stress. Data on the oxidative status of hypothyroidism are limited and controversial 15. Decreases in the metabolic reactions occur in parallel with hypothyroidism and decreased generation of free radicals 16. Asayama et al. observed growth inhibition of rats in an experimental hypothyroidism model with 0.05% PTU 17. In another study there was no change in oxidative stress parameter levels in the liver tissues of rats 18. According to experimental evidence, oxidative stress occurs in tissues from hyperthyroid animals 19, 20, 21, 22.
There are many reports that showed increased oxidative stress in hyperthyroid condition. The altered levels of lipid peroxides, glutathione, and activity of antioxidant enzymes are studied most often to show the oxidative stress 23. However, the oxidative damages of serum proteins in hyperthyroidism have not been explored previously. The carbonylation is a marker of oxidative damage of proteins. Goswami et al. demonstrated that the level of carbonylation of serum proteins in hyperthyroid patients was higher in comparison to control. This clearly indicated that blood proteins undergo oxidative changes in this condition 24.
When we searched the medical indexes, we have found two reports evaluating the relation between IMA levels and thyroid dysfunction. Ma et al. measured IMA levels in total of 35 untreated patients with overt hyperthyroid (Ohe), 35 untreated patients with overt hypothyroid (Oho), and 35 control subjects. IMA levels were higher in patients with Oho than in euthyroid or Ohe subjects (p = < 0.05). Basal IMA levels were reduced after treatments in all patients (p < 0.05). In Ohe patients, serum IMA levels were positively correlated with free triiodothyronine (r = 0.424, p = 0.011) and free thyroxine (r = 0.567, p < 0.001) levels. In Oho patients, serum IMA levels were inversely correlated with free triiodothyronine (r = −0.555, p = 0.001) and free thyroxine (r = −0.457, p = 0.006) but positively correlated with antithyroid peroxidase antibody. Linear regression analyses showed that free triiodothyronine was the most important factor affecting serum IMA levels in Ohe (β = 0.694, p = 0.019) and in Oho (β = −0.512, p = 0.025; 25). Ersoy et al. measured IMA levels in 85 patients: euthyroid (n = 48), subclinical hypothyroidism (n = 24), and overt hypothyroidism (n = 13). They stated that IMA levels did not differ between the groups 26.
In our study, IMA levels of hypothyroid group were significantly lower than hyperthyroid and euthyroid groups as well as IMA levels of hyperthyroid group were higher compared to the euthyroid group. When we have evaluated the relation between IMA and thyroid hormone levels, we observed a negative correlation between IMA and TSH levels and a positive correlation between IMA and FT4 and FT3 levels. Our results are different from these two previous reports. However, we suggest that the research of Ma et al. had some limitations. First, they did not measure albumin concentrations and did not calculate albumin‐adjusted IMA levels. As this was mentioned in many reports about IMA levels in different clinical researches, measuring albumin concentration and adjusting IMA levels due to albumin concentrations is necessary because IMA levels are inversely correlated with albumin concentrations. So when albumin levels tend to decrease IMA levels tend to increase. The results of Ma et al. are not reliable. Reddy mentioned about this fact in his letter to the editor in the same journal. He stated that in hyperthyroidism, degradation of proteins and lipolysis is increased to a greater extent resulting in hypoalbuminemia, increased plasma FFAs, enhanced FFA mobilization, and decrease in serum cholesterol, and triglyceride level. Whereas, in hypothyroidism, the degradation of both proteins and lipids is decreased to a greater extent than their synthesis with the net effect reflected as increased albumin pool, decreased plasma FFAs, impaired FFA mobilization. Albumin level interferes with the IMA estimation. It was also suggested that it would be very important to present IMA values corrected for albumin interference in thyroid disorders. This is of much importance as albumin‐adjusted IMA index has been reported to be more sensitive than IMA. Human serum albumin is the primary binder of free fatty acids (FFAs) that have a potential role in the formation of IMA. Therefore, variations in the concentration of FFA would affect IMA values 27. Although Ersoy et al. suggested that they did not find any significant difference between euthyroid, subclinical hypothyroid, and overt hypothyroid groups, IMA levels tended to decrease in hypothyroid group compared to euthyroid group. However they did not measure albumin concentrations and calculate albumin‐adjusted IMA levels, either. They did not mention about any correlation analysis between IMA levels and thyroid hormone levels. So, due to albumin‐adjusted IMA levels in our study we suggest that IMA levels were decreased in hypothyroid group compared to euthyroid and hyperthyroid groups. The results of the correlation analysis between IMA and thyroid hormone levels support this finding.
CONCLUSION
Hypothyroidism is a clinical status with hypometabolic state. As mentioned by previous reports and repeated by Reddy, hypoalbuminemia is in conjunction with increased IMA levels. So we suggest that albumin adjusted IMA levels are significantly lower in hypothyroid group than hyperthyroid and euthyroid groups. Further studies are needed for clarifying the exact mechanism of decreased albumin‐adjusted IMA levels in larger populations and in clinical researches with different stages of thyroid dysfunction and with addition of some other oxidative stress markers.
REFERENCES
- 1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metabol 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 2. Makay B, Makay O, Yenisey C, et al. The interaction of oxidative stress response with cytokines in the thyrotoxic rat: Is there a link? Mediators Inflam 2009;2009:391682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coria1 MJ, Pastrán AI, Gimenez MS. Serum oxidative stress parameters of women with hypothyroidism. Acta Biomed 2009;80:135–139. [PubMed] [Google Scholar]
- 4. Bar‐Or D, Lau E, Rao N, Bampos N, Winkler JV, Curtis CG. Reduction in the cobalt binding capacity of human albumin with myocardial ischaemia. Ann Emer Med 1999;34(Suppl 4):1–5. [Google Scholar]
- 5. Bar‐Or D, Curtis G, Rao N, Bampos N, Lau E. Characterization of the Co21 and Ni21 binding amino‐acid residues of the N‐terminus of human albumin. An insight into the mechanism of a new assay for myocardial ischemia. Eur J Biochem 2001;268:42–47. [DOI] [PubMed] [Google Scholar]
- 6. Wudkowska A, Goch J, Goch A. Ischemia−modified albumin in differential diagnosis of acute coronary syndrome without ST elevation and unstable angina pectoris. Kardiologia Polska 2010;68(4):431–437. [PubMed] [Google Scholar]
- 7. Roy D, Quiles J, Gaze DC, Collinson P, Kaski JC, Baxter GF. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart 2006;92:113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Senes M, Kazan N, Coskun O, Zengi O, Inan L, Yücel D. Oxidative and nitrosative stress in acute ischaemic stroke. Ann Clin Biochem 2007;44:43–47. [DOI] [PubMed] [Google Scholar]
- 9. Valle Gottlieb MG, da Cruz IB, Duarte MM, et al. Associations among metabolic syndrome, ischemia, inflammatory, oxidatives, and lipids biomarkers. J Clin Endocrinol Metab 2010;95:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaefer M, Piva SJ, de Carvalho JA, et al. Association between ischemia modified albumin, inflammation and hyperglycemia in type 2 diabetes mellitus. Clin Biochem 2010;43(4–5):450–454. [DOI] [PubMed] [Google Scholar]
- 11. Jia ZT, Liu CY, Li H. Changes of the concentration of serum ischemia modified albümin and high sensitivity C‐reactive protein in type 2 diabetic patients with retinopathy. Zhonghua Yan Ke Za Zhi 2009;45:805–808. [PubMed] [Google Scholar]
- 12. Kıyıcı A, Mehmetoğlu I, Karaoğlan H, Atalay H, Solak Y, Süleyman Türk S. Ischemia modified albumin levels in patients with end stage renal disease patients on hemodialysis: Does albumin analysis method affect albumin adjusted ischemia modified albumin levels? J Clin Lab Anal 2010;24:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bar–Or D, Lau E, Winkler JV. A novel assay for cobalt‐albumin binding and its potential as a marker for myocardial ischemia—A preliminary report. J Emerg Med 2000;19:311–315. [DOI] [PubMed] [Google Scholar]
- 14. Lippi G, Montagnana M, Salvagno GL, Guidi GC. Standardization of ischaemia modified albumin testing: Adjustment for serum albumin. Clin Chem Lab Med 2007;45:261–262. [DOI] [PubMed] [Google Scholar]
- 15. Kebapcilar L, Akinci B, Bayraktar F. Plasma thiobarbituric acid‐reactive substance levels in subclinical hypothyroidism. Med Princ Pract 2007;16:432–436. [DOI] [PubMed] [Google Scholar]
- 16. Yilmaz S, Ozan S, Benzer F, Canatan H. Oxidative damage and antioxidant enzyme activities in experimental hypothyroidism. Cell Biochem Funct 2003;21:325–330. [DOI] [PubMed] [Google Scholar]
- 17. Asayama K, Dobashi K, Hayashibe H, Megata Y, Kato K. Lipid peroxidation and free radical scavengers in thyroid dysfunction in the rat: A possible mechanism of injury to heart and skeletal muscle in hyperthyroidism. Endocrinology 1987;121:2112–2118. [DOI] [PubMed] [Google Scholar]
- 18. Venditti P, Balestrieri M, Meo S, Leo T. Effect of thyroid state on lipid peroxidation, antioxidant defences and susceptibility to oxidative stress in rat tissues. J Endocrinol 1997;155:151–157. [DOI] [PubMed] [Google Scholar]
- 19. Fernández V, Barrientos X, Kipreos K, Valenzuela A, Videla LA. Superoxide radical generation, NADPH oxidase activity and cytochrome P‐450 content of rat liver microsomal fractions in an experimental hyperthyroid state: Relation to lipid peroxidation. Endocrinology 1985;117:496–501. [DOI] [PubMed] [Google Scholar]
- 20. Venditti P, Daniele MC, Masullo P, Di Meo S. Antioxidant‐sensitive triiodothyronine effects on characteristics of rat liver mitochondrial population. Cell Physiol Biochem 1999;9:38–52. [DOI] [PubMed] [Google Scholar]
- 21. Das K, Chainy GBN. Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochim Biophys Acta 2001;1537:1–13. [DOI] [PubMed] [Google Scholar]
- 22. Tapia G, Cornejo P, Fernández V, Videla LA. Protein oxidation in thyroid hormone‐induced liver oxidative stress: Relation to lipid peroxidation. Toxicol Lett 1999;106:209–214. [DOI] [PubMed] [Google Scholar]
- 23. Dincer Y, Akca T, Alademir Z, Ilkova H. Effect of oxidative stress on glutathione pathway in red blood cells from patients with insulin dependent diabetes mellitus. Metabolism 2002;51:1360–1362. [DOI] [PubMed] [Google Scholar]
- 24. Goswami K, Nandakumar DN, Koner BC, Bobby Z, Sen SK. Oxidative changes and desialylation of serum proteins in hyperthyroidism. Clin Chim Acta 2003;337:163–168. [DOI] [PubMed] [Google Scholar]
- 25. Ma SG, Liu‐xue Yang LX, Bai F, Xu W, Hong B. Ischemia‐modified albumin in patients with hyperthyroidism and hypothyroidism. Eur J Intern Med 2012;23:e136–e140. [DOI] [PubMed] [Google Scholar]
- 26. Ersoy K, Anaforoğlu İ, Algün E. Serum ischemic modified albumin levels might not be a marker of oxidative stress in patients with hypothyroidism. Endocrine 2013;43(2):430–433. [DOI] [PubMed] [Google Scholar]
- 27. Reddy VS. Ischemia‐modified albumin in patients with hyperthyroidism and hypothyroidism. Eur J Intern Med 2013;25(3):e42–e43. [DOI] [PubMed] [Google Scholar]


