Abstract
Background
Risperidone (RIS) is a widely used atypical antipsychotic drug. We developed and validated a sensitive and accurate LC‐MS/MS method, which requires a small‐volume of plasma and small‐volume injection for measurement of RIS levels in ASD pediatric patients. We also investigated the relationship between RIS levels and RIS dosages, including prolactin levels.
Method
Blood samples were processed by protein precipitation extraction. Only 1 μl of sample was injected. Plasma samples were separated on a C18 column (4.6 cm × 50 mm; 1.8 μm particle size). Detection was by MS‐MS with an analytical run time of 6 min.
Results
The inter‐day accuracy of RIS was 101.33–107.68% and 95.24–103.67% for 9‐OH‐RIS. The inter‐day precision of RIS was ≤7.27% CV and ≤7.41% CV for 9‐OH‐RIS. The extraction recovery of RIS and 9‐OH‐RIS were 95.01 ± 7.31–112.62 ± 7.50% and 90.27 ± 11.15–114.00 ± 10.35%, respectively. This method was applied in the therapeutic drug monitoring of ASD pediatric patients. Higher RIS dosage has a tendency to produce higher RIS plasma levels. The high RIS plasma levels have a tendency to produce hyperprolactinemia.
Conclusion
The determination of RIS in individual patients might be clinically useful for monitoring and prediction of treatment response.
Keywords: 9‐hydroxyrisperidone, Autism spectrum disorders, LC–MS/MS, prolactin, risperidone
Introduction
Risperidone (RIS) is a benzisoxazole derivative belonging to the class of atypical antipsychotic drugs. It is a selective monoadrenergic antagonist with high affinity for dopamine (D2) and serotonin (5HT2) receptors 1 and has a lower potential to cause extrapyramidal side effects as compared to classic antipsychotics 2. RIS is effective in the treatment of serious behavioral problems in children with autistic spectrum disorders (ASD) and other psychiatric illnesses in adults and children, such as bipolar disorder and schizophrenia 3, 4, 5. RIS is extensively metabolized by cytochrome 2D6 in the liver to form 9‐hydroxyrisperidone (9‐OH‐RIS), which shows pharmacological activity similar to RIS 6, 7. The sum plasma concentrations of RIS and 9‐OH‐RIS have been referred to as the total plasma active moiety contributing to the clinical effect 8. Because there is high variability in plasma concentration of RIS among patients and within the same patient after oral intake 9, 10, 11, 12, 13, 14, 15, 16, determination of RIS levels in individual patients may be clinically useful to manage a patient's medication regimen and optimize outcome. Early methods for the determination of RIS and 9‐OH‐RIS have mostly used high‐performance liquid chromatography (HPLC) with UV detection 17, 18, 19 or electrochemical detection 20, 21. More recently, several liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) methods have been developed 22, 23, 24, 25, 26. Most of these methods require 200 μl of plasma and around 20 μl for injection. Moreover, these reports did not apply for the pediatric patients with ASD treated with risperidone. In this study, we summarized the development and validation of a sensitive, accurate and specific LC‐MS/MS method, which requires only 50 μl of plasma together with a small‐volume injection (1 μl) to measure the concentration of RIS and 9‐OH‐RIS in plasma of ASD pediatric patients taking RIS oral doses. Furthermore, this study aimed to investigate the relationship between RIS dosages and RIS plasma levels including possible correlation between RIS plasma levels and serum prolactin levels in ASD pediatric patients.
Materials and Methods
Chemicals and reagents
RIS (R 3030), 9‐OH‐RIS (C 6305), and the internal standards (Clozapine, P 0099) were obtained from Sigma‐Aldrich Co. (St. Louis, MO). Acetonitrile (ACN) and methanol (MeOH) were HPLC grade reagents (RCI Labscan limited, BKK, Thailand) and the other reagents, including ammonium acetate and formic acid, were analytical grades (Carlo Erba reagent SAS, Val de Reuil, France). Drug‐free (blank) EDTA plasma was obtained from the Hematology Division, Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, and was stored at −20°C prior to use.
Liquid chromatography and mass spectrometric conditions
The liquid chromatography was an Agilent 1260 HPLC system. The chromatographic system was connected to an API 3200 (Framingham, MA). Chromatographic separation was performed on an Agilent, USA C18 column (4.6 cm × 50 mm; 1.8 μm particle size). Mobile phase A consisted of ammonium acetate (10 mmol/l) containing 0.1% formic acid. Mobile phase B consisted of 100% acetonitrile. Separation of RIS and 9‐OH‐RIS derivatives was performed at a flow rate of 0.40 ml/min. The retention times of RIS, 9‐OH‐RIS, and IS were typically 1.6, 1.5, and 1.7 min, respectively. One sample with small volume (1 μl) was injected every 6 min. The mass spectrometer was operated in the multiple‐reaction monitoring mode (MRM) with the transitions m/z 411–191 for RIS and m/z 428–207 for 9‐OH‐RIS derivative (Fig. 1). The MS/MS conditions, that is, ion fragments, declustering potential (DP), entrance potential (EP), collision energy (CE), cell entrance potential (CEP), cell exit potential (CXP) for RIS, 9‐OH‐RIS, and internal standard (IS), are reported in Table 1. Integration of peak areas and determination of the concentrations was performed with Analyst 1.5.2 software (SCIEX). Quadratic regression with 1/x weighted concentrations was used.
Figure 1.
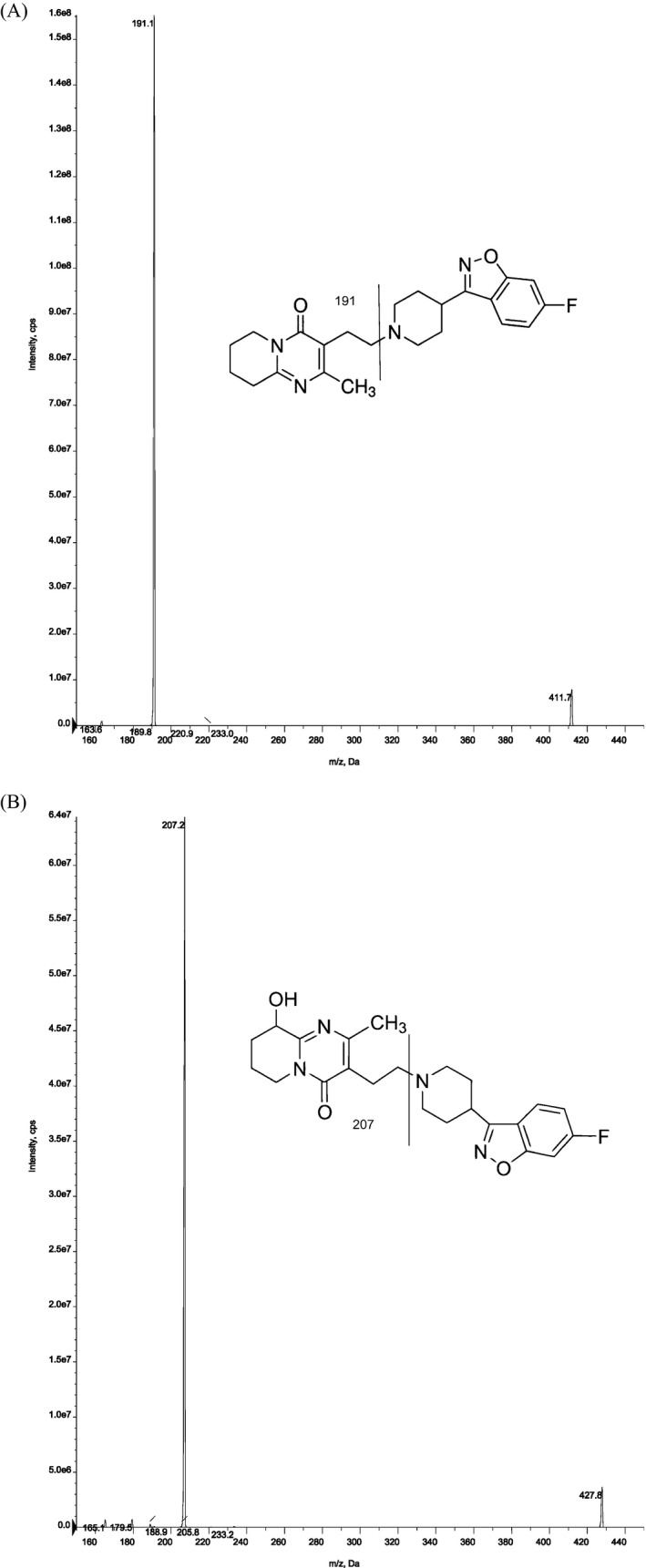
ESI product ion mass spectra for the precursor ions of (a) risperidone, (b) 9‐hydroxy risperidone.
Table 1.
Ion Source and Analyte‐Dependent MS Parameters
| Ion source | |||
|---|---|---|---|
| Spray voltage | 5500.0 | ||
| Capillary temperature | 500.0 | ||
| Curtain gas | 20.0 | ||
| Collision gas | 6.0 | ||
| Polarity mode | Positive | ||
| Ion source Gas1 (GS1) | 40.0 | ||
| Ion source Gas 2 (GS2) | 50.0 | ||
| Analyte‐dependent |
| Risperidone | 9‐OH‐risperidone | Clozapine | |
|---|---|---|---|
| Precursor ion (m/z) | 411.20 | 427.80 | 327.50 |
| Product ion (m/z) | 191.20 | 207.00 | 270.10 |
| DP (V) | 36.43 | 57.97 | 31.87 |
| EP (V) | 2.02 | 3.02 | 3.98 |
| CE (V) | 37.81 | 39.91 | 29.22 |
| CEP (V) | 26.69 | 27.28 | 24.09 |
| CXP (V) | 1.87 | 2.79 | 3.84 |
Preparation of standard solutions
Stock solutions of RIS and 9‐OH‐RIS were prepared in Acetonitrile (ACN) at a free base concentration of 1 mg/ml. The working standard solutions were prepared from stock solutions by dilution with ACN. The working standard solutions were used to prepare the calibration curve and quality control (QC) samples in human plasma. All stock and working solutions were stored at −20°C.
EDTA blank plasma, obtained from Hematology Division, Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, was screened before spike to ensure it was free of endogenous interference at the retention times of RIS, 9‐OH‐RIS and Clozapine (IS). The final concentrations of calibration standards were 0.2, 1, 3, 6, 12, 25, 50, 100 ng/ml for RIS and 0.5, 1, 3, 6, 12, 25, 50, 100 ng/ml for 9‐OH‐RIS. Quality controls were prepared at three concentrations; 0.6 ng/ml, low quality control level (QCL); 40 ng/ml, medium quality control level (QCM) and 80 ng/ml, high quality control level (QCH) for RIS and 1.5 ng/ml, low quality control level (QCL); 40 ng/ml, medium quality control level (QCM) and 80 ng/ml, high quality control level (QCH) for 9‐OH‐RIS. The concentration of the IS working solution was 1,000 ng/ml.
Plasma sample preparation
Blood samples were processed by protein precipitation extraction. A 50 μl aliquot of EDTA‐plasma sample was mixed with 5 μl of internal standard (IS) working solution (1,000 ng/ml of Clozapine). Then, 100 μl of ACN‐MeOH (70:30, v/v) was added to extract the analytes. The mixture was vortexed, then centrifuged at 21,380 g for 5 min to remove the protein pellet. Afterward, 150 μl of supernatant was transferred to another polypropylene tube and dried in a speed vacuum system. Samples were resuspended in 50 μl of a mixture of 0.1% formic acid in ammonium acetate (10 mmol/l) and 100% acetonitrile (50:50, v/v) as the mobile phase, and a volume of 1 μl was injected into the LC–MS/MS system for analysis.
LC‐MS/MS method validation
This method was validated in accordance with the US Food and Drug Administration (US‐FDA) guidelines for bioanalytical method validation 27 as follows:
Selectivity
Selectivity of the method was confirmed by analyzing six different lots of EDTA blank plasma to test for interference at the retention times of RIS, 9‐OH‐RIS, and IS.
Linearity and sensitivity
EDTA‐plasma samples spiked with RIS, 9‐OH‐RIS, and IS working solutions were processed for the construction of calibration curves at eight‐points; 0.2, 1.00, 3.00, 6.00, 12.00, 25.00, 50.00, 100.00 ng/ml for RIS and 0.5, 1.00, 3.00, 6.00, 12.00, 25.00, 50.00, 100.00 ng/ml for 9‐OH‐RIS. The LLOQ was defined as that amount of RIS and 9‐OH‐RIS which gives a signal to noise ratio of 5 and still has accuracy and precision within an acceptable range.
Accuracy and precision
The intra‐ and inter‐day accuracy (%) and precision (%CV) were analyzed by measuring five validation batches, each containing one set of calibration standards and six replicates of LLOQ and QC samples at low, medium, and high levels.
Extraction recovery
The percent extraction recovery of analytes was obtained by comparing the peak area of extracted analytes to the peak area of unextracted standards (standard spiked in extracted blank plasma) in six replicates of each level (low, medium, high) of quality controls. Each of the samples was spiked with IS at working concentration of 1,000 ng/ml.
Stability
The stability of RIS and 9‐OH‐RIS in spiked samples was investigated. The stability experiments aimed to test the effects of possible conditions that the analytes might experience during collection, storage, and analysis. Six aliquots of each QCL and QCH plasma sample were stored at room temperature (bench‐top stability) for 18 h and the stability determined. The processed sample stability was evaluated by comparing the extracted plasma samples that were injected immediately (time 0), with the samples that were re‐injected after storage in the autosampler at 25°C for 24 h. The freeze‐thaw stability was determined after three repeated freeze‐thaw cycles, following the US‐FDA guidelines. Plasma samples were stored at 4°C, −20°C and −80°C to evaluate long‐term stability.
Application of LC‐MS/MS method
One‐hundred and forty‐one Thai ASD pediatric patients who fulfilled the DSM‐IV criteria were enrolled. All the patients had received RIS for more than 1 month. Recommended RIS dosage for pediatric indications from the US‐FDA 28 was adjusted to classify patients into three groups, according to weight; low dose, recommended dose, and high dose. The recommended starting dose of RIS is 0.25–0.5 mg/day if body weight is less than 20 kg or 0.5–1 mg/day if body weight is greater than or equal to 20 kg. If patients took less or more than US‐FDA recommendations, this was classified as low or high dose, respectively. This study was approved by Ramathibodi Ethics Committee, Bangkok, Thailand. After the study was completely described, the parents of all children involved in the study gave informed written consent. Fasting morning blood samples from patients were collected by venipuncture into EDTA blood collection tubes. The blood was immediately centrifuged at ambient temperature, and the plasma layer separated and stored at −20°C until analysis.
Serum prolactin measurement
A fasting morning blood sample was analyzed with a chemiluminescent immunoassay system (IMMULITE1000; Siemens Healthcare Diagnostics Products Ltd, Llanberis, Gwynedd, UK) in the laboratory of Yuwaprasart Waithayopathum Child and Adolescent Psychiatric Hospital.
Statistical analyses
Statistical analyses were performed by using SPSS version 18.0 (SPSS Inc., Chicago, IL). Accordingly, nonparametric data were expressed as median (IQR). A Kruskal–Wallis test was used to make comparisons between the three groups. Spearman rank correlation test was used to measure relationships between two continuous random variables. P values <0.05 were considered statistically significant.
Results
LC‐MS/MS method validation
Selectivity
No endogenous interference was observed at the retention times of the analytes in six different lots of extracted blank plasma (Fig. 2), indicating that the developed LC–MS/MS method is highly selective.
Figure 2.
(a) Blank plasma from six different lots, (b) Blank plasma spiked with IS (100 ng/ml).
Linearity and LLOQ
Eight‐point calibration curves were prepared ranging from 0.2 to 100 ng/ml for RIS and 0.5 to 100 ng/ml for 9‐OH‐RIS. Quadratic regression with 1/x weighted concentrations was used to achieve homogeneity of variance. The calibration curves had highly reproducible correlation coefficients (r ≥ 0.9990) (n = 5) for RIS and 9‐OH‐RIS. The equations of Quadratic regression obtained for this value range were y = −2.07 × 10−4(±−0.0002)x 2 + 0.238(±0.052)x + −0.004(±0.005) (r = 0.9994) for RIS and y = −1.63 × 10−5(±2.27 × 10−5)x 2 + 0.022(±0.003)x + 0.000(±0.0003) (r = 0.9993) for 9‐OH‐RIS. The ranges of the calibration points’ accuracy for RIS and 9‐OH‐RIS were ±15% of the nominal value, and ±20% of the nominal value at LLOQ. LLOQ (signal‐to‐noise ratio ≥5) was 0.2 ng/ml with an accuracy 107.68% and a precision 7.27% CV for RIS and 0.5 ng/ml with an accuracy 101.13% and a precision 7.41% CV for 9‐OH‐RIS (Table 2). These results show the effectiveness of the present LC‐MS/MS method in the assay of RIS and 9‐OH‐RIS from low to high serum levels.
Table 2.
Accuracy and Precision of Determination of Risperidone and 9‐Hydroxyrisperidone in Human Plasma (n = 6)
| Analytes | Nominal conc. (ng/ml) | Intra‐day | Inter‐day | ||||
|---|---|---|---|---|---|---|---|
| Calculated conc. (ng/ml) | Accuracy (%) | Precision (% CV) | Calculated conc. (ng/ml) | Accuracy (%) | Precision (% CV) | ||
| Risperidone | 0.2 | 0.21 ± 0.01 | 104.00 | 3.85 | 0.22 ± 0.01 | 107.68 | 7.27 |
| 0.6 | 0.62 ± 0.02 | 103.28 | 3.17 | 0.64 ± 0.01 | 106.61 | 5.95 | |
| 40 | 41.95 ± 1.95 | 104.88 | 4.65 | 41.91 ± 0.92 | 104.77 | 5.08 | |
| 80 | 80.08 ± 0.08 | 100.10 | 0.10 | 81.06 ± 3.07 | 101.33 | 3.90 | |
| 9‐Hydroxyrisperidone | 0.5 | 0.51 ± 0.01 | 101.77 | 1.74 | 0.50 ± 0.03 | 101.13 | 7.41 |
| 1.5 | 1.47 ± 0.03 | 98.22 | 1.81 | 1.55 ± 0.04 | 103.67 | 4.99 | |
| 40 | 40.20 ± 0.20 | 100.50 | 0.50 | 39.26 ± 0.65 | 98.14 | 2.59 | |
| 80 | 80.13 ± 0.13 | 100.17 | 0.17 | 76.19 ± 3.55 | 95.24 | 5.25 | |
Accuracy and precision
Intra‐day accuracy and precision were determined by the replicate analyses of LLOQ (n = 6) and QC samples at three concentrations (n = 6 for each concentration). All replicates of the QC samples at each concentration level from five separate validation batches were used to evaluate inter‐day accuracy and precision. All intra‐ and inter‐day accuracy (%) and precision (%CV) measurements were within acceptable ranges prescribed by the US‐FDA guidelines for bioanalytical method validation (Table 2.)
Extraction recovery
Six replicates at low, medium, and high quality control concentrations for RIS and 9‐OH‐RIS were prepared for recovery determination. The results of extraction recovery (%) and precision (%CV) for RIS, 9‐OH‐RIS, and IS are shown in Table 3. The results indicate that the extraction efficacy for all the analytes as well as internal standard were consistent and reproducible.
Table 3.
Results of Extraction Recovery (%) (n = 6)
| Analytes | Nominal conc. (ng/ml) | Extraction recovery (%) | CV (%) |
|---|---|---|---|
| Risperidone | 0.6 | 112.62 ± 7.50 | 6.66 |
| 40 | 102.76 ± 6.34 | 6.17 | |
| 80 | 95.01 ± 7.31 | 7.69 | |
| 9‐Hydroxyrisperidone | 1.5 | 114.00 ± 10.35 | 11.76 |
| 40 | 95.73 ± 10.70 | 11.18 | |
| 80 | 90.27 ± 11.15 | 12.35 | |
| Internal standard (IS) | 100 | 90.91 ± 5.33 | 5.86 |
Stability
The stabilities of RIS and 9‐OH‐RIS were investigated at QCL and QCH levels (Table 4). The results reveal that RIS and 9‐OH‐RIS were stable in plasma for at least 18 h at room temperature and 24 h in the autosampler (25°C) (Table 4). Plasma samples were stable over at least three freeze‐thaw cycles (Table 4), indicating that plasma samples can be frozen and thawed at least three times prior to analysis. Long‐term stability experiments reveal that both compounds were stable for 3 months when stored at −20 and −80°C.
Table 4.
Stability Results for Risperidone (RIS) and 9‐Hydroxyrisperidone (9‐OH‐RIS) (n = 6)
| Stability | Risperidone | 9‐Hydroxyrisperidone | ||||
|---|---|---|---|---|---|---|
| Nominal conc. (ng/ml) | Calculated conc. (ng/ml) | Accuracy (%) | Nominal conc. (ng/ml) | Calculated conc. (ng/ml) | Accuracy (%) | |
| Auto sampler stability (24 h, n = 6) | 0.6 | 0.62 ± 0.04 | 100.43 | 1.5 | 1.40 ± 0.05 | 99.17 |
| 80 | 74.05 ± 0.85 | 101.00 | 80 | 72.87 ± 2.64 | 99.07 | |
| Stability of plasma samples at room temperature (18 h, n = 6) | 0.6 | 0.59 ± 0.04 | 95.23 | 1.5 | 1.32 ± 0.03 | 94.08 |
| 80 | 72.62 ± 1.38 | 99.05 | 80 | 74.93 ± 2.63 | 101.88 | |
| Freeze‐thaw stability (n = 6) | ||||||
| −20°C | 0.6 | 0.60 ± 0.03 | 96.50 | 1.5 | 1.437 ± 0.09 | 102.13 |
| 80 | 81.73 ± 6.06 | 111.48 | 80 | 82.1 ± 2.54 | 111.62 | |
| −80°C | 0.6 | 0.60 ± 0.06 | 97.58 | 1.5 | 1.37 ± 0.09 | 97.39 |
| 80 | 83.10 ± 4.80 | 113.34 | 80 | 82.2 ± 2.44 | 111.76 | |
| Long‐term stability (3 months, n = 6) | ||||||
| −20°C | 0.6 | 0.60 ± 0.05 | 96.58 | 1.5 | 1.37 ± 0.08 | 97.51 |
| 80 | 83.98 ± 2.94 | 114.55 | 80 | 83.17 ± 3.88 | 113.08 | |
| −80°C | 0.6 | 0.60 ± 0.03 | 96.79 | 1.5 | 1.49 ± 0.19 | 105.69 |
| 80 | 82.10 ± 5.64 | 111.98 | 80 | 82.73 ± 3.30 | 112.49 | |
LC‐MS/MS method application
Our goals in this study were to develop and validate a highly efficient LC‐MS/MS method to assess RIS levels in ASD pediatric patients and to investigate the relationship between RIS dosages with RIS levels including the effect of RIS treatment on prolactin levels. Based on the RIS dosing group, the patients were divided into three groups (low dose, recommended dose, and high dose). Most patients (52%) were treated with the recommended dose of RIS according to the US‐FDA guidelines (Table 5). The median concentrations of RIS, 9‐OH‐RIS, and active moiety (RIS+9‐OH‐RIS) at the high dose were significantly higher than at the recommended dose and low dose (Table 5). This finding confirmed that RIS doses are significantly positively related to the concentration of RIS, 9‐OH‐RIS, and active moiety (RIS+9‐OH‐RIS) (Table 6 and Fig. 3a). Moreover, our study found statistically significant positive correlations between the concentration of RIS, 9‐OH‐RIS and active moiety (RIS+9‐OH‐RIS) and prolactin levels (Table 6 and Fig. 3b).
Table 5.
Association Between Risperidone Levels (ng/ml) and Risperidone Dosage
| Risperidone dose | No. (n = 141, %) | Risperidone(ng/ml), Median (IQR) | 9‐OH‐Risperidone(ng/ml), Median (IQR) | Active moiety (RIS+9‐OH‐RIS) (ng/ml), Median (IQR) |
|---|---|---|---|---|
| Low dosea | 10 (7.09) | 1.09 (0.69–3.08) | 4.64 (3.02–5.56) | 6.02 (3.76–11.27) |
| Recommended doseb | 73 (51.77) | 2.34 (0.83–7.15) | 7.86 (4.65–13.89) | 10.58 (6.13–21.67) |
| High dosea | 58 (41.14) | 5.60 (1.86–14.46) | 21.20 (15.28–29.14) | 29.41 (22.64–42.79) |
| P‐value | 0.001* | <0.0001* | <0.0001* |
The low or high dose was classified if the patient took less or more than US FDA recommendations.
The recommended dose was classified according to the US FDA recommendations 28 as follows: 0.25–0.5 mg/day if the body weight <20 kg or 0.5–1 mg/day if the body weight ≥20 kg.
*P‐value < 0.05.
Table 6.
Correlation of Risperidone Plasma Levels with Risperidone Dosage and Prolactin Levels
| Plasma levels (ng/ml) | Risperidone doses (mg/dl) | Prolactin levels (ng/ml) | ||
|---|---|---|---|---|
| Correlation coefficient (r s) (n = 141) | P‐value | Correlation coefficient (r s) (n = 141) | P‐value | |
| Risperidone | 0.359 | <0.0001* | 0.314 | <0.0001* |
| 9‐OH‐risperidone | 0.660 | <0.0001* | 0.412 | <0.0001* |
| Active moiety(RIS+9‐OH‐RIS) | 0.642 | <0.0001* | 0.435 | <0.0001* |
*P‐value < 0.05.
Figure 3.
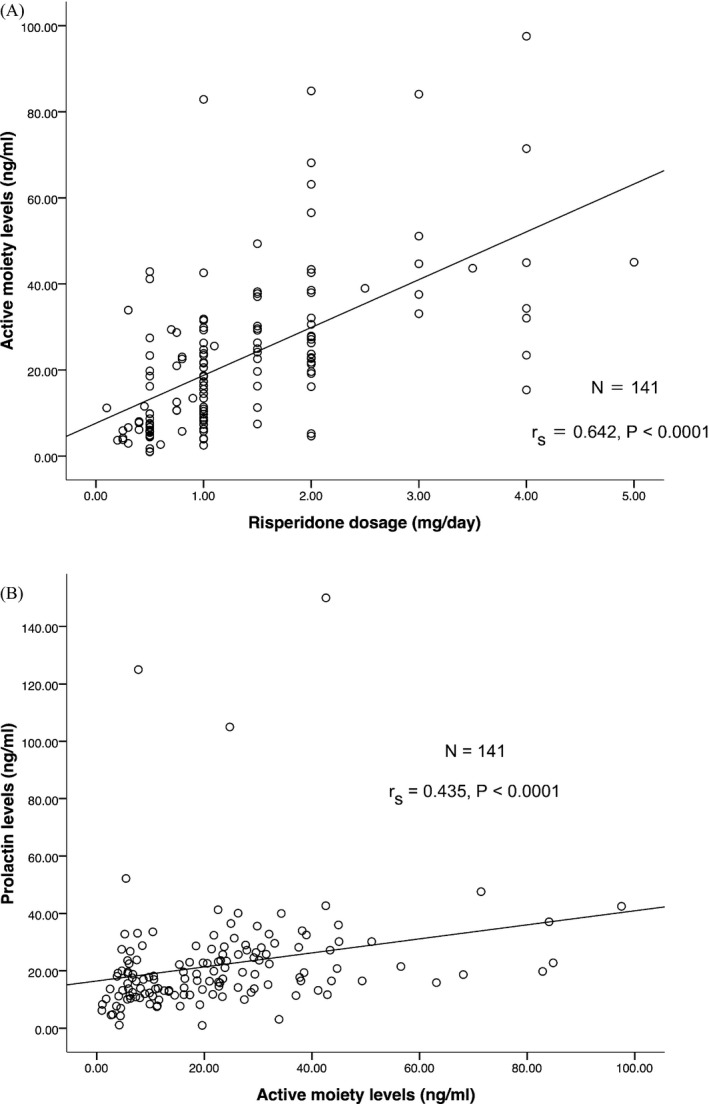
Correlation of active moiety (RIS+9‐OH‐RIS) plasma levels (ng/ml) with (a) risperidone dosage (mg/day), (b) prolactin levels (ng/ml).
Discussion
Different HPLC and LC–MS/MS assays were reported for RIS plasma concentration 18, 22, 23, 24, 25. In comparison to the HPLC method, LC‐MS/MS methods show lower LLOQ concentration 18, 22, 23, 24, 25. Most LC‐MS/MS methods provide LLOQ concentration around 0.2 ng/ml, whereas the HPLC method provides LLOQ concentration at 10 ng/ml. All of these LC‐MS/MS methods require larger plasma volume and larger injection volume than our method. Moreover, these reports are not readily applied to pediatric patients with ASD treated with risperidone. Our study is the first developed and thoroughly validated assay for determining RIS plasma concentration in ASD pediatric patients, and which requires only 50 μl of plasma together with a small‐volume injection (1 μl). The validation of this method (selectivity, linearity, LLOQ, intra‐ and inter‐day accuracy and precision, recovery, and stability) yielded fair results in accordance with previously studies of LC‐MS/MS assays 22, 23, 24, 25. All the variations were within acceptable ranges according to US‐FDA guidelines for bioanalytical method validation 27. The present study provides the LLOQ for RIS and 9‐OH‐RIS at 0.2 and 0.5 ng/ml, which have signal‐to‐noise ratios (S/N) greater than 5. The intra‐ and inter‐day accuracy for both RIS and 9‐OH‐RIS were within 15% of the nominal value. The intra‐ and inter‐day precision were within 15% of the coefficient of variation (CV). These results indicate that our method has desirable sensitivity, accuracy and precision, which is acceptable for monitoring of RIS levels in plasma samples from pediatric patients. It is possible to use small‐volume plasma and small‐volume injection in quantitative determination of risperidone plasma levels. The advantage of using small‐volume plasma and small‐volume injection is essentially to reduce of blood sample volume from pediatric patients. Moreover, it is helpful to require less chemicals and reagents. This method was applied to investigate the relationship between RIS dosages and RIS plasma levels and the relationship between RIS plasma levels and serum prolactin levels. The results indicate that the higher RIS dosage has a tendency to produce higher RIS plasma levels. Moreover, high RIS plasma levels have a tendency to produce hyperprolactinemia. Our findings support the previous studies in children and adults reporting that RIS plays a predominant role in elevating serum prolactin levels 29, 30, 31. Therefore, the determination of RIS levels in the individual patient might be useful predictor of clinical response, such as hyperprolactinemia in pediatric patients. The main limitation of this study is that the data analysis was restricted due to the cross‐sectional design of the study. Therefore, this method was not applied to a pharmacokinetic analysis in pediatric patient. A longitudinal prospective study is necessary to address this shortcoming.
Conclusion
In summary, to the best of our knowledge, this sensitive, accurate, and specific LC‐MS/MS method is the first developed and thoroughly validated assay for ASD pediatric patients treated with risperidone. Our method was sensitive and able to measure accurate drug levels from a small‐volume sample (50 μl), and a small‐volume injection (1 μl). This reduction in the amount of biological sample required for assay is an especially significant improvement for pediatric patients. Our results also suggest that plasma concentration of RIS, 9‐OH‐RIS and their sum at steady state is related to the dosage and prolactin levels. Therefore, we recommend the determination of RIS and 9‐OH‐RIS levels be routinely monitored to adjust the RIS dose and to achieve the optimum clinical outcome as well as to maintain awareness of the potential adverse drug reactions such as hyperprolactinemia.
Acknowledgments
This study was supported by grants of the (a) Pharmacogenomics for Autistic Child Project, Khoon Poom Foundation, The Project of Her Royal Highness Princess Ubonratana Rajakanya Siriwatana Bhanawadee, (b) Office of National Research Council of Thailand (c) Faculty of Medicine Ramathibodi Hospital and (d) Mahidol University. The authors thank all the staff in Yuwaprasart Waithayopathum Child and Adolescent Psychiatric Hospital and all the children and adolescents with ASD who participated in the study. The authors thank all pediatric patients with Autism spectrum disorders who participated in the study. The authors wish to thank Dr. Craig Campbell, The University of Sydney, who assisted in the proof‐reading of this manuscript.
References
- 1. Leysen JE, Gommeren W, Eens A, et al. Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther 1988;247:661–670. [PubMed] [Google Scholar]
- 2. Miller CH, Mohr F, Umbricht D, et al. The prevalence of acute extrapyramidal signs and symptoms in patients treated with clozapine, risperidone, and conventional antipsychotics. J Clin Psychiatry 1998;59:69–75. [DOI] [PubMed] [Google Scholar]
- 3. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: Results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142–1148. [DOI] [PubMed] [Google Scholar]
- 4. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314–321. [DOI] [PubMed] [Google Scholar]
- 5. Bishop JR, Pavuluri MN. Review of risperidone for the treatment of pediatric and adolescent bipolar disorder and schizophrenia. Neuropsychiatr Dis Treat 2008;4:55–68. [PMC free article] [PubMed] [Google Scholar]
- 6. Megens AAHP, Awouters FHL. In vivo pharmacological profile of 9‐hydroxyrisperidone, the major metabolite of the novel antipsychotic risperidone. Drug Dev Res 1994;33:399–412. [Google Scholar]
- 7. Scordo MG, Spina E, Facciola G, et al. Cytochrome P450 2D6 genotype and steady state plasma levels of risperidone and 9‐hydroxyrisperidone. Psychopharmacology 1999;147:300–305. [DOI] [PubMed] [Google Scholar]
- 8. Yasui‐Furukori N, Saito M, Nakagami T, et al. Clinical response to risperidone in relation to plasma drug concentrations in acutely exacerbated schizophrenic patients. J Psychopharmacol 2010;24:987–994. [DOI] [PubMed] [Google Scholar]
- 9. Kang RH, Jung SM, Kim KA, et al. Effects of CYP2D6 and CYP3A5 genotypes on the plasma concentrations of risperidone and 9‐hydroxyrisperidone in Korean schizophrenic patients. J Clin Psychopharmacol 2009;29:272–277. [DOI] [PubMed] [Google Scholar]
- 10. Mannheimer B, von Bahr C, Pettersson H, Eliasson E. Impact of multiple inhibitors or substrates of cytochrome P450 2D6 on plasma risperidone levels in patients on polypharmacy. Ther Drug Monit 2008;30:565–569. [DOI] [PubMed] [Google Scholar]
- 11. Youngster I, Zachor DA, Gabis LV, et al. CYP2D6 genotyping in paediatric patients with autism treated with risperidone: A preliminary cohort study. Dev Med Child Neurol 2014;56:990–994. [DOI] [PubMed] [Google Scholar]
- 12. Riedel M, Schwarz MJ, Strassnig M, et al. Risperidone plasma levels, clinical response and side‐effects. Eur Arch Psychiatry Clin Neurosci 2005;255:261–268. [DOI] [PubMed] [Google Scholar]
- 13. Calarge CA, del Miller D. Predictors of risperidone and 9‐hydroxyrisperidone serum concentration in children and adolescents. J Child Adolesc Psychopharmacol 2011;21:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roke Y, Buitelaar JK, Boot AM, et al. Risk of hyperprolactinemia and sexual side effects in males 10‐20 years old diagnosed with autism spectrum disorders or disruptive behavior disorder and treated with risperidone. J Child Adolesc Psychopharmacol 2012;22:432–439. [DOI] [PubMed] [Google Scholar]
- 15. Seto K, Dumontet J, Ensom MH. Risperidone in schizophrenia: Is there a role for therapeutic drug monitoring? Ther Drug Monit 2011;33:275–283. [DOI] [PubMed] [Google Scholar]
- 16. Aravagiri M, Marder SR, Nuechterlein KH, Gitlin MJ. Intra‐ and interindividual variations in steady‐state plasma concentrations of risperidone and 9‐hydroxyrisperidone in schizophrenic patients treated chronically with various doses of risperidone. Ther Drug Monit 2003;25:657–664. [DOI] [PubMed] [Google Scholar]
- 17. Avenoso A, Facciolà G, Salemi M, Spina E. Determination of risperidone and its major metabolite 9‐hydroxyrisperidone in human plasma by reversed‐phase liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl 2000;746:173–181. [DOI] [PubMed] [Google Scholar]
- 18. Titier K, Deridet E, Cardone E, et al. Simplified high‐performance liquid chromatographic method for determination of risperidone and 9‐hydroxyrisperidone in plasma after overdose. J Chromatogr B Analyt Technol Biomed Life Sci 2002;772:373–378. [DOI] [PubMed] [Google Scholar]
- 19. Nagasaki T, Ohkubo T, Sugawara K, et al. Determination of risperidone and 9‐hydroxyrisperidone in human plasma by high‐performance liquid chromatography: Application to therapeutic drug monitoring in Japanese patients with schizophrenia. J Pharm Biomed Anal 1999;19:595–601. [DOI] [PubMed] [Google Scholar]
- 20. Titier K, Bouchet S, Pehourcq F, et al. High‐performance liquid chromatographic method with diode array detection to identify and quantify atypical antipsychotics and haloperidol in plasma after overdose. J Chromatogr B Analyt Technol Biomed Life Sci 2003;788:179–185. [DOI] [PubMed] [Google Scholar]
- 21. Aravagiri M, Marder SR, Wirshing D, Wirshing WC. Plasma concentrations of risperidone and its 9‐hydroxy metabolite and their relationship to dose in schizophrenic patients: Simultaneous determination by a high performance liquid chromatography with electrochemical detection. Pharmacopsychiatry 1998;31:102–109. [DOI] [PubMed] [Google Scholar]
- 22. Cabovska B, Cox SL, Vinks AA. Determination of risperidone and enantiomers of 9‐hydroxyrisperidone in plasma by LC‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2007;852:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang MZ, Shentu JZ, Chen JC, et al. Determination of risperidone in human plasma by HPLC‐MS/MS and its application to a pharmacokinetic study in Chinese volunteers. J Zhejiang Univ Sci B 2008;9:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lostia AM, Mazzarini L, Pacchiarotti I, et al. Serum levels of risperidone and its metabolite, 9‐hydroxyrisperidone: Correlation between drug concentration and clinical response. Ther Drug Monit 2009;31:475–481. [DOI] [PubMed] [Google Scholar]
- 25. Zhang X, Zhao X, Zhang C, et al. Accuracy Profile Theory for the Validation of an LC–MS‐MS Method for the Determination of Risperidone and 9‐Hydroxyrisperidone in Human Plasma. Chromatographia 2010;71:1015–1023. [Google Scholar]
- 26. De Meulder M, Remmerie BM, de Vries R, et al. Validated LC‐MS/MS methods for the determination of risperidone and the enantiomers of 9‐hydroxyrisperidone in human plasma and urine. J Chromatogr B Analyt Technol Biomed Life Sci 2008;870:8–16. [DOI] [PubMed] [Google Scholar]
- 27. US Department of Health and Human Services . Food and Drug Administration Center for Drug Evaluation and Research. Center for Veterinary Medicine. Guidance for Industry Bioanalytical Method Validation, 2001.
- 28. Janssen Pharmaceuticals, Inc , Risperdal prescribing information, 2014.
- 29. Anderson GM, Scahill L, McCracken JT, et al. Effects of short‐ and long‐term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry 2007;61:545–550. [DOI] [PubMed] [Google Scholar]
- 30. Hongkaew Y, Ngamsamut N, Puangpetch A, et al. Hyperprolactinemia in Thai children and adolescents with autism spectrum disorder treated with risperidone. Neuropsychiatr Dis Treat 2015;11:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hellings JA, Zarcone JR, Valdovinos MG, et al. Risperidone‐induced prolactin elevation in a prospective study of children, adolescents, and adults with mental retardation and pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005;15:885–892. [DOI] [PubMed] [Google Scholar]


