Abstract
Intestinal parasitosis is highly prevalent worldwide, being among the main causes of illness and death in humans. Currently, laboratory diagnosis of the intestinal parasites is accomplished through manual technical procedures, mostly developed decades ago, which justifies the development of more sensitive and practical techniques. Therefore, the main objective of this study was to develop, evaluate, and validate a new parasitological technique referred to as TF‐Test Modified, in comparison to three conventional parasitological techniques: TF‐Test Conventional; Rugai, Mattos & Brisola; and Helm Test/Kato‐Katz. For this realization, we collected stool samples from 457 volunteers located in endemic areas of Campinas, São Paulo, Brazil, and statistically compared the techniques. Intestinal protozoa and helminths were detected qualitatively in 42.23% (193/457) of the volunteers by TF‐Test Modified technique, against 36.76% (168/457) by TF‐Test Conventional, 5.03% (23/457) by Helm Test/Kato‐Katz, and 4.16% (19/457) by Rugai, Mattos & Brisola. Furthermore, the new technique presented “almost perfect kappa” agreement in all evaluated parameters with 95% (P < 0.05) of estimation. The current study showed that the TF‐Test Modified technique can be comprehensively used in the diagnosis of intestinal protozoa and helminths, and its greater diagnostic sensitivity should help improving the quality of laboratory diagnosis, population surveys, and control of intestinal parasites.
Keywords: TF‐Test Modified, parasitological techniques, laboratory diagnosis, parasitology, intestinal parasitosis
INTRODUCTION
Intestinal parasitosis (synonym: enteroparasitosis) is highly prevalent worldwide, present in continents with tropical, subtropical, and equatorial climates, and has led annually thousands of individuals to debilitating conditions, or nevertheless, to deaths 1, 2, 3, 4, 5, 6. In Brazil, for more than a century, parasites of humans’ intestinal tract have become one of the major “public health” problems, mainly since it is a tropical and developing country, which enables favorable conditions for the establishment of these diseases—for example, poor sanitary conditions; inadequate housing; and expressive number of malnourished with poor hygiene, mostly children 7, 8. When present, these parasites imply immense damage to carriers’ health by causing many pathological symptoms such as intestinal mucosal lesions, diarrhea, and reduced absorption of liquids and nutrients, which can contribute to the establishment of organic deficit of both physical and mental origins 9, 10.
As a way of propagation, the fecal bolus of infected hosts contains eggs and larvae of helminths, and trophozoites, cysts, oocysts and spores of protozoa, which are most frequently diagnosed through stool examinations. For this diagnostic purpose, clinical analysis laboratories, both public and private, make use of conventional techniques and/or commercial kits that present several parasitic concentration principles with elimination of fecal debris 11, 12, 13, 14. However, according to scientific literature reports, these kits and techniques, as for diagnostic efficacy, are lower than expected 11, 12, 14, 15, 16. In order to overcome these deficiencies, over the last few years, new parasitological techniques were developed and validated in order to make enteroparasitosis diagnosis efficient and practical 12, 14.
In this circumstance, the TF‐Test Conventional parasitological technique (three fecal tests), created in 2004 with the assistance of the Foundation Research Support of the State of São Paulo (FAPESP, Proc. 99/06228‐4), has been providing effective and practical enteroparasitosis diagnosis for clinical analysis laboratories, especially by collecting three separate stool samples on alternate days (day in, day out) and processing them at once with the laboratory procedure of parasite concentration and elimination of fecal debris by the principle of centrifugal sedimentation 11, 14, 16. Throughout these years, the scientific literature has demonstrated a major diagnostic efficiency of this technique when compared to other conventional techniques and commercial kits 11, 12, 14, 15, 16, 17, 18, 19.
In order to enhance the conventional technique (in this study called TF‐Test Conventional), a new technique was validated, in 2010, sponsored by University of Campinas (UNICAMP), and referred to as TF‐Test Modified 20. This new technique introduces three unprecedented technical/laboratorial principles in its protocol: centrifugal sedimentation, spontaneous flotation, and spontaneous sedimentation. Coelho et al. (16), in a study with dogs in the veterinary parasitology field, demonstrated superiority of 20.63% of diagnostic sensitivity of TF‐Test Modified compared to conventional techniques of Faust et al., TF‐Test Conventional, Willis, and Direct Examination.
In order to prove the diagnostic effectiveness in humans, in the present work, which originated the conclusion of a master's degree dissertation project 19, we developed, evaluated, and validated the new parasitological TF‐Test Modified technique by comparing it with the techniques of TF‐Test Conventional, which, according to the evidence from the literature, demonstrates high range for enteroparasitosis detection; Rugai, Mattos & Brisola, specific for detection of intestinal nematodes larvae; and Helm Test/Kato‐Katz (a qualitative–quantitative kit based on the Kato‐Katz technique, manufactured by Institute of Technology in Immunobiological ‐ Bio‐Manguinhos/Fiocruz, Rio de Janeiro, Brazil), specific for the detection of five species of Nematoda and Trematoda classes, according to the scientific literature 24, 25.
MATERIALS AND METHODS
Volunteers and Study Field
A total of 457 stool samples were obtained in two ways, in enteroparasitosis endemic areas: (i) from 185 children aged between 9 months and 7 years, who were enrolled in two Municipal Elementary Schools (EMEI, in Portuguese) located in the southwestern region of Campinas, São Paulo, Brazil, and (ii) from 272 individuals aged between 4 and 72 years, these being users of public Basic Health Units (UBS, in Portuguese) located in the south and southeastern regions in the municipality of Campinas, São Paulo, Brazil.
Specimen Collection
In this step, the fecal samples were obtained in accordance with the operational protocols of each parasitological technique, as practiced in public and private laboratories. Thus, the volunteers were instructed to collect at home three stool samples on alternate days (day in, day out), not exceeding 10 days. Samples were collected in TF‐Test collector tubes for subsequent laboratory processing of TF‐Test Conventional and TF‐Test Modified techniques.
For the both other conventional techniques (Rugai, Mattos & Brisola and Helm Test), at the last collecting day of the TF‐Test tube, one collection was conducted separately in a universal container. After performing all collections, the samples were conducted to above‐mentioned municipal sectors (EMEI and UBS), where they were gathered and sent for processing at two clinical laboratories located at the University of Campinas (UNICAMP): Laboratory of Parasitology of the Animal Biology Department and Laboratory of Visual Informatics (LIV, in Portuguese) in Biomedical and Health of the Institute of Computing. Due to their distinct physical structures, the same team of researchers had to work in these two laboratories, processing TF‐Test Modified and TF‐Test Conventional in the Laboratory of Visual Informatics, and Rugai, Mattos & Brisola and Helm Test/Kato‐Katz in the Laboratory of Parasitology.
Parasitological Techniques
The TF‐Test Conventional technique was processed in laboratory according to the operational protocol defined in its patent application (INPI PI0104372‐2, 2001) 20. In turn, the processing of the TF‐Test Modified technique followed the operational protocol described in its patent application (PCT/BR2010/000340, 2010) 21, presented below.
The collected, homogenized, and preserved fecal material delivered to the laboratory had an ethyl acetate based solvent liquid (CH3COOCH2CH3) added for delipidation and separation of fecal debris, and afterward a centrifugal sedimentation principle was used in the parasitological technique. Soon after, about 600 μl of fecal suspension (sediment plus physiological saline) was obtained in the appropriate conical tube of the parasitological technique. This suspension was homogenized and separated into two parts (tubes) with approximately 300 μl each, for further laboratory processing.
In the first tube, or process, a spontaneous‐flotation technique was performed to detect protozoan cysts and light‐weight helminth eggs with specific densities inferior to the reagent solution used in this step, which was zinc sulfate (ZnSO4) based with specific gravity of 1.17 g/ml. This reagent solution was added in the tube to establish a meniscus on the top edge. In this region, a microscope slide was placed over the meniscus, so that it came in contact with the liquid suspension for a 15‐min period. Subsequently, with a reversing sudden movement, the slide was removed from the tube's edge and transferred to an appropriate place. A drop of Lugol‐based staining solution was added to the slide and it was analyzed under a microscope.
For the second tube, or processing, a spontaneous‐sedimentation technique was performed, with elimination of fecal debris in order to detect heavy structures, in other words, with specific densities greater than 1.17 g/ml. To this tube, three drops of a compound solution (composition = NaOH + NaCl + NaClO + water with active chlorine content) were added. This new suspension was homogenized for 30 sec and rested for 5 min. Subsequently, 3 ml of 7.5% neutral buffered formalin solution was added to this medium. This new suspension went through another homogenization and finally received the addition of 3 ml of ethyl acetate solvent liquid. A new homogenization was established, this time vigorously, for 30 sec, and the tube remained still for a 15‐min period, until most of fecal debris floated by specific gravity difference. The supernatant was carefully removed from top to bottom, so that a 0.5 ml fecal suspension was left in the tube. Afterward, four drops of saline were added to the suspension and so all material went through new homogenization. Two drops of the suspension were suctioned and conducted to a microscope slide. Over this material, a staining Lugol‐based solution was added. This stain solution was prepared with glycerin–water in the proportion of 12 volumes/drops of water (6 volumes of distilled water to 2 volumes of glycerin—glycerol or propane‐1,2,3‐triol—buffered) to 8 volumes/drops of Lugol. Finally, the slide and coverslip were conducted to microscope for the second microscopic analysis. Helm Test/Kato‐Katz and Rugai, Mattos & Brisola techniques were performed according to their operational protocols 13, 22.
Research Ethics
This study was analyzed and approved by the Research Ethics Committee of the Faculty of Medical Sciences, UNICAMP (proc. no. 570/2011), and received the judgment of the Health Municipal Secretary of Campinas on medical monitoring of positive‐diagnosed cases of volunteers for intestinal parasites.
RESULTS
Among the stool samples of 457 volunteers, 1,828 tests were performed by the four parasitological techniques resulting in detection of 14 species, from which six were related to protozoa and eight related to intestinal helminths (Tables 1, 2, 3). The sum (union) of positive cases of all four techniques was 308 parasitic structures, including cysts, eggs, and larvae. Among them, 303 were identified by TF‐Test Modified, 256 by TF‐Test Conventional, 23 by Helm Test, and 19 by Rugai, Mattos & Brisola technique.
Table 1.
Number of Parasitic Species Detected in the Study of 457 Individuals by TF‐Test Modified and TF‐Test Conventional Parasitological Techniques
| Parasitic species | TF‐Test Modified | TF‐Test Conventional |
|---|---|---|
| Protozoa | ||
| Blastocystis hominis | 84 | 61 |
| Entamoeba coli | 35 | 34 |
| Endolimax nana | 42 | 41 |
| Iodamoeba bütschlii | 5 | 5 |
| Giardia duodenalis | 25 | 25 |
| Entamoeba histolytica/E. dispar | 14 | 12 |
| Helminths | ||
| Ascaris lumbricoides | 21 | 20 |
| Trichuris trichiura | 7 | 5 |
| Ancylostomatidae | 12 | 8 |
| Strongyloides stercoralis | 25 | 22 |
| Schistosoma mansoni | 14 | 10 |
| Hymenolepis nana | 2 | 1 |
| Enterobius vermicularis | 13 | 8 |
| Taenia spp. | 4 | 4 |
| Total | 303 | 256 |
Table 2.
Number of Helminths Detected by TF‐Test Modified, TF‐Test Conventional, and Helm Test/Kato‐Katz Parasitological Techniques, Considering Only the Species Detected by Helm Test/Kato‐Katz
| Parasitic species | TF‐Test Modified | TF‐Test Conventional | Helm Test/Kato‐Katz |
|---|---|---|---|
| Helminths | |||
| Ascaris lumbricoides | 21 | 20 | 12 |
| Trichuris trichiura | 7 | 5 | 1 |
| Ancylostomatidae | 12 | 8 | 0 |
| Schistosoma mansoni | 14 | 10 | 9 |
| Taenia spp. | 4 | 4 | 1 |
| Total | 58 | 47 | 23 |
Table 3.
Positivity and Type of Infection Recorded in the Study of 457 Individuals by Four Different Parasitological Techniques
| Individuals per type of infection | |||||
|---|---|---|---|---|---|
| Technique | Infected individuals No. | Simple | Double | Triple | Multiple |
| TF‐Test Modified | 193 | 105 | 70 | 15 | 3 |
| TF‐Test Conventional | 168 | 98 | 54 | 14 | 2 |
| Helm Test/Kato‐Katz | 23 | 23 | 0 | 0 | 0 |
| Rugai, Mattos & Brisola | 19 | 19 | 0 | 0 | 0 |
The numbers expressed in Table 1 indicate 98.38% (303/308) of diagnostic sensitivity for the new TF‐Test Modified technique, against 83.12% (256/308) for TF‐Test Conventional. Considering only the species detected by the Helm Test/Kato‐Katz technique (Table 2), the total number of infections was 58, resulting in a diagnostic sensitivity of 100% (58/58) for the TF‐Test Modified technique, 81.03% (47/58) for TF‐Test Conventional, and 39.66% (23/58) for Helm Test. For the specific qualitative detection of Schistosoma mansoni eggs, TF‐Test Modified detected 14 individuals with this parasitosis, and Helm Test detected the eggs in only nine individuals. For the specific detection of Strongyloides stercoralis larvae, with 26 positive individuals in total, the new technique was more efficient than Rugai, Mattos & Brisola, detecting six more parasitic structures: 25 cases for the former against 19 for the latter technique.
In turn, according to Table 3, the new TF‐Test Modified technique identified 42.23% (193/457) of infected volunteers, followed by TF‐Test Conventional; Helm Test; and Rugai, Mattos & Brisola techniques, which were able to detect parasitism in 36.76% (168/457), 5.03% (23/457), and 4.16% (19/457) of the volunteers, respectively.
When considering the diagnostic accuracy for the detection of all parasitic species, and the sum of positive cases of all four techniques as reference, TF‐Test Modified exhibited high kappa index (0.975), “almost perfect” degree of agreement, high concordance index (CI) that ranged from 0.954 to 0.997 with 95% (P < 0.05) of estimate, and standard deviation (SD) value of 0.011. The diagnostic efficacy of TF‐Test Conventional showed moderate kappa index (0.762), a “substantial” degree of agreement, which ranged from 0.703 to 0.822 with an estimate of 95% (P < 0.05), and SD value of 0.030.
Considering only the species detected by the Helm Test/Kato‐Katz technique (Ascaris lumbricoides, Trichuris trichiura, Ancylostomatidae, Schistosoma mansoni, and Taenia spp.) 24, 25, and the sum of positive cases of these species in all four techniques as reference, TF‐Test Modified showed a high kappa concordance index (1.000), almost perfect rank, and 95% confidence interval of 1.000—being superior than Helm Test/Kato‐Katz that exhibited a kappa concordance index of 0.534, moderate rank, 95% confidence interval from 0.403 to 0.666, and SD value of 0.067.
Considering the specific identification of Strongyloides stercoralis larvae, and the sum of positive cases of this species in all four techniques as reference, the TF‐Test Modified technique showed a kappa concordance index of 0.979, almost perfect rank, 95% confidence interval from 0.939 to 1.000, and SD value of 0.021—being better than the Rugai, Mattos & Brisola technique that exhibited a kappa index of 0.837, almost perfect rank, 95% confidence interval from 0.718 to 0.955, and SD value of 0.060.
DISCUSSION
The high positive findings rate, 43.33% (198/457), was certainly due to three basic factors present in this current study's regions: population's poor hygiene conditions, lack of sanitary service, and inadequate housing. Also, the high positivity rate can be assigned by the use of a high diagnostic efficacy technique (TF‐Test Modified).
Ecological environment provided by the studied regions assuredly has been allowing, through the years, the perfect propagation of enteroparasitosis among its residents. Despite the high number of helminth species found in our study, the protozoa were those that demonstrated greater intensity of infection in volunteers by eliminating 209 (67.86%) cystic structures, against 99 (32.14%) of helminths, when considering the positive results of all techniques. More than 95% of the samples were collected and returned correctly to the responsible for municipal authorities involved in the study, which, regardless of the social‐economic condition, reinforces the conception that any citizen, exceedingly when well informed, would have the ability to perform a good stool sample collection, regardless of the recommended number of days and quantity (volume) of sampling. Gomes et al. reached the same conclusion in a study accomplished with 1,244 individuals with low purchasing power in four distinct regions of the state of São Paulo.
Considering the results in a comprehensive and qualitative way, the TF‐Test Modified technique was more effective than TF‐Test Conventional in 25 (193 − 168) infected volunteers. The new technique excelled when compared to Helm Test/Kato‐Katz and Rugai, Mattos & Brisola in the specific detection of S. mansoni eggs (Table 2) and S. stercoralis larvae, with five 14 − 9 and six 25 − 19 more infected volunteers, respectively. These numbers demonstrate the high capacity of the new technique to concentrate parasitic structures.
Moreover, the new technique presented greater number of parasitological infection type when compared to other conventional techniques, for monoparasitism (single) and poliparasitism (double, triple, and multiple, e.g.) cases, especially by demonstrating broad diagnostic range characteristics. Also, the new technique was able to present high intensity of infection for intestinal parasites, which reflected in 98.38% (303/308) of total infection. These numbers are able to express how an endemic region for this parasitosis type is compromised when low and moderate diagnostic efficacy techniques are utilized. This reinforces the need of using broader techniques, not only in clinical laboratories’, both public and private, routine but also in programs of municipal, state, and federal scope.
The TF‐Test Modified technique stood out when compared to TF‐Test Conventional for the detection of four species of protozoa, and obtained the same detection number for Giardia duodenalis (synonyms: G. lamblia and G. intestinalis) and Iodamoeba bütschlii (Table 1). Figure 1 shows a vacuolar shape of the B. hominis parasite obtained by TF‐Test Modified technique. This test was able to precisely identify the resistance structures of this parasite species, by utilizing, in its operational protocol, neutral formalin preservative solution based liquid in collection and parasite concentration procedure with fecal debris elimination.
Figure 1.
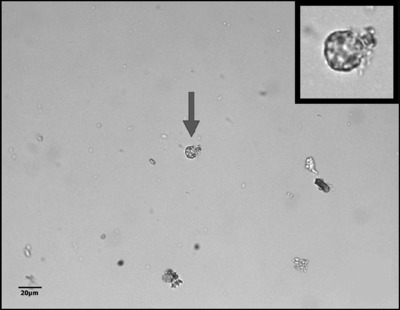
Image of a vacuolar structure of B. hominis stained with Lugol's iodine, indicated by arrow and frame, obtained by the TF‐Test Modified technique in the spontaneous‐flotation step, with little fecal debris.
Currently, B. hominis parasite is considered by some authors 13, 18, 19 as the most prevalent among human intestinal protozoan infections, but its finding has become unusual due to lack of professional microscopy practice to identify their various structures, which reflects in diagnostic interpretation error. In the laboratory, TF‐Test Modified allowed the preparation of temporary smears stained with Lugol's iodine solution, with a high concentration of this parasite species, and largely free of fecal debris (Fig. 1). The microscope slide with little fecal debris along with the parasitic morphology favored the identification of this species without the use of permanent smears with specific stains, as suggested by the scientific literature.
Similarly, the same characteristic image was observed by this new technique for the finding of other protozoa species, for example, G. duodenalis, E. coli, E. nana, I. bütschlii, and E. histolytica/E. dispar. The sharpness on which the protozoa structures were visualized in the image field obtained by the TF‐Test Modified technique certainly has been attributed to spontaneous‐flotation stage utilizing ZnSO4 saturated solution, which was responsible for the elimination of most fecal debris. It is worth emphasizing that excessive fecal debris on a microscope slide can promote false‐negative and/or false‐positive results.
For the helminth finding, more precisely to detect eight parasitic species, the TF‐Test Modified demonstrated, in a qualitative way, diagnostic superiority when compared to TF‐Test Conventional, with the detection of 20 more positive cases, and still only equaling with this conventional technique for Taenia spp. eggs concentration, even though the parasitological stool analysis not being recommended as a tool for the detection of this parasite species (Table 1).
From our experience, we observed that many helminth eggs presented specific densities lower than 1.18 g/ml, for example: Ascaris lumbricoides (fertile), Ancylostomatidae, Hymenolepis nana, and Enterobius vermicularis. Literature reports that the densities of these eggs may range from 1.10 to 1.15 g/ml 13 and were obtained in high concentration by the TF‐Test Modified due to the utilization of spontaneous‐flotation step using a zinc sulfate saturated solution at 1.17 g/ml (Fig. 2), so as to overcome TF‐Test Conventional in diagnostic efficacy (Table 1). In a different situation, our studies have also shown that some helminth species presented specific densities greater than 1.18 g/ml, such as, in cases of A. lumbricoides (infertile); Trichuris trichiura, S. mansoni, and Taenia spp. eggs; and S. stercoralis larvae. In this circumstance where the literature estimates that these species‐specific densities might vary from 1.19 to 1.25 g/ml, the new parasitological technique also demonstrated a qualitative diagnostic superiority when compared to TF‐Test Conventional (Table 1) due to the use of spontaneous‐sedimentation stage and a compound solution to eliminate debris and parasitic concentration.
Figure 2.
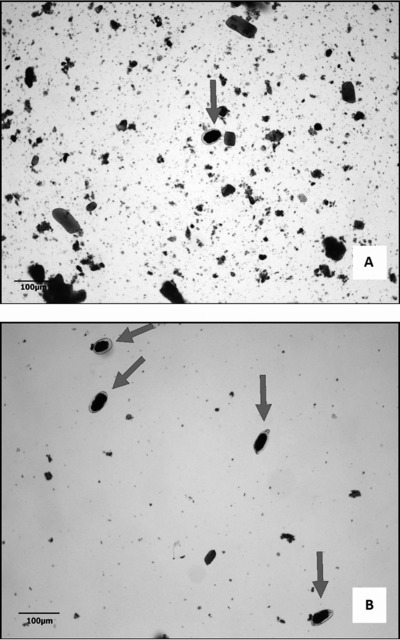
Ancylostomatidae eggs stained with Lugol's iodine. (A) Egg obtained by the TF‐Test Conventional technique, with 10× objective magnification. (B) Eggs obtained by the TF‐Test Modified technique in the spontaneous‐flotation step with 10× objective magnification.
In the qualitative and specific diagnosis assessment of S. stercoralis larvae, the TF‐Test Modified exhibited high diagnostic sensitivity of 96.15% (25/26), according to Table 3. The sensitivities demonstrated by the other techniques were 84.15% (22/26) for TF‐Test Conventional and 73.08% (19/26) for the specific technique of Rugai, Mattos & Brisola. These numbers express the necessity of utilizing a technique characterized by multiple stool sample collection and parasitic concentration to detect S. stercoralis larvae, which have irregular biological cycle for their elimination in feces, so as to resemble with the disharmonic elimination shown by protozoa of vertebrate's intestinal lumen 13.
Figure 3A and B exhibits images of S. stercoralis larvae obtained by the laboratory processing of two parasitological techniques using stool sample from the same volunteer. We can observe that the specific technique of Rugai, Mattos & Brisola (Fig. 3A) demonstrated an image field with less fecal debris, although with lower larvae concentration (nine larvae per field) when compared with TF‐Test Modified technique (more than 40 larvae per field). This can be explained by the fact that the conventional and specific technique is performed by a single stool sample collection and does not contain a centrifugation step toward a higher larvae concentration. The slide obtained by the new technique exhibited a field with high larvae concentration and moderate fecal debris (Fig. 3B).
Figure 3.
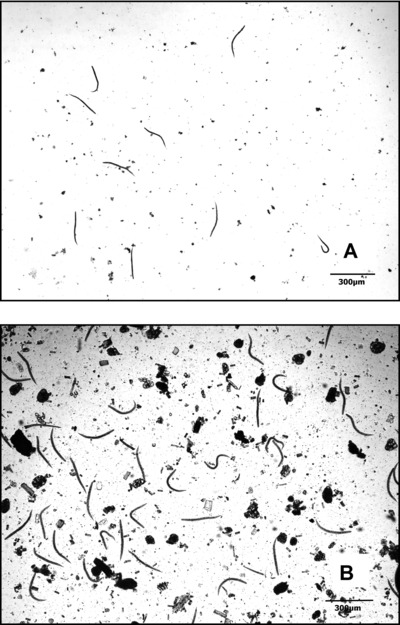
S. stercoralis larvae identified by different parasitological techniques and stained with Lugol's iodine. (A) Larvae detected by Rugai, Mattos & Brisola with 10× objective magnification. (B) Larvae obtained by TF‐Test Modified in the spontaneous‐sedimentation step with 10× objective magnification.
Still in a specific way, an evaluation of qualitative diagnosis performance was applied for the recognition of another parasitic species: S. mansoni (Fig. 4A and B). In this circumstance, the new TF‐Test Modified parasitological technique detected four more positive cases than TF‐Test Conventional, and detected five more positive cases than Helm Test/Kato‐Katz (Table 2). These results revel that the specific and conventional Kato‐Katz technique, despite being widely used in governmental programs, may be lower than expected in diagnostic efficiency, especially for lower eggs eliminations by S. mansoni female parasite, a condition often found in areas of low endemicity of S. mansoni infection. To reinforce our review, in the study performed by Berquist et al. 23, in 2009, the authors concluded that the Kato‐Katz technique was able to concentrate nearly 50% of the eggs in endemic regions with low endemicity characteristic for this parasite. Kongs et al. 24, in 2001, reached to a similar conclusion in a study conducted with 1,255 individuals in an endemic area for S. mansoni. In this same condition, Luis Rey 25 refers loss up to two‐thirds in stool examinations when performed by Kato‐Katz technique, mainly due to the fact that this method is capable of processing only about 50 mg of feces in the laboratory. It is worth emphasizing that, according to the operational protocol, the conventional and modified TF‐Test techniques, individually, are capable to process about 5 g of feces at once, in other words, and almost 100 times more fecal material than Kato‐Katz technique. Furthermore, our findings allow us to realize that depending on the collected stool consistency, for example, semi‐pasty and/or liquid, with very dark coloring and overly withered, it can become an obstacle for detection of S. mansoni eggs by the conventional Kato‐Katz technique.
Figure 4.
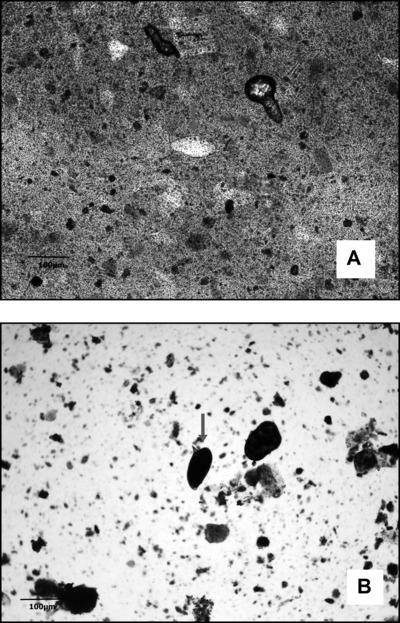
Images of S. mansoni eggs. (A) Egg obtained through Kato‐Katz/Helm Test, with 10× objective magnification. (B) Egg obtained by TF‐Test Modified in the spontaneous‐sedimentation step, with 10× objective magnification and stained with Lugol's iodine.
The TF‐Test Modified technique presented high kappa coefficient and an almost perfect agreement in all qualitative evaluations: demonstrated index of 0.975 for comprehensive evaluation; index of 1.000 for the helminth species detected by Helm Test/Kato‐Katz, including S. mansoni eggs, and index of 0.979 for the specific assessment of S. stercoralis larvae. In general, the new parasitological TF‐Test Modified technique allowed the finding, with high diagnostic efficiency, of all parasitic structures studied, and presented a remarkably reliable diagnosis, especially when analyzed comparatively with relevant techniques from literature, providing a suitable applicability in clinical laboratories’, whether public or private, routine.
The current study leads to the conclusion that among the four different evaluated parasitological techniques, the new TF‐Test Modified revealed, qualitatively, high diagnostic efficacy results, presenting microscopic slides with high parasite concentration and lesser quantities of fecal debris. The modifications added to this new diagnostic technique provided high diagnostic values, increasing the sensitivity in 15.26% when compared to the second‐best technique: TF‐Test Conventional.
Lastly, the present study demonstrated that the TF‐Test Modified technique can be comprehensively used in qualitative diagnosis of humans’ intestinal helminths and protozoa. The gain in diagnostic sensitivity provided by this new technique should have an estimable contribution to the laboratorial diagnosis, population surveys, and intestinal parasites control, in order to reflect on social contribution.
REFERENCES
- 1. Britton E, Hales S, Venugopal K, Baker MG. The impact of climate variability and change on cryptosporidiosis and giardiasis rates in New Zealand. J Water Health 2010;8:561–571. [DOI] [PubMed] [Google Scholar]
- 2. Bacon KM, Shah M, Taylor L, Macatangay BJ, Veldkamp P, Belizario VY Jr. Assessment of a school‐based mass treatment for soil‐transmitted helminth infections in Capiz, the Philippines. Southeast Asian J Trop Med Public Health 2012;43:589–600. [PubMed] [Google Scholar]
- 3. Cañete R, Díaz MM, Avalos García R, Laúd Martinez PM, Manuel Ponce F. Intestinal parasites in children from a day care centre in Matanzas City, Cuba. PLoS One 2012;7:e51394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palacios MJ, Valera F, Barbosa A. Experimental assessment of the effects of gastrointestinal parasites on offspring quality in chinstrap penguins (Pygoscelis antarctica). Parasitology 2012;1390:819–824. [DOI] [PubMed] [Google Scholar]
- 5. Amare B, Ali J, Moges B, et al Nutritional status, intestinal parasite infection and allergy among school children in Northwest Ethiopia. BMC Pediatr 2013;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouchaud O. Circumstances for diagnosis and treatment of intestinal parasitosis in France. Presse Med 2013;42: 84–92. [DOI] [PubMed] [Google Scholar]
- 7. Muniz‐Junqueira MI, Queiroz EF. Relationship between protein‐energy malnutrition, vitamin A, and parasitoses in living in Brasília. Rev Soc Bras Med Trop 2002;35:133–141. [DOI] [PubMed] [Google Scholar]
- 8. Barreto ML, Genser B, Strina A, et al Impact of a citywide sanitation program in Northeast Brazil on intestinal parasites infection in young children. Environ Health Perspect 2010;118:1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ajjampur SS, Koshy B, Venkataramani M, et al Effect of cryptosporidial and giardial diarrhoea on social maturity, intelligence and physical growth in children in a semi‐urban slum in south India. Ann Trop Paediatr 2011;31:205–212. [DOI] [PubMed] [Google Scholar]
- 10. Aronson SM. Poverty, malnutrition and worms. Med Health R I 2011;94:59. [PubMed] [Google Scholar]
- 11. Gomes JF, Hoshino‐Shimizu S, Dias LC, Araujo AJ, Castilho VL, Neves FA. Evaluation of a novel kit (TF‐Test) for the diagnosis of intestinal parasitic infections. J Clin Lab Anal 2004;18:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes JF, Hoshino‐Shimizu S, Falcão, AX . Recentes Avanços Tecnológicos no Exame Parasitológico de Amostras de Fezes. Bio Farma: Rev Téc Cient Farm Bioquím Anal Clín Toxicol 2008;3:44–53. [Google Scholar]
- 13. Garcia LS. Practical Guide to Diagnostic Parasitology, second edition Washington, DC: ASM Press; 2009. [Google Scholar]
- 14. Carvalho GL, Moreira LE, Pena JL, Marinho CC, Bahia MT, Machado‐Coelho GL. A comparative study of the TF‐Test®, Kato‐Katz, Hoffman‐Pons‐Janer, Willis and Baermann‐Moraes coprologic methods for the detection of human parasitosis. Mem Inst Oswaldo Cruz 2012;107:80–84. [DOI] [PubMed] [Google Scholar]
- 15. Hoshino‐Shimizu S, Gomes JF, Dias LCS, Araujo AJUS, Castilho VL, Neves FAMA. Detecção de Enteroparasitoses em Amostras Fecais Provenientes de Diferentes Localidades do Estado de São Paulo, Utilizando a Técnica de TF‐Test. Rev Bras Anal Clín 2003;35:46. [Google Scholar]
- 16. Coelho WMD, Gomes JF, Lumina G, et al A new technique for the diagnosis of gastrointestinal parasites in dogs. Rev Bras de Parasit Vet 2013;22:1–5. [DOI] [PubMed] [Google Scholar]
- 17. Gomes JF, Suzuki CTN, Papa JP, Hoshino‐Shimizu S, Falcão, AX . Toward automation of the diagnosis of enteroparasitosis via computational image analysis In XII International Congress of Parasitology ICOPA, Melbourne, 2010. pp. 169–174. [Google Scholar]
- 18. Rebolla MF. Inquérito parasitológico, comparação de técnicas de diagnóstico fecal, controle e prevenção de Giardia em creches e pré‐escolas, São Sebastião da Grama, São Paulo. M.S. Thesis, Institute of Biology, University of Campinas – UNICAMP, SP, Brazil, 2012. http://www.bibliotecadigital.unicamp.br/document/?code=000876870 (accessed Apr 28, 2015).
- 19. Carvalho JB. Avaliação de uma nova técnica (TF‐Test Modified) destinada ao diagnóstico de parasitoses intestinais em amostras fecais M.S. Thesis, Institute of Biology, University of Campinas – UNICAMP, SP, Brazil, 2013. http://www.bibliotecadigital.unicamp.br/document/?code=000914761 (accessed Apr 28, 2015).
- 20. Hoshino‐Shimizu S., Gomes JF, Dias LCS. Multiple Collection Kit for Stool Laboratory Examination (International application PCT/BR/WO/014705), 2003, EUA. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2003014705 (accessed Apr 28, 2015).
- 21. Falcão AX, Gomes JF, Hoshino‐Shimizu S, Suzuki CTN. Method for Preparing a Faecal Coproparasitological Specimen and Clarifying Composition. (International application PCT/BR2010/000340), 2010, EUA. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2011044649 (accessed Apr 28, 2015).
- 22. Katz N, Coelho PMZ, Pellegrino J. Evaluation of Kato's quantitative method through the recovery of Schistosoma mansoni eggs added to human feces. J Parasitol 1970;56:1030–1033. [PubMed] [Google Scholar]
- 23. Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: What tools to use and when? Trends Parasitol 2009;25:151–156. [DOI] [PubMed] [Google Scholar]
- 24. Kongs A, Marks G, Verlé P, van der Stuyft P. Limitations of Kato‐Katz technique for evaluating S . mansoni infections. Trop Med Int Health 2001;6:163–169. [DOI] [PubMed] [Google Scholar]
- 25. Rey L. Parasitologia: Parasitos e doenças parasitárias do homem nos trópicos ocidentais, fourth edition São Paulo: Guanabara Koogan; 2008. [Google Scholar]


