Abstract
Background
Resistin is an adipocytokine associated with inflammation and insulin resistance. Recent studies have shown that resistin plays an important role in the pathogenesis and progression in osteoarthritis (OA) patients. The current study was aimed at investigating the relationship between resistin in serum and synovial fluid (SF) and disease severity in patients with knee osteoarthritis.
Method
Seventy‐four patients diagnosed with knee OA and 79 healthy controls receiving regular body check in our hospital were recruited in the study. The Noyes score method was used to assess articular cartilage damage arthroscopically. The symptomatic severity was evaluated according to the Western Ontario McMaster University Osteoarthritis (WOMAC) scores. The radiographic disease severity of OA was assessed by the Kellgren‐Lawrence (K–L) grading system. The resistin levels in serum and SF were determined by enzyme‐linked immunosorbent assay. Cartilage degradation marker CTX–II in SF was also examined.
Results
SF but not serum resistin levels are positively associated with Noyes scores, K‐L grading scores WOMAC pain scores, physical functional scores and WOMAC total scores. In addition, SF resistin correlated positively with CTX‐II.
Conclusion
Resistin in SF might serve as a potential biomarker for reflecting the disease severity and cartilage degenerative extent of knee OA.
Keywords: osteoarthritis, resistin, disease severity, catilage damage
INTRODUCTION
Osteoarthritis (OA) is one of the most common degenerative joint diseases in aging population. It is characterized by articular cartilage destruction, osteophyte formation, and subchondral bone abnormalities 1. Epidemiological studies showed that around 15% of the world population 2 and 37% of people over 60 years 3 suffer from OA and the morbidity is estimated reaching 100,000 new cases per year 3. Osteoarthritis affects any articular with the knee joints most commonly involved 4. The increased OA morbidity calls for the urgency to make early diagnosis, prevention, and treatment. In recent years, biochemical markers represent a possible nonivasive method to assess the risk for the progression of the disease, detect early changes of the disease and evaluate the response to such potential disease‐modifying OA drugs 5, 6.
Adipocyte‐derived molecules, also known as adipokines, have prompted much interest in OA pathogenesis studies over the past years in that they play crucial roles in bone and cartilage homeostasis 7, 8. In addition, the association of adipokines with obesity, along with their pro‐ or anti‐inflammatory functions indicates that adipokines might represent another crucial mediators that links inflammation with obesity and OA 9, 10.
Resistin is a newly identified adipocytokine that has demonstrated links between obesity and insulin resistance in rodents 11. It is mainly expressed and secreted by adipose tissue, but has also been proved in other tissues in rodents 12. In contrast with animal models, immunocompetent cells rather than adipocytes seem to be the major source of resistin in humans that is in consistent with the phenomenon that proinflammatory properties of resistin are superior to its insulin resistance‐inducing effects 13.
Recently, resistin has been investigated with its occurrence and potential involvement in the pathophysiology of OA. It is reported that injection of resistin into knee joints of healthy mice induced cartilage destruction and synovial inflammation 14. Morever, resistin could direct effect on cartilage matrix and cytokine production to upregulate expression of MMP‐1, MMP‐13 as well as IL‐6 and TNF‐alpha, etc. 15. In human studies, it has also been proved that synovial fluid and serum resistin levels increase in patients after knee injury 16. Resistin levels have also been identified elevated in both serum and synovial fluid in OA patients, indicating its role involved in inflammatory changes of OA 17, 18. In a previous study, Koskinen et al. found levels of resistin synovial fluid are positively associated with IL‐6 and matrix metalloproteinases MMP‐1, MMP‐3 in knee OA patients 19.
All these data indicate that resistin may play crucial roles in OA progression. However, to our knowledge, there have been no studies available illustrating the correlation between serum and SF concentrations of resistin and disease severity as well as degree of cartilage degeneration in knee OA patients. Therefore, the present study was carried out to explore whether serum and SF levels of resistin are related to the disease severity and extent of cartilage injury of the disease.
MATERIALS AND METHODS
Patients and Controls
This study was approved by the ethical committee of the Second Hospital of Hebei Medical University and performed in conformity with the declaration of Helsinki principles. Written informed consent was obtained from all participants. Seventy‐four patients with primary knee OA visited our hospital undergoing arthroscopic irrigation and debridement from July 2014 to March 2015 were enroll in the present study. All patients met the American College of Rheumatology criteria for the diagnosis of OA. The criteria are: patients suffer articular knee pain with at least three of the below items: 1 Active joint motion with crepitus, 2 morning stiffness no more than half an hour, 3 age >50 years, 4 bony enlargement of the knee on examination, and 5 bony tenderness of the knee 6. No palpable warmth. Laboratory criteria: 1 Erythrocyte sedimentation rate (ESR) < 40 mm/h, 2 rheumatoid factor (RF) < 1:40, and 3 synovial fluid analysis: clear, viscous, white blood cell count < 2000/μL. Radiographic criteria: Presence of osteophytes. Seventy‐nine sex and age matched healthy individuals receiving routine body check in our hospital during the same period were recruited as controls. All the controls had no signs of OA‐related symptoms and radiographic changes. Participants were excluded if they suffer from knee injury, joint infection, secondary posttraumatic arthritis, systemic inflammatory, or rheumatoid disorders, malignant disease, progressive renal disease, gout, hydroxyapatite, or calcium pyrophosphate deposition disease. In addition, patients were also excluded if any anti‐inflammatory drugs, corticosteroids as well as sodium hyaluronate were used within the past 2 weeks.
Definition of Cartilage Damage
Noyes method 20 was utilized to evaluate articular cartilage damage arthroscopically while performing arthroscopic irrigation and debridement. This rating system was based on depth and area of cartilage damage. Cartilage damage < 10 mm in diameter is regarded clinically insignificant and marked 0 points. One point is designated if the diameter of cartilage damage ranges from 10 to 15 mm. Two points are given for an open lesion less than 50% thickness, three points are given for an open lesion more than 50% thickness of the articular cartilage and 5 points are given for bone exposure. If the cartilage damage is > 15 mm in diameter, twice the number of points are assigned as for damage 10–15 mm in diameter. The knee is divided into six anatomical areas: medial femur, medial tibia, lateral femur, lateral tibia, patella, and femoral sulcus. After evaluating cartilage damage in each area, patellofemoral, medial tibiofemoral, and lateral tibiofemoral compartment scores are calculated. The total scores were then expressed as a percent of normal using a standard formula. We used the sum of the scores for each part for convenience. All arthroscopic operations and evaluation were performed by one expert in our hospital.
Definition of Disease Severity
The symptomatic severity of OA was assessed by the Western Ontario McMaster University Osteoarthritis Index (WOMAC) including three subscales 21: pain, stiffness, and physical function. The psychometric properties of the WOMAC score have been shown to be valid and reliable in patients with primary knee OA 22, 23.
Antero‐posterior weight‐bearing radiographs of both knees were taken before the operation 24. Radiographic severity was evaluated according to the Kellgren–Lawrence grading system: Grade 1, doubtful narrowing of joint space and possible osteophytic lipping; Grade 2, definite osteophytes and possible narrowing of joint space; Grade 3, moderate multiple osteophytes, definite narrowing of joints space, some sclerosis and possible deformity of bone contour; Grade 4, large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour 25. The grade used for analysis was the higher of the two knees. The scores of radiographs were assessed by an experienced radiologist in our hospital who was blinded to the selection of subjects.
ELISA
Synovial fluid was aspirated from the affected knee using sterile knee puncture just before the arthroscopic irrigation and debridement was performed, centrifuged to remove cells and joint debris and stored immediately at –80°C until the day of measurement. Each synovial fluid sample from patients was observed by two experienced pathologists in our hospital to decide whether abnormal deposits were existed. No synovial fluid was extracted from the controls due to ethical concerns. Venous blood samples were obtained from the same patients on the day of arthroscopic operation and all healthy individuals receiving regular physical examination. The specimen were then centrifuged and stored at –80°C until utilized. Double‐blind quantitative analysis of resistin in serum and synovial fluid was performed by sandwich enzyme‐linked immunosorbent assay (ELISA) using a commercially kit according to the manufacturer's protocol (Santa Cruz Biotechnology, Santa Cruz, USA). SF CTX‐II levels were also examined with the same procedure (Santa Cruz Biotechnology, Santa Cruz, USA).
Statistic Analysis
Resistin concentration, WOMAC scores, and Noyes scores were expressed as median (range) whereas the basic clinical data from participants were expressed as mean ± SD. Serum resistin levels between OA patients and controls were analyzed using unpaired Student's t test. The correlation of serum or SF resistin concentrations with WOMAC scores, Noyes scores, or levels of CTX‐II was determined by Pearson or Spearman correlation analysis, where appropriate. P < 0.05 was considered significant.
RESULTS
Basic Clinical Data
Characteristics of the study population are demonstrated in Table 1. There are no clinical meaningful differences between OA patients and controls in age (65.8 ± 7.3 years vs. 66.2 ± 6.9 years, P = 0.451), gender (40/34 vs. 42/37 F/M, Chi‐square P = 0.298), or body mass index (26.7 ± 1.5 kg/m2 vs. 26.8 ± 1.2 kg/m2, P = 0.353).
Table 1.
Baseline Characteristics
| OA patients (n = 74) | Healthy controls (n = 79) | P value | |
|---|---|---|---|
| Age (Y) | 65.8 ± 7.3 | 66.2 ± 6.9 | 0.451 |
| Gender (F/M) | 40/34 | 42/37 | 0.298 |
| BMI (kg/m2) | 26.7 ± 1.5 | 26.8 ± 1.2 | 0.353 |
| Diabetes suffering (Yes/No) | 17/57 | / | |
| WOMAC pain scores | 9 (4–17) | / | |
| WOMAC stiffness scores | 5 (2–8) | / | |
| WOMAC physical function scores | 43 21–66) | / | |
| WOMAC total scores | 61 (29–90) | / | |
| Noyes scores | 11 (2–36) | / | |
| KL grading (2/3/4) | 22/29/23 | / | |
| Serum resistin levels (ng/ml) | 19.5 (10.2–36.7) | 17.2 (8.79–32.1) | 0.035 |
| SF resistin levels (ng/ml) | 8.7 (2.7–15.6) | / |
Basic values are expressed as the mean value ± SD or n (%). WOMAC index, Noyes scores and serum/SF resistin levels were given as median (interquartile range).
Resistin levels in serum and SF
Serum fluid resistin expression of knee OA patients and controls was also showed in Table 1. Compared with healthy controls, knee OA patients had a slightly higher level of resistin concentration in serum [19.5 10.2–36.7)ng/ml vs. 17.2 8.79–32.1)ng/ml, P = 0.035]. The serum levels of resistin were considerably higher compared to the SF levels [19.5 10.2–36.7)vs. 8.7 2.7–15.6) ng/ml, P < 0.001)] (Table 1).
Association between serum and SF resistin levels and disease severity
SF levels of resistin were positively associated with the radiographic severity of knee OA (r = 0.642, P < 0.001) (Fig. 3). In addition, SF concentrations of resistin were positively correlated with WOMAC pain (r = 0.514, P < 0.001) (Fig. 1A), WOMAC function (r = 0.533, P < 0.001) (Fig. 1B) and WOMAC total scores (r = 0.491, P < 0.001) (Fig. 1D). However, the correlation between SF resistin levels and WOMAC stiffness scores did not reach significance(r = 0.066, P > 0.05) (Fig. 1C). And we surprisingly found, there were no significant associations between serum resistin concentrations and WOMAC subscale or total scores (P < 0.05) as well as K‐L Grading system in OA patients (P < 0.05) (Figs. 2 and 4).The correlation between serum and SF resistin levels and WOMAC scores was further evaluated by multinomial logistic regression analysis. Multiple regression analysis showed that the correlation between SF resistin concentrations and WOMAC scores was still significant after adjusting for other confounding factors (Table 2.
Figure 3.
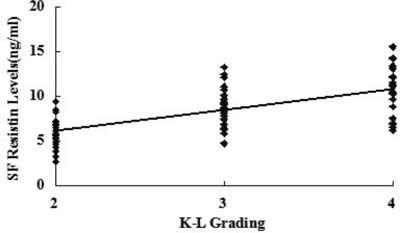
Correlation of SF resistin levels (ng/ml) in knee OA patients with radiographic severity measured by K–L grading scale.
Figure 1.
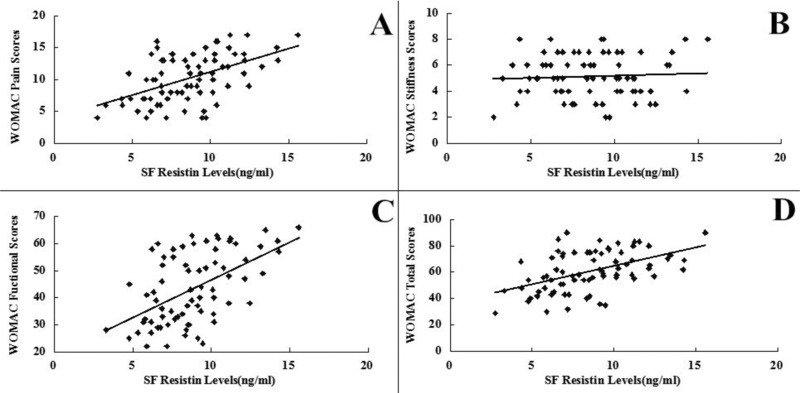
Correlation of SF resistin levels (ng/ml) with WOMAC pain (A), stiffness (B), physical function (C), and total (D) scores in OA patients.
Figure 2.
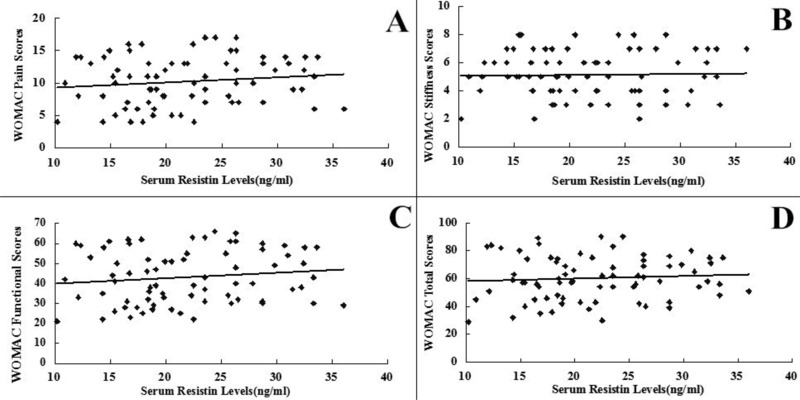
Correlation of serum resistin levels (ng/ml) with WOMAC pain (A), stiffness (B), physical function (C), and total (D) scores in knee OA patients.
Figure 4.
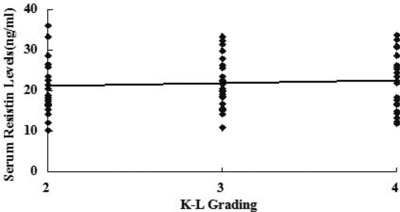
Correlation of SF resistin levels (ng/ml) in knee OA patients with radiographic severity measured by K–L grading scale.
Table 2.
Multivariate Linear Regression
| (WOMAC P) | (WOMAC S) | (WOMAC F) | (WOMAC T) | |||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Age | −0.035 | 0.810 | −0.027 | 0.854 | −0.056 | 0.732 | ‐0.041 | 0.772 |
| BMI | 0.126 | 0.142 | 0.142 | 0.117 | 0.156 | 0.101 | 0.130 | 0.138 |
| Gender | 0.079 | 0.31 | 0.085 | 0.294 | 0.053 | 0.734 | 0.064 | 0.661 |
| Diabetes suffering | 0.096 | 0.188 | 0.092 | 0.190 | 0.051 | 0.740 | 0.083 | 0.298 |
| SF resistin | 1.663 | 0.001 | 0.127 | 0.142 | 1.746 | 0.001 | 1.528 | 0.001 |
| Serum resistin | 0.053 | 0.733 | 0.031 | 0.818 | 0.067 | 0.667 | 0.058 | 0.728 |
WOMAC P/S/F/T = WOMAC pain/stiffness/functional/total.
Association between synovial fluid resistin levels and cartilage damage degree
In order to identify the role that resistin plays in the cartilage damage process, we explored the relationship between synovial fluid resistin levels and Noyes rating scores as well as cartilage degeneration mark CTX‐II. Interestingly, we found synovial fluid resistin concentrations were positively correlated with Noyes scores (r = 0543 P < 0.001) and CTX‐II (r = 0.411, P = 0.001) (Figs. 5 and 6).
Figure 5.
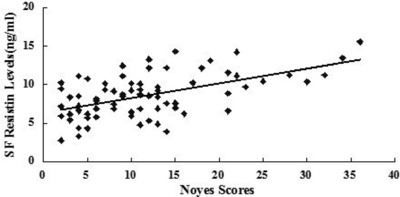
Correlation of SF resistin levels (ng/ml) in knee OA patients with cartilage damage extent according to Noyes scores.
Figure 6.
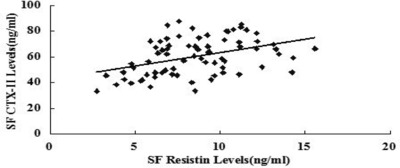
. Correlation of SF resistin levels (ng/ml) in knee OA patients with SF CTX‐II levels.
DISCUSSION
The current study investigated the relationship between resistin expression in SF and serum and disease severity as well as cartilage damage degree. Notably for the first time, in SF instead of serum, positive correlations were found between resistin and the Noyes Scores, SF CTX‐II concentrations as well as K‐L Radiographic Grading, WOMAC pain, WOMAC Functional and WOMAC total scores. These results indicate that resistin in SF plays an important role in the progression of knee OA and may serve as a novel and reliable biomarker for reflecting disease severity and cartilage degree.
Osteoarthritis (OA) is a debilitating degenerative joint disease particularly affecting weight‐bearing joints within the body, principally the knees. In recent years, biochemical markers show promise in determination of the disease severity and monitoring of the efficacy and safety of disease‐modifying OA drugs, with the potential to act as diagnostic and prognostic tools 26. The presence of local joint inflammation and altered cartilage and bone turnover in OA implicates a potential role for a range of biomarkers in OA progression.
Adipokines are protein mediators secreted by adipose tissue. Recently, adipokines have also been involved in the regulation of inflammation and catabolic process of in various arthritis including osteoarthritis 27, 28. Resistin, known as one of adipocyte‐secreted factors (ADSF), was proposed as a potential link between obesity and diabetes 29. It has been proved that resistin in humans is synthesized predominantly by mononuclear cells both within and outside adipose tissue 13. Recently, the involvement of resistin in bone metabolism has been widely studied. Resistin could upregulate expression of MMP‐1, MMP‐13, and ADAMTS‐4 in human articular chondrocytes 16. Furthermore, resistin is capable of stimulating proteoglycan degradation, as well as inhibiting the production of proteoglycan and type Ⅱ collagen in mouse and human cartilage explants 16.
In the present study, serum resistin levels were found significantly higher than matched synovial levels, which is consistent with the previous study 30. Most controversial studies focused on the relationship between systemic resistin concentration and hand OA. One study showed serum resistin levels was associated with radiographic subchondral erosion in patients suffering from hand OA 31. However, resistin was not correlated with radiographic progression in hand OA in another small cross‐sectional study 32. In our study, the correlation between serum levels of resistin and radiographic, cartilage damage, and WOMAC scores in knee OA did not reach significance, indicating that hand osteoarthritis tends to be systemic whereas the involvement of resistin in knee OA is local and the pathological process is probably taken place in the joint. This implicates that resistin could be locally released and may participate the inflammation process merely within the articular joint.
In contrast with the serum resistin, we notably found that elevated resistin concentrations in SF were positively related to greater pain and worse physical disability in OA patients. In addition, increased SF resistin levels also correlated with cartilage damage defined by Noyes scores, SF levels CTX‐II as well as K‐L Grading. This result is concordant with a previous study that SF resistin concentration is associated with inflammation markers IL‐6 and matrix metalloproteinases MMP‐1, MMP‐3 in knee OA patients 19. In a respective study, SF resistin levels are positively associated with systemic markers of inflammation 33. It should be noted that levels of resistin in the OA synovial fluid was significant lower than in matched OA serum levels in that resistin is widely secreted in various organs as mentioned above.
There several limitations should be taken into account. First, the sample size was relatively small among Chinese individuals. Further multicentral study in a larger sample is required to identify the results. Second, this study was cross‐sectional; therefore, our findings should be validated in long‐term prospective studies. Third, we only examed resistin, which is one of the adipokines involved in the progression of OA, other biomarkers like leptins and adiponectin as well as their correlations with OA deserve further intensive study. Last, we did not examine the resistin levels after arthroscopic operations.
In summary, patients with primary knee OA had elevated levels of serum resistin compared with healthy controls. We further performed this study aiming at relating serum and SF levels of resistin to the disease severity knee OA. We demonstrated that SF but not serum resistin concentrations significantly correlated with the magnitude of OA radiographic progression and symptomatic severity as well as cartilage damage. Resistin in SF measurement may serve as a novel biochemical marker of disease progression with the potential to contribute to the fundamental processes underlying the pathogenesis of knee OA. Further intensive studies are warranted to elucidate the influence of resistin on disease outcome.
REFERENCES
- 1. Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum 2000;43:1916–1926. [DOI] [PubMed] [Google Scholar]
- 2. Lawrence RC, Felson DT, Helmick CG, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am 2004;42:1–9. [DOI] [PubMed] [Google Scholar]
- 4. Toda Y, Toda T, Takemura S, et al. Change in body fat, but not body weight or metabolic correlates of obesity, is related to symptomatic relief of obese patients with knee osteoarthritis after a weight control program. J Rheumatol 1998;25:2181–2186. [PubMed] [Google Scholar]
- 5. Kraus V, Nevitt M, Sandell L. Summary of the OA Biomarkers Workshop 2009‐biochemical biomarkers: biology, validation, and clinical studies. Osteoarthritis Cartilage 2010;18:742–745. [DOI] [PubMed] [Google Scholar]
- 6. Kraus V, Burnett, B , Coindreau, et al. OARSI FDA Osteoarthritis Biomarkers Working Group. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage 2011;19:515–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang EH, Lee YJ, Kim TK, et al. Adiponectin is a potential catabolic mediator in osteoarthritis cartilage. Arthritis Res Ther 2010;12:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNulty AL, Miller MR, O'Connor SK, et al. The effects of adipokines on cartilage and meniscus catabolism. Connect Tissue Res 2011;52:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poonpet T, Honsawek S. Adipokines: Biomarkers for osteoarthritis? World J Orthop 2014;18:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu PF, Bao JP, Wu LD. The emerging role of adipokines in osteoarthritis: A narrative review. Mol Biol Rep 2011;38:873–878. [DOI] [PubMed] [Google Scholar]
- 11. Steppan CM, Brown EJ, Wright CM, Bhat S, et al. A family of tissue‐specific resistin‐like molecules. Proc Natl Acad Sci USA 2001;98:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nogueiras R, Gallego R, Gualillo O, et al. Resistin is expressed in different rat tissues and is regulated in a tissue‐and gender‐specific manner. FEBS Lett 2003;548:21–27. [DOI] [PubMed] [Google Scholar]
- 13. Savage DB, Sewter CP, Klenk ES, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator‐activated receptor‐gamma action in humans. Diabetes 2001;50:2199–2202. [DOI] [PubMed] [Google Scholar]
- 14. Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin, an adipokine with potent proinflammatory properties. J Immunol 2005;174:5789–5795. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Z, Xing X, Hensley G, et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum 2010;62:1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JH, Ort T, Ma K, et al. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis Cartilage 2009;17:613–620. [DOI] [PubMed] [Google Scholar]
- 17. Filková M, Lisková M, Hulejová H, et al. Increased serum adiponectin levels in female patients with erosive compared with non‐erosive osteoarthritis. Ann Rheum Dis 2009;68:295–296. [DOI] [PubMed] [Google Scholar]
- 18. Perruccio A, Mahomed N, Chandran V, et al. Plasma adipokine levels and their association with overall burden of painful joints among individuals with hip and knee osteoarthritis. J Rheumatology 2014;. 41:334–337. [DOI] [PubMed] [Google Scholar]
- 19. Koskinen A, Vuolteenaho K, Moilanen T, et al. Resistin as a factor in osteoarthritis: Synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP‐1 and MMP‐3. Scand J Rheumatol 2014;43:249–253. [DOI] [PubMed] [Google Scholar]
- 20. Noyes FR, Stabiler CL. A system for grading anicular cartilage lesions at arthroscopy. Am J Sports Med 1989;17:505–513. [DOI] [PubMed] [Google Scholar]
- 21. Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatology 1988;15:1833–1840. [PubMed] [Google Scholar]
- 22. Bellamy N. WOMAC: A 20‐year experiential review of a patient‐centered self‐reported health status questionnaire. J Rheumatol 2002;29:2473–2476. [PubMed] [Google Scholar]
- 23. Ackerman IN, Tacey MA, Ademi Z, et al. Using WOMAC Index scores and personal characteristics to estimate Assessment of Quality of Life utility scores in people with hip and knee joint disease. Qual Life Res 2014;23:2365–2374. [DOI] [PubMed] [Google Scholar]
- 24. Leach RE, Gregg T, Siber FJ. Weight‐bearing radiography in osteoarthritis of the knee. Radiology 1970;97:265–268. [DOI] [PubMed] [Google Scholar]
- 25. Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blanco FJ. Osteoarthritis year in review 2014: We need more biochemical biomarkers in qualification phase. Osteoarthritis Cartilage 2014;22:2025–2032. [DOI] [PubMed] [Google Scholar]
- 27. Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–783. [DOI] [PubMed] [Google Scholar]
- 28. Thommesen L, Stunes AK, Monjo M, et al. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem 2006;99:824–834. [DOI] [PubMed] [Google Scholar]
- 29. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature 2001;409:307–312. [DOI] [PubMed] [Google Scholar]
- 30. Degawa‐Yamauchi M, Bovenkerk JE, Juliar BE, et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab 2003;88:5452–5455. [DOI] [PubMed] [Google Scholar]
- 31. Choe JY, Bae J, Jung HY, et al. Serum resistin level is associated with radiographic changes in hand osteoarthritis: Cross‐sectional study. Joint Bone Spine 2012;79:160–165. [DOI] [PubMed] [Google Scholar]
- 32. Massengale M, Lu B, Pan JJ, et al. Adipokine hormones and hand osteoarthritis: Radiographic severity and pain. PLoS One 2012;7:e47860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schäffler A, Ehling A, Neumann E, et al. Adipocytokines in synovial fluid. JAMA 2003;290:1709–1710. [DOI] [PubMed] [Google Scholar]


