Abstract
Background
The human epididymis protein 4 (HE4) may have high specificity in the detection of malignant diseases, making the development of an immunoassay for HE4 essential.
Methods
In our study, a fusion gene was constructed encoded with the HE4 protein. This protein was then produced in the bacterial cells (Escherichia coli) and used to immunize mice in order to eventually generate hybridomas specific to HE4. The hybridoma supernatants were then screened, and four positive anti‐HE4 cell lines were selected. These cell lines produce monoclonal antibodies against HE4 epitopes, as demonstrated in the Western blot as well as by direct enzyme‐linked immunosorbent assay (ELISA). Using the developed antibodies, we successfully identified several good antibody pairs from the hybridomas, which allowed for the development of a sandwich ELISA to measure HE4 levels. By using the HE4 ELISA, we measured HE4 levels of 60 clinical human serum samples.
Results
Compared with the Food and Drug Administration (FDA) approved kit (Roche), our results showed a strong positive correlation to those of the FDA‐approved kit.
Conclusions
In summary, highly sensitive antibody pairs were screened against HE4, and a sandwich ELISA was developed as an accurate analytical tool for the detection of HE4 in human serum, which could be especially valuable for diagnosing ovarian carcinomas.
Keywords: HE4, monoclonal antibody, sandwich ELISA, ovarian cancer, immunoassay
INTRODUCTION
The lifetime prevalence of ovarian cancer is 1–2% in the developing world. Ovarian cancer is one of the gynecological malignancies with the highest mortality rate, and the second most commonly diagnosed cancer in the United States 1, 2. It is often described as a silent killer. However, early symptoms of abdominal bloating, changes in urinary frequency, sensation of fullness, and pelvic/abdominal pain are common. As these symptoms are nonspecific, they are often dismissed by patients and healthcare professionals, and this disease remains undiagnosed. This cancer meets the World Health Organization's standard for a disease where women's health would benefit from early screening 3. More than 70–80% of women with ovarian cancer are diagnosed with advanced stages of this disease, and the cure rate is less than 30%. The survival rate of a patient with ovarian cancer depends strongly upon the stage of the cancer when the cancer is initially identified. As such, making earlier detection is rather critical to improve the patient's outcome. Therefore, increased efforts are underway to identify screening strategies that can detect ovarian cancer at earlier stages when it is more treatable.
Serum biomarkers are of great importance for their potential to provide an effective, low‐cost, and noninvasive screening modality that can be applied on populations all over the world 4. For many years, CA125 has been used as the most common ovarian cancer biomarker 5. Indeed, it is a valuable tool to assess responses to ovarian cancer treatment and monitor for disease recurrence. However, CA125 is associated with a high false‐positive rate. In fact, CA125 antigen is not exclusively expressed in the tumor cells of ovarian cancer, but its concentration is also grossly increased in nonmalignant abdominal conditions not related to ovarian cancer.
Several reports have demonstrated that human epididymis protein 4 (HE4) may be a promising biomarker for ovarian cancer. The recent use of techniques such as the serial analysis of gene expression or coda microarrays have identified that HE4 was amplified in ovarian carcinomas, but not increased in normal tissues 6, 7. Mary et al. have performed comprehensive research and analysis on HE4 expression in normal and malignant human tissues and summarized the findings of the HE4 protein expression patterns in a survey of wide‐ranging carcinomas, which is useful for its application in a histopathological diagnosis 8, 9. At present, detected expression levels of HE4 were not increased in patients with ovarian endometrioma, while their CA125 levels were increased during advanced stages of endometrioma 10, 11. Huhtinen et al. have suggested that the HE4 concentration within the sample was a valuable marker to better distinguish patients with ovarian malignancies from those suffering from benign ovarian endometriotic cysts 12.
Abdel‐Azeez's findings have indicated that HE4 could aid the diagnosis of ovarian carcinoma, and HE4 has an advantage over the classical CA125 test by providing fewer false positives in women who have benign forms of the diseases 13. HE4 has also been approved by the Food and Drug Administration (FDA) for use in the United States to monitor ovarian cancer patients for disease recurrence 14, and Shah et al. have shown that the diagnostic accuracy of HE4 of healthy controls is similar to that of high‐risk and average‐risk women (AUC = 0.931 and AUC = 0.928, respectively, P = 0.94) 15.
A method that fulfills the requirement for speed, sensitivity, accuracy, and practicality for routine testing in clinical application is becoming more and more urgent. Accordingly, a simple and accurate method targeting the detection of HE4 in clinical diagnoses is necessary. Based on the importance of HE4 in this aspect, and especially for ovarian cancer, the principal aims of our research are to establish a useful sandwich enzyme‐linked immunosorbent assay (ELISA) to detect HE4, and to demonstrate its utility for screening or measuring HE4 levels in human clinical samples.
MATERIALS AND METHODS
Expression and Purification of His‐tagged Recombinant HE4
cDNA encoding HE4 (gene ID: 10406, Tax ID: 9606, NCBI reference sequences: NM_080733.1) was purchased from Harvard University PlasmID database(http://plasmid.med.harvard.edu/PLASMID/Home.xhtml;jsessionid = C086920F5C2D4AF7146ADF34C50D1B62.plasmid2). The gene was amplified by Polymerase Chain Reaction (PCR) using a pair of primers, 5′‐AAAGAATTCGAGAAGACTGGCGTGTGC‐3′ (nucleotides 91–118) and 5′‐AAACTCGAGTCAGAAATTGGGAGTGACACA‐3′ (nucleotides 355–372), which contain the restriction sites EcoRI and Xho1, respectively.
The following steps were performed as described by Han et al. with some modifications 16. PCR was carried out with PrimeSTAR HS DNA polymerase (Takara Bio Inc., Shiga, Japan) under the following conditions: after initial denaturation of DNA at 94°C for 3 min using a BioRad T100TM Thermal Cycler PCR (BioRad), a program was set at 94°C for 30 s, 57°C for 10 s, and 72°C for 30 s for a total of 30 cycles and then finally 72°C for 5 min. The amplified HE4 DNA of 284 bp, corresponding to the mature HE4 protein with deletion of the N‐terminal signal peptide segment (amino acid residues 1–30), was treated with EcoR1 and Xho1 and then ligated to the pET32a vector (Invitrogen). Escherichia coli top10 (Invitrogen) was placed in Luria‐Bertani (LB) agar containing 50 μg/ml of ampicillin. Escherichia coli strain (DE3)/(Invitrogen) was transformed with the pET32a‐HE4‐plasmid prepared with Plasmid Miniprep (Omega) from the positive‐colony cells.
The transformants were cultured in LB agar containing 50 μg/ml of ampicillin at 37°C until absorbance at 600 nm reached to 0.6. The expression of His‐tagged HE4 was induced by adding 0.5 mM Isopropyl β‐D‐1‐thiogalactopyranoside (IPTG), and the culture was then incubated at 22°C for an additional 16 h. Cells were harvested by centrifugation at 5,000 × g for 10 min and washed twice with PBS (pH 7.4). The pellets were resuspended in a solution of 20 mM Tris‐HCl (pH 8.0) and subjected to ultrasonic oscillation for a total of 10 min with 3 s exposure and 30 s intermittent cooling using JY92‐11 sonicator (Ningbo, China), and then centrifuged at 20,000 × g for 30 min at 4°C. The clear soluble fraction was applied to a nickel ion immobilized metal affinity chromatography resin column (GE Healthcare), equilibrated with a solution of 20 mM Tris‐HCl, 0.05 M NaCl, and 10 mM imidazole (pH 8.0), and then washed with the same solution. The column was eluted with a solution of 20 mM Tris‐HCl, 0.5 M NaCl, and 800 mM imidazole (pH 8.0). Neighboring fractions containing the HE4 protein were pooled and dialyzed with a large excess of phosphate‐buffered saline (PBS). The protein concentration was quantified by the Lowry method using bovine serum albumin (BSA) as the standard (Thermo Fisher Scientific K.K., Kanagawa, Japan).
Mouse Immunization
Three female BALB/c mice (8–10 weeks old) were immunized with recombinant HE4. In the first intraperitoneal injection, 50 μg of recombinant HE4 were dissolved in PBS (pH 7.4) and then emulsified with an equal volume of Freund's complete adjuvant. The subsequent second injection, emulsified in incomplete Freund's adjuvant, was given at 2‐week intervals. Seven days after the third injection, the mice were tail‐bled, and their antisera were tested by indirect ELISA using recombinant HE4 as a coating antigen as described below. The mouse with the highest titer was selected as the donor of spleen cells for cell fusion. Three days before the fusion, the selected mouse was given another 50 μg of antigen in PBS without any adjuvant.
Cell Fusion
SP2/0 murine myeloma cells were cultured in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Cell fusion procedures were carried out as described by Liu et al. 17 and Kishiro et al. 18 with some modifications. Mouse splenocytes were mixed with the myeloma cells at the ratios ranging from 3:1 to 5:1 and centrifuged. PEG 1500 (1 ml, 50% in water, Sigma) preheated to 37°C was dropped into the mixture of cells within 1 min. After standing for 60 s, 3 ml of incomplete media (DMEM) of 37°C was added within 60 s. Then after 3 min, 20–30 ml DMEM was added into the mixture. The cells were then left aside for 10 min at 37°C. After the fused cells were centrifuged at 800 rpm for 5 min and resuspended in Hypoxanthine Aminopterin Thymidine (HAT) selection media, the suspensions of fused cells were transferred into five 96‐well culture plates coated with feeding cells from the peritoneal cavity of nonimmunized mice, and cultivated in the specified incubator (Thermo) with the conditions of 5% CO2 at 37°C. After 3 days, half of the media in the wells was replaced with fresh HAT media. When most of the nonfused cells were eliminated, the HAT media were substituted with HT media without aminopterin on the eighth day. Eventually, the screened cells were cultivated in complete media.
Selection and Subclone of Hybridoma Cells
Approximately 10 days after cell fusion, when the hybridoma cells were grown to approximately 30–40% confluency, the culture supernatants were collected and screened for the presence of anti‐HE4 antibodies by indirect ELISA using HE4 as the coating antigen. The cells of interest in the 96‐well plates were then expanded in 24‐well plates previously coated with feeding cells. After approximately 14 days, when the hybridoma cells were approximately 60% confluency in a 24‐well plate, the cell supernatant we collected was used in indirect ELISA with His‐tag as a control to exclude the cells against His‐tag. The positive cells were expanded into dishes (100 mm). The selected hybridomas were subcloned by limiting dilution assay, and the stable antibody‐producing clones were expanded. Indirect ELISA was then employed to determine whether the antibodies from the expanded clones could specifically recognize recombinant HE4. The monoclonal antibody (mAb) against HE4 was obtained from ascites of mice injected with the suspensions of subclonal cells. Ascites fluid was collected after 1–2 weeks. Monoclonal Immunoglobulin G (IgG) and Immunoglobulin M (IgM) were purified from the ascites fluid using a Hitrap protein A HP column (GE Healthcare) and a Kaptiv‐M column (GE Healthcare), respectively, according to the manufacturer's protocol. The antibody isotype and the IgG subclass were determined by Rapid Isotyping Arrays (Raybiotech, Inc.).
Indirect ELISA
The anti‐HE4 antibody was detected by indirect ELISA 19. Microtiter plates were coated overnight with 1 μg/ml of recombinant HE4 in 10 mM of sodium carbonate buffer (pH 9.6) at 4°C or 37°C for 2 h, and then blocked with PBS containing 5% skim milk at 37°C after three washes. After washing the wells three times with Phosphate Buffered Saline Tween‐20 (PBST), 100 μl per well of mouse antiserum, culture supernatants, or mAbs diluted by PBS were added to the plates and incubated for 1.5 h at 37°C. After the plates were washed thoroughly with PBST, 100 μl of a secondary antibody (biotinylated rat anti‐mouse antibody) at 1:10,000 diluted in PBS was added into each well. After a second incubation for 1 h, the plates were washed six times; and then 50,000‐fold diluted streptavidin‐HRP in PBS was added and incubated at 37°C for 1 h. The tetramethylbenzidine substrate system (100 μl/well) was then added and incubated away from light for 3–5 min at 37°C. After the color developed, the reaction was halted by 50 μl/well of 2 mol/l sulfuric acid (H2SO4), and then the absorbance at 450 nm was determined with an ELISA reader (Biotek).
Specificity Test of Monoclonal Antibody by Western Blotting
Western blotting was performed according to previous publication 20 with some modifications. Tissue lysates or serum samples containing equal amounts of protein were analyzed by Sodium Dodecyl Sulfate Polyacrylamide Gel Electropheresis (SDS‐PAGE) and proteins were transferred to nitrate cellulose (NC) membranes (Millipore Corp., Bedford, MA). First, the antigen and samples were subjected to a 12% SDS‐PAGE, and then the protein bands were transferred into a NC membrane. Then the membrane was blocked with Tris‐Buffered Saline (TBS) containing 5.0% skim milk and 0.05% Tween 20 for 1.5 h at room temperature, washed four times with TBS containing 0.1% Tween 20, and incubated with the primary antibody (HE4 antibody clone 1E10) at room temperature for 1 h or at 4°C overnight; washing followed. The bound antibody was detected by IRDye 800CW Donkey anti‐mouse IgG (H + L) (Odyssey), which was used as a secondary antibody, diluted 1:15,000, was added, and then incubated for 1 h at room temperature, washed, and scanned by Odyssey reader (Odyssey).
Cross‐Reactivity of the Monoclonal Antibody
HE4 and its analogues were used for the cross‐reactivity study. Other related substances, such as Alpha Fetoprotein (AFP), CA125, CA15‐3, CA19‐9, Carcino‐embryonic Antigen (CEA), β‐Human Chorionic Gonadotropin (HCGb), pepsinogen 1 (PGI), GP73, and CA724, were also used as important cancer biomarkers. These proteins were provided by Raybiotech, Inc. (Norcross, GA), were dissolved in 10 mM of sodium carbonate buffer (pH 9.6), coated on the plate, and examined as potential interfering molecules using indirect ELISA with the same steps as described above.
Sandwich ELISA
A 96‐well microtiter plate was coated overnight with 100 μl per well of 1 μg/ml of HE4 antibodies for eventual use as capture antibodies at 4°C. The next day, after having been washed with a PBST solution, the plate was then blocked with 5% skim milk in PBS, 200 μl/well, and incubated for 2 h at 37°C. Then, 100 μl of serially diluted HE4 standard solution (200 ng/ml, 100 ng/ml, 50 ng/ml, 25 ng/ml, 12.5 ng/ml, 6.25 ng/ml, and 3.125 ng/ml) with dilution buffer (1% BSA in PBS) was applied to each well in duplicate and allowed to react with the captured antibodies for 2 h at 37°C. After washing the wells with a PBST solution, 100 μl of appropriately diluted biotin‐labeled anti‐mouse IgG were applied to each well for 2 h at 37°C. Then streptavidin‐HRP that had been diluted 50,000‐fold with PBS was added and incubated at 37°C for 1 h after having been washed. The tetramethylbenzidine substrate system (100 μl/well) was then added and incubated away from light for 15–30 min at 37°C. After the color developed, the reaction was stopped by 50 μl/well of 2 mol/l sulfuric acid (H2SO4), and then the absorbance at 450 nm was measured on a spectrophotometer (Biotek).
Preparation of Serum Samples
Sixty human serum samples (anonymous samples) from patients suffering from ovarian cancer were randomly obtained from the Second Affiliated Hospital of Sun Yat‐Sen University (Guangzhou, China). In addition, according to procedures supervised by local authorities responsible for ethical research, the protocols were conducted with full respect of the individual's rights to confidentiality. The serum samples were aliquoted and stored at −80°C until required. Table 1 shows the baseline characteristics of the patients.
Table 1.
Ovarian Cancer Characteristics
| Disease | Number of cases | Median of age | Range of age |
|---|---|---|---|
| Ovarian cancer | 60 | 52 | 19–79 |
RESULTS
Preparation of Recombinant HE4
The constructed gene cloning fraction is depicted in Figure 1. The recombinant plasmid pET32‐HE4 fraction sequences containing an N‐terminal 6× histidine and thioredoxin tag for affinity purification was verified by DNA sequencing. The expression of the recombinant HE4 protein was apparent in the soluble fraction as shown by the ∼35 kDa band on the SDS‐PAGE gel (Fig. 2A). Meanwhile, SDS‐PAGE analysis for each step of the purification process of the HE4 fusion protein is shown in Figure 2B. Purity was extremely improved through the use of a Ni sepharose column, to greater than 80% purity. Finally, all proteins collected from eluted solutions were mixed together and made to be used as the purification test for this process, whose result is shown in Figure 2C for the reducing conditions of an SDS‐PAGE gels and as visualized by Coomassie blue staining. Two milligrams of the protein were finally produced and showed a purity of no less than 90%, and a molecular weight of over 35 kDa.
Figure 1.
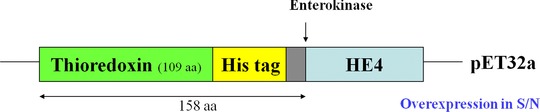
The design of the recombinant HE4 gene cloning.
Figure 2.
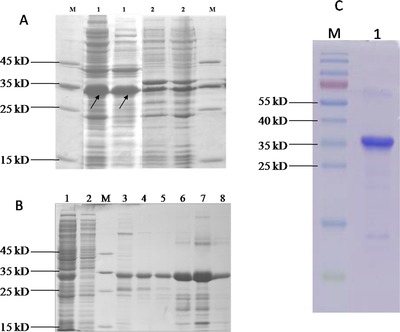
(A) The analysis of expressed fusion HE4 protein by SDS‐PAGE. SDS‐PAGE protein gel stained with Coomassie Blue. M: molecular mass marker. Lane 1#: soluble fraction following overexpression of recombinant HE4 protein in bacterial cells, the target HE4 fusion protein (∼35 kDa) highlighted by arrow. Lane 2#: insoluble fraction. (B) Purification of SDS‐PAGE of fusion protein following Ni+ sepharose column. Lane 1#: fusion protein before purification; Lane 2#: flow through protein; M: molecular mass marker; Lane 3#: washing 1 proteins; Lane 4#: washing 2 proteins; Lane 5#: washing 3 proteins; Lane 6#: elution 1 protein; Lane 7#: elution 2 protein; Lane 8#: elution 3 protein. (C) The purification of the recombinant HE4 protein (∼35 kDa). M: molecular mass marker; 1: the HE4 protein after purification.
Immune Response of Mice Towards Recombinant HE4
Mice were immunized with the purified recombinant HE4, and their serum titer against HE4 was measured by indirect ELISA after the second immunization. The results showed that the titer optical density (OD) values from these mice were 0.480–0.846 at 1/100,000 dilution from the second immunization (data not shown), whereas the titer OD values were 1.712–1.867 at 1/100,000 dilution after 7 days from the third immunization (Fig. 3). Clearly, the immune response by our protocol of consecutive immunizations achieved good results, and improved titer over each immunization. The results in Figure 3 indicate that two of the mice performed well in both titer and specificity, and that Mouse #2 was much more sensitive against HE4 as seen by our dilution testing. Therefore, Mouse #2 was chosen for splenocyte cell fusion and monoclonal antibody development.
Figure 3.
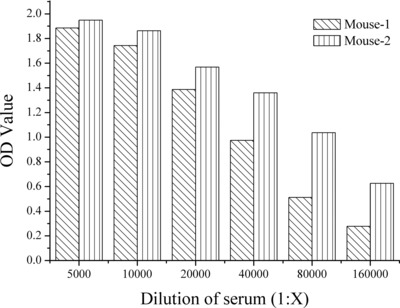
The titer of the serum from two mice after 7 days post third immunization.
Specificity Analysis of the HE4 Antibodies
The culture supernatants with anti‐HE4 antibodies secreted by cells were tested by indirect ELISA against HE4, and among the 480 test wells, we found that more than 400 (OD value >1) supernatants were able to recognize HE4. We chose 48‐wells cell of the targeted hybridoma cells from these 400, expanded them into 24‐well plates, and screened them via ELISA to exclude the cells against the His‐tag of HE4. In the end, after several rounds of subclones, only four positive hybridomas wells could stably secrete the specific antibodies against HE4. The hybridomas in these four wells were subcloned by limiting dilution assay to obtain a monoclonal population of cells that secreted anti‐HE4 mAbs. Thus, these four monoclonal cell lines, named HE4‐1E10‐D10‐E6 (1E10), HE4‐2B8‐G6‐E11 (2B8), HE4‐2G2‐E3‐E10‐G9‐F12 (2G2), and HE4‐3B1‐F8‐G2‐E10 (3B1), were obtained, all of which showed a significant binding in the presence of HE4. Table 2 summarizes the characterizations of these four antibodies against HE4 in terms of cell lines, subclass, and titer values (the titer of positive mouse serum is equal to or more than 2.1‐folds than their negative mouse serum). Isotyping analysis revealed that the antibodies were of the IgG1 and IgM subclasses.
Table 2.
Key Parameters of Four Mouse Anti‐HE4 Monoclonal Cell Lines
| Cell line | Isotype | Titer value |
|---|---|---|
| HE4‐1E10‐D10‐E6 (1E10) | IgG1 | 160,000 |
| HE4‐2B8‐G6‐E11 (2B8) | IgG1 | 160,000 |
| HE4‐2G2‐E3‐E10‐G9‐F12 (2G2) | IgM | 10,000 |
| HE4‐3B1‐F8‐G2‐E10 (3B1) | IgG1 | 160,000 |
To grow larger amounts of these antibodies in vivo, suspensions of these four specific cell lines were injected intraperitoneally into mineral‐oil‐primed mice in order to produce mAb ascites. To evaluate the specificity of the anti‐HE4 antibody, the reactivity of the mouse ascites fluid was next examined for use in Western blot detection of HE4 within the human serum samples. After optimizing the assay conditions such as blotting time, and blocking reagent, specificity of the antibodies was confirmed for HE4 and was shown in Figure 4. The monoclonal antibody (3B1) can specifically recognize HE4 proteins, as well as with human serum samples from ovarian cancer patients. But the HE4 levels in normal serum were below limit of detection in Western blotting with the same loading amount of ovarian cancer samples (date was not shown). From Figure 4, we can see that the molecule weight of HE4 in human serum is much bigger than the recombinant HE4, because it may represent N‐glycosylated form 6. These results indicated that the monoclonal antibodies had a high level of specificity and could be applied to detect the HE4 protein in human serums.
Figure 4.
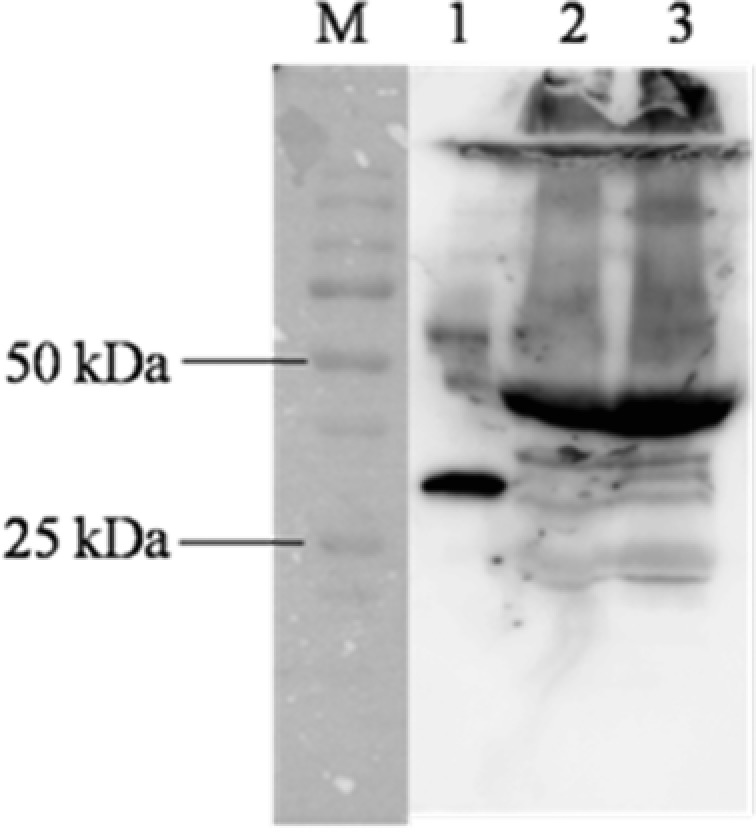
Specificity test of the mouse anti‐HE4 monoclonal antibody (3B1). M: marker (standard proteins), 1: recombinant HE4 protein, 2: G690 (human ovarian cancer serum), 3: G403 (human ovarian cancer serum).
Cross‐Reactivity Analysis
To test the specificity of these four anti‐HE4 antibodies more directly, nine cancer biomarkers just like HE4 were chosen and examined by indirect ELISA. The four purified monoclonal antibodies: 1E10, 2B8, 2G2, and 3B1 can only recognize the HE4 target, not with the other nine cancer targets, which were shown in Figure 5.
Figure 5.
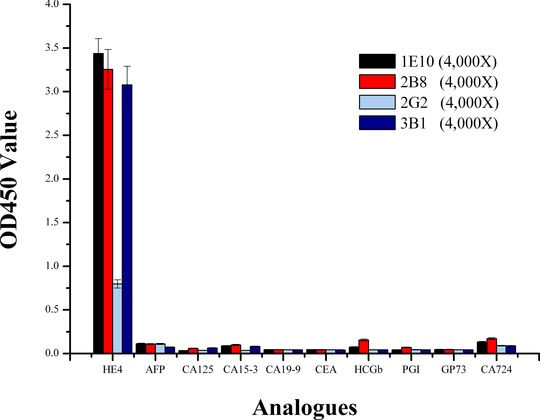
Cross‐reactivity of monoclonal antibodies tested by indirect ELISA. The antigens of the test were immobilized in the microplate wells and subjected to ELISA using the monoclonal antibodies (1E10, 2B8, 2G2, and 3B1).
Pair Testing of the HE4 Antibodies
Since we identified four mAbs against HE4, we next sought to identify functional antibody pairs for an ELISA‐based test. For finding the pairs against the HE4 protein, we first biotin‐labeled all four of these anti‐human HE4 antibodies as detection antibodies, and then coated these four antibodies without labels on the ELISA plate. We tested all 12 potential combinations of antibody pairs, and eventually identified four good pairs for HE4: 1E10 is a pair with 2G2, and 2B8 with 2G2, 3B1 with 1E10, 3B1 with 2B8. The calibration curves of these pairs were plotted under the optimal test conditions, which are shown in Figure 6. The correlation coefficient (R 2 > 0.99) of the standard curves showed a good linear relationship for each pair against HE4. According to the slopes of these standard curves (0.5633, 1.3435, 0.6128, and 0.8584), one pair, 1E10 and 2G2, was the most sensitive against HE4 protein, and was selected as the pair to develop our ELISA testing platform measuring serum HE4 levels.
Figure 6.
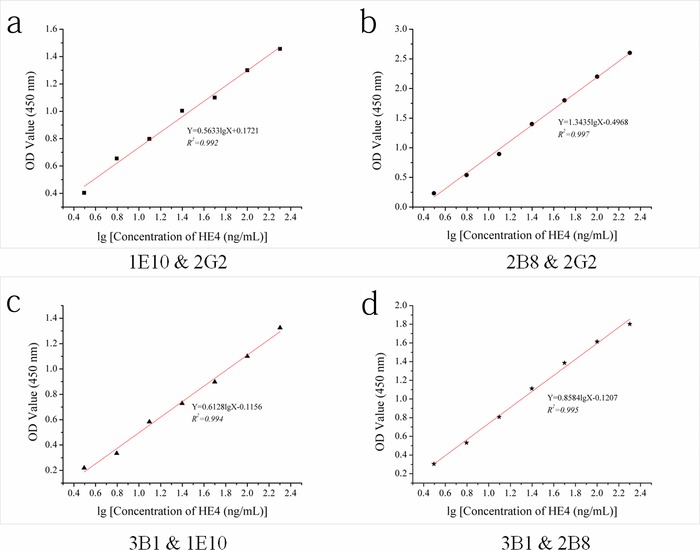
The pair test's good results of the anti‐HE4 antibodies (1E10, 2B8, 2G2, and 3B1).
Reliability of the Present ELISA Kit and Comparison With the Results of Roche
Using the 1E10 and 2G2 antibody pair in a sandwich ELISA, we set out to test 60 clinical samples for the presence of HE4. These samples (normal or abnormal human ovarian cancer samples) were simultaneously analyzed to assess the feasibility of using our method in clinical applications. Meanwhile, the samples were assayed in parallel in the hospital, which used the FDA‐approved kit for HE4 detection (Roche). The results from our method correlated with these from the hospital with a Spearman's rank correlation coefficient of 0.945, which indicates a strong positive correlation between these methods. Also, the linear relationship (y = 1.03906x − 1.03583, R 2 = 0.891) of these two methods is shown in Figure 7, which clearly shows a strong comparable relationship between our ELISA kit and the FDA‐approved kit. In summary, our present ELISA kit is reliable in detecting HE4 levels in human serum, and the sensitivity of this method in these clinical samples reaches to 100% (28/28) with a specificity of 96.88% (31/32) by using 150 pmol/l as the cutoff value.
Figure 7.
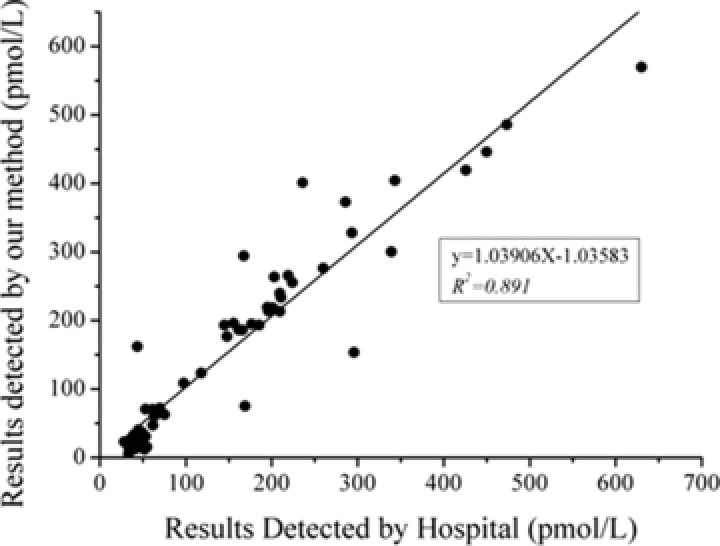
Comparison of HE4 in serum, measured with our sandwich ELISA and the FDA‐approved HE4 test kit (Roche) method in hospital for 60 samples from patients (normal or abnormal HE4 concentration).
DISCUSSION
As a promising biomarker for ovarian cancer, HE4 was approved by the FDA to monitor disease recurrence or progressive disease in epithelial ovarian cancer patients in conjunction with CA125. In our study, we focused on finding the best immune detection reagents and to develop an ELISA testing platform to recognize HE4 in human serum samples in order to help improve diagnosis of ovarian cancer.
Our work here describes our methods for creating a validated sandwich ELISA against HE4, and for quantification of HE4 in human serum samples. We have proven that the recombinant HE4 protein used in the immunization procedure contains the same epitope with that of the human serum samples, and generated four highly sensitive and specific mAb producing hybridomas. Upon determining the antibody pairs suitable for a sensitive ELISA platform, we then tested 60 clinical human serum samples for HE4 levels, and confirmed our results by a hospital performed, FDA‐approved, HE4 kit. The results showed a strong positive correlation, which indicates the functionality and accuracy of our method. Therefore, this highly specific, reliable, and rapid sandwich ELISA against HE4 is suitable for accurate analysis of HE4 in actual human serum samples. This platform could expand the current testing methodologies for HE4, and will be a good tool for academic and clinical development of ovarian cancer diagnostic tests.
ACKNOWLEDGMENTS
We would like to express our thanks for the support of the RayBiotech innovative research fund, the leading scientist project for Guangzhou economic development district (2013L‐P255), Program of hundred leading innovators and entrepreneurs (LCY201111), Guangdong innovative research team program (201001s0104659419), UK‐China (Guangzhou) Healthtech Open Innovation (2012Q‐P182), Guangzhou Municipal Innovation Fund (2013J4400170), Foundation of Enterprise University Research Institute Cooperation of Guangdong Province and Ministry of Education of China (2012B090600021), Special program for the development of technology business incubators in Guangzhou (2013J4200016), and research grants from the Guangzhou economic development district (2010Q—P450).
Grant sponsor: RayBiotech innovative research fund; Grant number: 2013L‐P255; Grant sponsor: Program of hundred leading innovators and entrepreneurs; Grant number: LCY201111; Grant sponsor: Guangdong innovative research team program; Grant number: 201001s0104659419; Grant sponsor: UK‐China (Guangzhou) Healthtech Open Innovation; Grant number: 2012Q‐P182; Grant sponsor: Guangzhou Municipal Innovation Fund; Grant number: 2013J4400170; Grant sponsor: Foundation of Enterprise University Research Institute Cooperation of Guangdong Province and Ministry of Education of China; Grant number: 2012B090600021; Grant sponsor: Special program for the development of technology business incubators in Guangzhou; Grant number: 2013J4200016; Grant sponsor: Guangzhou economic development district; Grant number: 2010Q—P450.
Correction added on 28th March 2016, after first online publication date: corresponding address of Ruopan Huang is added in the paper.
Contributor Information
Chaohui Duan, Email: 1725012289@qq.com.
Ruopan Huang, Email: rhuang@raybiotech.com.
REFERENCES
- 1. Goff BA, Mandel LS, Drescher CW, et al. Development of an Ovarian Cancer Symptom Index. Cancer 2007;109:221–227. [DOI] [PubMed] [Google Scholar]
- 2. Li JP, Dowdy S, Tipton T, et al. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev Mol Diagn 2009;9:555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menon U, Jacobs IJ. Ovarian cancer screening in the general population: Current status. Int J Gynecol Cancer 2001;11:3–6. [DOI] [PubMed] [Google Scholar]
- 4. Lowe KA, Shah C, Wallace E, et al. Effects of personal characteristics on serum CA125, mesothelin, and HE4 levels in healthy post‐menopausal women at high‐risk for ovarian cancer. Cancer Epidemiol Biomarker Prev 2008;17:2480–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urban N, Drescher C. Current and future developments in screening for ovarian cancer. Womens Health 2006;2:733–742. [DOI] [PubMed] [Google Scholar]
- 6. Drapkin R, Von Horsten HH, Lin YF, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res 2005;65:2162–2169. [DOI] [PubMed] [Google Scholar]
- 7. Hellstrom I, Heagerty PJ, Swisher EM, et al. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms. Cancer Lett 2010;296:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galgano MT, Hapmton GM, Frierson HF, Jr . Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol 2006;19:847–853. [DOI] [PubMed] [Google Scholar]
- 9. Molina R, Escudero JM, Ange JM, et al. HE4 a novel tumour marker for ovarian cancer: Comparison with CA125 and ROMA algorithm in patients with gynaecological diseases. Tumor Biol 2011;32:1078–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Gorp T, Cadron I, Despierre E, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: Prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer 2011;104:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacob F, Meier M, Caduff R, et al. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol Oncol 2011;121:487–491. [DOI] [PubMed] [Google Scholar]
- 12. Huhtinen K, Suvitie P, Hiissa J, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer 2009;100:1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdel‐Azeez HA, Labib HA, Sharaf SM, Refaie AN. HE4 and mesothelin: Novel biomarkers of ovarian carcinoma in patients with pelvic masses. Asian Pac J Cancer Prev 2010;11:111–116. [PubMed] [Google Scholar]
- 14. Andersen MR, Goff BA, Lowe KA, et al. Use of a Symptom Index, CA125 and HE4 to predict ovarian cancer. Gynecol Oncol 2010;116:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah CA, Lowe KA, Paley P, et al. Influence of ovarian cancer risk status on the diagnostic performance of the serum biomarkers mesothelin, HE4, and CA125. Cancer Epidemiol Biomarkers Prev 2009;18:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han Q, Fang JM, Li JY. Kynurenine aminotransferase and glutamine transaminase K of Escherichia coli: Identity with aspartate aminotransferase. Biochem J 2001;360:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Lou Y, Xu D, et al. Production and characterization of monoclonal antibody for class‐specific determination of O,O‐dimethyl organophosphorus pesticides and effect of heterologous coating antigens on immunoassay sensitivity. Microchem J 2009;93:36–42. [Google Scholar]
- 18. Kishiro Y, Kagawa M, Naito I, Sado Y. A novel method of preparing rat‐monoclonal antibody‐producing hybridomas by using rat medial iliac lymph node cells. Cell Struct Funct 1995;20:151–156. [DOI] [PubMed] [Google Scholar]
- 19. Matsui H, Hanaki H, Inoue M, et al. Development of an immunochromatographic strip for simple detection of penicillin‐binding protein 2. Clin Vaccine Immunol 2011;18:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Y, Huang RC, Chen LP, et al. Profiling of cytokine expression by biotin‐labeled protein arrays. Proteomics 2003;3:1750–1757. [DOI] [PubMed] [Google Scholar]


