Abstract
Background
Laboratory diagnosis of neurosyphilis is complicated especially when it is asymptomatic, no single laboratory test result being appropriate to diagnose central nervous system infectivity caused by Treponema pallidum. Our objective was to evaluate two polymerase chain reaction (PCR) techniques for the detection of T. pallidum DNA in the cerebrospinal fluid (CSF) of patients with syphilis.
Methods
One hundred twenty‐four CSF samples from patients with reactive blood tests for syphilis were obtained. Two PCR techniques (47‐PCR, polA‐PCR) were used to detect T. pallidum DNA. The laboratory criteria used for the diagnosis of neurosyphilis to which the PCR techniques were compared were those recommended by the IUSTI: 2008 European guidelines on the management of syphilis.
Results
Treponema pallidum DNA was detected amplified in 37 of 124 (29.8%) and 30 of 124 (24.2%) samples with the 47‐PCR and polA‐PCR, respectively. Sensitivities were 75.8% and 69.7% and specificities 86.8% and 92.3%, respectively, for 47‐PCR and polA‐PCR techniques, respectively. The three CSF samples of patients with primary syphilis did not fulfill the criteria of neurosyphilis and DNA was only detected in one by the 47‐PCR. In samples from secondary syphilis and neurosyphilis, three of nine and nine of nine respectively, results were coincident for the two PCR techniques and neurosyphilis criteria. Major discrepancies between the two PCR techniques and neurosyphilis diagnostic criteria were observed in latent syphilis.
Conclusion
Beyond some limitations of the study, which are discussed here, both PCR techniques seem to be useful for the diagnosis of neurosyphilis, although 47‐PCR presents a higher sensitivity and polA‐PCR a higher specificity.
Keywords: neurosyphilis, T. pallidum, CNS infection
INTRODUCTION
Treponema pallidum sp. pallidum, the bacterium that causes syphilis, disseminates through the body, including cerebral spinal fluid (CSF), within hours of infection (1). Neuroinvasion occurs early in the course of syphilis and T. pallidum can be spontaneously cleared from the CNS or the infection may persist as asymptomatic syphilis meningitis or progress to acute syphilis meningitis. In the absence of therapy, the meningeal infection may develop to meningo‐vascular syphilis or to the late form of neurosyphilis 2, 3.
Neurosyphilis may be symptomatic and asymptomatic 2, 3. It can be divided into early and late forms. The first occurs weeks or few years after primary infection and may affect the CSF, meninges, or blood vessels of the central nervous system (CNS). Late forms involving brain and spinal cord parenchyma can occur some years or decades after initial infection. The diagnosis of symptomatic neurosyphilis is based on the following criteria: clinical evidence, reactive serological tests for syphilis, and CSF abnormalities. When it is asymptomatic, its diagnosis relies only on the latter two 2, 3, 4.
In relation with CSF abnormalities, some authors believe that a reactive CSF‐VDRL/RPR test confirms the diagnosis of neurosyphilis 5, 6 and that CSF pleocytosis (>5–10 white cell count/mm3) and raised protein level are suggestive of this infection 7, 8, 9. Other authors state that when the CSF‐FTA‐Abs test is nonreactive, the diagnosis should be excluded 5, 9, 10, 11. Although the rabbit infectivity test (RIT) has been considered the gold standard for T. pallidum detection, since it is only positive in the context of viable organisms, it is cumbersome and requires animals’ availability and longtime procedures 4. This is why it became impracticable in the routine of clinical laboratories 12, 13. Taking that into account, the CSF‐VDRL is considered the standard test for the diagnosis of neurosyphilis, since it is highly specific in the CSF 5, 6. However, because its sensitivity can be as low as 30%, active disease may be present with a nonreactive CSF‐VDRL 5, 6, 9. False‐positive CSF‐VDRL/RPR reactions also occur, especially when there is contamination with blood 14, 15. Other diseases, such as HIV, can produce pleocytosis and raised protein concentration that may be indistinguishable from that due to neurosyphilis 16, but a CSF pleocytosis of more than 20 cell/mm3 is probably related to neurosyphilis and not to HIV infection alone 17.
Molecular biology techniques, such as polymerase chain reaction (PCR), amplifying a segment of the 47‐kDa protein gene 18, 19, 20, 21, 22, 16S RNA 23, 24, and T. pallidum polA gene 25, 26, 27, 28 have been used for the diagnosis of syphilis. The objective of the present study was to evaluate the accuracy of two PCR techniques, for the detection of T. pallidum DNA in the CSF of patients with syphilis.
MATERIALS AND METHODS
Cerebral spinal fluid samples were obtained from 124 patients with reactive blood tests for syphilis during 4 years. Diagnosis of neurosyphilis is based on positive CSF THPA and/or FTA‐Abs test together with an increased white blood cell count (>5–10/mm3) or the positive RPR/VDRL 29. We decided to use these criteria despite the fact the new guidelines for syphilis have been issued, because no specific criteria are used in the 2014 Syphilis Guidelines 6.
Patients’ syphilis stages, according to the 2014 European guidelines 6, are described in Table 1. Latent syphilis was not differentiated into early and latent, since the duration of the disease was not known. One hundred CSF samples from individuals with neurological symptoms but no history of syphilis and nonreactive serology for this infection were used as negative controls. None of the patients received any antibiotic treatment for CSF infection.
Table 1.
Characterization of Patients’ Syphilis in Accordance to the 2014 European Guidelines 6
| Syphilis stage | No./total number of patients (%) |
|---|---|
| Primary syphilis | 3/124 |
| (2.41%) | |
| Secondary syphilis | 5/124 |
| (4.03%) | |
| Neurologic syphilis | 9/124 |
| (7.25%) | |
| Latent syphilis | 80/124 |
| (64.51%) | |
| Treated syphilisa | 27/124 |
| (21.77%) |
Considered when patients had been treated for syphilis.
Two trained microbiologists did all the laboratory work of this study and were blinded to the results of the other tests. The Scientific Council of the Instituto de Higiene e Medicina Tropical approved the study, since it represents the research committee on human subjects.
White blood cell count, RPR, VDRL, TPHA, and FTA‐Abs tests and two PCR techniques were performed in every CSF sample. DNA extraction was performed with the Mini‐kit QIAamp Blood Qiagen Blood/tissue (Qiagen, UK) in 200 μl of CSF samples. Before the precipitation step with ethanol, 3 μl of a dilution of calf thymus DNA (10.5 mg/ml; Sigma, Germany) was added to serve as carrier. Primers, sequence bases, and target used in the PCR techniques were described by Orle et al. 20 and Liu et al. 25.
The first PCR technique (47‐PCR) was performed with the primers KO3A/KO4 to amplify a fragment of the T. pallidum 47‐kDa protein gene, as referred by Orle et al. To perform the other PCR test (polA‐PCR), the primers polA‐F/polA‐R were used to amplify a fragment of the DNA polymerase I gene, according to Liu et al.
DNA amplification was performed in a reaction mixture with a total of 25 μl containing PCR buffer with 3 mM of MgCl2 (Citomed, Bioline UK), 10 mmol/l each of the deoxynucleoside triphosphates (Ultrapure dNTP set, Amersham Pharmacia Biotech), 25 pmol/l of each primer, and 1.5 U of Taq polymerase (Imolase, Citomed). DNA extracted from the clinical specimen (5 μl), 2 μl of T. pallidum Nichols strain DNA (extracted from a suspension with 10 × 102 T. pallidum Nichols strain), and 5 μl of DNA extracted from CSF samples from individuals with nonreactive syphilis serology were each added to the previous mixture in three different tubes. The last two were used as positive and negative controls, respectively. Distilled water instead of DNA served as a negative control for the PCR conditions.
The PCR reaction was performed in a thermal cycler (Master cycler personal, Eppendorf) with an initial cycle at 95ºC during 5 min to activate the polymerase. This initial cycle was followed by 40 cycles—94ºC for 20 s, 60ºC for 20 s, and 72ºC for 20 s—when performing 47‐PCR—and 45 cycles—94ºC for 20 s, 62ºC for 20 s, and 72º C for 30 s—for polAPCR. Final extension consisted of one cycle at 72ºC for 5 (47‐PCR) or 15 min (polA‐PCR) and then tubes were stored at 4ºC until analyzed. The amplicons were detected by electrophoresis using a 1.5% agarose gel (Bio‐Rad) and the gels were run with a 50‐bp ladder (Citomed) at 100 V for 1 h. After staining with ethidium bromide (0.5 mg/ml, Sigma), bands were visualized on a UV transilluminator Eagle Eye II (Stratagene). The specimens were scored as positive if a band of 260 bp was visualized for the 47‐PCR, and a band of 378 bp for the polA‐PCR.
RESULTS
All samples studied were from adult individuals and all tests were performed in every one of them. All CSF samples (100) from individuals serving as negative controls had nonreactive CSF syphilis serology (both nontreponemal and treponemal tests) and T. pallidum DNA was not amplified in any of the two PCR tests.
Results from 124 CSF samples from patients with syphilis discriminated by PCR type and neurosyphilis criteria are discriminated in Figure 1. Treponema pallidum DNA was amplified in 37 of 124 (29.8%) and 30 of 124 (24.2%) with the 47‐PCR and polA‐PCR techniques, respectively. Among those, 33 had neurosyphilis criteria, 25 (75.8%) with DNA detected by 47‐PCR and 23 (69.7%) by polA‐PCR. In total, among the 91 samples, which did not present any neurosyphilis criteria, 12 were positive with the PCR‐47 and 7 with the polA‐PCR.
Figure 1.
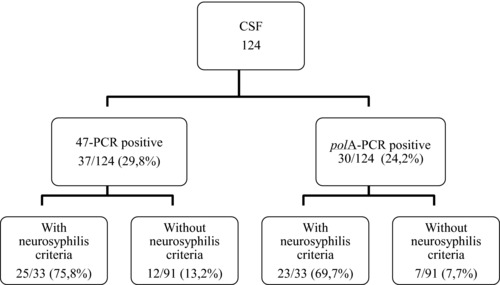
Syphilis patients’ results discriminated by PCR type and neurosyphilis criteria.
The sensitivity and specificity of the two PCR techniques were determined taking into account the criteria for neurosyphilis. The results obtained are discriminated in Table 2. Sensitivities was 75.8% and 69.7% and specificities were 86.8% and 92.3%, respectively, for the 47‐PCR and polA‐PCR techniques.
Table 2.
Sensitivity and Specificity of Each PCR Technique
| 47‐PCR | polA‐PCR | ||||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Neurosyphilis criteria | Present (33) | 25 | 8 | 23 | 10 |
| Not present (91) | 12 | 79 | 7 | 84 | |
| Sensitivity (25/33)—75.8% | Sensitivity (23/33)—69.7% | ||||
| Specificity (79/91)—86.8% | Specificity (84/91)—92.3% | ||||
Results from each PCR technique discriminated by syphilis stage are described in Table 3. In primary syphilis, one CSF sample, with no criteria of neurosyphilis, was only positive in the PCR‐47, while in secondary and neurologic syphilis, CSF samples positive in both PCR techniques, respectively three and nine samples, also had neurosyphilis criteria. The remaining primary and secondary syphilis samples did also not present any neurosyphilis criteria.
Table 3.
PCR Techniques’ Results Obtained in the CSF Samples From Patients With Syphilis
| 47‐PCR No. +/total (%) | polA‐PCR No. +/total (%) | |
|---|---|---|
| Primary syphilis | 1/3 | 0/3 |
| (33.3%) | (0%) | |
| Secondary syphilis | 3/5 | 3/5 |
| (60%) | (60%) | |
| Neurologic syphilis | 9/9 | 9/9 |
| (100%) | (100%) | |
| Latent syphilis | 22/80 | 16/80 |
| (27.5%) | (20%) | |
| Treated syphilisa | 2/27 | 2/27 |
| (7.4%) | (7.4%) |
Considered when patients had been treated for syphilis.
“+” signifies reactive.
In latent syphilis, T. pallidum DNA was detected in 22 samples with the 47‐PCR and in 16 with the polA‐PCR; however, in seven CSF samples DNA was only detected by 47‐PCR. Among those, five did not present neurosyphilis laboratory criteria, while in two, criteria were present. PolA‐PCR alone was only positive in one sample, which did not have neurosyphilis laboratory criteria. Three samples, from patients with treated syphilis, were positive in the PCR techniques as follows: one in both, and the remaining two in the 47‐PCR and polA‐PCR techniques, respectively. Neurosyphilis laboratory criteria were not found in any of these samples.
DISCUSSION AND CONCLUSIONS
The diagnosis of neurosyphilis, especially when it is asymptomatic, is still difficult, since none of the tests used alone are reliable. Therefore, different laboratory parameters should be evaluated together for the diagnosis of neurosyphilis 5. In this study, we decided to compare two PCR techniques with the laboratory diagnostic criteria recommended in the IUSTI European guidelines on the management of syphilis 29. Our diagnostic criteria did not consider the clinical manifestations, since it is known that when those are included, the sensitivity varies enormously, between 22.2% and 100% 30. We have decided to use both RPR/VDRL tests, since they have been shown to be comparable for the diagnosis of neurosyphilis in a previous paper 31. Either of them is considered in the 2014 European guidelines on the management of syphilis 6.
In this study, T. pallidum DNA was identified in a higher number of samples with the 47‐PCR technique than when the polA‐PCR technique was used. However, taking into account the laboratory diagnostic criteria used in this study, a higher number of CSF samples positive in the 47‐PCR (11) had no neurosyphilis criteria than the polA‐PCR (7). In accordance with that, when sensitivity was calculated using those criteria as the gold standard, the 47‐PCR technique showed to have a higher sensitivity than the polA‐PCR (75.8% vs. 69.7%), the specificity being higher for the latter PCR (92.3%) when compared with the first (86.8%).
As expected, results obtained with the PCR techniques varied in accordance with the stage of syphilis. All samples from patients considered to have neurologic syphilis, previously classified by clinics as symptomatic neurosyphilis, were positive in both PCR techniques and the neurosyphilis diagnostic criteria used in the present study. It seems to us that all of these were true neurosyphilis cases. Therefore, clinicians’ suspicion of neurosyphilis in the basis of clinical syphilis symptoms seem to be highly associated with reactive syphilis tests and positive PCR techniques. On the contrary, the positive 47‐PCR sample in primary syphilis seems to be a false positive as the remaining PCR technique and neurosyphilis diagnostic criteria were negative. In secondary syphilis, from the five suspected neurosyphilis cases, three were positive in all tests used (neurosyphilis laboratory criteria, 47‐PCR and polA‐PCR), giving the PCR technique a high sensitivity and specificity when compared with the neurosyphilis diagnostic criteria. The major problem found was in patients with latent syphilis, in whom a higher number of discrepant results were observed: seven samples being positive in the 47‐PCR alone, two having neurosyphilis criteria (probably true positives) and five with no neurosyphilis criteria (probably false positives), and one in which DNA was only detected by the polA‐PCR with no neurosyphilis criteria (probably false positive). This is not surprising, since latent syphilis has generally less T. pallidum in circulation 1, 12, 14 and therefore DNA may not be detected. Referring to samples from treated patients that were positive in any of the PCR techniques used in these study, none of them had neurosyphilis criteria. In view of these results, the 47‐PCR technique seems to be more sensitive and the polA more specific.
The limitations of the present study should also be discussed. We did not have any information regarding if the patients were in the early or late latent stage of syphilis, which could interfere in the differentiation between the immunological reaction of late neurosyphilis and the presence of organisms in the CSF, which may happen in meningitis or early neurosyphilis. The number of non‐HIV‐infected individuals was small, thus not allowing a comparison between the two groups. One of the criteria for neurosyphilis was the number of cells greater than or equal to 10/mm3 and not greater than or equal to 20/mm3, as recommended by Marra et al. 24 in HIV‐infected patients, since in the first place we have always considered the cells together with a reactive FTA‐Abs and we were not looking at the number of cells as criteria on its own. Most importantly, we had to establish criteria for neurosyphilis and we decided to choose the maximum number recommended in the IUSTI guidelines 29, in which the cell number range from 5 to 10/mm3. Therefore, and although these PCR techniques seem useful in the diagnosis of neurosyphilis, further studies are needed with a high number of patients and with long follow‐up periods, in view of a better clarification of the results obtained here.
REFERENCES
- 1. Larsen SA, Norris SJ, Steiner BM, Rudolph AH. Syphilis and related treponematoses In: Hausler WJ, Jr., Sussman M. (Eds.), Microbiology and Microbial Infections, volume 3, New York: Oxford University Press; 1998. p. 641–668. [Google Scholar]
- 2. Golden MR, Marra CM, Holmes KK. Update on syphilis: Resurgence of an old problem. JAMA 2003;290:1510–1514. [DOI] [PubMed] [Google Scholar]
- 3. Marra CM. Update on neurosyphilis. Curr Infect Dis Rep 2009;11:127–134. [DOI] [PubMed] [Google Scholar]
- 4. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev 1995;8:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC) . Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010;59(RR‐12):1–110. [PubMed] [Google Scholar]
- 6. Janier M, Hegyi V, Dupin N, et al. European guideline on the management of syphilis. J Eur Acad Dermatol Venereol 2014;28:1581–1593. [DOI] [PubMed] [Google Scholar]
- 7. Hooshmand H, Escobar MR, Kopf SW. Neurosyphilis. A study of 241 patients. JAMA 1972;219:726–728. [DOI] [PubMed] [Google Scholar]
- 8. Larsen SA, Johnson RE. Diagnostic test In: Larsen S, Pope V, Johnson RE, Kennedy ED. (Eds.), Manual of Tests for Syphilis, ninth edition, Washington, DC: American Public Health Association; 1998. p. 1–52. [Google Scholar]
- 9. Luger AF, Schmidt BL, Kaulich M. Significance of laboratory findings for the diagnosis of neurosyphilis. Int J STD AIDS 2000;11:224–234. [DOI] [PubMed] [Google Scholar]
- 10. Jaffe HW, Larsen SA, Peters M, Jove DF, Lopez B, Schroeter AL. Tests for treponemal antibody in CSF. Arch Intern Med 1978;138:252–255. [PubMed] [Google Scholar]
- 11. Marra CM, Critchlow CW, Hook EW, Hook EW 3rd, Collier AC, Lukehart SA. Cerebrospinal fluid treponemal antibodies in untreated early syphilis. Arch Neur 1995;52:68–72. [DOI] [PubMed] [Google Scholar]
- 12. Sanchez PJ, Wendel GD, Grimprel E, et al. Evaluation of molecular methodologies and rabbit infectivity testing for diagnosis of congenital syphilis and neonatal central nervous invasion by Treponema pallidum . J Infect Dis 1993;67:148–157. [DOI] [PubMed] [Google Scholar]
- 13. Wicher K, Horowitz HW, Wicher V. Laboratorial methods of diagnosis of syphilis for the beginning of the third millennium. Microbes Infect 1999;1:1035–1049. [DOI] [PubMed] [Google Scholar]
- 14. Izzat NN, Bartruf JK, Glicksman JM, Holder WR, Knox JM. Validity of the VDRL test on cerebrospinal fluid contaminated by blood. Br J Vener Dis 1971;47:162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh AE, Romanowski B. Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev 1999;12:187–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marshall DW, Brey RL, Butzin CA, Lucey DR, Abbadessa SM, Boswell RN. CSF changes in a longitudinal study of 124 neurologically normal HIV‐1‐infected U.S. Air Force personnel. J Acquir Immune Defic Syndr 1991;4:777–781. [PubMed] [Google Scholar]
- 17. Collier AC, Marra C, Coombs RW, et al. Central nervous system manifestations in human immunodeficiency virus infection without AIDS. J Acquir Immune Defic Syndr 1992;5(3):229–241. [PubMed] [Google Scholar]
- 18. Hay PE, Clark JR, Taylor‐Robinson D, Golmeier D. Detection of treponemal DNA in the CSF of patients with syphilis and HIV infection using the polymerase chain reaction. Genitourin Med 1990;66:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung KY, Lee MG, Lee JB. Detection of Treponema pallidum by polymerase chain reaction in the cerebrospinal fluid of syphilis patients. Yonsei Med J 1994;35:190–197. [DOI] [PubMed] [Google Scholar]
- 20. Orle KA, Gates CA, Martin DH, Body BA, Weiss JB. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol 1996;34:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gayet‐Ageron A, Ninet B, Toutous‐Trellu L, et al. Assessment of a real‐time PCR test to diagnose syphilis from diverse biological samples. Sex Transm Dis 2012. 39(4):291–297. [DOI] [PubMed] [Google Scholar]
- 22. Grange PA, Gressier L, Dion PL, et al. Evaluation of a PCR test for detection of Treponema pallidum in swabs and blood. J Clin Microbiol 2012;50:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centurion‐Lara A, Castro C, Shaffer JM, van Voorhis WC, Marra CM, Lukehart SA. Detection of Treponema pallidum by a sensitive reverse transcriptase PCR. J Clin Microbiol 1997;35:1348–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marra CM, Maxwell CL, Smith SL, et al. Cerebrospinal fluid abnormalities in patients with syphilis: Association with clinical and laboratory features. J Infect Dis 2004;189:369–376. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Rodes B, Chen CY, Steiner B. New tests for syphilis: Rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J Clin Microbiol 2001;39:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leslie DE, Azzato F, Karapanagiotidis T, Leydon J, Fyfe J. Development of a real‐time PCR assay to detect Treponema pallidum in clinical specimens and assessment of the assay's performance by comparison with serological testing. J Clin Microbiol 2007;45(1):93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castro R, Prieto E, Aguas MJ, et al. Detection of Treponema pallidum sp pallidum DNA in latent syphilis. Int J STD AIDS 2007;18:842–845. [DOI] [PubMed] [Google Scholar]
- 28. Shields M, Guy RJ, Jeoffreys NJ, Finlayson RJ, Donovan B. A longitudinal evaluation of Treponema pallidum PCR testing in early syphilis. BMC Infect Dis 2012;12:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. French P, Gomberg M, Janier M Schmidt B, van Voorst Vader P, Young H. IUSTI: 2008 European guidelines on the management of syphilis. Int J STD AIDS 2009;20:300–309. [DOI] [PubMed] [Google Scholar]
- 30. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal‐specific antibody tests in neurosyphilis: A systematic review. Sex Transm Dis 2012;39(4):291–297. [DOI] [PubMed] [Google Scholar]
- 31. Castro R, Prieto ES, da Luz Martins Pereira F. Nontreponemal tests in the diagnosis of neurosyphilis: An evaluation of the Venereal Disease Research Laboratory (VDRL) and the Rapid Plasma Reagin (RPR) tests. J Clin Lab Anal 2008;22(4):257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]


