Abstract
Background
Allergic rhinitis has been suspected to be a risk factor for asthma in several studies but this association is not firmly established. The objective of this study was to synthesize the evidence of the association between allergic rhinitis and the risk of asthma through a systematic review and meta-analysis.
Methods
We performed a search in Medline, Scopus, ISI Proceedings databases and other databases from inception until February 2019, followed by manual search to identify potentially relevant case-control and cohort studies that reported relative risk estimates and confidence intervals of the association between allergic rhinitis and asthma. Cross-sectional studies were excluded. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using fixed and random effects models and quality of studies was assessed through a modified version of the Newcastle-Ottawa scale.
Results
Twenty-nine eligible studies, 22 cohort and 7 case-control studies, with a total of 274489 subjects, were included in the meta-analysis. The results show that history of allergic rhinitis is significantly associated with the occurrence of asthma (OR = 3.82; 95% CI: 2.92–4.99). European studies showed a stronger association (OR = 4.35; (95% CI: 3.12–6.06) than non-European studies (OR = 2.75; 95% CI: 2.16–3.50), and case-control studies showed a stronger association (OR = 4.71; 95% CI: 3.58–6.17) than cohort studies (OR = 3.42; 95% CI: 2.60–4.50).
Conclusions
This meta-analysis shows that allergic rhinitis is strongly associated with asthma. Further prospective studies on the effect of treatment of allergic rhinitis on the development of asthma are needed. Relief of airway allergic manifestations may need dual control of allergic rhinitis and asthma.
Registration
PROSPERO database with registration number CRD42017055156.
Keywords: Allergic rhinitis, Asthma, Meta-analysis
Introduction
Asthma is an important health problem. Its prevalence is increasing worldwide, especially in low and middle income countries.1 The Global Burden of Disease 2015 estimated that nearly 400 million people around the world suffer from this condition. Asthma is considered as the 11th highest cause of Years Lived with Disability (YLDs) worldwide.2, 3
Allergic Rhinitis (AR) is a disease the prevalence of which is increasing in a similar fashion to that of asthma.1 It affects 10–30% of the general population and is suspected to be a risk factor for onset of asthma in several epidemiologic studies.4, 5, 6, 7 However, most studies of this relation are of a cross-sectional nature, a design that could prove inadequate for causal inference. Furthermore, the remaining case-control and cohort studies evaluating this association show effect estimates the magnitude of which vary considerably from study to study, swaying between absence of statistically significant association,8 and extremely strong relationships.9 Yet in 2012, the ARIA (Allergic Rhinitis and its Impact on Asthma) guidelines stated that sophisticated statistical methods should be implemented in order to have a more objective view of the link between AR and asthma.10
To provide a global assessment and a quantitative summary of the association between AR and asthma, we performed a systematic review and meta-analysis of cohort and case-control studies.
Methods
Search strategy
We searched MEDLINE via Pubmed, Scopus, the Conference Proceedings Citation Index-Science (CPCI–S) database, the Open Access Theses and Dissertations (OATD), and the 5 regional bibliographic databases of the World Health Organization (WHO) (African Index Medicus, Latin American and Caribbean Health Science Literature Database, Index Medicus for the Eastern Mediterranean Region, Index Medicus for South-East Asia Region, Western Pacific Region Index Medicus), from inception of each database to the end of February 2019, without any language limitation. The search strategy was as follows: ((((("Rhinitis, Allergic, Seasonal"[Mesh]) OR ("Rhinitis, Allergic"[Mesh]) OR "allergic rhinitis" OR rhinitis)))) AND (("Asthma"[Mesh]) OR asthma) AND (((("Case-Control Studies"[Mesh]) OR "Cohort Studies"[Mesh]) OR case-control) OR cohort) for Medline and equivalent wording in other databases. Due to the nature of exposure, rhinitis, our meta-analysis had to be based on observational studies only, in spite of their limitations. No experimental studies can be carried out on this topic. To find any additional published study, the reference lists of all retrieved articles and related review articles were searched manually. All searches were performed by two independent reviewers (HRT and NM) and disagreements about inclusion of some studies were resolved by consensus and, when needed, by consulting with a third party (BT). The review protocol was registered in PROSPERO with registration number CRD42017055156.
Inclusion criteria
Case-control and cohort studies were included if: 1) the main or secondary outcome was asthma, 2) the exposure of interest or one of the covariates was allergic rhinitis, 3) they reported a measure of association between allergic rhinitis and asthma such as Risk Ratio, Rate Ratio, Odds Ratio and their variance, standard error or 95% confidence intervals (CI), or provided enough raw data to calculate them. Whenever necessary, we contacted the authors to provide more information for the calculation of the effect measure or to clarify some methodologic aspects of the study.11, 12, 13, 14 Studies without comparison group (allergic rhinitis-free in cohort studies and asthma-free in case-control studies) and those that did not report measures of effect nor provide enough information for calculating them were excluded.13 If a study did not adjust for any factor, this study was not excluded but instead, we used its crude estimate.8, 15 In case of duplication of a publication, we included the most complete one. When we were unable to assess whether two publications were from the same study, we contacted their authors to consult about this issue.7, 16, 17, 18, 19, 20, 21
Data extraction
Data were extracted using a predefined form with the following information: first author, year of publication, country, study design, source of data, type of population, number of participants, mean age of participants, measure of association, and adjustment, restriction or matching factors. The data were extracted following the same procedure as that used for the bibliographic searches. The extraction was carried out independently by 2 authors and discrepancies were resolved by consensus.
Quality assessment
We assessed the quality of the studies using a modified version of the Newcastle-Ottawa scale that we adapted to the needs of this meta-analysis. We used a common quality assessment form for both case-control and cohort studies to be able to use their score in a comprehensive fashion. We first performed an analysis based on the global score and then subsequent analyses based on each criterion. The following 5 criteria were considered on a yes/no basis: 1) whether the target population was clearly defined or just based on convenience sampling, 2) whether asthma diagnosis is based on clinical features and spirometry (flow rate) and/or reversal after treatment or was only based on clinical examination, 3) whether rhinitis diagnosis is based on clinical and lab examination or was based on questionnaire only, 4) whether or not asthma symptoms clearly occurred after rhinitis onset, and 5) whether or not results were adjusted for age, sex and family history of asthma. Each item was scored as 0 or 1. Quality assessment was independently conducted by two investigators (HRT and NM) and disagreements were solved by a third investigator (BT).
Statistical methods
Risk Ratios and Rate Ratios in cohort studies and Odds Ratios in case control studies were considered as measures of effect in this meta-analysis. Odds Ratios from case-control studies were assumed to be unbiased estimates of the Rate Ratios.22 Heterogeneity among studies was evaluated via the Ri statistic (the proportion of total variance due to between-studies variance)23 as well as the DerSimonian & Laird Q test, in which a p-value<0.1 was considered as statistically significant. For pooling the effect size estimates, the inverse of variance was considered as the weight for each study. We computed both fixed effects and random effects estimates, but as heterogeneity was frequent in subgroup analyses, we presented random effects data only. For each study we used the estimate that was adjusted for the largest number of variables.
We performed subgroup analysis according to the design of studies, region, age group, quality score, and items of quality assessment. Cumulative meta-analysis was conducted to identify any trend in estimates across time. The Begg's rank correlation test and the Egger regression test were used to statistically assess publication bias. We also visually assessed publication bias using a funnel plot and performed the Trim-and-Fill procedure to estimate the number of potential missing studies in our meta-analysis and their effect on the outcome.24 HEpiMA software25 and STATA 12 (Stata Corp, College Station, TX, USA) were used for statistical analysis.
Results
We found a total of 29 eligible studies, including 22 cohort studies and 7 case-control studies with a total of 274,489 subjects.6, 7, 8, 9, 11, 12, 15, 16, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45
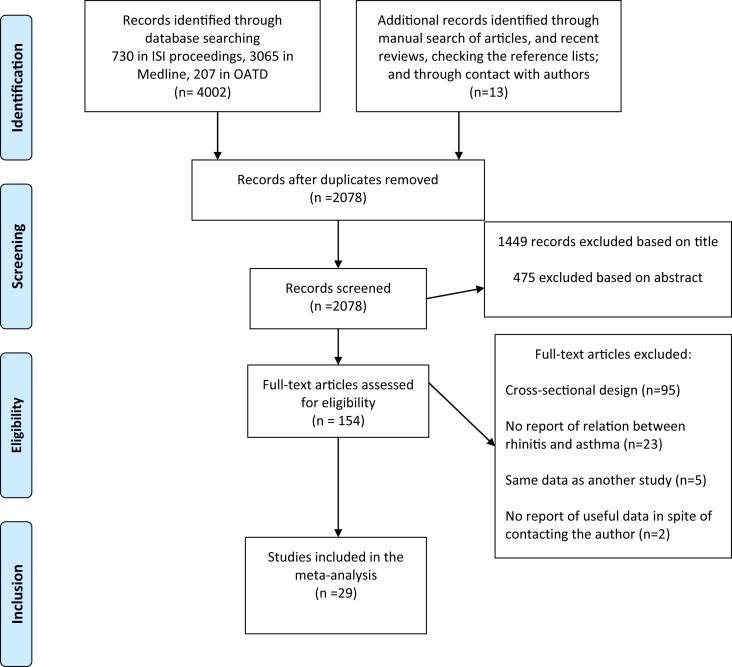
Twenty one studies were conducted in European countries and 8 in non-European countries between 1994 and 2019. Except for 2 articles published in Spanish44 and German,9 the rest were written in English. One article comprised 2 independent case-control studies carried out in different countries. We, therefore, considered these 2 studies separately.45 Among the studies that could have been potentially included in our meta-analysis, we excluded 3 articles17, 18, 19 because of overlap of data with a more comprehensive study.7 Furthermore, we found 3 articles from the same author,16, 20, 21 2 of which were excluded after consulting with the author.20, 21 We also excluded 1 conference abstract,46 as after contacting the authors, we were informed that the corresponding study was published later as a full paper in another language.9 One study was excluded as we could not trace its authors to collect the necessary information to calculate effect measures.13 Data from 2 studies were obtained through contact with the authors.11, 12 We did not find any relevant study published in a book or dissertation. The main characteristics of the remaining 29 studies included in this meta-analysis are summarized in Table 1, Table 2. The flow diagram presenting the screening process and the reasons for exclusion is presented in Fig. 147
Table 1.
Main characteristics of cohort studies included in the meta-analysis of association between allergic rhinitis and asthma.
| Author (Year) | Country | Source of data | Nº of cases of asthma/cohort size | Nº of allergic rhinitis patients | Age at start of cohort | Follow-up duration | RR (95%CI) | Adjusting or matching variables |
|---|---|---|---|---|---|---|---|---|
| Segura-Navas et al. (2018)40 | Spain | Cohort of schoolchildren in Castellón, Spain | 44/1280 | 106 | 13–15 years | 10 years | 4.05 (1.71–9.60) | Age, sex, family history asthma, family history of allergic rhinitis, history of respiratory diseases, breastfeeding, living place, passive smoking, mother's age at time of birth, and pet at home |
| Moshe et al. (2015)41 | Israel | All-male Israel Defense Forces (IDF)a | 1984/128591 | 4891 | 18–21 years | 3 years | 1.86 (1.57–2.21) | Sex |
| Tormanen et al. (2018)42 | Finland | Cohort of infant hospitalized for bronchiolitis | 18/138 | 39 | less than 6 months | 11–13 years | 4.06 (1.35–12.25) | Age, maternal asthma, maternal allergy, parental asthma, paternal allergy, maternal and paternal smoking |
| Carr et al. (2019)43 | USA | Tucson Children's Respiratory Study (TCRS) | 117/521 | 87 | 6 years | 26 years | 3.7 (2.2–6.1) | age, sex, ethnicity, maternal asthma, maternal education, maternal smoking at enrolment, and history of 4 + colds per year |
| Arnedo et al. (2007)44 | Spain | Cohort of schoolchildren in Castellón | 108/1698 | 107 | 6–7years | 8 years | 3.21 (l.45–7.09) | Age, sex, family history of asthma, family history of allergic rhinitis, history of bronchitis, mother's age, other respiratory diseases, passive smoking, pet at home, social class. |
| Verlato et al. (2016)27 | Italy | Italian Study on Asthma in young Adults (ISAYA) and Italian Study on the Incidence of Asthma (ISIA) | 145/5241 | 517 | 20–54 years | 9 years | 4.00 (3.68–4.35) | Age, sex, occupation, asthma-like symptoms, chronic bronchitis, smoking |
| Yeh et al. (2016)12 | Taiwan | National Health Insurance Research Database | 187/7599 | 2130 | 35–65 years | 11 years | 1.84 (1.38–2.45) | Age, sex, co-morbidities |
| Pallasaho et al. (2011)28 | Finland | FinEsS study, Helsinki | 157/6062 | 1387 | 20–69 years | 11 years | 2.00 (1.40–2.87) | Age, sex, family history of asthma, smoking |
| Rochat et al. (2010)29 | Germany | German Multicentre Allergy Study (MAS) birth cohort. | 50/1314 | 178 | 0 | 13 years | 6.46 (2.93–14.23) | Age, sex, study center, parental atopy, parental education, maternal smoking during pregnancy, siblingship |
| Ekerljung et al. (2008)31 | Sweden | Random sample of general population from Stockholm | 159/4479 | Not given | 20–69 years | 10 years | 2.21 (1.41–3.48) | Age, sex, family history of asthma, smoking |
| Shaaban et al. (2008)7 | 14 European countries | European Community Respiratory Health Survey (ECRHS) | 140/6461 | 1217 | 20–44 years | 9 years | 3.53 (2.11–5.91) | Age, sex, country, body mass index, IgE, FEV1, family history of asthma, history of respiratory infection, smoking |
| Rzehak et al. (2008)30 | Germany | Bitterfeld study | 142/3199 | 124 | 5–13 years | 12 years | 4.34 (2.73–6.9) | Age, sex, parental asthma |
| Morais-Almeida et al. (2007)32 | Portugal | Hospital database | 70/308 | 156 | 6 month-6 year old | 8 years | 15.76 (6.1–40.8) | Parental asthma, history of atopic eczema, history of food allergy, attendance of kindergarten, IgE, age of onset of symptoms, allergen sensitization |
| Burgess et al. (2007)37 | Australia | Tasmanian Asthma Study | 299/8583 | 1061 | 7 years | 37 years | 3.16 (2.46–4.05). | Age, history of allergy, impaired lung function, socioeconomic status |
| Porsbjerg et al. (2006)8 | Denmark | Civil Registry | 45/291 | 38 | 7–17 years | 12 years | 1.7 (0.70–3.90) | None |
| Thomsen et al. (2005)16 | Denmark | Danish Twin Registry | 838/19349 | 1667 | 12–41 years | 8 years | 3.90 (3.31–4.61) | Sex |
| Polosa et al. (2005)38 | Italy | University Allergy Clinic database | 161/436 | 332 | 18–40 years | 10 years | 7.81 (3.05–20.04) | Sex, family history of allergy, pet ownership, sensitization to allergens, parental smoking at home |
| Ronmark et al. (2002)34 | Sweden | Obstructive Lung Disease in Northern Sweden (OLIN) studies | 51/3247 | 156 | 7–8 years | 2 years | 5.91 (2.76–12.65) | Sex, family history of asthma, respiratory infections, breast feeding, mother's smoking, pets, dampness |
| Linneberg et al. (2002)11 | Denmark | Copenhagen Allergy Study | 48/1112 | Not given | 15–69 years | 8 years | 9.3 (4.38–19.74) | Age, sex |
| Huovinen et al. (1999)39 | Finland | Finnish Twin Cohort Study | 261/11540 | 656 | 18–45 years | 15 years | 3.14 (1.61–6.12) | Age, sex, dizygotic/monozygotic |
| Strachan et al. (1996)36 | England, Scotland and Wales | British 1958 cohort study | 2488/18559 | 1855 | 0 | 33 years | 1.42 (1.33–1.51) | Age, sex, maternal age, birth order, social class, smoking, gestation, albuminuria, bleeding in pregnancy, history of pneumonia, whooping cough, tonsillectomy, eczema, abdominal pain, vomiting, and migraine. |
| Settipane et al. (1994)15 | USA | Brown University cohort study | 97/1836 | 162 | 18 years | 23 years | 2.92 (1.55–5.48) | None |
Retrospective cohort study, RR; relative risk, CI; Confidence Interval
Table 2.
Main characteristics of case-control studies included in the meta-analysis of association between allergic rhinitis and asthma.
| Author (Year) | Country | Source of data | Cases/controls | Nº of allergic rhinitis patients | Age Mean ± SD | OR (95%CI) | Adjusting or matching variables |
|---|---|---|---|---|---|---|---|
| Jacob et al. (2016)6 | Germany | German pediatricians database | 34305/34305 | 24463 | 6–17 years | 5.38 (5.17–5.59) | Age, sex, co-morbidities |
| Coelho et al. (2016)26 | Brazil | Students registered in Family Health Strategy (FHS) program | 172/379 | 326 | 6–14 years | 3.35 (2.24–5.01) | Pets, passive smoking, consumption of cooked vegetables, consumption of fish, history of eczema, family history of allergy, skin tests for immediate hypersensitivity (STIH) |
| Tomic Spiric et al. (2007)9 | Serbia | Hospital database | 134/134 | Not given | Cases: 38.96 ± 12.37 years Controls: 39.37 ± 12.38 years |
30.74 (7.62–123.98) | Age, sex, living place, outpatient/inpatient status, history of childhood lower respiratory infection, Sinusitis |
| Guerra et al. (2002)35 | USA | Tucson Epidemiologic Study of Obstructive Lung Diseases | 173/2177 | 1086 | Cases: 50.81 ± 19.3 years Controls: 52.96 ± 21.2 years |
3.21 (2.19–4.71) | Age, sex, atopic status, smoking status, presence of COPD |
| Toren et al. (2002)33 | Sweden | MAP-study | 235/2044 | 104 | Cases:39.6 ± 0.82 years Controls: 36.9 ± 0.1 years |
4.8 (3.4–6.7) | Age, atopy, year of diagnosis |
| Szentpetery et al. (A) (2017)45 | Puerto Rico | Population-based case control | 312/297 | 67 | Age range: 6–14 years; Cases: 10.06 ± 2.6 years Controls: 10.51 ± 2.7 years |
3.9 (1.9–7.6) | Age, sex, household income, parental asthma, early life second hand smoking, obesity, diet, lifetime exposure to gun violence. |
| Szentpetery et al. (B) (2017)45 | Sweden | The Children, Allergy, Milieu, Stockholm, Epidemiological Survey [BAMSE] Study | 121/2169 | 32 | Cases: 12.92 ± 0.77 Controls: 12.97 ± 0.83 |
7.8 (3.5–17.6) | Age, sex, parental asthma, early life second hand smoking, obesity |
OR; Odds Ratio, CI; Confidence Interval, SD; Standard Deviation
Fig. 1.
Flow diagram of study selection in systematic review and meta-analysis of history of allergic rhinitis and risk of asthma
Association of allergic rhinitis and asthma
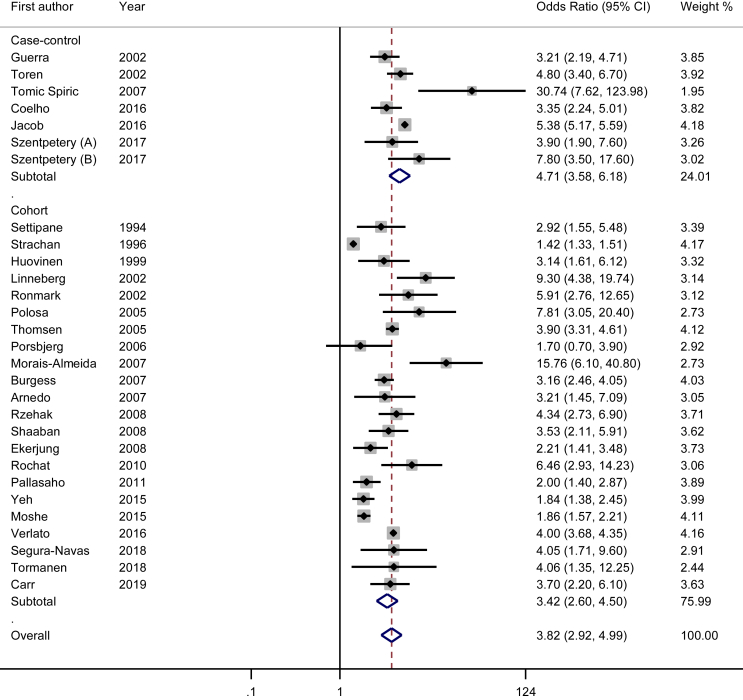
After pooling the data using the random effect model, we observed that history of allergic rhinitis is strongly associated with asthma (pooled Odds Ratio = 3.82; 95% CI: 2.92–4.99). Subgroup analysis showed that this association was weaker for non European studies (OR = 2.75; 95% CI: 2.16–3.50) than for European studies (OR = 4.35; 95% CI: 3.12–6.06). Although the association was significant in both designs, case-control studies showed a stronger association (OR = 4.71; 95% CI: 3.58–6.17) than cohort studies (OR = 3.42; 95% CI: 2.60–4.50). The association was similar in studies with low and high quality scores (OR = 3.86; 95% CI: 2.49–6.00 versus 3.61; 95% CI: 2.97–4.39, respectively), while studies on children showed a stronger relation (OR = 4.10; 95% CI: 2.56–6.56) than those conducted on adults (OR = 3.37; 95% CI: 2.64–4.30). The detailed information as well as results of other subgroup analyses are presented in Table 3. In all analyses (overall and subgroups) there was evidence of heterogeneity between studies (Table 3, Fig. 2). Based on the proportion of total variance due to between-study variance, this heterogeneity was low for studies with full adjustment and moderate for the following subgroups: case-control studies, good quality studies, non-European studies and studies with adequate diagnosis of asthma and rhinitis, while this heterogeneity was high for the rest of subgroups.
Table 3.
Random effects Pooled Odds Ratios (ORs) and 95% confidence intervals (CIs) of allergic rhinitis and asthma in different subgroups.
| Groups | Number of studies | OR (95% CI) | Ria | p- value of Q test |
|---|---|---|---|---|
| All studies | 29 | 3.82 (2.92–4.99) | 0.98 | <0.001 |
| Design | ||||
| Cohort studies | 22 | 3.42 (2.60–4.50) | 0.96 | <0.001 |
| Case-control studies | 7 | 4.71 (3.58–6.17) | 0.70 | 0.003 |
| Region | ||||
| European | 21 | 4.35 (3.12–6.06) | 0.98 | <0.001 |
| Non European | 8 | 2.75 (2.16–3.50) | 0.73 | <0.001 |
| Age group | ||||
| Children | 15 | 4.10 (2.56–6.56) | 0.99 | <0.001 |
| Adults | 14 | 3.37 (2.64–4.30) | 0.89 | <0.001 |
| Quality Score | ||||
| <3 | 11 | 3.86 (2.49–6.00) | 0.99 | <0.001 |
| ≥3 | 18 | 3.61 (2.97–4.39) | 0.51 | 0.006 |
| Items of quality assessment: | ||||
| Proper definition of target population | ||||
| Yes | 23 | 3.42 (2.62–4.48) | 0.96 | <0.001 |
| No | 6 | 6.26 (3.18–12.30) | 0.97 | <0.001 |
| Proper diagnosis of asthma | ||||
| Yes | 9 | 3.67 (2.52–5.34) | 0.81 | <0.001 |
| No | 20 | 3.82 (2.92–4.99) | 0.98 | <0.001 |
| Proper diagnosis of Allergic Rhinitis | ||||
| Yes | 12 | 5.06 (3.58–7.15) | 0.64 | 0.001 |
| No | 17 | 3.18 (2.26–4.46) | 0.99 | <0.001 |
| Clear temporal trend between rhinitis and occurrence of asthma | ||||
| Yes | 24 | 3.55 (2.74–4.61) | 0.96 | <0.001 |
| No | 5 | 4.89 (3.26–7.35) | 0.68 | 0.015 |
| Adjustment | ||||
| Full adjustment | 12 | 3.82 (2.92–4.98) | 0.43 | 0.057 |
| Incomplete adjustment | 17 | 3.94 (2.77–5.61) | 0.99 | <0.001 |
Proportion of total variance due to between-study variance
Fig. 2.
Forest plot of the association between history of allergic rhinitis and risk of asthma (random effects)
Publication bias
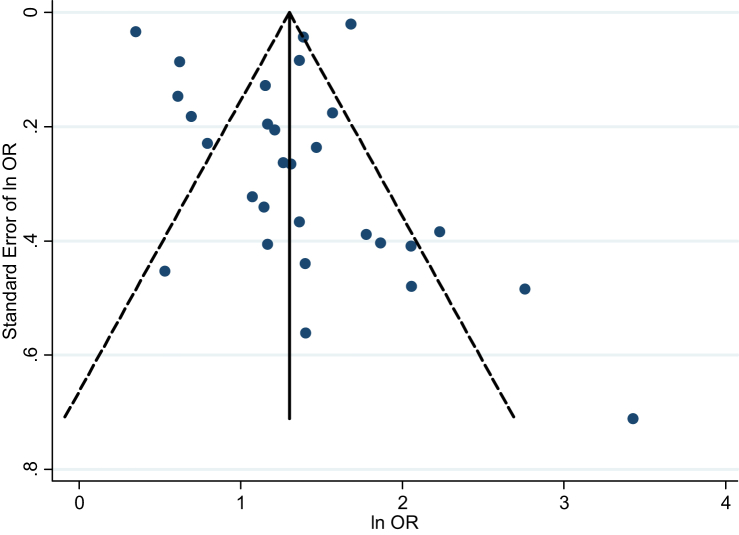
The funnel plot of effect size of the studies was slightly skewed to the right (Fig. 3), and the Begg's test (p-value = 0.023) provided some evidence for the presence of small-study effects. However, the Egger's test showed no evidence of publication bias (p-value = 0.66). The Trim-and-Fill procedure did not impute any study when we applied the random effects model, while one study was imputed when the fixed effects model was used. The pooled OR after inclusion of this study was 3.67 (95% CI: 3.56–3.78), very close to the original pooled OR of 3.82 (95% CI: 2.92–4.99).
Fig. 3.
Funnel plot of association between history of allergic rhinitis and risk of asthma
To evaluate the possibility of publication bias in case–control studies, the design most susceptible to be rejected by journals when results lack statistical signification, we recalculated our pooled estimates under the following extreme assumptions: 1) published studies listed in Table 2 are only half of the case-control studies on allergic rhinitis and risk of asthma ever conducted, 2) all studies of the unpublished half found null associations (ie, OR = 1) between allergic rhinitis and asthma, and 3) on average, the unpublished studies included as many cases and controls as the published ones. Under these assumptions, the observed pooled OR was 2.89 (95% CI: 2.19–3.81) that was still large enough to support the findings of our study.
We performed another simulation to evaluate the robustness of our results to the publication bias. We calculated how many unpublished negative (OR = 1) case-control studies with as many cases and controls as the average number of the published studies were necessary to reverse our conclusions. Our simulation showed that 187 negative studies were needed to obtain a pooled OR of 1.10 (95% CI: 1.06–1.14) and 945 studies to obtain an OR of 1.01 (95% CI: 1.003–1.018).
Cumulative meta-analysis
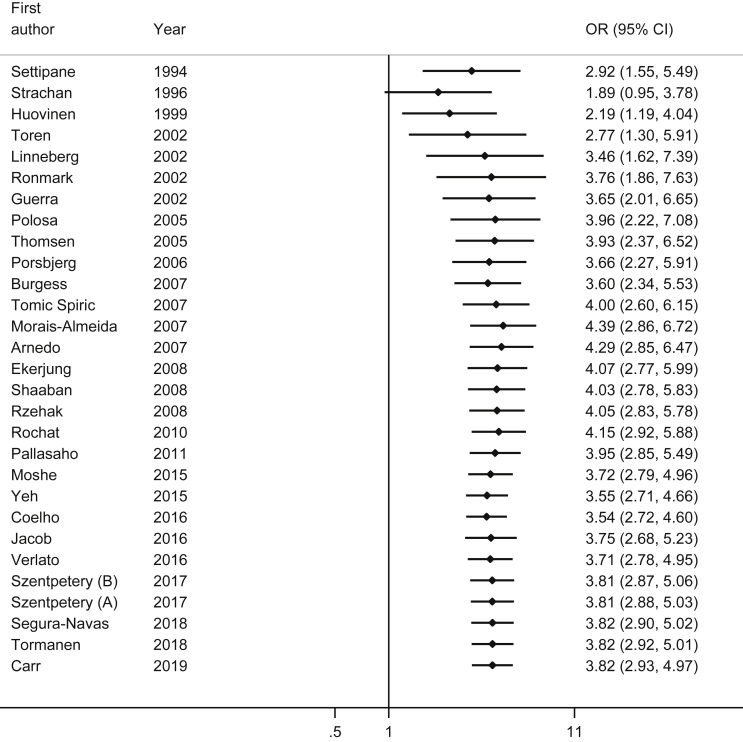
Cumulative meta-analysis by year of publication showed that the first stable and statistically significant effect was observed after including the study by Huovinen et al. published in 1999 (pooled OR = 2.19; 95% CI: 1.19–4.04).39 The pooled OR increased up to a value of 3.46 (95% CI: 1.62–7.39) after adding the study of Linneberg et al., in 2002,11 but subsequent studies did not change the strength of association in a meaningful fashion and just increased the precision of the pooled estimate (Fig. 4).
Fig. 4.
Cumulative forest plot of the association between history of allergic rhinitis and risk of asthma
Discussion
Our meta-analysis showed that allergic rhinitis is associated with the occurrence of asthma. This association was observed in all subgroups, albeit with different magnitudes. A plausible explanation of this association is provided by the “atopic march” hypothesis that considers atopic disorders as a series of consecutive clinical manifestations which start with atopic dermatitis, follow with allergic rhinitis, and end up with asthma as sensitizations in upper and lower airways, respectively.48 A similar view is represented by the “one airway, one disease” concept, in which AR and asthma are believed to be but one unique disease.49
In a study in human subjects, Braunstahl et al. revealed that the provocation of nasal mucosa with allergens results in an increase in lower airways eosinophilia in patients with AR.50 In addition, the increase in the risk of asthma after exposure to AR is supported by some experimental animal studies. Li et al. showed that exposure of nasal mucosa to allergens increases the concentration of factors that are related to asthma such as eosinophils, interleukin-5 and CD34 cells in peripheral blood and bone marrow in mice.51
Another explanation of the relation of allergic rhinitis and asthma could be represented by the existence of a common cause for these two diseases, which affects upper and lower airways in different periods and could play a role of confounder in this association. Some studies showed that polymorphisms in the filaggrin gene, such as R501X and 2282del4, are related to both AR and asthma.52, 53 Also, inhalant allergens, such as pollen, pets, house dust mites, and moulds could be predictors of AR and asthma. Nevertheless, the existence of a strong confounder that could explain the total of the association observed is unlikely. Even if this unidentified factor (either a polymorphism or an environmental factor) could multiply by 5 the risk of asthma among subjects exposed to it (Relative Risk confounder–disease = 5) and, at the same time, this factor is 5 times more prevalent among people suffering from AR than among the rest of the population (Relative Risk confounder–exposure = 5), the adjusted Relative Risk of the relation rhinitis-asthma would still be 2.30 (assuming one-third of people are exposed to this unknown factor).54 The existence of a factor so strongly associated with rhinitis on one side, and with asthma on the other side, is highly improbable.
This meta-analysis showed a large amount of heterogeneity that persisted after stratification in subgroups. We therefore focused our interpretation on the random effects estimates as recommended.55, 56 Meta-analysis experts claim that there is no amount of heterogeneity that is unacceptable provided the eligibility criteria are sound and the data are correct,55 and that heterogeneity in meta-analysis, due to the differences in methods and populations, should be viewed as the “expectation, rather than the exception”.57
Publication bias is unlikely to happen in this meta-analysis because the association remained strong even after extremely conservative assumptions about the potentially related but unpublished studies. In addition, although the Begg's test showed some evidence of publication bias, Egger's test did not confirm it and the Trim-and-Fill procedure did not modify the results obtained previously. Furthermore, to prevent this bias, we conducted a thorough electronic and manual search, without any language restriction, in virtually every available database that could provide articles, dissertations, and conference abstracts.
Furthermore, the results observed do not depend on the intrinsic quality of the studies as the magnitude of the association is similar among studies deemed to be of high quality and studies with a lower quality score.
To the best of our knowledge, this study is the first meta-analysis on this topic. However, there are previous narrative reviews which have not retrieved all related studies or which have included cross sectional studies, a type of studies which cannot provide reliable evidence for cause-effect relationship due to its inability to assess a temporal trend between the occurrence of AR and that of asthma. To avoid this bias, our meta-analysis excluded cross-sectional designs and used cohort and case-control studies only.
In conclusion, the magnitude of the associations, and the consistency of the results in different settings provide strong epidemiological evidence that people with allergic rhinitis have a higher odds of asthma occurrence than healthy people. Further prospective studies on the effect of treatment of allergic rhinitis on the development of asthma are needed. Relief of airway allergic manifestations may need dual control of allergic rhinitis and asthma.
Potential competing interests
The authors report no competing interests.
Ethics statement
This article is a meta-analysis. It does not use any patients or personal data that could be submitted to the evaluation of an Ethics committee.
Contributions
Hamid Reza Tohidinik took the lead, made substantial contributions to conception, design and writing. Narmeen Mallah performed data extraction and contributed to the drafting of the article. Bahi Takkouche revised the study critically, contributed substantially to the interpretation of data and drafting of the article.
Acknowledgments
This work did not receive any specific public or private funding. Dr Tohidinik’s scientific stay at the University of Santiago de Compostela was funded through the Research Excellence Programme USC – IRAN. Dr Takkouche’s work is funded by Grant ED431C 2018/20 from the Regional Ministry of Education, Universities and Vocational Training (Consellería de Educación, Universidades y Formación Profesional). Santiago de Compostela, Spain.
All authors of this paper have read the manuscript, agree that the work is ready for submission to a journal, and accept responsibility for the manuscript’s content. All authors certify that they do not have any conflict of interest.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100069.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Asher M.I., Montefort S., Bjorksten B. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Flaxman A.D., Naghavi M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawankar R., Canonica G.W., Holgate S., Lockey R. World Allergy Organization; 2013. WAO White Book on Allergy: Update 2013. [Google Scholar]
- 5.Leynaert B., Bousquet J., Neukirch C., Liard R., Neukirch F. Perennial rhinitis: an independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol. 1999;104(2 Pt 1):301–304. doi: 10.1016/s0091-6749(99)70370-2. [DOI] [PubMed] [Google Scholar]
- 6.Jacob L., Keil T., Kostev K. Comorbid disorders associated with asthma in children in Germany - national analysis of pediatric primary care data. Pediatr Allergy Immunol. 2016;27(8):861–866. doi: 10.1111/pai.12656. [DOI] [PubMed] [Google Scholar]
- 7.Shaaban R., Zureik M., Soussan D. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372(9643):1049–1057. doi: 10.1016/S0140-6736(08)61446-4. [DOI] [PubMed] [Google Scholar]
- 8.Porsbjerg C., von Linstow M.L., Ulrik C.S., Nepper-Christensen S., Backer V. Risk factors for onset of asthma: a 12-year prospective follow-up study. Chest. 2006;129(2):309–316. doi: 10.1378/chest.129.2.309. [DOI] [PubMed] [Google Scholar]
- 9.Tomic Spiric V., Jankovic S., Bogic M., Maksimovic N., Djuric V., Bolpacic J. Atopic asthma and related risk factors. Allergologie. 2007;30(2):41–49. [Google Scholar]
- 10.Bousquet J., Schunemann H.J., Samolinski B. Allergic rhinitis and its Impact on asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130(5):1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 11.Linneberg A., Henrik Nielsen N., Frolund L., Madsen F., Dirksen A., Jorgensen T. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002;57(11):1048–1052. doi: 10.1034/j.1398-9995.2002.23664.x. [DOI] [PubMed] [Google Scholar]
- 12.Yeh J.J., Wang Y.C., Hsu W.H., Kao C.H. Incident asthma and Mycoplasma pneumoniae: a nationwide cohort study. J Allergy Clin Immunol. 2016;137(4):1017–1023. doi: 10.1016/j.jaci.2015.09.032. e1011-1016. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Larsen V., Potts J.F., Del Giacco S. Changes in symptoms of asthma and rhinitis by sensitization status over ten years in a cohort of young Chilean adults. BMC Pulm Med. 2016;16(1):116. doi: 10.1186/s12890-016-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izuhara Y., Matsumoto H., Nagasaki T. Mouth breathing, another risk factor for asthma: the Nagahama Study. Allergy. 2016;71(7):1031–1036. doi: 10.1111/all.12885. [DOI] [PubMed] [Google Scholar]
- 15.Settipane R.J., Hagy G.W., Settipane G.A. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc. 1994;15(1):21–25. doi: 10.2500/108854194778816634. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen S.F., Ulrik C.S., Kyvik K.O. The incidence of asthma in young adults. Chest. 2005;127(6):1928–1934. doi: 10.1378/chest.127.6.1928. [DOI] [PubMed] [Google Scholar]
- 17.Anto J.M., Sunyer J., Basagana X. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy. 2010;65(8):1021–1030. doi: 10.1111/j.1398-9995.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 18.de Marco R., Locatelli F., Cazzoletti L., Bugianio M., Carosso A., Marinoni A. Incidence of asthma and mortality in a cohort of young adults: a 7-year prospective study. Respir Res. 2005;6:95. doi: 10.1186/1465-9921-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toren K., Gislason T., Omenaas E. A prospective study of asthma incidence and its predictors: the RHINE study. Eur Respir J. 2004;24(6):942–946. doi: 10.1183/09031936.04.00044804. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen S.F., Ulrik C.S., Kyvik K.O. Risk factors for asthma in young adults: a co-twin control study. Allergy. 2006;61(2):229–233. doi: 10.1111/j.1398-9995.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen S.F., Duffy D.L., Kyvik K.O., Backer V. Genetic influence on the age at onset of asthma: a twin study. J Allergy Clin Immunol. 2010;126(3):626–630. doi: 10.1016/j.jaci.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Rothman K., Greenland S., Lash T. third ed. Lippincott, Williams and Wilkins; Philadelphia: 2008. Modern Epidemiology; p. 61. [Google Scholar]
- 23.Takkouche B., Cadarso-Suarez C., Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150(2):206–215. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 24.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Costa-Bouzas J., Takkouche B., Cadarso-Suarez C., Spiegelman D. HEpiMA: software for the identification of heterogeneity in meta-analysis. Comput Methods Progr Biomed. 2001;64(2):101–107. doi: 10.1016/s0169-2607(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 26.Coelho M.A., de Pinho L., Marques P.Q., Silveira M.F., Sole D. Prevalence and factors associated with asthma in students from Montes Claros, Minas Gerais, Brazil. Ciência Saúde Coletiva. 2016;21(4):1207–1216. doi: 10.1590/1413-81232015214.04572015. [DOI] [PubMed] [Google Scholar]
- 27.Verlato G., Nguyen G., Marchetti P. Smoking and new-onset asthma in a prospective study on Italian adults. Int Arch Allergy Immunol. 2016;170(3):149–157. doi: 10.1159/000446509. [DOI] [PubMed] [Google Scholar]
- 28.Pallasaho P., Juusela M., Lindqvist A., Sovijarvi A., Lundback B., Ronmark E. Allergic rhinoconjunctivitis doubles the risk for incident asthma--results from a population study in Helsinki, Finland. Respir Med. 2011;105(10):1449–1456. doi: 10.1016/j.rmed.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Rochat M.K., Illi S., Ege M.J. Allergic rhinitis as a predictor for wheezing onset in school-aged children. J Allergy Clin Immunol. 2010;126(6):1170–1175. doi: 10.1016/j.jaci.2010.09.008. e1172. [DOI] [PubMed] [Google Scholar]
- 30.Rzehak P., Schoefer Y., Wichmann H.E., Heinrich J. A prospective study on the association between hay fever among children and incidence of asthma in East Germany. Eur J Epidemiol. 2008;23(1):17–22. doi: 10.1007/s10654-007-9205-3. [DOI] [PubMed] [Google Scholar]
- 31.Ekerljung L., Ronmark E., Larsson K. No further increase of incidence of asthma: incidence, remission and relapse of adult asthma in Sweden. Respir Med. 2008;102(12):1730–1736. doi: 10.1016/j.rmed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Morais-Almeida M., Gaspar A., Pires G., Prates S., Rosado-Pinto J. Risk factors for asthma symptoms at school age: an 8-year prospective study. Allergy Asthma Proc. 2007;28(2):183–189. doi: 10.2500/aap.2007.28.2953. [DOI] [PubMed] [Google Scholar]
- 33.Toren K., Olin A.C., Hellgren J., Hermansson B.A. Rhinitis increase the risk for adult-onset asthma--a Swedish population-based case-control study (MAP-study) Respir Med. 2002;96(8):635–641. doi: 10.1053/rmed.2002.1319. [DOI] [PubMed] [Google Scholar]
- 34.Ronmark E., Perzanowski M., Platts-Mills T., Lundback B. Incidence rates and risk factors for asthma among school children: a 2-year follow-up report from the obstructive lung disease in Northern Sweden (OLIN) studies. Respir Med. 2002;96(12):1006–1013. doi: 10.1053/rmed.2002.1391. [DOI] [PubMed] [Google Scholar]
- 35.Guerra S., Sherrill D.L., Martinez F.D., Barbee R.A. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109(3):419–425. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 36.Strachan D.P., Butland B.K., Anderson H.R. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312(7040):1195–1199. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess J.A., Walters E.H., Byrnes G.B. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120(4):863–869. doi: 10.1016/j.jaci.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Polosa R., Al-Delaimy W.K., Russo C., Piccillo G., Sarva M. Greater risk of incident asthma cases in adults with allergic rhinitis and effect of allergen immunotherapy: a retrospective cohort study. Respir Res. 2005;6:153. doi: 10.1186/1465-9921-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huovinen E., Kaprio J., Laitinen L.A., Koskenvuo M. Incidence and prevalence of asthma among adult Finnish men and women of the Finnish Twin Cohort from 1975 to 1990, and their relation to hay fever and chronic bronchitis. Chest. 1999;115(4):928–936. doi: 10.1378/chest.115.4.928. [DOI] [PubMed] [Google Scholar]
- 40.Segura-Navas L., Arnedo-Pena A., Tosca-Segura R. Incidence of asthma in young adults from Castellon, Spain: a prospective cohort study. Allergol Immunopathol (Madr). 2018;46(2):112–118. doi: 10.1016/j.aller.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Moshe S., Slodownik D., Yagev Y. Atopy as a risk factor for the development of asthma in young recruits. J Asthma. 2015;52(5):453–457. doi: 10.3109/02770903.2014.982287. [DOI] [PubMed] [Google Scholar]
- 42.Tormanen S., Lauhkonen E., Riikonen R. Risk factors for asthma after infant bronchiolitis. Allergy. 2018;73(4):916–922. doi: 10.1111/all.13347. [DOI] [PubMed] [Google Scholar]
- 43.Carr T.F., Stern D.A., Halonen M., Wright A.L., Martinez F.D. Non-atopic rhinitis at age 6 is associated with subsequent development of asthma. Clin Exp Allergy. 2019;49(1):35–43. doi: 10.1111/cea.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnedo A., Bellido J.B., Pac M.R. [Incidence of asthma and risk factors in a cohort of schoolchildren aged from 6-7 years old to 14-15 years old in Castellon (Spain) following the International Study of Asthma and Allergies in Childhood (ISAAC)] Med Clínica. 2007;129(5):165–170. doi: 10.1157/13107792. [DOI] [PubMed] [Google Scholar]
- 45.Szentpetery S.S., Gruzieva O., Forno E. Combined effects of multiple risk factors on asthma in school-aged children. Respir Med. 2017;133:16–21. doi: 10.1016/j.rmed.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomic Spiric V., Bogic M., Peric-Popadic A. Allergic rhinitis as a risk factor of atopic asthma. Allergy. 2008;63 71-71. [Google Scholar]
- 47.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spergel J.M. Atopic march: link to upper airways. Curr Opin Allergy Clin Immunol. 2005;5(1):17–21. doi: 10.1097/00130832-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Grossman J. One airway, one disease. Chest. 1997;111(2 Suppl):11s–16s. doi: 10.1378/chest.111.2_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 50.Braunstahl G.J., Overbeek S.E., Kleinjan A., Prins J.B., Hoogsteden H.C., Fokkens W.J. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107(3):469–476. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 51.Li J., Saito H., Crawford L., Inman M.D., Cyr M.M., Denburg J.A. Haemopoietic mechanisms in murine allergic upper and lower airway inflammation. Immunology. 2005;114(3):386–396. doi: 10.1111/j.1365-2567.2005.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Oord R.A., Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433. doi: 10.1136/bmj.b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziyab A.H., Karmaus W., Zhang H. Association of filaggrin variants with asthma and rhinitis: is eczema or allergic sensitization status an effect modifier? Int Arch Allergy Immunol. 2014;164(4):308–318. doi: 10.1159/000365990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenland S. Basic methods for Sensitivity analysis of biases. Int J Epidemiol. 1996;25(6):1107–1116. [PubMed] [Google Scholar]
- 55.Higgins J.P. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 56.Council N.R. National Academy Press; Washington, DC: 1992. Combining Information: Statistical Issues and Opportunities for Research; p. 52. [Google Scholar]
- 57.Berlin J.A. Invited commentary: benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol. 1995;142(4):383–387. doi: 10.1093/oxfordjournals.aje.a117645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.